Abstract
An example is provided where, with antagonistic selection and epistatic interaction of alleles at two loci, an autosomal allele can rise in frequency, persist in the population, and even continue to fixation, despite having an apparently lower average fitness than the alternative allele, in a process similar to Parrondo's paradox.
A recent discovery in game theory known as Parrondo's paradox (Harmer and Abbott 1999a,b), developed to illustrate Brownian ratchet models (Magnasco 1993), has shown that, in certain circumstances if one can switch between two states (or games), even if they have a constant or losing expectation, a counterintuitive “winning” gain or “uphill” increase can result (Harmer and Abbott 1999a,b; Parrondo et al. 2000; see for review Harmer and Abbott 2002). However, the general applicability of this effect to real world processes, including biological systems, has been both speculated and criticized (e.g., Harmer and Abbott 1999a,b; Harmer et al. 2001; Iyengar and Kohli 2004; Wolf et al. 2005; Mihailovic and Rajkovic 2006). Here I show an example where, with reasonable assumptions a Parrondo-like process may make a contribution to changes in allele frequencies in a population genetic system and result in allele frequency dynamics that are unexpected given single-locus expectations.
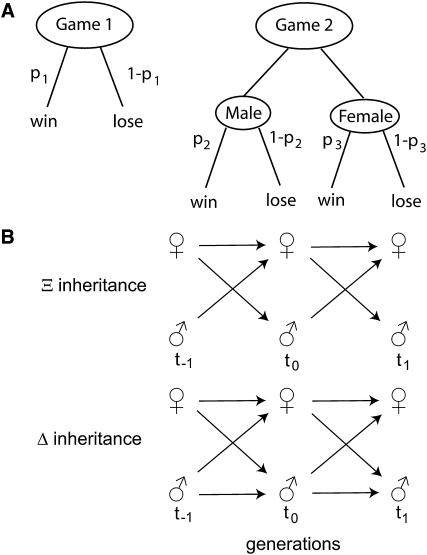
The typical construction of Parrondo's games is that in discrete time steps there are two or more games from which to choose; this choice can be systematic or random. Typically one game has a near equal probability of winning or losing (p1 in Figure 1A). The other game consists of subgames, typically one with favorable and another with unfavorable odds of winning (p3 and p2, respectively, in Figure 1A; cf. Harmer and Abbott 1999b), and a rule that decides which subgame to play. These rules can be dependent on the current “capital” or sum of games won and lost (Harmer and Abbott 1999a,b), the recent history of winning or losing (Parrondo et al. 2000), or other more complicated criteria (such as the winning or losing states of other players; Toral 2001). According to Iyengar and Kohli (2004) a necessary condition for Parrondo's paradox to operate is that one of the games must be a second-order (or higher) Markov chain process (i.e., the transition probabilities for the next state are dependent on at least two current or previous states). Another condition for Parrondo's paradox to operate is a convex probability space, where a mix of two nonwinning strategies can shift the overall game into an intermediate area with a winning expectation (Harmer and Abbott 2002).
Figure 1.—
(A) A diagram of a Parrondo's game (cf. Harmer and Abbott 1999b). Here game 1 represents a neutral genetic background without selection, and winning or losing can be thought of as passing or not passing an allele on to the next generation, in this case 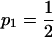 . Game 2 represents the sexually antagonistic selection that results from the interaction of two alleles, in this case
. Game 2 represents the sexually antagonistic selection that results from the interaction of two alleles, in this case 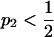 and
and 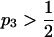 . (B) A diagram of the inheritance pattern for the X-linked Ξ- and autosomal Δ-loci.
. (B) A diagram of the inheritance pattern for the X-linked Ξ- and autosomal Δ-loci.
A two-locus system under selection can be constructed in a manner similar to Parrondo's games. Consider a gene, Ξ, with an allele ξ at frequency x, on an X chromosome in an XX/XY sex determination system and another gene, Δ, with an allele δ at frequency a, on an autosome. The interaction of ξ and δ is required for selection to act. For example, consider Δ a trans-acting factor that affects the expression of Ξ in a particular tissue or stage of development. Ξ is expressed only if the ξ- and δ-alleles are present in the same organism and can interact. Furthermore, the expression of Ξ is under sexually antagonistic selection (e.g., Rice and Chippindale 2001) and is deleterious in males but beneficial in females. From this point of view, the δ-allele is neutral in the non-ξ genetic background with an equal probability (with the non-δ-allele) of transmission to the next generation. This provides the first equal probability game (game 1 in Figure 1A). When δ is in the ξ background, selection can either favor or disfavor the probability of transmission depending on the sex of the organism. This provides the second set of subgames (game 2 in Figure 1A). Males receive their X chromosomes from females in the previous generation while females receive X chromosomes from both males and females (Figure 1B). A difference in allele frequencies between the sexes can create an oscillation in allele frequencies in succeeding generations. Thus, the frequency of ξ in females (xf) in the next (t1) generation depends not only on xf in the current (t0) generation, but also on xf in the t−1 generation (via the t0 males), making x over time a second-order Markov chain. The autosomal locus Δ is inherited from both the male and the female parents each generation so the frequency of δ also depends on two states in the preceding generation, its frequency in males, am, and in females, af (Figure 1B). Furthermore, with selection the frequencies of δ and ξ are interdependent.
The model:
Building upon earlier work (e.g., Owen 1953; Parsons 1961; Haldane 1962; Mandel 1971), Rice (1984) provides the expected frequencies of X-linked and autosomal alleles in the next generation when they are affected by selection independently. Building upon this and assuming independent association of alleles between the two loci, the male frequencies of ξ and δ in the next generation are
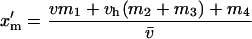 |
and
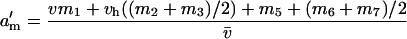 |
(see Table 1), where v is the relative fitness of male individuals containing ξ- and homozygous δ-alleles, vh is the fitness of ξ- and δ-heterozygotes, all other fitnesses are set to 1, and
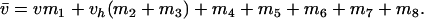 |
The female frequencies in the next generation are
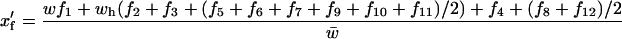 |
and
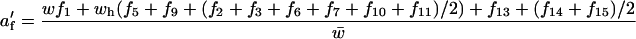 |
(see Table 2), where w is the relative fitness of female individuals homozygous for both ξ- and δ-alleles, wh is the fitness of females heterozygous at either or both of these loci (for simplicity all types of heterozygotes were assumed to have the same fitness), all other fitnesses are set to 1, and
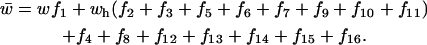 |
TABLE 1.
Expected compound genotype states in males
| X chromosome
|
||
|---|---|---|
| Autosome | xf | 1 − xf |
| amaf | m1 = xfamafa | m5 = (1 − xf)amaf |
| am(1 − af) | m2 = xfam(1 − af)b | m6 = (1 − xf)am(1 − af) |
| af(1 − am) | m3 = xfaf(1 − am)b | m7 = (1 − xf)af(1 − am) |
| (1 − am)(1 − af) | m4 = xf(1 − am)(1 − af) | m8 = (1 − xf)(1 − am)(1 − af) |
This genotype combination has a fitness of v.
These genotype combinations have a fitness of vh. The relative fitness of all other compound genotypes is set to one.
TABLE 2.
Expected compound genotype states in females
| X chromosome
|
||||
|---|---|---|---|---|
| Autosome | xmxf | xm(1 − xf) | xf(1 − xm) | (1 − xm)(1 − xf) |
| amaf | f1 = xmxfamafa | f5 = xm(1 − xf)amafb | f9 = xf(1 − xm)amafb | f13 = (1 − xm)(1 − xf)amaf |
| am(1 − af) | f2 = xmxfam(1 − af)b | f6 = xm(1 − xf)am(1 − af)b | f10 = xf(1 − xm)am(1 − af)b | f14 = (1 − xm)(1 − xf)am(1 − af) |
| af(1 − am) | f3 = xmxfaf(1 − am)b | f7 = xm(1 − xf)af(1 − am)b | f11 = xf(1 − xm)af(1 − am)b | f15 = (1 − xm)(1 − xf)af(1 − am) |
| (1 − am)(1 − af) | f4 = xmxf(1 − am)(1 − af) | f8 = xm(1 − xf)(1 − am)(1 − af) | f12 = xf(1 − xm)(1 − am)(1 − af) | f16 = (1 − xm)(1 − xf)(1 − am)(1 − af) |
This genotype combination has a fitness of w.
These genotype combinations have a fitness of wh. The relative fitness of all other compound genotypes is set to one.
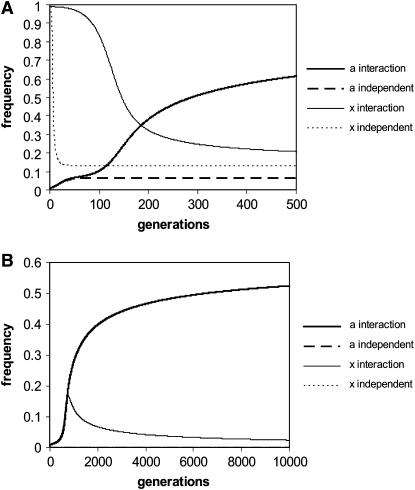
Using these recursion formulas the expected change in allele frequencies over time can be plotted. When the ξ-allele is common, δ behaves much as it would as if it acted independently from the ξ-allele (i.e., if selection could act upon δ without the epistatic interaction as in the single-locus case, which is equivalent to x = 1; see <100 generations in Figure 2A). However, when ξ drops in frequency in the population δ begins moving more commonly onto different backgrounds (ξ and non-ξ) and rises dramatically in frequency above what would be expected if δ acted independently (>100 generations in Figure 2A). This is analogous to switching between different games in Figure 1A. Furthermore, despite having a lower sex-averaged fitness than the alternative allele 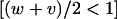 , δ can avoid removal and ratchet up to a high frequency in the population (Figure 2B). This is not predicted for a single-locus autosomal allele acting independently, even with antagonistic selection (Rice 1984). In fact, δ can rise to a persistent polymorphic frequency even when the ξ–δ interaction is male lethal (Figure 3). Also note that the conditions (in the example given in Figure 2B) for a single-locus X-linked allele to increase when rare are not met; the minimum female fitness required with full dominance is
, δ can avoid removal and ratchet up to a high frequency in the population (Figure 2B). This is not predicted for a single-locus autosomal allele acting independently, even with antagonistic selection (Rice 1984). In fact, δ can rise to a persistent polymorphic frequency even when the ξ–δ interaction is male lethal (Figure 3). Also note that the conditions (in the example given in Figure 2B) for a single-locus X-linked allele to increase when rare are not met; the minimum female fitness required with full dominance is
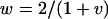 |
(1) |
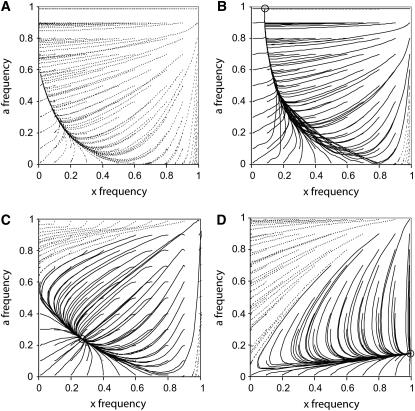
(Parsons 1961; see Equation 6 in Rice 1984). It is also interesting that with the starting conditions of Figure2B, ξ also initially rises in frequency until the point where ξ is approximately in a δ background one-half of the time 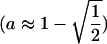 but then reverses and drops in frequency as a passes this area, suggesting that switching between δ and non-δ backgrounds (games 1 and 2 in Figure 1A) allows a boost to the ξ-alleles frequency. For a more complete picture, the trajectories of x and a are plotted from a grid of starting points (Figure 4A). Ultimately ξ will be lost, selection will no longer act, and a will remain at a frequency of ∼0.6; with stochastic drift this is also the probability that δ will ultimately become fixed in the population (Fisher 1930). Under slightly different fitness parameters, with dominance (vh = v, wh = w) δ can reach fixation in the population (Figure 4B). If codominance is assumed
but then reverses and drops in frequency as a passes this area, suggesting that switching between δ and non-δ backgrounds (games 1 and 2 in Figure 1A) allows a boost to the ξ-alleles frequency. For a more complete picture, the trajectories of x and a are plotted from a grid of starting points (Figure 4A). Ultimately ξ will be lost, selection will no longer act, and a will remain at a frequency of ∼0.6; with stochastic drift this is also the probability that δ will ultimately become fixed in the population (Fisher 1930). Under slightly different fitness parameters, with dominance (vh = v, wh = w) δ can reach fixation in the population (Figure 4B). If codominance is assumed 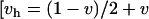 ,
, 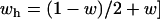 , a doubly balanced polymorphism can result (Figure 4C).
, a doubly balanced polymorphism can result (Figure 4C).
Figure 2.—
(A) The trajectory of x and a frequencies with the following starting conditions: x = 0.99, a = 0.01, w = wh = 2, v = vh = 0.2. The two-locus system is indicated with solid lines and the single-locus expectation (which is not dependent on an epistatic interaction) is indicated with dashed lines. (B) The trajectory of x and a frequencies with sex-averaged fitness of 0.9 for the autosomal allele δ (relative to a fitness of 1 for non-δ-, non-ξ-genotypes). The starting conditions were a = 0.01, x = 0.01, w = wh = 1.5, v = vh = 0.3. Note that the single-locus curves are also plotted but they quickly drop to a frequency of zero.
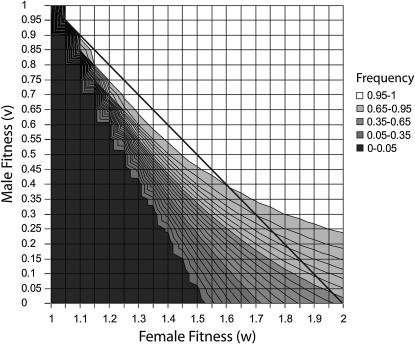
Figure 3.—
The frequency of δ after 10,000 generations starting with a = x = 0.01 and assumed dominance. The area below the diagonal line has an autosomal sex-averaged fitness <1. In the interaction described here, δ can rise in frequency and persist in the population over a larger area including when the sex-averaged fitness is lower than that of the non-δ-allele. (Note the jaggedness of the line at a = 0.05 is an artifact of the plotting program.) Also, the p2 and p3 probabilities of winning (game 2, Figure 1A) are related to v and w, respectively. This suggests a convex nature of the winning curve, a necessary condition for Parrondo's games (cf. Figure 14 of Harmer and Abbott 2002).
Figure 4.—
A plot of the changes in allele frequencies from a grid of starting points over 10,000 generations. (A) Fitness parameters identical to Figure 2B are used, w = wh = 1.5, v = vh = 0.3. (B) Fitness parameters w = wh = 1.5 and v = vh = 0.4 are used. (C) Fitness parameters w = 1.5, wh = 1.25, v = 0.4, and vh = 0.7 are used. (D) Fitness parameters w = 0.1, wh = 0.55, v = 0.1, and vh = 2 are used. Dashed lines indicate trajectories that result in the loss of the interaction between the ξ- and δ-alleles. Solid lines indicate trajectories that maintain the ξ–δ interaction at equilibrium; these equilibrium points are indicated by an open circle.
Stability analysis:
A linear approximation (a Jacobian matrix of the partial derivatives) was used to conduct a stability analysis of the two-locus system at each of the four trivial equilibria fixation points (see supplemental files for a Mathematica 5.2 notebook at http://www.genetics.org/supplemental/; Wolfram Research, Champaign, IL). The first point (x = 0, a = 0) yields a leading eigenvalue of λ(0,0) = 1, which suggests that a linear approximation is insufficient to describe the model at this point and the stability is controlled by higher-order terms. However, numerically it can be shown that this can be an unstable equilibrium and that δ and ξ can co-invade (Table 3). Note that the initial rise in frequency is disproportionately slower as x and a become very small, suggesting why the linear approximation is insufficient here. The probability of these two alleles coarising in the population is the product of the population scaled mutation rates, 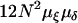 , where N is the population size, μξ is the mutation rate to the ξ-allele, and μδ is the mutation rate to the δ-allele; so co-invasion is much more likely, but initially much slower, in larger populations.
, where N is the population size, μξ is the mutation rate to the ξ-allele, and μδ is the mutation rate to the δ-allele; so co-invasion is much more likely, but initially much slower, in larger populations.
TABLE 3.
Changes in allele frequencies near x = 0, a = 0
| Starting frequency: x and a | Ending frequencya
|
|
|---|---|---|
| x | a | |
| 0.01 | 0.087 | 0.85 |
| 0.001 | 0.091 | 0.80 |
| 0.0001 | 0.000135 | 0.000126 |
| 0.00001 | 0.0000103 | 0.0000102 |
| 0.000001 | 0.000001003 | 0.000001002 |
Selection parameters w = wh = 1.5 and v = vh = 0.4 are used.
Ending frequencies are after 10,000 generations have passed.
The opposing fixation point (x = 1, a = 1) has two eigenvalues of interest (the model is four-dimensional so each equilibrium has four eigenvalues). One eigenvalue is 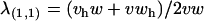 , which is equivalent to the conditions of instability found in the single-locus autosomal case analyzed by Kidwell et al. (1977). The second eigenvalue is
, which is equivalent to the conditions of instability found in the single-locus autosomal case analyzed by Kidwell et al. (1977). The second eigenvalue is 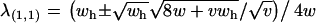 . Setting λ(1,1) = 1 and solving for v yields
. Setting λ(1,1) = 1 and solving for v yields 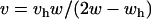 in the first case and
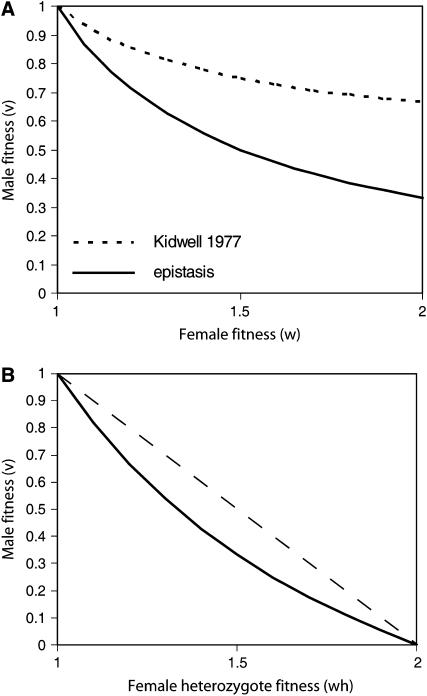
in the first case and 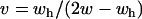 in the second. This yields a plot of the minimum (noninclusive) v required to make (x = 1, a = 1) an unstable equilibrium and that the newly discovered eigenvalue in this two-locus system is more permissible for the invasion of alternate alleles than the single-locus case (Figure 5A).
in the second. This yields a plot of the minimum (noninclusive) v required to make (x = 1, a = 1) an unstable equilibrium and that the newly discovered eigenvalue in this two-locus system is more permissible for the invasion of alternate alleles than the single-locus case (Figure 5A).
Figure 5.—
(A) A plot of the minimum male fitness vs. female fitness required to make the fixation point x = 1, a = 1 an unstable equilibrium. In this plot wh and vh were set equal to one (which assumes recessive effects of the heterozygotes). The line labeled Kidwell 1977 refers to the single-locus, harmonic mean result of Kidwell et al. (1977). (B) A plot of the minimum male fitness vs. female fitness required to make the fixation point x = 0, a = 1 an unstable equilibrium. The solid line indicates the minimum male fitness (v). The area below the dashed line has an average fitness of less than one.
The leading eigenvalue at (x = 1, a = 0) is 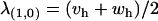 , which simply means that the sex-averaged fitness of the heterozygotes must be greater than one for this equilibrium point to become unstable. Finally, the leading eigenvalue at (x = 0, a = 1) is
, which simply means that the sex-averaged fitness of the heterozygotes must be greater than one for this equilibrium point to become unstable. Finally, the leading eigenvalue at (x = 0, a = 1) is 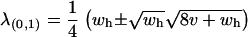 . Setting λ(0,1) = 1 and solving for v yields
. Setting λ(0,1) = 1 and solving for v yields
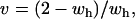 |
(2) |
which means that very low male fitnesses as wh = 2 is approached will allow unstable equilibria (Figure 5B). Furthermore, this equilibrium allows the interacting alleles to invade the population at different points in time. The δ-allele can fix by stochastic drift prior to the appearance of the ξ-allele and the ξ-allele can invade, even with a lower sex-averaged fitness for δ (Figure 5B). Note also that with complete dominance Equation 2 is equivalent to the requirement found by Parsons (1961) and Rice (1984) for an X-linked allele to increase when rare; see Equation 1 above.
Monte Carlo simulation:
To understand how much of the fitness parameter space might be affected by this type of epistatic interaction, random fitness values between 0 and 1 were assigned to each of the six fitnesses (w, wh, wn, v, vh, and vn, where wn is the noninteracting female fitness and vn is the noninteracting male fitness). The starting frequencies x and a were also assigned a random value between 0 and 1. The recursion equations were iterated until the maximum change in allele frequencies between generations was <10−12 (cf. Rand et al. 2001). This was repeated independently 50,000 times. Of these 50,000 replicates 7421 (14.8%) remained polymorphic at both interacting loci at the end of the run; this is larger than the one-ninth proportion of runs (5556) that are expected to be doubly overdominant. Within this doubly polymorphic class, 292 results (0.58%) had fitness values that did not meet the condition for polymorphism with sexually differential fitness found by Kidwell et al. (1977) (i.e., that the heterozygous fitness exceeds the harmonic mean of the homozygote fitnesses). Of these 292 “Kidwell violations” all male fitnesses were either directionally lower for the interacting alleles (consistent with the Parrondo formulation here) or in a pattern of underdominance; all female fitnesses were either directionally higher for the interacting alleles (also consistent with the formulation here) or were in a pattern of overdominance. In these cases all examples of male underdominance (13 cases) were paired with female overdominance (cf. Mérat 1969). Interestingly, 4232 (8.5%) replicates had ξ-alleles that reached fixation despite a lower sex-averaged fitness than the noninteracting state (2w + v < 2wn + vn) and without fitness underdominance in either sex (the majority of these cases had male overdominance; Figure 4D). Similarly, 153 (0.31%) replicates had δ-alleles that reached fixation with a lower sex-averaged fitness (w + v < wn + vn) and without fitness underdominance.
Discussion:
The epistatic-antagonistic system presented here, while not identical to the original Parrondo formulation, may be viewed as being in the class of Parrondo effects—this is supported by the necessary condition of state dependence, shown in Figure 1B, and convexity shown in Figure 3. Furthermore, this system has many interesting properties in its own right—selective sweeps of apparently less fit alleles. At first it seems counterintuitive that an allele with lower sex-averaged fitness can increase in frequency in the population, even if it is neutral in the noninteracting genetic background. However, it is easy to see that because of the sexually antagonistic selection, it is most beneficial for δ to be in a non-ξ male background and in a ξ female background. Because females have two chances of having a ξ-allele, while males have only one, δ is in a ξ background more often in females than in males at all frequencies of ξ (although this difference is greatest at intermediate frequencies of ξ and near zero at very low and high frequencies, which suggests why the change in frequency is disproportionately smaller at lower values; Table 3). Thus δ can initially rise in frequency while switching between male and female states by taking advantage of the epistatic interaction to minimize the deleterious effect in males and maximize the advantageous effect in females, in effect “ratcheting” upward between the sexes. However, when ξ and δ are more common, there are an increasing number of deleterious interactions in males and the effect diminishes.
If both loci were located far apart (so independent association can still be assumed) on the same X chromosome, with the reasoning above, it might make the rise in interacting allele frequencies, when rare, more efficient and rapid, because the advantageous interaction would be more common in females for both loci. Including linkage would make the model more complex. However, it can be seen that in-phase linkage (positive linkage disequilibrium) between the interacting alleles (both alleles are present on the same chromosome) would always expose the alleles to deleterious fitness when the chromosome was present in males, and out-of-phase linkage (negative linkage disequilibrium) would prevent the deleterious fitness interaction in males but allow the advantageous interaction to continue to occur at some frequency in females. Thus, I speculate that linkage will, in general, act to remove in-phase haplotypes, promote out-of-phase haplotypes in the population, and make the initial rise in frequency of the alleles more efficient.
Here I have cast the functional relationship between Ξ and Δ in terms of the evolution of expression regulation. Adaptive changes in expression are predicted (e.g., Stone and Wray 2001) and inferred (e.g., Oleksiak et al. 2002) to be commonly occurring in natural populations. Furthermore, sexually antagonistic genetic conflict appears to be common in the genome, especially on the X chromosome (e.g., Rice 1998; Chippendale et al. 2001; Gibson et al. 2002). So it is reasonable to suspect the counterintuitive scenario outlined here—that an apparently less fit allele can deterministically rise in frequency—may occur in natural populations. Furthermore, this type of interaction is not necessarily limited to the example given but may apply in more general cases where alleles interact in different genetic backgrounds and antagonistic selection is occurring. However, it may be more difficult for this process to act between two autosomal loci unless they are in a haplodiploid organism. Perhaps unlinked alleles could also take advantage of cases where alleles cycle in frequency over time (e.g., Dybdahl and Lively 1998; Axenovitch et al. 2007) to ratchet to higher frequencies. The increased area of parameter space for δ to persist in a polymorphic state (Figure 3) is similar to the maintained nuclear–cytoplasmic polymorphism when antagonistic selection and X chromosomes are involved (Rand et al. 2001). However, in general only a small fraction of the fitness parameter space appears to result in similar effects to those described here; but, the fixation of less fit, interacting, X-linked, alleles, particularly when there is male overdominance at the autosomal locus, was quite common (8.5%). Also, the case where both loci are present on an X chromosome might be more powerful and should be further explored. Perhaps these effects could also contribute to explanations of the imbalanced male–female expression patterns observed on the X chromosome (Parisi et al. 2003).
Finally, although it might be challenging to artificially construct a similar epistatic–antagonistic system, these results suggest a novel approach to transform natural populations. More importantly, these results also illustrate how what is actually a very simple system can have strikingly counterintuitive results. This underscores the caution urged in strategies to use genetic constructs to transform natural populations in a controlled manner (see Sinkins and Gould 2006 for review), particularly when epistasis and differential fitness in different genetic backgrounds may exist. Extensive theoretical and empirical testing prior to release, to maximize the likelihood of the desired effect and avoid unexpected, uncontrolled dynamics, is necessary.
Acknowledgments
I thank Derek Abbott and two anonymous reviewers for helpful suggestions. F.A.R. is supported by National Institutes of Health grant F32HG003801.
Note added in proof: The author has recently become aware of Trojak and Murphy (J. E., Trojak and E. A. Murphy, 1983, Paradoxical fixation of deleterious alleles in two-locus systems with epistasis. Am. J. Med. Genet. 16: 493–502) who describe the deterministic fixation of a deleterious allele in an autosomal two-locus epistatic system. In contrast to the present article, the model Trojak and Murphy present is not formulated as a Parrondo's game and the deleterious allele's rise in frequency is driven by asymmetrical mutation rates and the masking of its phenotypic effect by epistasis.
References
- Axenovitch, T. I., I. V. Zorkolsteva, I. R. Akberdin, S. T. Beketov, S. N. Kashtanov et al., 2007. Inheritance of litter size at birth in farmed artic foxes (Alopex lagopus, Canidae, Carnivora). Heredity 98: 99–105. [DOI] [PubMed] [Google Scholar]
- Chippendale, A. K., J. R. Gibson and W. R. Rice, 2001. Negative genetic correlation for adult fitness between the sexes reveals ontogenetic conflict in Drosophila. Proc. Natl. Acad. Sci. USA 98: 1671–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybdahl, M. F., and C. M. Lively, 1998. Host-parasite coevolution: evidence for rare advantage and time-lagged selection in a natural population. Evolution 52: 1057–1066. [DOI] [PubMed] [Google Scholar]
- Fisher, R. A., 1930. The Genetical Theory of Natural Selection. Clarendon Press, Oxford.
- Gibson, J. R., A. K. Chippendale and W. R. Rice, 2002. The X chromosome is a hot spot for sexually antagonistic fitness variation. Proc. R. Soc. Lond. Ser. B 269: 499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldane, J. B. S., 1962. Conditions for stable polymorphisms at an autosomal locus. Nature 193: 1108. [DOI] [PubMed] [Google Scholar]
- Harmer, G. P., and D. Abbott, 1999. a Parrondo's paradox. Stat. Sci. 14: 206–213. [Google Scholar]
- Harmer, G. P., and D. Abbott, 1999. b Losing strategies can win by Parrondo's paradox. Nature 402: 864. [Google Scholar]
- Harmer, G. P., and D. Abbott, 2002. A review of Parrondo's paradox. Fluctuation Noise Lett. 2: R71–R107. [Google Scholar]
- Harmer, G. P., D. Abbott, P. G. Taylor and J. M. R. Parrondo, 2001. Brownian ratchets and Parrondo's games. Chaos 11: 705–714. [DOI] [PubMed] [Google Scholar]
- Iyengar, R., and R. Kohli, 2004. Why Parrondo's paradox is irrelevant for utility theory, stock buying, and the emergence of life: Can losses be combined to give gains? Complexity 9: 23–27. [Google Scholar]
- Kidwell, J. F., M. T. Clegg, F. M. Stewart and T. Prout, 1977. Regions of stable equilibria for models of differential selection in the two sexes under random mating. Genetics 85: 171–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnasco, M. O., 1993. Forced thermal ratchets. Phys. Rev. Lett. 71: 1477–1481. [DOI] [PubMed] [Google Scholar]
- Mandel, S. P. H., 1971. Owen's model of a genetical system with differential viability between the sexes. Heredity 26: 49–63. [DOI] [PubMed] [Google Scholar]
- Mérat, P., 1969. Selection différente dans les deux sexes. Discussion générale des possibilités d'equilibre pour un locus autosomal a deux alléles. Ann. Génét. Sel. Anim. 1: 49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailovic, Z., and M. Rajkovic, 2006. Cooperative Parrondo's games on a two-dimensional lattice. Physica A 365: 244–251. [Google Scholar]
- Oleksiak, M. F., G. A. Churchill and D. L. Crawford, 2002. Variation in gene expression within and among natural populations. Nat. Genet. 32: 261–266. [DOI] [PubMed] [Google Scholar]
- Owen, A. R. G., 1953. A genetical system admitting of two distinct stable equilibria under natural selection. Heredity 7: 97–102. [Google Scholar]
- Parisi, M., R. Nuttall, D. Naiman, G. Bouffard, J. Malley et al., 2003. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science 299: 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrondo, J. M. R., G. P. Harmer and D. Abbott, 2000. New paradoxical games based on Brownian ratchets. Phys. Rev. Lett. 85: 5226–5229. [DOI] [PubMed] [Google Scholar]
- Parsons, P. A., 1961. The initial progress of new genes with viability differences between the sexes and sex-linkage. Heredity 16: 103–107. [Google Scholar]
- Rand, D. M., A. G. Clark and L. M. Kann, 2001. Sexually antagonistic cytonuclear fitness interactions in Drosophila melanogaster. Genetics 159: 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. R., 1984. Sex chromosomes and the evolution of sexual dimorphism. Evolution 38: 735–742. [DOI] [PubMed] [Google Scholar]
- Rice, W. R., 1998. Male fitness increases when when females are eliminated from the gene pool: implications for the Y chromosome. Proc. Natl. Acad. Sci. USA 95: 6217–6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice, W. R., and A. K. Chippindale, 2001. Intersexual ontogenetic conflict. J. Evol. Biol. 14: 685–693. [Google Scholar]
- Sinkins, S. P., and F. Gould, 2006. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7: 427–435. [DOI] [PubMed] [Google Scholar]
- Stone, J. R., and G. A. Wray, 2001. Rapid evolution of cis-regulatory sequences via local point mutations. Mol. Biol. Evol. 18: 1764–1770. [DOI] [PubMed] [Google Scholar]
- Toral, R., 2001. Cooperative Parrondo's games. Fluctuation Noise Lett. 1: L7–L12. [Google Scholar]
- Wolf, D. M., V. V. Vazirani and A. P. Arkin, 2005. Diversity in times of adversity: probabilistic strategies in microbial survival games. J. Theor. Biol. 234: 227–253. [DOI] [PubMed] [Google Scholar]