Abstract
Multi-modality imaging is rapidly becoming an essential tool in oncology. Clinically, the best example of multimodality imaging is seen in the rapid evolution of hybrid positron emission tomography (PET)/computed tomography (CT) and single positron emission computed tomography (SPECT)/CT scanners. However, use of multi-modality imaging is prone to artefacts and pitfalls. Important artefacts that may lead to clinical misinterpretation result from the use of CT data to correct for attenuation and the existence of mismatches between the fused images, for example due to respiratory movement. Furthermore, for institutions who proceed from a standalone PET to a hybrid PET-CT, there is an issue of interchangeability between these systems, especially for quantitative studies. Another issue is visualisation: hospital PACS is not sufficiently capable of adequately viewing integrated images. This article reviews and illustrates the most common artefacts and pitfalls that can be encountered in multi-modality nuclear medicine imaging. For correct management of oncological patients it is essential to be able to detect and correctly interpret these artefacts and pitfalls. Therefore, solutions and recommendations to these problems are provided.
Keywords: Multi-modality imaging, PET/CT, pitfalls, artefacts, recommendations
Introduction
Integrating anatomic and functional information potentially allows improved lesion localisation and characterisation. Better diagnostic accuracy and staging are fundamental in the management of oncological patients and can directly influence therapeutic decisions. Hence, multimodality imaging is an important achievement in the fields of oncology, nuclear medicine, radiology and radiation oncology.
In the past few years there has been a strong migration from dedicated positron emission tomography (PET) scanners to hybrid positron emission tomography/computed tomography (PET/CT) scanners in hospitals worldwide. These integrated systems have led to more accurate information in investigations using [18F]fluorodeoxyglucose (FDG), in particular with regard to lesion localisation[1–3] and in radiotherapy treatment planning[4,5]. Also single positron emission computed tomography (SPECT) systems combined with CT have gained in popularity within the nuclear medicine community.
CT-based attenuation correction is usually performed with combined PET/CT or SPECT/CT systems. As CT scans are more rapid than conventional transmission scans using electronically windowed rotating rod sources such as germanium-68/gallium-68 (Ge-68/Ga-68), this reduces the time needed for overall whole-body PET scanning by up to 50%[6].
However, especially due to the use of CT for attenuation correction and movement of (organs of) patients, the use of multi-modality systems in clinical practice is not free from pitfalls and imaging artefacts. Moreover, when patients get follow-up PET scans on both a standalone PET and PET-CT, this may lead to misinterpretation in semi-quantitative studies due to a difference in attenuation correction. Since an increasing number of institutions will proceed from a standalone PET to a hybrid PET-CT scanner, this issue becomes increasingly relevant. Furthermore, current standard picture archiving and communication systems (PACS) do not provide adequate and reliable tools for visualisation of multi-modality image sets, which prevents hospital-wide reviewing and demonstration of image fusion. Finally, multi-modality imaging implies an increase in imaging data. Optimised protocols have to prevent an overwhelming volume of data being presented to referring specialists.
In this article, we review the imaging artefacts and pitfalls that can be encountered when using combined PET/CT or SPECT/CT systems. Furthermore, we quote solutions and recommendations on how to deal with these issues and how to apply multi-modality systems into the multi-disciplinary environment of a hospital.
Imaging artefacts and pitfalls
Inevitably, the technology of hybrid PET/CT suffers from artefacts of its own[7,8]. Artefacts may occur on CT images due to metallic implants, contrast agents and truncation. When CT data are used for scatter and attenuation corrections of the PET images, these artefacts can have subsequent effects in PET/CT images. Furthermore, due to patient movement or respiratory movement, additional imaging problems such as misregistration or erroneous attenuation correction, may occur.
CT-induced artefacts
Due to high photon absorption, metallic implants such as dental fillings or hip prosthetics, generate streaking artefacts on CT images[9,10]. As attenuation for electron dense areas at CT energies (up to 140 keV) is typically an order of magnitude higher than attenuation at the PET photon energy (511 keV), an overestimation of PET activity can be expected in those areas after CT-based attenuation correction. Hence, this may lead to a false-positive PET finding. An illustration of this phenomenon is given in Fig. 1. The same type of artefacts and PET misinterpretation may result from the use of CT contrast agents such as iodine and barium sulphate, which are used to delineate vessels and soft tissue on CT[11–13]. The magnitude of the error induced by CT contrast agents in the corrected PET images is not significant in many cases in clinical practice[14–16]. Because of the more similar photon energies of CT and SPECT radiopharmaceuticals, the issues of these CT-induced artefacts are much less of a problem than they are for PET/CT.
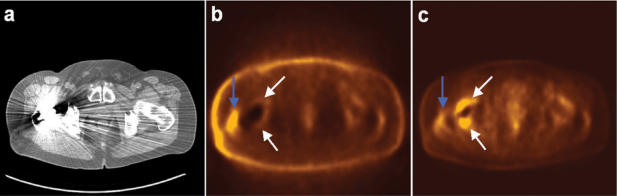
Figure 1.
Hybrid PET/CT (Siemens LSO Biograph 2, Siemens Medical Solutions, USA) of the hip area. Shown are transverse slices of CT (a), uncorrected PET (b) and attenuation-corrected PET (c). The hip prosthetic results in severe artefacts on CT, which subsequently results in a seemingly high uptake on the corrected PET (white arrows), not present on the uncorrected PET. The PET hot-spot at the far right side of the patient (blue arrow) is a true-positive (bursitis).
Awareness of these possible artefacts is the first step in dealing with them. It is recommended to always use PET images that are not corrected for attenuation (uncorrected PET), as they never manifest these types of artefacts. They should be interpreted in conjunction with the corrected images. Furthermore, algorithms are proposed to properly transform CT attenuation coefficients in the electron dense regions from CT energies to 511 keV. In general, segmenting the region of a CT dense area and replacing it by lower CT numbers, forms the basis of these algorithms[13,17].
Truncation artefacts may also occur[18,19] when the field of view (FOV) of the PET is larger than the FOV of the CT[20]. These artefacts arise when positioning patients away from the in-plane centre of the PET/CT gantry or when imaging large patients. As a patient extends beyond the CT FOV, no attenuation values exist for the PET data in the corresponding region. This results in an underestimation of activity concentration on the attenuation-corrected PET images, potentially resulting in misinterpretation of the PET scan.
An algorithm to compensate for truncation artefacts on corrected PET images is suggested by Beyer et al.[18]. By artificially creating an extended FOV of the CT, which matches the PET FOV, the truncated regions can be recovered to a high degree.
Motion-induced artefacts
Patient motion leads to artefacts in the reconstructed PET images as there is a difference in acquisition time of the PET and CT. Although use of fixation masks, belts or cushions and good instructions by technologists may help in minimising the overall patient movement, one cannot prevent artefacts due to respiratory movement, as demonstrated by several investigators[20–23]. Mainly in the upper abdomen and thorax one has to be aware of severe problems as a discrepancy exists in the position of organs (and thus of lesions) in those regions between the CT and the uncorrected PET image. PET images are acquired during free breathing, as the acquisition time is relatively long (several minutes per bed position). CT is usually acquired during a specific stage of the breathing cycle. Especially in the region around the diaphragm, where a sharp transition exists on CT between liver and lung tissue, severe misinterpretations may occur[21]. For example, an apparent contour change of the liver on CT-based attenuation-corrected PET images may be observed. In addition, there can be a reduced sensitivity for lesions in the affected area and the PET signal will no longer be quantitative in the regions of attenuation correction artefacts, which may compromise follow-up measurements. An example of such an induced artefact is demonstrated in Fig. 2.
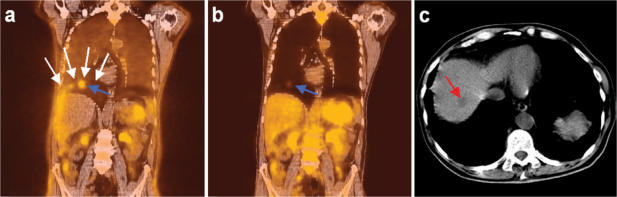
Figure 2.
Hybrid PET/CT (Siemens LSO Biograph 2, Siemens Medical Solutions, USA) of a liver metastasis (blue and red arrows), with CT acquired during inspiration breathhold. Coronal slices of uncorrected PET/CT (a), attenuation corrected PET/CT (b) and a transverse CT slice (c) are shown. A difference in diaphragm position between CT and uncorrected PET (white arrows) is visible (a). (b) demonstrates a change in shape of the liver on PET after attenuation correction, to falsely match CT. The liver metastasis appears in the lung on the corrected PET image (blue arrow), and suffers from severe loss of signal intensity. (c) shows the lesion is within the liver (red arrow).
Without a comprehensive breathing protocol, motion artefacts occur frequently[22]. A recommendation that can easily be implemented in clinical practice is that patients hold their breath at mid-expiration or mid-inspiration[20,24]. However, maintaining an unforced expiration breathhold is easily underestimated. Especially elderly or diseased patients will fail to sustain breathhold during actual scanning, causing artefacts on CT such as deformities throughout the liver. In the lungs, this causes small lung nodules to be missed in up to 34% of cases[25]. This problem is obviously related to the acquisition time for CT during whole-body scanning. The faster the CT scanner, or the more CT detector rows, the better breathhold compliance will be. For patients who are unable to comply with any breathhold instructions, respiration induced artefacts are reduced when using PET/CT systems employing CT components with an increasing amount of detector rows[26].
Even in an ideal situation, with a fast, multi-slice CT scanner, accurate breathhold instructions and an exemplary patient, the exact position of the diaphragm during instructed breathhold cannot be predicted. Furthermore, the shape of the diaphragm may differ from free breathing, because breathhold generates different muscle tension. This implies that differences in position and shape of the liver between PET and breathhold CT may be unavoidable and severe. This is illustrated by Goerres et al.[27] where an optimised breathing protocol results in differences in diaphragm position on expiration breathhold CT and free breathing PET between −25 and +19 mm. A solution to this unpredictable problem is respiratory gating. Several investigators[28–30] propose a phased attenuation correction in respiration correlated CT/PET which can lead to a more precise lesion localisation and quantification of the activity.
Another way to address motion-induced artefacts on PET is to perform attenuation correction on the basis of transmission scans using, e.g. rotating Ge-68/Ga-68 rod sources, although most current hybrid PET/CT scanners lack this option. Respiratory motion during such transmission scans is similar to that during the emission acquisition. Consequently, a better correlation of the diaphragm position is expected and hence a better attenuation correction in regions with sharp tissue/air transition[21,31]. Disadvantages of using traditional rod sources are the longer scanning time with respect to CT and the higher statistical noise than CT data in the final attenuation-corrected PET image.
Finally, there could be a role for software fusion. When image registration is performed with uncorrected PET and CT images, followed by the attenuation correction procedure, artefacts to respiratory motion can be reduced. Unfortunately, current hybrid PET/CT scanners do not provide this option. However, software fusion is foreseen to become an important tool, not only for the correction of residual motion-induced misregistration within combined PET/CT data sets, but also for follow-up studies involving, for example, dedicated CT, dedicated PET, PET/CT, and magnetic resonance imaging (MRI)[32,33].
Additional pitfalls of hybrid PET/CT and SPECT/CT
Although integrated PET/CT and SPECT/CT systems are advertised extensively as ‘state-of-the-art’ and as the latest achievement in modern medicine technology, some obvious pitfalls should not be forgotten. These hybrid scanners result in unnecessary duplication of CT scans, since in most cases, a patient will already have had a diagnostic CT exam before the PET study. To avoid poor radiation safety practice, one can implement an adapted diagnostic strategy by considering that in certain clinical conditions, obtaining a diagnostic CT before PET/CT may not be necessary. Furthermore, for many patients integrated PET/CT (or SPECT/CT) provides the same information that could be obtained from side-by-side imaging of a standalone CT and PET (or SPECT). Finally, a pitfall of multi-modality systems is that lesions can be overlooked when merely focusing on fused (overlaid) image results. The information that is depicted in the separate images is not fully present in an overlaid representation. For optimal reviewing of integrated image sets, visualisation of adjacent separate image sets as well as an overlaid image set is needed. The overlaid image set should be used for localisation purposes only.
Ge-68/Ga-68-based versus CT-based attenuation correction
Traditionally, for standalone PET imaging, attenuation correction is performed using transmission scans with electronically windowed rotating rod sources such as Ge-68/Ga-68, or rotating point sources such as caesium-137 (Cs-137). Therefore, if the activity concentration in a certain region within the patient is sufficiently high, e.g. in a tumour, the photons emitted by the radiopharmaceutical that was injected into the patient, disturbs the rod- or point-source-based attenuation correction for that region. For multi-modality PET-CT scanners, emission photons will not be measured during the CT acquisition as it normally operates at an energy below 140 keV. Hence, misinterpretation might occur when patients get follow-up PET scans on both a standalone PET and PET-CT. As institutions proceed from a single-modality PET to a multi-modality PET-CT scanner, this issue will become increasingly relevant, especially when quantitative values are compared across imaging systems.
Emission contamination is easily demonstrated. Fig. 3 shows a Ge/Ga-based attenuation map and an attenuation-corrected PET image of a sphere with a diameter of 60 mm, using a full-ring dedicated PET scanner (Siemens ECAT Exact 47, Siemens/CTI, USA). The activity concentration in the sphere was 100 kBq/ml FDG. Instead of a close to uniform attenuation map, we observe a depletion in the attenuation coefficient in the sphere, due to the emission contamination. Consequently, the activity concentration derived from the corrected PET image is underestimated by tens of percent. Moreover, the activity concentration in the centre of the sphere is approximately 30% lower with respect to the concentration at the border of the sphere. This could erroneously be interpreted as central necrosis. Using CT for attenuation correction, we would not observe these biases.
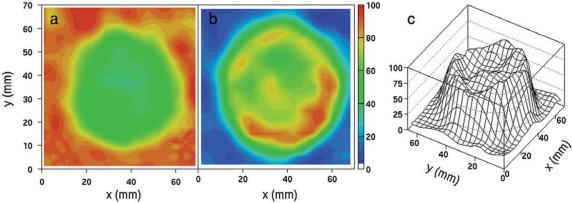
Figure 3.
A Ge/Ga-based attenuation map (a) and a attenuation-corrected FDG-PET image of a sphere with a diameter of 60 mm in a water environment (b,c) (Siemens ECAT Exact 47, Siemens/CTI, USA). The scale is such that ‘zero’ corresponds to no recovery and ‘100’ to full recovery. The activity concentration in the sphere was 100 kBq/ml. Instead of a close to uniform attenuation map, we observe a depletion in the attenuation coefficient in the sphere (a), due to the emission contamination. Consequently, the activity concentration derived from the corrected PET image is underestimated by tens of percent (b,c).
The literature confirms these phenomena. Nakamoto et al.[34] showed that CT-based attenuation correction produced activity concentration values significantly higher than the Ge/Ga-based corrected values: mean and maximum radioactivity concentrations were 4.3–15.2% higher for CT-corrected images than for Ge/Ga-corrected images. By performing a Ge/Ga-based transmission scan before injection of the radiopharmaceutical, which is obviously the ideal situation, but not always practical, one can prevent emission contamination to occur. With this gold standard, Van der Weerdt et al.[35] showed that post transmission scans for attenuation correction in cardiac FDG-PET scans resulted in substantial underestimation of FDG activity up to 15%.
One method is suggested to perform a correction for the emission contamination, using a dwell profile[35–37]. Although the correction method is not extremely complicated, it is generally not, or insufficiently, implemented in clinical PET. Therefore, another possibility to partially compensate for the emission contamination would be to perform a cross calibration: using a phantom close to the patient's geometry, translations can be derived from a measured standardized uptake value (SUV) on a standalone PET to a measured SUV on a PET/CT. Obviously, for follow-up studies, it is best that scans are acquired on the same system for each patient.
What about hybrid PET/MRI?
MRI is superior to CT in visualisation and delineation of soft tissue anatomy. The combination of PET with MRI may therefore be preferable over PET/CT, also in terms of radiation protection. However, breathing issues remain an issue. Current MRI techniques do not allow whole-body imaging during breathhold, and free breathing during MRI can severely distort the images. At this moment, the best approach to hybrid PET/MRI is unclear. Still, due to the potential advantages of a combination of PET and MRI over combined PET/CT systems, clinical PET/MRI systems are eagerly awaited. Initial measurements with one PET detector module in a 7 T field during application of MRI sequences by Pichler et al.[38] were encouraging. Recently, the design of a micro-PET/MRI system has been completed, and assembly and testing is underway[39].
Multi-modality imaging and PACS
Visualisation of fused multi-modality imaging
For optimal interpretation and discussion of multi-modality imaging, visualisation of both the separate images and the fused images is necessary. Dedicated image fusion software, that runs on a single or limited number of dedicated computer stations in the departments where the actual scanning is done, e.g. the radiology and nuclear medicine departments, provides this functionality. Although availability of fused multi-modality images through a picture archiving and communication system (PACS) benefits the entire hospital[40,41], exporting of the integrated image sets to a PACS is usually not provided, or in a very limited way only. This omission in functionality can first be explained by the lack of a Digital Imaging and Communications in Medicine (DICOM) standard for integrated image sets, and second by the lack of visualisation and linking tools for integrated image sets in current PACS systems. Therefore, there is no generally accepted method to transfer these image sets to a PACS, or for subsequent visualisation of the images. This blocks access to the integrated image sets from computer systems other than the fusion workstation itself, and prevents presentation of the fused images to physicians in other departments. Also, this seriously limits interdisciplinary discussions in, for example, surgical or oncological workgroups. In the current era of growing connectivity and accessibility, this is not acceptable.
Until PACS vendors provide easy and adequate image fusion software in their systems, a useful alternative would be to produce an alternating DICOM series file that contains alternating separate and fused representations of anatomically corresponding slices from different scans[42]. Subsequently, such a file can then be displayed in standard PACS either ‘serialized’ or ‘side-by-side’. In the serial configuration, stepping through the images in one viewing frame will reveal different representations of a single slice, before moving on to the next slice. Stepping backwards and forwards through the images will suggest a useful toggle mechanism. Alternatively, the alternating images can be displayed adjacently by showing multiple slices on a single screen. An example, based on software fusion of MRI and SPECT, is given in Fig. 4.
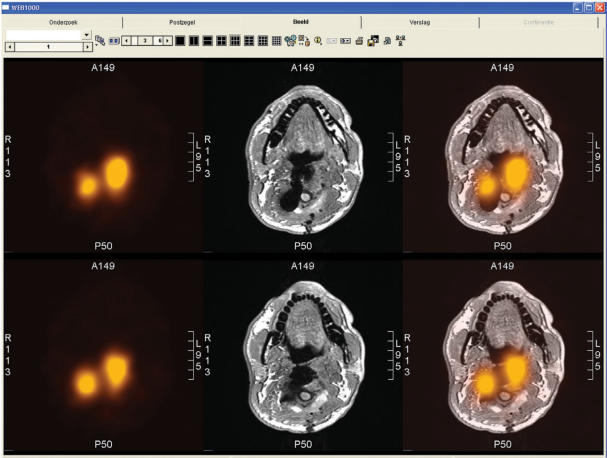
Figure 4.
Example of alternating fused images visualised in a PACS (AGFA Web 1000). Transverse slices of the neck region are shown in fused SPECT (indium-111 octreotide) and MRI (t1-weighted sequence) images. The series consists of three alternating representations of each slice: separate SPECT (left), separate MRI (middle) and combined SPECT-MRI (right). The MRI is difficult to interpret due to multiple surgery and the presence of metal. The SPECT shows suspected regions for remaining tumour tissue. The fused images provide the location of these regions.
Increased imaging data
The amount of acquired imaging data per patient is growing each year. Better resolution and new imaging techniques result in an enormous amount of data, which is difficult for referring physicians to handle. Moreover, potentially every image can be fused with all other images, by which the amount of data would even grow more. Hence, a strategy is necessary to limit the amount of available images for physicians, such that all pertinent information is still present.
First, it should be realised that image fusion impacts on the management of patients in only a limited amount of cases. Based on clinical experience with PET and CT, Jager et al.[43] showed that there is no need to look at CT images in about 80% of cases. They also showed that in the majority of the remaining 20% of cases, side-by-side imaging of the two modalities provides sufficient information. Hence, only in a small part of the patient population and only in a small number of lesions does image fusion have additional value. Therefore, image fusion should not be performed in all situations.
The following protocol is suggested. After acquiring the images, both the uncorrected and corrected PET images, and the CT image, should be used to generate the clinical report. At the end, one or more summary screenshots are created, indicating the location of a lesion on PET, the corresponding CT and the fused result. These summaries are the only fused images that should be sent to PACS and thus can be used during multi-disciplinary discussions hospital-wide.
Conclusion
Better diagnostic accuracy and staging are of pivotal importance for the adequate management of oncological patients. Multi-modality imaging, for example using hybrid PET/CT, contributes to this goal. However, the use of integrated imaging modalities in clinical practice is prone to imaging artefacts and pitfalls. Patient and respiratory movement, resulting in misalignment of the images and biases in CT-based attenuation correction, are important issues in combined PET/CT systems. Lack of adequate and reliable tools for visualisation of multi-modality image sets in hospital PACS, which prevents hospital-wide reviewing and demonstration of image fusion, is another important issue. Awareness of possible artefacts and pitfalls and being able to detect, correctly interpret and possibly solve them, is necessary for a maximum diagnostic yield.
References
- 1.Chung HH, Jo H, Kang WJ, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol. 2007;104:529–34. doi: 10.1016/j.ygyno.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Metser U, Golan O, Levine CD, Even-Sapir E. Tumor lesion detection: when is integrated positron emission tomography/computed tomography more accurate than side-by-side interpretation of positron emission tomography and computed tomography? J Comput Assist Tomogr. 2005;29:554–9. doi: 10.1097/01.rct.0000164671.96143.c2. [DOI] [PubMed] [Google Scholar]
- 3.Keidar Z, Haim N, Guralnik L, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med. 2004;45:1640–6. [PubMed] [Google Scholar]
- 4.Wang D, Schultz CJ, Jursinic PA, et al. Initial experience of FDG-PET/CT guided IMRT of head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:143–51. doi: 10.1016/j.ijrobp.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Van Baardwijk A, Baumert BG, Bosmans G, et al. The current status of FDG-PET in tumour volume definition in radiotherapy treatment planning. Cancer Treat Rev. 2006;32:245–60. doi: 10.1016/j.ctrv.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Czernin J, Schelbert H. PET/CT imaging: facts, opinions, hopes, and questions. J Nucl Med. 2004;45:S1–3. [Google Scholar]
- 7.Bockisch A, Beyer T, Antoch G, et al. Positron emission tomography/computed tomography-imaging protocols, artifacts, and pitfalls. Mol Imaging Biol. 2004;6:188–99. doi: 10.1016/j.mibio.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 8.Sureshbabu W, Mawlawi O. PET/CT imaging artifacts. J Nucl Med Technol. 2005;33:156–61. [PubMed] [Google Scholar]
- 9.Goerres GW, Ziegler SI, Burger C, Berthold T, von Schulthess GK, Buck A. Artifacts at PET and PET/CT caused by metallic hip prosthetic material. Radiology. 2003;226:577–84. doi: 10.1148/radiol.2262012141. [DOI] [PubMed] [Google Scholar]
- 10.Kamel EM, Burger C, Buck A, von Schulthess GK, Goerres GW. Impact of metallic dental implants on CT-based attenuation correction in a combined PET/CT scanner. Eur Radiol. 2003;13:724–8. doi: 10.1007/s00330-002-1564-2. [DOI] [PubMed] [Google Scholar]
- 11.Antoch G, Freudenberg LS, Egelhof T, et al. Focal tracer uptake: a potential artifact in contrast-enhanced dual-modality PET/CT scans. J Nucl Med. 2002;43:1339–42. [PubMed] [Google Scholar]
- 12.Cohade C, Osman M, Nakamoto Y, et al. Initial experience with oral contrast in PET/CT: phantom and clinical studies. J Nucl Med. 2003;44:412–16. [PubMed] [Google Scholar]
- 13.Nehmeh SA, Erdi YE, Kalaigian H, et al. Correction for oral contrast artifacts in CT attenuation-corrected PET images obtained by combined PET/CT. J Nucl Med. 2003;44:1940–4. [PubMed] [Google Scholar]
- 14.Berthelsen AK, Holm S, Loft A, Klausen TL, Andersen F, Hojgaard L. PET/CT with intravenous contrast can be used for PET attenuation correction in cancer patients. Eur J Nucl Med Mol Imaging. 2005;32:1167–75. doi: 10.1007/s00259-005-1784-1. [DOI] [PubMed] [Google Scholar]
- 15.Dizendorf E, Hany TF, Buck A, von Schulthess GK, Burger C. Cause and magnitude of the error induced by oral CT contrast agent in CT-based attenuation correction of PET emission studies. J Nucl Med. 2003;44:732–8. [PubMed] [Google Scholar]
- 16.Yau YY, Chan WS, Tam YM, et al. Application of intravenous contrast in PET/CT: does it really introduce significant attenuation correction error? J Nucl Med. 2005;46:283–91. [PubMed] [Google Scholar]
- 17.Mirzaei S, Guerchaft M, Bonnier C, Knoll P, Doat M, Braeutigam P. Use of segmented CT transmission map to avoid metal artifacts in PET images by a PET-CT device. BMC Nucl Med. 2005;5:3. doi: 10.1186/1471-2385-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beyer T, Bockisch A, Kuhl H, Martinez MJ. Whole-body 18F-FDG PET/CT in the presence of truncation artifacts. J Nucl Med. 2006;47:91–9. [PubMed] [Google Scholar]
- 19.Schaller S, Sembritzki O, Beyer T, Fuchs TO, Kachelriess M, Flohr TG. An algorithm for virtual extension of the CT field of measurement for application in combined PET/CT scanners. Radiology. 2002;225:497. [Google Scholar]
- 20.Beyer T, Antoch G, Blodgett T, Freudenberg LF, Akhurst T, Mueller S. Dual-modality PET/CT imaging: the effect of respiratory motion on combined image quality in clinical oncology. Eur J Nucl Med Mol Imaging. 2003;30:588–96. doi: 10.1007/s00259-002-1097-6. [DOI] [PubMed] [Google Scholar]
- 21.Papathanassiou D, Becker S, Amir R, Meneroux B, Liehn JC. Respiratory motion artefact in the liver dome on FDG PET/CT: comparison of attenuation correction with CT and a caesium external source. Eur J Nucl Med Mol Imaging. 2005;32:1422–8. doi: 10.1007/s00259-005-1868-y. [DOI] [PubMed] [Google Scholar]
- 22.Osman MM, Cohade C, Nakamoto Y, Wahl RL. Respiratory motion artifacts on PET emission images obtained using CT attenuation correction on PET-CT. Eur J Nucl Med Mol Imaging. 2003;30:603–6. doi: 10.1007/s00259-002-1024-x. [DOI] [PubMed] [Google Scholar]
- 23.Cohade C, Osman M, Marshall LN, Wahl RN. PET-CT: accuracy of PET and CT spatial registration of lung lesions. Eur J Nucl Med Mol Imaging. 2003;30:721–6. doi: 10.1007/s00259-002-1055-3. [DOI] [PubMed] [Google Scholar]
- 24.Goerres GW, Burger C, Schwitter MR, Heidelberg TN, Seifert B, von Schulthess GK. PET/CT of the abdomen: optimizing the patient breathing pattern. Eur Radiol. 2003;13:734–9. doi: 10.1007/s00330-002-1548-2. [DOI] [PubMed] [Google Scholar]
- 25.len-Auerbach M, Yeom K, Park J, Phelps M, Czernin J. Standard PET/CT of the chest during shallow breathing is inadequate for comprehensive staging of lung cancer. J Nucl Med. 2006;47:298–301. [PubMed] [Google Scholar]
- 26.Beyer T, Rosenbaum S, Veit P, et al. Respiration artifacts in whole-body (18)F-FDG PET/CT studies with combined PET/CT tomographs employing spiral CT technology with 1 to 16 detector rows. Eur J Nucl Med Mol Imaging. 2005;32:1429–39. doi: 10.1007/s00259-005-1879-8. [DOI] [PubMed] [Google Scholar]
- 27.Goerres GW, Kamel E, Heidelberg TNH, Schwitter MR, Burger C, von Schulthess GK. PET-CT image co-registration in the thorax: influence of respiration. Eur J Nucl Med Mol Imaging. 2002;29:351–60. doi: 10.1007/s00259-001-0710-4. [DOI] [PubMed] [Google Scholar]
- 28.Lang N, Dawood M, Buther F, Schober O, Schafers M, Schafers K. Organ movement reduction in PET/CT using dual-gated list-mode acquisition. Z Med Phys. 2006;16:93–100. doi: 10.1078/0939-3889-00296. [DOI] [PubMed] [Google Scholar]
- 29.Nagel CC, Bosmans G, Dekker AL, et al. Phased attenuation correction in respiration correlated computed tomography/positron emitted tomography. Med Phys. 2006;33:1840–7. doi: 10.1118/1.2198170. [DOI] [PubMed] [Google Scholar]
- 30.Nehmeh SA, Erdi YE, Pan T, et al. Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys. 2004;31:3179–86. doi: 10.1118/1.1809778. [DOI] [PubMed] [Google Scholar]
- 31.Chin BB, Nakamoto Y, Kraitchman DL, Marshall L, Wahl R. PET-CT evaluation of 2-deoxy-2-[18F]fluoro-D-glucose myocardial uptake: effect of respiratory motion. Mol Imaging Biol. 2003;5:57–64. doi: 10.1016/s1536-1632(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 32.Pietrzyk U. Does PET/CT render software registration obsolete? Nuklearmedizin. 2005;44(Suppl 1):S13–17. [PubMed] [Google Scholar]
- 33.Vogel WV, Oyen WJG, Barentsz JO, Kaanders JHAM, Corstens FHM. PET/CT: panacea, redundancy, or something in between? J Nucl Med. 2004;45:S15–24. [PubMed] [Google Scholar]
- 34.Nakamoto Y, Osman M, Cohade C, et al. PET/CT: comparison of quantitative tracer uptake between germanium and CT transmission attenuation-corrected images. J Nucl Med. 2002;43:1137–43. [PubMed] [Google Scholar]
- 35.van der Weerdt AP, Boellaard R, Knaapen P, Visser CA, Lammertsma AA, Visser FC. Postinjection transmission scanning in myocardial 18F-FDG PET studies using both filtered backprojection and iterative reconstruction. J Nucl Med. 2004;45:169–75. [PubMed] [Google Scholar]
- 36.Carson RE, Ube-Witherspoon ME, Green MV. A method for postinjection PET transmission measurements with a rotating source. J Nucl Med. 1988;29:1558–67. [PubMed] [Google Scholar]
- 37.Meikle SR, Bailey DL, Hooper PK, et al. Simultaneous emission and transmission measurements for attenuation correction in whole-body PET. J Nucl Med. 1995;36:1680–8. [PubMed] [Google Scholar]
- 38.Pichler BJ, Judenhofer MS, Catana C, et al. Performance test of an LSO-APD detector in a 7-T MRI scanner for simultaneous PET/MRI. J Nucl Med. 2006;47:639–47. [PubMed] [Google Scholar]
- 39.Lucas AJ, Hawkes RC, Ansorge RE, et al. Development of a combined microPET-MR system. Technol Cancer Res Treat. 2006;5:337–341. doi: 10.1177/153303460600500405. [DOI] [PubMed] [Google Scholar]
- 40.Alyafei S, Inoue T, Zhang H, et al. Image fusion system using PACS for MRI, CT, and PET images. Clin Positron Imaging. 1999;2:137–43. doi: 10.1016/s1095-0397(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 41.Pohjonen H. Image fusion in open-architecture PACS-environment. Comput Methods Programs Biomed. 2001;66:69–74. doi: 10.1016/s0169-2607(01)00137-7. [DOI] [PubMed] [Google Scholar]
- 42.Vogel WV, van Dalen JA, Huisman H, Oyen WJ, Karssemeijer N. Sliced alternating DICOM series: convenient visualisation of image fusion on PACS. Eur J Nucl Med Mol Imaging. 2005;32:247–8. doi: 10.1007/s00259-004-1711-x. [DOI] [PubMed] [Google Scholar]
- 43.Jager PL, Slart RHJA, Corstens F, Oyen WJG, Hoekstra O, Teule J. PET-CT: a matter of opinion? Eur J Nucl Med Mol Imaging. 2003;30:470–1. doi: 10.1007/s00259-002-1081-1. [DOI] [PubMed] [Google Scholar]