Abstract
Escherichia coli PolIV, a DNA polymerase capable of catalyzing synthesis past replication-blocking DNA lesions, belongs to the most ubiquitous branch of Y-family DNA polymerases. The goal of this study is to identify spontaneous DNA damage that is bypassed specifically and accurately by PolIV in vivo. We increased the amount of spontaneous DNA lesions using mutants deficient for different DNA repair pathways and measured mutation frequency in PolIV-proficient and -deficient backgrounds. We found that PolIV performs an error-free bypass of DNA damage that accumulates in the alkA tag genetic background. This result indicates that PolIV is involved in the error-free bypass of cytotoxic alkylating DNA lesions. When the amount of cytotoxic alkylating DNA lesions is increased by the treatment with chemical alkylating agents, PolIV is required for survival in an alkA tag-proficient genetic background as well. Our study, together with the reported involvement of the mammalian PolIV homolog, Polκ, in similar activity, indicates that Y-family DNA polymerases from the DinB branch can be added to the list of evolutionarily conserved molecular mechanisms that counteract cytotoxic effects of DNA alkylation. This activity is of major biological relevance because alkylating agents are continuously produced endogenously in all living cells and are also present in the environment.
DESPITE the proficiency of DNA repair, some DNA lesions persist. Because persistent lesions often block the replication apparatus, natural selection has favored the emergence of damage tolerance systems that allow complete replication in the presence of DNA damage. Damage tolerance is a measure of last resort to rescue cells from DNA damage. Without it, cells would become highly sensitive to killing by external and endogenously generated DNA-damaging agents. DNA lesions can be tolerated via different pathways, of which the two best studied are homologous recombination and replicative lesion bypass. Replicative lesion bypass requires specialized DNA polymerases (Rattray and Strathern 2003), most of which belong to the Y-family of DNA polymerases that are found in prokaryotes, eukaryotes, and archaea (Ohmori et al. 2001). The characteristics of the Y-family DNA polymerases are the lack of the 3′ → 5′ exonuclease activity and a more open catalytic site compared to the replicative polymerases (Yang 2003). These features enable the Y-family DNA polymerase to successfully bypass lesions, but also compromise the accuracy of the replication of a nondamaged template. Lesion bypass can be either error free or error prone when the correct or incorrect nucleotide, respectively, is incorporated opposite the damage.
The most ubiquitous branch of the Y-family of DNA polymerases, a DinB branch, is typified by Escherichia coli PolIV, human Polκ, and the archaeal Dbh/Dpo4 enzymes (Ohmori et al. 2001). Such remarkable conservation throughout evolution strongly suggests that the Y-family DNA polymerases from the DinB branch are extremely important for cell survival and fitness. In addition to PolIV, encoded by the dinB gene, E. coli possesses two more DNA polymerases capable of bypassing lesions: PolV, encoded by the umuDC genes and belonging to the Y-family, and PolII, encoded by the polB gene and belonging to the B-family of DNA polymerases (Nohmi 2006). In the unstressed, growing cell, there are 30–50 molecules of PolII and 250 of PolIV, whereas PolV is undetectable. For comparison, under such conditions there are ∼30 molecules/cell of replicative DNA polymerase PolIII. Such a high spontaneous expression level of dinB gene indicates that PolIV performs an important metabolic function, which remains to be elucidated at the molecular level. It is intriguing that inactivation of the dinB gene has no strong phenotype in unstressed cells (McKenzie et al. 2001; Kuban et al. 2004; Wolff et al. 2004). However, the overexpression of the dinB gene substantially increases spontaneous mutagenesis (Kim et al. 1997), probably by competing with PolIII for binding to the β-clamp (Lenne-Samuel et al. 2002).
In stressed cells, PolIV was shown to contribute considerably to mutagenesis. For example, PolIV is responsible for the untargeted mutagenesis of nonirradiated λ-phage in UV-irradiated cells (Brotcorne-Lannoye and Maenhaut-Michel 1986) and for the increased generation of mutations under carbon source starvation and stationary phase (Foster 2000; McKenzie et al. 2001; Tompkins et al. 2003). PolIV was also shown to be required for long-term survival in stationary phase (Yeiser et al. 2002). Genes coding for PolII, PolIV, and PolV are positively regulated by the SOS system (Fernandez De Henestrosa et al. 2000; Courcelle et al. 2001); the number of PolII and PolIV rapidly increases to 250 and 2500 molecules/cell, respectively, while PolV reaches ∼60 molecules/cell 1 hr after SOS induction (Nohmi 2006). In addition, the transcription of the dinB gene is controlled by RpoS, a σ-subunit of RNA polymerase, which regulates a general stress response (Layton and Foster 2003). PolIV is also regulated by the heat-shock chaperone GroE (Layton and Foster 2005). Therefore, PolIV is a component of several cellular stress responses.
In vitro, E. coli PolIV can perform DNA synthesis across a variety of base modifications, but in vivo it is involved in the bypass of only a subset of these base modifications, i.e., those induced by benzo[a]pyrene, 4-nitroquinolone N-oxide, nitrofurazone, and reactive oxygen species (Fuchs et al. 2004; Jarosz et al. 2006). For example, PolIV bypasses abasic sites in vitro but not in vivo (Maor-Shoshani et al. 2003). Such discrepancies indicate that the access to the DNA damage and the activity of PolIV and other bypass DNA polymerases is regulated in vivo. When replicative DNA polymerase is blocked, other DNA polymerases have access to the lesion site in the hierarchical order (Delmas and Matic 2006). In addition, depending on the type of DNA damage, different polymerases can compete or collaborate at the lesion site (Fuchs et al. 2004). The bypass of a given lesion is expected to be error free or error prone, depending on which DNA polymerase is involved; i.e., bypass of a cognate lesion is expected to be predominantly error free and that of noncognate lesion predominantly error prone (Friedberg et al. 2002). The cognate lesion for a given DNA repair enzyme is a DNA lesion that is specifically and preferentially recognized and processed by this enzyme. It was recently proposed that N2-deoxyguanosine adducts are cognate lesions for PolIV, because it catalyzes accurate error-free bypass of these replication-blocking lesions (Jarosz et al. 2006). This hypothesis is based on results from the studies using chemical DNA-damaging agents. The aim of our study is to try to identify cognate lesion(s) for PolIV polymerase by investigating the consequence of PolIV-mediated bypass of different types of spontaneous DNA damage in vivo. To increase the amount of one specific lesion in the genome, and to prevent other DNA repair systems from removing the lesion before PolIV has an opportunity to perform the bypass, an exhaustive set of mutants affected in their DNA repair ability was constructed. The results indicate that E. coli PolIV polymerase is involved in tolerance of cytotoxic alkylating DNA lesions in vivo. More specifically, PolIV is involved in the error-free processing of 3-methyladenine (3-meA) and 3-methylguanine (3-meG). We propose that this might be one of the major biological functions of PolIV.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media:
All strains used in this study (Table 1) were derivatives of the E. coli MG1655 attλ∷cI (Ind−) λpR tetA Δara∷FRT ΔmetRE∷FRT strain designated as the parental strain. The construction of this strain, as well as of its derivatives carrying the forward mutation assay that scores mutations in the λ cI (Ind−) repressor gene inserted into the λ attachment site on the E. coli chromosome, is described below. Strains were constructed using P1-mediated transduction of alleles kindly provided by colleagues or constructed using a previously described PCR-based method (Datsenko and Wanner 2000). Alleles constructed using the PCR-based method for gene deletion are Δara∷Cm (constructed by M. Elez); ΔdinB∷Cm, ΔmutS∷Cm, ΔpolB∷Cm, ΔumuDC∷Cm (constructed by M. Vulic); ΔmutM∷Cm; (constructed by L. Le Chat); and ΔalkA∷Cm, ΔmetRE∷Cm, ΔmutY∷Cm, Δnei∷Cm, Δnfo∷Cm, Δnth∷Cm, Δtag∷Phleo, Δung∷Cm, ΔuvrA∷ Cm, Δxth∷Cm, ΔdinByafNOP∷Cm, ΔyafNOP∷Cm (this work). ΔmutS∷spec/strep and mutS∷Tn5 alleles are from our laboratory collection. Δada-25∷Cm and ogt-1∷Kan alleles are a generous gift from L. Samson (Mackay et al. 1994). pYG768 plasmid (Kim et al. 1997), pGB2 vector plasmid, pGB2-dinBΔC5, and pGB2-dinB+ were kindly provided by R. Fuchs (Lenne-Samuel et al. 2002). The pY-2P-intC plasmid was kindly provided by A. Lindner.
TABLE 1.
Strains used in this study
| Designation in this article | Genotypea |
|---|---|
| Parental strain | MG1655 attλ∷cI (Ind−) λpR tetA Δara∷FRT ΔmetRE∷FRTb |
| dinB | ΔdinB∷FRT |
| mutS | mutS∷Tn5 met+ |
| mutS dinB | mutS∷Tn5 ΔdinB∷FRT met+ |
| ada ogt mutS | Δada-25∷Cm ogt-1∷Kan mutS∷Spec/Strep |
| ada ogt mutS dinB | Δada-25∷Cm ogt-1∷Kan mutS∷Spec/Strep ΔdinB∷FRT |
| mutM mutY mutS | mutM∷FRT; mutY∷Cm; mutS∷Tn5 |
| mutM mutY mutS dinB | mutM∷FRT; mutY∷Cm; mutS∷Tn5; dinB∷FRT |
| xth nfo mutS | Δxth∷Cm Δnfo∷FRT mutS∷Tn5 |
| xth nfo mutS dinB | Δxth∷Cm Δnfo∷FRT mutS∷Tn5 ΔdinB∷FRT |
| ung mutS | Δung∷Cm mutS∷Tn5 |
| ung mutS dinB | Δung∷Cm mutS∷Tn5 ΔdinB∷FRT |
| nei nth mutS | Δnei∷FRT Δnth∷Cm mutS∷Tn5 |
| nei nth mutS dinB | Δnei∷FRT Δnth∷Cm mutS∷Tn5 ΔdinB∷FRT |
| alkA mutS | ΔalkA∷FRT ΔmutS∷Cm |
| alkA mutS dinB | ΔalkA∷FRT ΔmutS∷Cm ΔdinB∷FRT |
| tag mutS | Δtag∷Phleo ΔmutS∷Cm |
| tag mutS dinB | Δtag∷Phleo ΔmutS∷Cm ΔdinB∷FRT |
| alkA tag | ΔalkA∷FRT Δtag∷Phleo |
| alkA tag dinB | ΔalkA∷FRT Δtag∷Phleo ΔdinB∷FRT |
| alkA tag dinB intC∷dinB+ | ΔalkA∷FRT Δtag∷Phleo ΔdinB∷FRT intC∷dinB+ Cm |
| alkA tag mutS | ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 |
| alkA tag mutS dinB | ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 ΔdinB∷FRT |
| alkA tag mutS dinB intC∷dinB+ | ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 ΔdinB∷FRT intC∷dinB+ Cm |
| umuDC | ΔumuDC∷FRT met+ |
| umuDC dinB | ΔumuDC∷FRT ΔdinB∷FRT ara+ met+ |
| umuDC alkA tag | ΔumuDC∷Cm ΔalkA∷FRT Δtag∷Phleo |
| umuDC alkA tag dinB | ΔumuDC∷Cm ΔalkA∷FRT Δtag∷Phleo ΔdinB∷FRT |
| umuDC mutS alkA tag | ΔumuDC∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 |
| umuDC mutS alkA tag dinB | ΔumuDC∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 ΔdinB∷FRT |
| polB | ΔpolB∷Cm ara+ met+ |
| polB dinB | ΔpolB∷Cm ΔdinB∷FRT ara+ met+ |
| polB mutS alkA tag | ΔpolB∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 |
| polB mutS alkA tag dinB | ΔpolB∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 ΔdinB∷FRT |
| polB umuDC | ΔpolB∷FRT ΔumuDC∷FRT |
| polB umuDC dinB | ΔpolB∷FRT ΔumuDC∷FRT ΔdinB∷FRT |
| uvrA mutS | ΔuvrA∷FRT mutS∷Spect/Strep |
| uvrA mutS dinB | ΔuvrA∷FRT mutS∷Spect/Strep ΔdinB∷FRT |
| uvrA mutS alkA tag | ΔuvrA∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 |
| uvrA mutS alkA tag dinB | ΔuvrA∷Cm ΔalkA∷FRT Δtag∷Phleo mutS∷Tn5 ΔdinB∷FRT |
| yafNOP | ΔyafNOP∷Cm |
| yafNOP dinB | ΔdinB yafNOP∷Cm |
Because all strains are derivatives of parental strain, in the genotype column, only differences compared to the genotype of parental strain are indicated.
FRT indicates that a scar was left upon elimination of the antibiotic resistance cassette using the FLP recombinase.
Bacterial strains were grown in LB, supplemented when needed with ampicillin (100 μg/ml), tetracycline (12.5 μg/ml), chloramphenicol (30 μg/ml), kanamycine (50 or 100 μg/ml), spectinomycine (50 μg/ml), streptomycine (25 μg/ml), phleomycine (10 μg/ml), methyl methanesulfonate (MMS; Acros Organics), ethyl methanesulfonate (EMS; Acros Organics) and N-methyl-N′-nitro-N-nitrosoguanidine (MNNG; Aldrich).
Construction of the forward mutation assay and its integration into the E. coli chromosome:
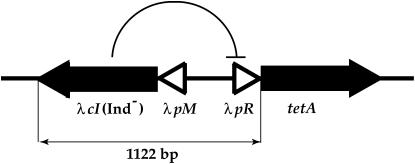
For this study, we constructed a forward mutation assay that scores mutations in the λ cI (Ind−) repressor gene (Figure 1). This repressor, which cannot be cleaved upon SOS induction, represses the tetA gene whose native promoter was replaced by the λ pR promoter. This construction was inserted in the λ attachment site at the E. coli chromosome. Any mutation that inactivates cI derepresses the λpRtetA gene, which confers resistance to tetracycline. Tetracycline-resistant clones can be selected for and mutations inactivating cI identified by sequencing the 1122-bp region using the following primers for PCR: 5′-TCAGCCAAACGTCTCTTCAG-3′ and 5′-GCCAATCCCCATGGCATCGAGTAAC-3′.
Figure 1.—
Forward mutation assay. For this study we constructed a forward mutation assay that scores mutations in the λ cI (Ind−) repressor gene. This repressor, which cannot be cleaved upon SOS induction, represses the tetA gene whose native promoter was replaced by the λ pR promoter. This construction was inserted in the λ attachment site at the E. coli chromosome. Any mutation that inactivates cI derepresses the λpRtetA gene, which confers resistance to tetracycline. Tetracycline-resistant clones can be selected for and mutations inactivating cI identified by sequencing the 1122-bp region.
The cI(Ind−)-λpRtetA mutation assay was constructed as follows: (i) the cI-λpRtetA fragment from the pGBG1 plasmid (Schneider et al. 2000) was excised using SacI and SmaI restriction enzymes and cloned into the pUC18 plasmid, subsequently named pUC18-cI-λpRtetA and (ii) the SOS noninducible cIind1 allele, called cI(Ind−) further in the text, was PCR amplified from λDNA using 5′-TCAGCCAAACGTCTCTTCA-3′ and 5′-ATGAGCACAAAAAAGAAACC-3′ primers. The PCR-amplified fragment was digested by PshI and BclI and used to replace cI with cI(Ind−), thus generating the pUC18-cI(Ind−)-λpRtetA plasmid.
To integrate the cI(Ind−)-λpRtetA construct in the λ attachment site (attλ) on the E. coli chromosome, a previously described method was used (Haldimann and Wanner 2001). The integration plasmid pAH143 was modified:
the attHK site of pAH143 was replaced by the attλ site, obtained by NheI and NcoI restriction of pAH63 plasmid. The gentamycin resistance cassette of pAH143 was replaced by the kanamycin resistance cassette from the SphI- and NotI-digested pAH125 plasmid. Thus modified, pAH143 was named pAH143-attλ-Kan plasmid.
cI(Ind−)-λpRtetA was PCR amplified using 5′-ACTACGTAAGCATGCTCAGCCAAACGTCTCTTCAG-3′ and 5′-TACAGAGGATCCATCGCAATTGATATTTGGTGACGAAATAACTAAG-3′ primers from the pUC18-cI(Ind−)-λpRtetA plasmid. Amplified DNA was cloned into SphI- and BamHI-restricted pAH143-attλ-Kan plasmid.
The resulting integration plasmid, pAH143- attλ-Kan-cI(Ind−)-λpRtetA, was inserted into the attλ site of the pINTts plasmid-transformed MG1655 E. coli strain, according to the modified previously described protocol (Haldimann and Wanner 2001). Integration protocol was adapted for our usage because integrase on the pINTts plasmid is under cI857 control, and its expression at 42° is diminished due to the presence of CI(Ind−) in our construct. Therefore, the integrase expression was induced (1 hr at 37° and 30 min at 42°) prior to transformation with pAH143-attλ-Kan-cI(Ind−)-λpRtetA plasmid. Transformants were selected on LB plates supplemented with 10 μg/ml kanamycin and verified for the multiple inserts as in Haldimann and Wanner (2001).
Subsequently, the origin of replication of the plasmid and the kanamycin resistance cassette were deleted from the plasmid inserted into chromosome and replaced by the FRT-flanked chloramphenicol resistance cassette (Datsenko and Wanner 2000).
Finally, the chloramphenicol resistance cassette was then removed as previously described (Datsenko and Wanner 2000). At least 30 candidates were taken for removal of the chloramphenicol resistance cassette, because the gene coding for the FLP recombinase on the pCP20 is under cI857 control. Primers used for the deletion of origin of replication of the plasmid and the kanamycin resistance cassette were 5′-CAGAGAAGCACAAAGCCTCGCAATCCAGTGCAAACCATGGGTGTAGGCTGGAGCTGCTTC-3′ and 5′-TAATCTAGTGGATCAAGAGACAGGATGAGGATCGTTTCGCCATATGAATATCCTCCTTAG-3′, whereas plasmids for verification of the deletions were 5′-ATGGTATTAGTGACCTGTAAC-3′ and 5′-CATTCAAATATGTATCCGCTC-3′.
E. coli strains used for cloning were DH5α (laboratory strain collection) and BW23474 without a plasmid (obtained from E. coli Genetic Stock Center, Yale University). BW23474 carries the pir-116 allele required for the propagation of pir-dependent plasmids (Haldimann and Wanner 2001). PCR amplifications were performed with Pfu Ultra DNA polymerase (Stratagene, La Jolla, CA). All restriction enzymes were from New England Biolabs (Beverly, MA) and T4 DNA ligase was from Roche.
Integration of dinB+ in the intC chromosomal site:
Integration of the functional dinB gene in the intC site at E. coli chromosome was performed using the p-intC-Cm-dinB+ plasmid, which carries two regions homologous to the intC site flanking the chloramphenicol resistance cassette (Cm) and the functional dinB gene. This plasmid was constructed as follows: (i) the pYG768 plasmid (Kim et al. 1997) was cut with SacI; (ii) the resulting linear DNA was rendered blunt ended using the PolI Klenow fragment; (iii) the linear DNA was subsequently cut with EcoRI producing the DNA fragment carrying the functional dinB gene with its native promoter; and (iv) this DNA fragment was ligated with the fragment of the pY-2P-intC plasmid (kindly provided by A. Lindner) carrying the chloramphenicol resistance cassette flanked by the two intC fragments. This pY-2P-intC plasmid fragment was produced by (i) cutting pY-2P-intC with KpnI, (ii) blunt ending the linearized DNA using the PolI Klenow fragment, and (iii) finally cutting the linearized DNA with EcoRI.
The p-intC-Cm-dinB+ plasmid was cut with AhdI and SphI enzymes, and the fragment carrying the dinB+ gene and the chloramphenicol resistance cassette flanked by the two intC fragments was introduced into the intC site of the E. coli alkA tag dinB mutS+/−strains' chromosome using a previously described method (Datsenko and Wanner 2000).
Spontaneous mutagenesis assay:
For each genetic background, dinB-proficient and dinB-deficient derivatives were always tested in parallel. Each experiment was repeated 4–11 times. In addition, different genetic backgrounds were tested in parallel with mutS strains in at least two independent experiments. Bacterial cultures were started with <100 cells to make sure that no preexisting mutants were present in the starting inoculum. Cells were grown in LB, supplemented with antibiotics when needed, and shaken overnight at 37°. Appropriate dilutions of cells were plated on selective media (LB containing 12.5 μg/ml tetracycline) to detect tetracycline-resistant mutants and on LB to determine the total number of colony-forming units. Colonies were scored after 24 hr of incubation at 37°. Mutation frequency was calculated by dividing the number of tetracycline-resistant mutants by the number of plated colony-forming units.
Measurement of sensitivity to, and frequency of mutations induced by, alkylating agents:
The sensitivity to different alkylating agents was estimated by spotting 8 μl of 10-fold serial dilutions of overnight cultures of different strains onto LB plates with and without alkylating agents and by plating serial dilutions of overnight cultures of different strains onto LB plates with and without alkylating agents.
The frequency of mutations induced by alkylating agents was measured by plating dilutions of overnight cultures of different strains on LB plates supplemented with MMS and on LB plates supplemented with MMS and 12.5 μg/ml of tetracycline. Colonies were scored after 24 hr of incubation at 37°.
Statistical analysis:
All statistical analyses were performed using Statview 5.0 software (SAS Institute). A P-value <0.05 was considered to indicate statistical significance.
RESULTS
Mutation assay:
As PolIV was shown to promote base substitutions and frameshifts (Kim et al. 1997; Fuchs et al. 2004), we constructed a forward mutation assay that scores mutations in the λ cI (Ind−) repressor gene (Figure 1). This repressor, which cannot be cleaved upon SOS induction, represses the tetA gene whose native promoter was replaced by the λ pR promoter. This construction was inserted in the λ attachment site at the E. coli chromosome. Any mutation that inactivates cI derepresses the tetA gene, which confers resistance to tetracycline. Tetracycline-resistant clones can be selected for and mutations inactivating cI can be identified by sequencing. The mutation spectrum shows that this assay allows detection of all types of mutations (data not shown). In the wild-type strain, ∼50% of spontaneous mutations are base substitutions (all possible transitions and transversions are represented), ∼38% are single-base deletions/insertions, and ∼10% are small rearrangements.
Spontaneous mutagenesis:
To identify cognate lesion(s) for PolIV polymerase, we diminished redundancy in DNA repair pathways and increased the amount of different spontaneous DNA lesions by using a set of mutants deficient for different DNA repair pathways (for reviews, see Friedberg et al. 2006). Because several DNA repair enzymes can act on the same lesions, in some cases we inactivated two enzymes that exhibit overlapping functions. Alkylation damage is increased by inactivating ada and ogt, which code for O6-methylguanine-DNA methyltransferases, and alkA and tag, which code for 3-methyladenine-DNA glycosylases. The former two enzymes remove premutagenic lesions, while the latter two enzymes remove cytotoxic lesions. Repair of abasic sites is hindered by inactivation of the xth and nfo genes coding for exonuclease III and endonuclease IV, respectively. Inactivation of nei and nth coding for endonuclease VIII and endonuclease III, respectively, reduces the cell's ability to repair oxidized bases. Inactivation of mutM and mutY genes abolishes the removal of 8-oxoguanine (8-oxoG) from DNA. MutM (formamidopyrimidine DNA glycosylase) removes 8-oxoG from 8-oxoG-C pairs, giving the repair DNA polymerase a chance to put in a G. If 8-oxoG is not removed before DNA replication occurs, it can mispair with an A. MutY glycosylase removes A in 8-oxoG-A mispairs. Disruption of the ung gene coding for uracil-DNA glycosylase results in the accumulation of uracil in DNA. Inactivation of uvrA renders the cell deficient for nucleotide excision repair, which removes a variety of bulky DNA damage. All mutants used were also mismatch repair deficient (mutS mutants) because mismatch repair has been shown to correct PolIV-generated errors (Strauss et al. 2000).
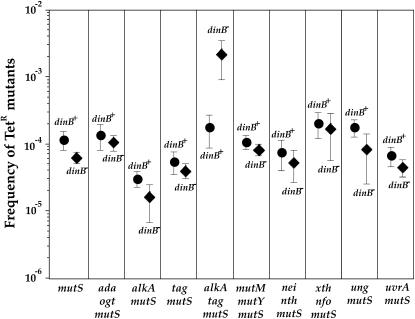
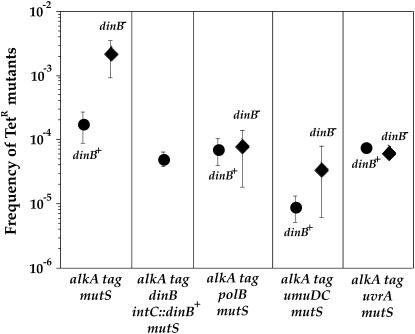
Because PolIV is expected to bypass its cognate lesion in an error-free fashion, the inactivation of dinB should significantly increase the mutation frequency in a background where this type of mutation is increased. Among the mutants tested, this was observed only in the alkA tag mutS background where an 11-fold (Mann–Whitney P = 0.0037) increase in mean value of mutation frequency occurred (Figure 2). An increase in the mean value of mutation frequency was also observed in a alkA tag mismatch-repair-proficient background but the effect of dinB gene inactivation was smaller, i.e., 4-fold (Mann–Whitney P = 0.019). The increase in mutation frequency was not observed in alkA mutS and tag mutS mutants. The complementation of the dinB-deficient mutant by a dinB-proficient gene, inserted in trans into the intC site of E. coli chromosome, reduced mutation frequency in the alkA tag mutS-deficient background (Figure 3). The increase in mutation frequency in the absence of PolIV results from the activity of PolII and nucleotide excision repair (Figure 3). PolV is the major error-prone bypass polymerase regardless of the presence of DinB. Because the PolV mutator effect is smaller in the presence than in the absence of PolIV (25- and 46-fold, respectively), it seems that PolIV competes with PolV for processing of alkylating lesions. These data indicate that PolIV participates in error-free processing of cytotoxic alkylation damage.
Figure 2.—
Effect of dinB gene deletion on the frequency of spontaneously arisen TetR mutants in different genetic backgrounds. Only a significant increase in mutation frequency was observed in the alkA tag mutS strain. Each point represents the mean (± standard error) values from 4–11 independent experiments.
Figure 3.—
Effect of dinB gene deletion on the frequency of spontaneously arisen TetR mutants in alkA tag mutS background. Data for the alkA tag mutS- and alkA tag mutS dinB strains are from Figure 2. The increase in mutation frequency observed in the alkA tag mutS strain is abolished by complementation with the functional dinB gene, as well as by inactivation of polB, umuDC, and uvrA genes. Each point represents the mean (± standard error) values from six to nine independent experiments.
Sensitivity to alkylating agents:
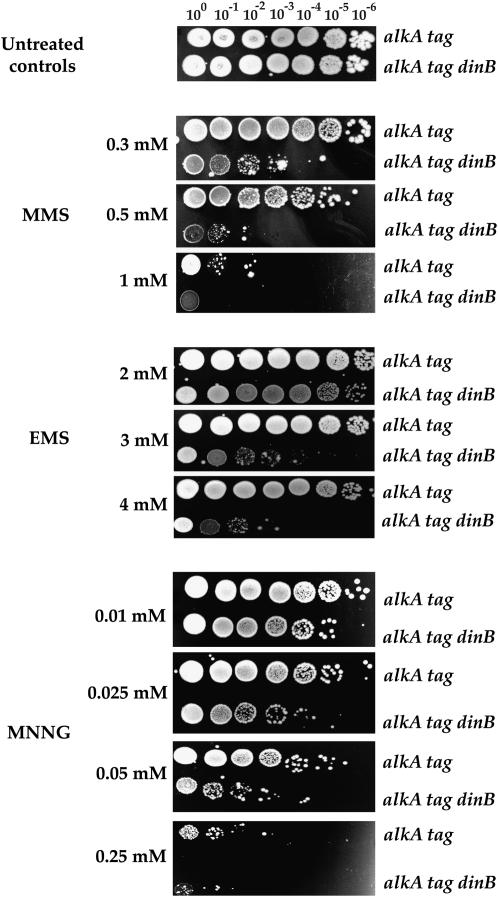
If PolIV participates in error-free bypass of cytotoxic lesions generated by endogenous alkylating agents, than it should also confer resistance to the killing effect of exogenous alkylating agents. To test this hypothesis, we investigated the sensitivity of alkA tag, mismatch-proficient, PolIV-proficient or deficient strains to MMS, EMS, and MNNG (Figure 4). In all cases, the PolIV-deficient strain was more sensitive than the proficient one.
Figure 4.—
Sensitivity of E. coli alkA tag PolIV-proficient and -deficient mutants to different alkylating agents. Sensitivity to MMS, EMS, and MNNG was estimated by spotting 8 μl of 10-fold serial dilutions of overnight cultures of the alkA tag and alkA tag dinB strains onto LB plates containing alkylating agents at the indicated concentrations.
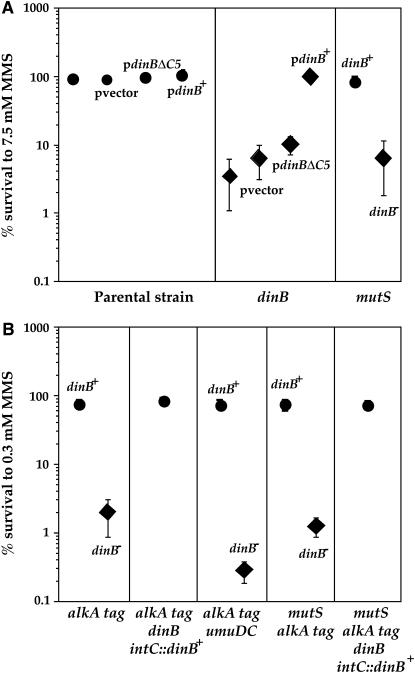
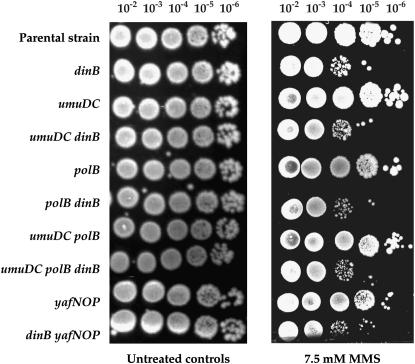
PolIV is also required for the survival of alkA tag-proficient strains exposed to high doses of MMS in mismatch-proficient and -deficient strains (Figures 5 and 6). PolIV bypasses MMS-induced cytoxic lesions without the assistance of PolII and PolV (Figures 5 and 6). The requirement of interaction of PolIV with the β-clamp to perform alkylation damage bypass indicates that MMS-induced cytoxic lesions block the replication fork (Figure 5A) (Larson et al. 1985; Wyatt et al. 1999; Sedgwick et al. 2007). This result was obtained by using PolIV with a C-terminal deletion of five amino acids, which are required for targeting PolIV to the β-clamp (Lenne-Samuel et al. 2002). The observed sensitivity of the dinB mutant is not due to a polar effect of the dinB gene deletion on the expression of three downstream genes, yafN, yafO, and yafP, from the same operon (Figure 6). This is also confirmed by the complementation of dinB-deficient mutants with the dinB-proficient gene, which restores resistance to the wild-type level (Figure 5).
Figure 5.—
Sensitivity of different PolIV-proficient and -deficient E. coli strains to MMS. (A) Sensitivity of parental and dinB strains with and without the pGB2 vector plasmid, pGB2 carrying the dinBΔC5 allele (coding for PolIV that cannot interact with the β-clamp), and pGB2 carrying the functional dinB gene, as well as of the mismatch-repair-deficient mutS strain with or without the functional dinB gene to 7.5 mm MMS were tested. (B) Sensitivity of different dinB-proficient and -deficient derivatives of the alkA tag mutant strains to 0.3 mm MMS were tested. alkA tag dinB intC∷dinB+ and alkA tag mutS dinB intC∷dinB+ strains carried the functional dinB gene and a chloramphenicol resistance cassette inserted in trans in the intC site on the E. coli chromosome. Each point represents the mean (± standard error) values from four to seven independent experiments. There is no difference in the viability of tested strains without the alkylation agents.
Figure 6.—
Sensitivity of different E. coli polymerase mutants to 7.5 mm MMS. Sensitivity to MMS was estimated by spotting 8 μl of 10-fold serial dilutions of overnight cultures of different dinB-proficient and -deficient strains onto LB plates containing MMS.
MMS-induced mutations:
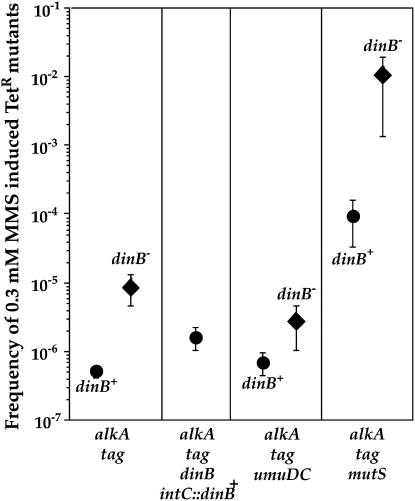
In the alkA tag-proficient background, there is no increase in mutation frequency upon treatment with MMS (data not shown), even at the dose that kills >90% of dinB-deficient cells (Figure 5A). However, in the alkA tag-deficient background, inactivation of the dinB gene results in an increase of the mean value of 0.3 mm MMS-induced mutation frequency in mismatch-repair-proficient and -deficient backgrounds (17- and 106-fold, respectively). This increase was significant in mismatch-repair-proficient and -deficient backgrounds (Mann–Whitney P = 0.006 and 0.014, respectively). In the absence of PolIV, mutation frequency increases due to PolV activity (Figure 7).
Figure 7.—
Effect of dinB gene deletion on the frequency of 0.3 mm MMS-induced TetR mutants in alkA tag background. Inactivation of the dinB gene results in a significant increase of mutation frequency in mismatch-repair-proficient and -deficient backgrounds. This increase is abolished by complementation with the functional dinB gene or by inactivation of the umuDC genes. Each point represents the mean (± standard error) values from four to seven independent experiments. There is no difference in the viability of tested strains without the alkylation agents.
DISCUSSION
Strong selective constraints imposed on the genes coding for PolIV observed in natural populations of E. coli (Bjedov et al. 2003), as well as the fact that there is a high amount of PolIV molecules even in nonstressed cells (Nohmi 2006), suggest that the activity of this DNA polymerase is very important for cell fitness and survival. PolIV was shown to perform DNA synthesis across a variety of base modifications in vitro and in vivo (Fuchs et al. 2004), which indicates that the biological role of this polymerase is to bypass DNA lesions that block replicative DNA polymerases. However, much of this DNA damage can also be bypassed by two other SOS polymerases: PolII and PolV (Fuchs et al. 2004). Therefore, we tried to identify DNA damage that is bypassed specifically and accurately by PolIV, which, by doing so, prevents other SOS polymerases from performing lesion bypass. As other DNA repair pathways can act on the same DNA lesions, we increased the amount of spontaneous DNA lesions using mutants deficient for different DNA repair pathways and measured the spontaneous mutation frequency of PolIV-proficient and -deficient backgrounds. The advantage of this approach is that we increased only DNA damage and not the damage of other cell constituents, as is frequently the case when cells are treated with chemical agents. The DNA lesions present at high concentration in these genetic backgrounds are presumably the most relevant for the evolutionary conservation of DNA repair proteins. We were looking for a genetic background in which inactivation of dinB would significantly increase mutation frequency (Figure 2). No significant increase or decrease in spontaneous mutation frequency was observed in the absence of PolIV in genetic backgrounds in which the repair of oxidative DNA damage (nei nth and mutM mutY mutants), abasic sites (xth nfo mutant), and bulky DNA adducts (uvrA mutant) was inactived in the absence of a functional mismatch repair system.
However, we found that E. coli PolIV polymerase participates in the error-free processing of DNA damage that accumulates in the genome of the alkA tag double-mutant strain (Figures 2 and 3). The fact that there is no such phenotype in alkA and tag single mutants indicates that each glycosylase eliminates DNA damage bypassed by PolIV and that only the absence of both AlkA and Tag provides enough substrate for PolIV-mediated bypass to be detected. tag is a constitutively expressed gene, while the expression of the gene coding for AlkA is controlled by an adaptive response, an inducible alkylation-specific DNA repair response (Volkert and Landini 2001; Sedgwick 2004). The adaptive response is under the positive control of the Ada protein, which removes alkyl groups from DNA and stimulates the expression of the ada, alkB, alkA, and aidB genes. In the absence of the Ada protein, the alkA gene cannot be induced, but Tag removes the substrate for PolIV, which explains why there is no significant effect of inactivation of dinB in the ada ogt double-mutant background (Figure 2).
Tag glycolysase excises 3-meA and, to a much lesser extent, 3-meG from DNA. AlkA has a much broader range of substrates, but it also excises 3-meA and 3-meG from DNA (Wyatt et al. 1999; Sedgwick et al. 2007). 3-meA and 3-meG are cytotoxic lesions that block both replication and transcription due to the aberrant alkyl group protruding into the minor groove of DNA (Wyatt et al. 1999; Sedgwick et al. 2007). Therefore, these two DNA lesions are the most likely candidates for the substrate for PolIV. PolIV contributes to the tolerance of cytotoxic alkylating DNA lesions induced by methylating and ethylating agents (Figure 4), which indicates that its activity is not limited to methyl adducts. It was recently shown that PolIV accurately bypasses N2-deoxyguanosine adducts (Jarosz et al. 2006), which are frequently formed from by-products of diverse cellular processes such as lipid peroxidation. Alkylating agents can alkylate the N2 site in guanine, but there is no evidence that this damage is recognized by AlkA and Tag (Wyatt et al. 1999; Friedberg et al. 2006); therefore the N2 alkylguanine lesion is probably not a major contributor to spontaneous mutagenesis in our study.
3-meG and 3-meA are mutagenic probably because they block DNA replication, induce the SOS response, and consequently induce the expression of genes coding for SOS polymerases (Boiteux et al. 1984). In addition, SOS induction is enhanced in bacteria deficient for the repair of alkylation cytotoxic lesions. In our experiments, mutations are generated by the activity of PolII and PolV (Figure 3). It was previously known that mutations induced by alkylating agents depend on PolV activity (Foster and Eisenstadt 1985), but this is the first report concerning the involvement of PolIV in the error-free processing of 3-meA and 3-meG. By doing this, PolIV prevents access of PolII and PolV to these lesions. Increase of mutation frequency in the alkA tag dinB strain relative to the alkA tag strain is dependent on the activity of nucleotide excision repair as well (Figure 3). Interestingly, nucleotide excision repair is also required for untargeted mutagenesis of nonirradiated λ-phage in UV-irradiated cells, which is PolIV dependent and PolV independent (Brotcorne-Lannoye and Maenhaut-Michel 1986). The exact role of nucleotide excision repair in promotion of mutagenesis is unclear. One possible explanation is that upon excision of an oligonucleotide carrying a damaged base, SOS polymerases generate mutations by participating in a resynthesis step. Their activity can be mutagenic because they (i) exhibit high error rates when copying normal DNA, (ii) because of the error-prone bypass of the lesion on the template strand, and/or (iii) because SOS polymerases have higher tendencies to incorporate damaged nucleotides. Second mechanisms would be similar to the involvement of PolII in the nucleotide-excision-repair-dependent repair of interstrand crosslinks (Berardini et al. 1999).
PolIV also contributes resistance to the killing effect of high doses of MMS in the alkA tag-proficient background (Figures 5A and 6). This may be one of the reasons why the dinB gene is expressed at a high level in unstressed cells; i.e., when cells are suddenly exposed to high doses of alkylating agents, a constitutive level of 3-methyladenine DNA glycosylases is not sufficient to ensure survival. To resist high doses of alkylating agents, bacteria must induce adaptive response (Sedgwick 2004). This response protects cells best when they are first exposed to low doses of alkylating agents, which, by inducing an adaptive response, allow cells to become resistant to the lethal and mutagenic effects of the subsequent high-level challenge from alkylating agents. Therefore, PolIV may be important for survival of cells exposed to high doses of alkylating agents prior to induction of an adaptive response.
In the light of our results, it is interesting that the expression of the dinB gene is elevated under carbon source starvation and stationary phase (Layton and Foster 2003). The induction of dinB gene transcription during stationary phase is controlled by RpoS. RpoS also upregulates the expression of ada and downregulates the expression alkA in stationary phase (Taverna and Sedgwick 1996; Landini and Busby 1999). Importantly, treatment with MMS does not induce expression of alkA in stationary phase cells, while, in rpoS mutant cells, alkA expression is significantly increased (Landini and Busby 1999). Such dual regulation of alkA gene expression by RpoS and Ada may result from the fact that the activity of AlkA may be deleterious in stationary phase. The overproduction of AlkA, unlike the overproduction of Tag, was shown to sensitize growing E. coli cells to alkylating agents (Kaasen et al. 1986) probably because AlkA generates more abasic sites and strand breaks as base-excision repair intermediates than can be efficiently repaired. Because the repair of abasic sites may be difficult in starving stationary phase cells, RpoS represses the alkA gene (AlkA produces abasic sites) but induces expression of the dinB gene. Intriguingly, PolIV cannot bypass abasic sites in vivo (Maor-Shoshani et al. 2003), but it can bypass 3-meA and 3-meG (this work). Furthermore, unlike replicative polymerase PolIII, PolIV and PolV have the potential to operate efficiently at low dNTP concentrations (Godoy et al. 2006), a condition encountered during stationary phase (Walker et al. 2004). Interestingly, it was recently proposed, on the basis of in vitro data, that the PolIV human homolog, Polκ, might also be utilized in repair replication under conditions of low nucleotide concentrations, for example, in nondividing cells (Ogi and Lehmann 2006).
In stationary phase E. coli cells, spontaneous generation of an endogenous DNA alkylating agent increases considerably, as suggested by the enhanced generation of mutations in stationary phase E. coli ada ogt cells (Mackay et al. 1994; Taverna and Sedgwick 1996; Bharatan et al. 2004). This may be true also for eukaryotes, because transcriptional profiles of Saccharomyces cerevisiae show that a large number of genes that were regulated in response to MMS are also regulated in response to being held at stationary phase (Fry et al. 2005). Consequently, a high amount of PolIV might help cells to survive cytotoxic alkylation DNA damage during stationary phase. This is particularly important in stationary phase when the synthesis of translation apparatus is inhibited and the number of ribosomes and rRNA gene expression decreases, resulting in a reduction in the rate of global protein synthesis (Saint-Ruf et al. 2004; Saint-Ruf and Matic 2006). If dinB were only under regulation of the SOS system, the induction of which requires new protein synthesis, it would be difficult to synthesize enough PolIV to survive exposure to alkylating agents during stationary phase. This may explain why PolIV is required for long-term survival in stationary phase.
What would the biological relevance of our observation be? All examined organisms possess DNA repair mechanisms that can specifically counteract the deleterious effects of DNA alkylation, which indicates that they are continuously exposed to alkylating agents and that this was also the case during their evolution. Alkylating agents are produced endogenously in cells and present in the environment. For E. coli, there are many possible sources of endogenous alkylating agents. S-adenosylmethionine, a methyl donor in many biochemical reactions, is a weak methylating agent (Sedgwick and Lindahl 2002; Sedgwick et al. 2007). Endogeneous nitrosation of amides, amines, amino acids, and related compounds can also generate alkylating agents, particularly during stationary phase (Sedgwick and Lindahl 2002). E. coli is exposed to exogenous alkylating agents in its primary habitat, the gastrointestinal tract of warm-blooded animals. Nitrosation of bile acids and food compounds that generate alkylating agents is mediated by bacterial flora, but also by a spontaneous chemical reaction in the stomach, where low pH facilitates this process (Lijinsky 1999; de Kok and van Maanen 2000; Drablos et al. 2004). It is therefore intriguing that the mouse PolIV homolog Polκ, similarly to its E. coli homolog (this work), is involved in translesion DNA synthesis across cytotoxic alkylation and that Polκ is present in epithelial cells lining the stomach (Velasco-Miguel et al. 2003; Takenaka et al. 2006). For humans, the involvement of Polκ in tolerance of alkylating DNA damage is, in addition to the above-mentioned examples, also relevant for cancer therapy because alkylating agents are used as cytostatic drugs. It can therefore be proposed that the capacity of the Y-family DNA polymerases from the DinB branch to bypass cytotoxic alkylating lesions in an error-free fashion is of major biological relevance.
Acknowledgments
We are grateful to M. Elez, R. Fuchs, L. Le Chat, A. Lindner, M. A. Petit, L. Samson, and M. Vulic for generous gifts of strains and plasmid. We thank Andy Barnes and Stéphane Delmas for comments on the manuscript. This work was supported by a grant from the Agence Nationale de la Recherche. I.B. was supported by a grant from the Association pour la Recherche sur le Cancer. D.S. held a Federation of European Biochemical Societies Summer Fellowship.
References
- Berardini, M., P. L. Foster and E. L. Loechler, 1999. DNA polymerase II (polB) is involved in a new DNA repair pathway for DNA interstrand cross-links in Escherichia coli. J. Bacteriol. 181: 2878–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharatan, S. M., M. Reddy and J. Gowrishankar, 2004. Distinct signatures for mutator sensitivity of lacZ reversions and for the spectrum of lacI/lacO forward mutations on the chromosome of nondividing Escherichia coli. Genetics 166: 681–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjedov, I., G. Lecointre, O. Tenaillon, C. Vaury, M. Radman et al., 2003. Polymorphism of genes encoding SOS polymerases in natural populations of Escherichia coli. DNA Rep. 2: 417–426. [DOI] [PubMed] [Google Scholar]
- Boiteux, S., O. Huisman and J. Laval, 1984. 3-Methyladenine residues in DNA induce the SOS function sfiA in Escherichia coli. EMBO J. 3: 2569–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcorne-Lannoye, A., and G. Maenhaut-Michel, 1986. Role of RecA protein in untargeted UV mutagenesis of bacteriophage lambda: evidence for the requirement for the dinB gene. Proc. Natl. Acad. Sci. USA 83: 3904–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courcelle, J., A. Khodursky, B. Peter, P. O. Brown and P. C. Hanawalt, 2001. Comparative gene expression profiles following UV exposure in wild-type and SOS-deficient Escherichia coli. Genetics 158: 41–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko, K. A., and B. L. Wanner, 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kok, T. M., and J. M. van Maanen, 2000. Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat. Res. 463: 53–101. [DOI] [PubMed] [Google Scholar]
- Delmas, S., and I. Matic, 2006. Interplay between replication and recombination in Escherichia coli: impact of the alternative DNA polymerases. Proc. Natl. Acad. Sci. USA 103: 4564–4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drablos, F., E. Feyzi, P. A. Aas, C. B. Vaagbo, B. Kavli et al., 2004. Alkylation damage in DNA and RNA-repair mechanisms and medical significance. DNA Rep. 3: 1389–1407. [DOI] [PubMed] [Google Scholar]
- Fernandez De Henestrosa, A. R., T. Ogi, S. Aoyagi, D. Chafin, J. J. Hayes et al., 2000. Identification of additional genes belonging to the LexA regulon in Escherichia coli. Mol. Microbiol. 35: 1560–1572. [DOI] [PubMed] [Google Scholar]
- Foster, P. L., 2000. Adaptive mutation in Escherichia coli. Cold Spring Harb. Symp. Quant. Biol. 65: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, P. L., and E. Eisenstadt, 1985. Induction of transversion mutations in Escherichia coli by N-methyl-N′-nitro-N-nitrosoguanidine is SOS dependent. J. Bacteriol. 163: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg, E. C., R. Wagner and M. Radman, 2002. Specialized DNA polymerases, cellular survival, and the genesis of mutations. Science 296: 1627–1630. [DOI] [PubMed] [Google Scholar]
- Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz et al. (Editors), 2006. DNA Repair and Mutagenesis, Ed. 2. American Society for Microbiology, Washington, DC.
- Fry, R. C., T. J. Begley and L. D. Samson, 2005. Genome-wide responses to DNA-damaging agents. Annu. Rev. Microbiol. 59: 357–377. [DOI] [PubMed] [Google Scholar]
- Fuchs, R. P., S. Fujii and J. Wagner, 2004. Properties and functions of Escherichia coli: Pol IV and Pol V. Adv. Protein Chem. 69: 229–264. [DOI] [PubMed] [Google Scholar]
- Godoy, V. G., D. F. Jarosz, F. L. Walker, L. A. Simmons and G. C. Walker, 2006. Y-family DNA polymerases respond to DNA damage-independent inhibition of replication fork progression. EMBO J. 25: 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldimann, A., and B. L. Wanner, 2001. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J. Bacteriol. 183: 6384–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz, D. F., V. G. Godoy, J. C. Delaney, J. M. Essigmann and G. C. Walker, 2006. A single amino acid governs enhanced activity of DinB DNA polymerases on damaged templates. Nature 439: 225–228. [DOI] [PubMed] [Google Scholar]
- Kaasen, I., G. Evensen and E. Seeberg, 1986. Amplified expression of the tag+ and alkA+ genes in Escherichia coli: identification of gene products and effects on alkylation resistance. J. Bacteriol. 168: 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. R., G. Maenhaut-Michel, M. Yamada, Y. Yamamoto, K. Matsui et al., 1997. Multiple pathways for SOS-induced mutagenesis in Escherichia coli: an overexpression of dinB/dinP results in strongly enhancing mutagenesis in the absence of any exogenous treatment to damage DNA. Proc. Natl. Acad. Sci. USA 94: 13792–13797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban, W., P. Jonczyk, D. Gawel, K. Malanowska, R. M. Schaaper et al., 2004. Role of Escherichia coli DNA polymerase IV in in vivo replication fidelity. J. Bacteriol. 186: 4802–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landini, P., and S. J. Busby, 1999. Expression of the Escherichia coli ada regulon in stationary phase: evidence for rpoS-dependent negative regulation of alkA transcription. J. Bacteriol. 181: 6836–6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson, K., J. Sahm, R. Shenkar and B. Strauss, 1985. Methylation-induced blocks to in vitro DNA replication. Mutat. Res. 150: 77–84. [DOI] [PubMed] [Google Scholar]
- Layton, J. C., and P. L. Foster, 2003. Error-prone DNA polymerase IV is controlled by the stress-response sigma factor, RpoS, in Escherichia coli. Mol. Microbiol. 50: 549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layton, J. C., and P. L. Foster, 2005. Error-prone DNA polymerase IV is regulated by the heat shock chaperone GroE in Escherichia coli. J. Bacteriol. 187: 449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenne-Samuel, N., J. Wagner, H. Etienne and R. P. Fuchs, 2002. The processivity factor beta controls DNA polymerase IV traffic during spontaneous mutagenesis and translesion synthesis in vivo. EMBO Rep. 3: 45–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijinsky, W., 1999. N-Nitroso compounds in the diet. Mutat. Res. 443: 129–138. [DOI] [PubMed] [Google Scholar]
- Mackay, W. J., S. Han and L. D. Samson, 1994. DNA alkylation repair limits spontaneous base substitution mutations in Escherichia coli. J. Bacteriol. 176: 3224–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maor-Shoshani, A., K. Hayashi, H. Ohmori and Z. Livneh, 2003. Analysis of translesion replication across an abasic site by DNA polymerase IV of Escherichia coli. DNA Rep. 2: 1227–1238. [DOI] [PubMed] [Google Scholar]
- McKenzie, G. J., P. L. Lee, M. J. Lombardo, P. J. Hastings and S. M. Rosenberg, 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7: 571–579. [DOI] [PubMed] [Google Scholar]
- Nohmi, T., 2006. Environmental stress and lesion-bypass DNA polymerases. Annu. Rev. Microbiol. 60: 231–253. [DOI] [PubMed] [Google Scholar]
- Ogi, T., and A. R. Lehmann, 2006. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat. Cell Biol. 8: 640–642. [DOI] [PubMed] [Google Scholar]
- Ohmori, H., E. C. Friedberg, R. P. Fuchs, M. F. Goodman, F. Hanaoka et al., 2001. The Y-family of DNA polymerases. Mol. Cell 8: 7–8. [DOI] [PubMed] [Google Scholar]
- Rattray, A. J., and J. N. Strathern, 2003. Error-prone DNA polymerases: when making a mistake is the only way to get ahead. Annu. Rev. Genet. 37: 31–66. [DOI] [PubMed] [Google Scholar]
- Saint-Ruf, C., and I. Matic, 2006. Environmental tuning of mutation rates. Environ. Microbiol. 8: 193–199. [DOI] [PubMed] [Google Scholar]
- Saint-Ruf, C., F. Taddei and I. Matic, 2004. Stress and survival of aging Escherichia coli rpoS colonies. Genetics 168: 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider, D., D. Faure, M. Noirclerc-Savoye, A. C. Barriere, E. Coursange et al., 2000. A broad-host-range plasmid for isolating mobile genetic elements in gram-negative bacteria. Plasmid 44: 201–207. [DOI] [PubMed] [Google Scholar]
- Sedgwick, B., 2004. Repairing DNA-methylation damage. Nat. Rev. Mol. Cell Biol. 5: 148–157. [DOI] [PubMed] [Google Scholar]
- Sedgwick, B., and T. Lindahl, 2002. Recent progress on the Ada response for inducible repair of DNA alkylation damage. Oncogene 21: 8886–8894. [DOI] [PubMed] [Google Scholar]
- Sedgwick, B., P. A. Bates, J. Paik, S. C. Jacobs and T. Lindahl, 2007. Repair of alkylated DNA: recent advances. DNA Rep. 6: 429–442. [DOI] [PubMed] [Google Scholar]
- Strauss, B. S., R. Roberts, L. Francis and P. Pouryazdanparast, 2000. Role of the dinB gene product in spontaneous mutation in Escherichia coli with an impaired replicative polymerase. J. Bacteriol. 182: 6742–6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takenaka, K., T. Ogi, T. Okada, E. Sonoda, C. Guo et al., 2006. Involvement of vertebrate Polkappa in translesion DNA synthesis across DNA monoalkylation damage. J. Biol. Chem. 281: 2000–2004. [DOI] [PubMed] [Google Scholar]
- Taverna, P., and B. Sedgwick, 1996. Generation of an endogenous DNA-methylating agent by nitrosation in Escherichia coli. J. Bacteriol. 178: 5105–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins, J. D., J. L. Nelson, J. C. Hazel, S. L. Leugers, J. D. Stumpf et al., 2003. Error-prone polymerase, DNA polymerase IV, is responsible for transient hypermutation during adaptive mutation in Escherichia coli. J. Bacteriol. 185: 3469–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Miguel, S., J. A. Richardson, V. L. Gerlach, W. C. Lai, T. Gao et al., 2003. Constitutive and regulated expression of the mouse DinB (Polkappa) gene encoding DNA polymerase kappa. DNA Rep. 2: 91–106. [DOI] [PubMed] [Google Scholar]
- Volkert, M. R., and P. Landini, 2001. Transcriptional responses to DNA damage. Curr. Opin. Microbiol. 4: 178–185. [DOI] [PubMed] [Google Scholar]
- Walker, K. A., P. Mallik, T. S. Pratt and R. Osuna, 2004. The Escherichia coli Fis promoter is regulated by changes in the levels of its transcription initiation nucleotide CTP. J. Biol. Chem. 279: 50818–50828. [DOI] [PubMed] [Google Scholar]
- Wolff, E., M. Kim, K. Hu, H. Yang and J. H. Miller, 2004. Polymerases leave fingerprints: analysis of the mutational spectrum in Escherichia coli rpoB to assess the role of polymerase IV in spontaneous mutation. J. Bacteriol. 186: 2900–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, M. D., J. M. Allan, A. Y. Lau, T. E. Ellenberger and L. D. Samson, 1999. 3-Methyladenine DNA glycosylases: structure, function, and biological importance. BioEssays 21: 668–676. [DOI] [PubMed] [Google Scholar]
- Yang, W., 2003. Damage repair DNA polymerases Y. Curr. Opin. Struct. Biol. 13: 23–30. [DOI] [PubMed] [Google Scholar]
- Yeiser, B., E. D. Pepper, M. F. Goodman and S. E. Finkel, 2002. SOS-induced DNA polymerases enhance long-term survival and evolutionary fitness. Proc. Natl. Acad. Sci. USA 99: 8737–8741. [DOI] [PMC free article] [PubMed] [Google Scholar]