Abstract
Maturation of auditory perceptual and discrimination process within the first two years of life is investigated in healthy infants by examining event-related potentials (ERPs). High-density EEG signals were recorded from the scalp monthly between 3 and 24 months of age. Two types of stimuli (100 vs. 100 Hz for standard stimuli; 100 vs. 300 Hz for deviant stimuli; occurrence rate: 85:15%) were presented using an oddball paradigm. Latencies and amplitudes were compared across development. The results showed that latencies of P150, N250, P350, and N450 components gradually decreased with increasing age. Amplitudes of the N250 and P350 components gradually increased and reached the maximum at 9 months, and then gradually decreased with the increase of age. Mismatch negativity was not obvious at 3 months of age, but was seen at 4–5 months and became robust after 6 months. Robust late positivity was recorded at all ages. These mismatch responses were noticeable in the frontal, central, and parietal areas, and the maximal MMN amplitude distribution gradually moved from the parietal area to the frontal area across the age range. Two important periods—one around 6 months and the other around 9 months are suggested in the maturation of auditory central system. Dynamical changes in the underlying source strengths and orientations may be principal contributors to ERP morphological changes in infants within the first 24 months.
Keywords: EEG, Auditory event-related potentials, Child, Development, Mismatch negativity
1. Introduction
The examination of auditory event-related potentials (ERPs) is a useful tool of studying cognitive functions in infants because non-invasive methods available for assessing brain psychophysiological functions at this early age are very rare and behavioral methods provide rather limited information of central sensory processing [1]. It has been shown that with the increase of age, infant ERP waveforms gradually become complex. Amplitudes of the ERP components progressively increase while latencies gradually decrease [2–4]. In most of the previous ERP studies of infants, ERP signals were usually obtained from one study visit. Although some longitudinal studies have been conducted recently, ERPs are often less frequently recorded. The relatively longer intervals between two ERP recordings can result in difficulties in interpretations of morphological changes seen in ERP components. Developmental evidence from monthly examined ERPs in the same subjects has not been made available.
Auditory ERPs recorded from the scalp consist of a series of waves at different latencies that are thought to reflect neural activity at different levels of the central nervous systems. In adults, early brain response components, such as P1 and N1 components, are thought to be exogenous and are associated with neural activity involved in the lateral lemniscus nuclei, the inferior colliculus and the thalamic nuclei, whereas activations underlying adult P2 component may involve unspecific projections to auditory cortex. Relatively longer latencies of ERP components are measured in infants and children. Brain responses to tonal changes in infants have been investigated in a number of studies, but the observations have not yet agreed upon each other. For example, some investigators showed that brain activations induced by tonal stimuli are predominant in the right hemisphere [5–6], whereas in some other studies, the highest amplitude of brain responses to tones are observed in the left hemisphere [7–9]. Dehaene-Lambertz [10] found that discrimination responses to tones were detected in the left frontal area in infants at 4 months of age, while these brain responses induced by tones were seen bilaterally by some other researchers [11]. The above differences in observations had been thought to be caused by insufficient spatial resolution (e.g. small electrode numbers), which made it difficult to distinguish a genuine effect from a spurious artificial effect [10]. Therefore, high-density EEG/ERP recordings are required for evaluation of brain regions involved in auditory processing in infants.
One approach to investigation of brain discrimination process to tonal changes is to induce mismatch responses, such as mismatch negativity (MMN). MMN is elicited in an oddball paradigm, where a difference between a deviant sound input and a sensory-memory trace representing physical features of repetitively presented standard sounds is detected [12]. Since the MMN response does not require active attention of subjects, it is well suited for acquisition in infants and very young children. Infant MMN has only received attention in the recent years [2–3,5,9–10,13–15]. Cheour et al. [16] compared brain responses to familiar and unfamiliar languages in infants at 6 and 12 months of age, suggesting that language-dependent memory traces may emerge in the brain sometime between those two ages.
Because of the above facts, we think that it is essential to conduct a longitudinal study in which high-dense ERPs are frequently acquired from infants to investigate the development of auditory processing. In this study, we are interested in the following questions. (1) At what age is the auditory cortex organized into functional neural network to detect changes in sounds? (2) How do the auditory discrimination functions change across age? (3) How do topographic distributions change across development of brains, and (4) what possible neural mechanisms contribute to those changes? Therefore, in order to compare waveforms across development, ERP signals were monthly recorded in the present study from the same five subjects until 2 years of age. Moreover, in order to compare ERP topographical features at critical developmental stages, ERP signals were also collected from some additional subjects at each of five ages.
2. Methods
2.1. Participants
ERP waveforms were obtained from five healthy infants (two males) monthly between the age of 3 months and 24 months. In addition to these data, ERPs from another 14 healthy infants (seven males) were also recorded at each of five ages (6, 9, 12, 16, and 24 months). At each of these ages, the data from these 14 subjects and the data from five subjects were pooled for topographical comparisons. All infants were full term (gestation age 39–42 weeks, weight 2600–4400 g, length 46–56 cm), with no history of neurological or developmental disorders reported. These infants were selected from those who participated in a longitudinal study. Infants who had a risk of hearing or other brain dysfunctions (e.g. from families having a history of language impairment) were not included in this study. Prior to participation, informed parental consents were obtained. The protocol of this study was approved by the Institutional Review Board of Rutgers University, where the study was conducted.
EEG/ERP signals were recorded while the infants were awake and seated comfortably on their parent’s lap in a soundproof and electrically shielded room. Silent movies were played on a monitor in front of the children to engage them and minimize their movement. An experimenter would attract the children using a puppet show or other toys if they lost interest in the video.
2.2. Stimuli
Each of the stimuli used in this study contained two complex tones that consisted of all harmonics with a fundamental frequency of 100 or 300 Hz and a 6 dB roll-off per octave. Each tone lasted for 70 ms, with a 5 ms rise time and 5 ms fall time. The two tones were separated by a silent period of 300 ms. Two types of stimuli were presented by using an oddball paradigm. The frequent stimuli (standard, 85%) contained two 100 Hz tones, and the infrequent stimuli (deviant, 15%) had 100 and 300 Hz tones. These stimuli were played binaurally at a rate of 1140 ms per stimulus through two loudspeakers (intensity 75 dB sound pressure level at the ears). Therefore, the standard and deviant stimuli only differed for the frequencies of the second tones. A total of 833 stimuli were presented. The presentation sequence was randomized and deviant stimuli were separated by at least three standard stimuli. About 8–10 standard stimuli were presented at the beginning of the presentation sequence.
These stimuli were used because our previous studies reveal that the ability to efficiently and accurately process rapidly presented sequential auditory stimuli reflects fundamental skills underlying the development of language [17]. Stimuli with a 300 ms inter-stimulus interval (ISI) can be easily discriminated at an early age as compared to stimuli with shorter ISIs [18]. The frequencies of tones used were close to the fundamental frequencies of human speech sounds.
2.3. EEG recordings
EEG signals were recorded at 62 electrode sites from the scalp using Geodesic Sensor Nets (Electric Geodesic, Inc., Eugene, OR, USA). The vertex was used as a common reference during recording. Electrodes placed above the canthus of both eyes were used for monitoring eye movement. A bandpass filter from 0.1 to 100 Hz was applied before digitization at 250 Hz. The data were re-referenced off-line to both mastoid electrodes.
The EEGs were segmented and averaged according to the stimulus types. The duration of each EEG segment was 1140 ms, including a 50 ms pre-stimulus segment for baseline correction. EEG segments containing signals higher than ±100 μV were excluded from averaging.
2.4. Measures and statistical analyses
The development of ERP morphology was described based on both monthly recorded ERPs and those collected at five ages. In addition to inspections of grand averaged waveforms, signals from each individual subject were also examined for inter-subject variations. Results presented here are based on the group data. However, individual data will also be reported if all the subjects did not show the same changes. ERP components were labeled according to their peak latencies. The peak latency was measured as the time point where the maximal positive (or negative) amplitude was recorded. Amplitudes were measured relative to the baseline. For example, the positive component peaked around 150 ms was termed as P150. Latencies and amplitudes of the ERP components were measured and compared across development. Results from prefrontal, frontal, central, parietal, occipital, anterior temporal, mid-temporal, and posterior temporal areas are presented here. In addition to the statistical comparisons of latencies and amplitudes for all ages, the measures obtained at the five ages from all 19 subjects were also examined. A repeated-measure analysis of variance (ANOVA) with the factors of age, stimulus type (standard vs. deviant), hemisphere, and brain area was employed. To measure mismatch responses, difference waves were obtained by subtracting waves to standard stimuli from those to deviants. Main effects of factors (i.e. age, hemisphere, and brain area) were examined by repeated-measure ANOVA. Greenhouse–Geisser adjustments were performed when applicable. The Newman–Keuls test was used as a post hoc for comparisons of individual values. Significance level was set at P<0.05.
3. Results
Dynamical changes in ERP waveforms across development were revealed by visual inspections. Congruous changes were observed in all the infants. Similar waveforms were elicited by each of the standard tones. A prominent positive component was induced from the prefrontal, frontal, central, parietal, and anterior temporal areas in all infants at each age. The amplitude was low at 3 months of age. The latency ranged between 110 and 180 ms (refer to P150). After the P150 component, a negative deflection was induced and the peak latency was measured between 220 and 300 ms. This component (N250) was clearly seen in infants after 4 months of birth, and had similar maximal distributions to those of the P150 component.
More complex waveforms were elicited by the deviant tones. Besides the P150 and N250 components, another positive component was induced, with the peak latency measured between 310 and 410 ms (called P350 here). The maximal distribution was seen in the frontal, central, and parietal areas. The P350 amplitude gradually increased, making the P350 component more prominent after 7 months of age. A negative deflection was elicited between 410 and 590 ms (called N450 here). This low-amplitude wave was recorded from all infants at all ages. An example of waveforms to standard and deviant tones recorded at 12 months is demonstrated in Fig. 1.
Fig. 1.
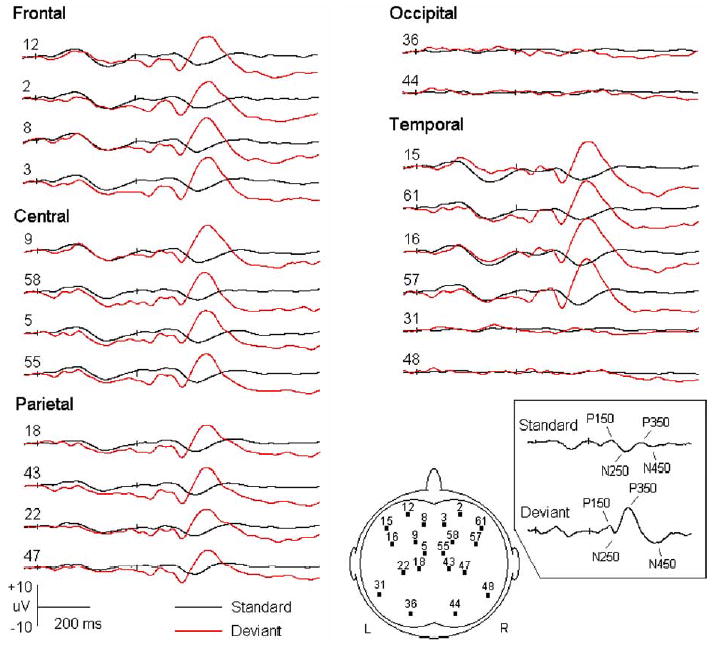
Grand averaged event-related potentials (ERPs) to standard and deviant stimuli (five subjects at 12 months of age). The onsets of the tone pairs are indicated by small ticks (at 0 and 370 ms, respectively). Out of the 62 channels, only a few waveforms are displayed to represent different brain areas. The number in front of each wave indicates the position of corresponding electrode. Its location is also demonstrated in the figure that represents the scalp. The ERP components induced by the standard and deviant tones are indicated in the box (those to the first tones are not shown).
The most prominent wave seen from difference waves was a positive deflection (late positivity). Inspections of the late positivity revealed that its amplitude gradually increased and reached the maximum at 9 months of age, and then turned to decrease gradually. A MMN appeared at 4–6 months of age and became robust after 6 months of age. Dynamical changing patterns were seen on the MMN and late positivity across development. The changes in waveforms across age are demonstrated in Fig. 2. In order to clearly demonstrate the changes of waveforms, the waves to standard and deviant tones are displayed separately (i.e. not superimposed for direct comparisons). The difference waves (deviant–standard) are also shown in this figure.
Fig. 2.
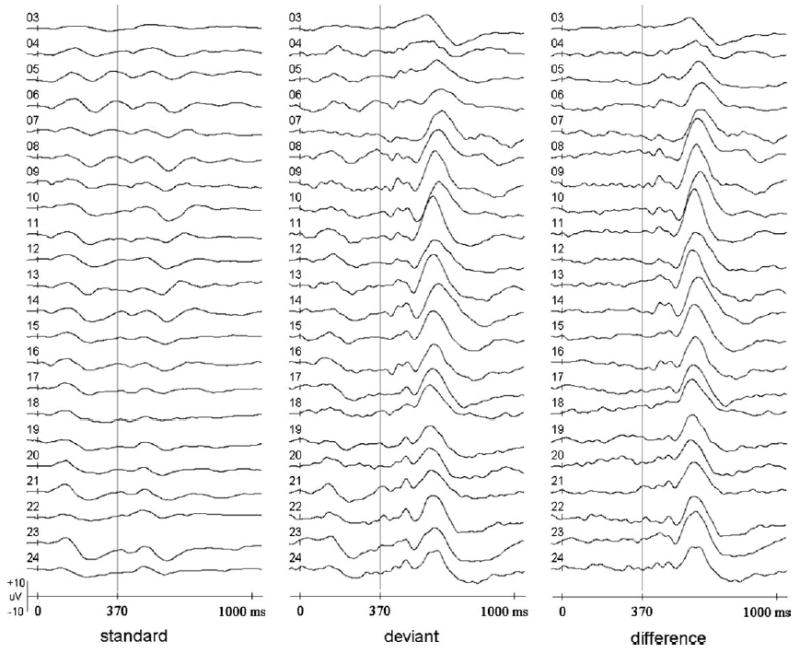
Comparisons of event-related potential waveforms across age. The onset of the first tone is indicated at 0 ms. The onset of the second tone (at 370 ms) is represented by vertical lines. The waves displayed were recorded from the same electrode site at each of the ages (Channel 9, also see Fig. 1). The ages in months are shown in front of the waves. The signals were averaged from all the five subjects.
Detailed statistical results for each ERP component are reported below. To facilitate comparisons, the major findings are also summarized in Table 1.
Table 1.
Summary of major changes seen in ERP components
| Latency | Amplitude | |
|---|---|---|
| P150 | Gradually decrease across age. Shorter in the central areas than in the frontal areas | Maximal at 10–11 months. Fronto-central maximal distributions |
| N250 | Gradually decrease across development. Shorter in the centro-parietal areas than in the frontal areas
Shorter to the deviant tones than to the standard tones |
Maximal at 9 months. Vary among the brain areas. Greater to the deviant tones than to the standard tones |
| P350 | Relatively shorter at two periods (3–4 and 17–21 months)
Shorter to the deviant tones than to the standard tones |
Maximal at 9 months (to the deviant tones) and 16 months (to the standard tones) |
| N450 | Shorter in the centro-parietal areas than in the frontal areas
Shorter to the deviant tones than to the standard tones |
Maximal at 9 months. Change as a function of brain area |
| Mismatch negativity | Relatively shorter in the frontal and central areas | More negative at 9–12 months than at other ages
Maximal scalp distributions gradually shift from the parietal areas to the frontal areas across age |
| Late positivity | Shorter in the frontal and central areas at each age | Frontal maximal distributions |
3.1. The P150 component
The P150 latencies significantly differed across age (F= 62.9, P<0.001) indicating that the P150 latency changes as a function of age. The post hoc test revealed that the latency gradually decreased across age (P<0.05). From 3 to 24 months, the latency decreased by 41 ms in the frontal area (P<0.01) and by 36 ms in the central area (P<0.01). Pearson Product–Moment Correlation analysis showed that changes in the P150 latency were significantly correlated to age (r=−0.89, P<0.05). A significant effect was detected for brain area (F=5.73, P<0.01). The latency measured in the central areas was significantly shorter than that measured in the frontal areas (P<0.05). This effect was not modified by age (F=0.83, P>0.6). The P150 latency significantly changed between hemispheres (F=6.33, P<0.05). There was a trend that in the mid-temporal areas, the P150 component was present earlier in the left hemisphere (i.e. shorter latency) than that in the right hemisphere at all ages. At 9 months of age, the P150 component appeared earlier in the right frontal area than that in the left frontal. These changes, however, did not reach significance (P>0.05, see Fig. 3A and B).
Fig. 3.
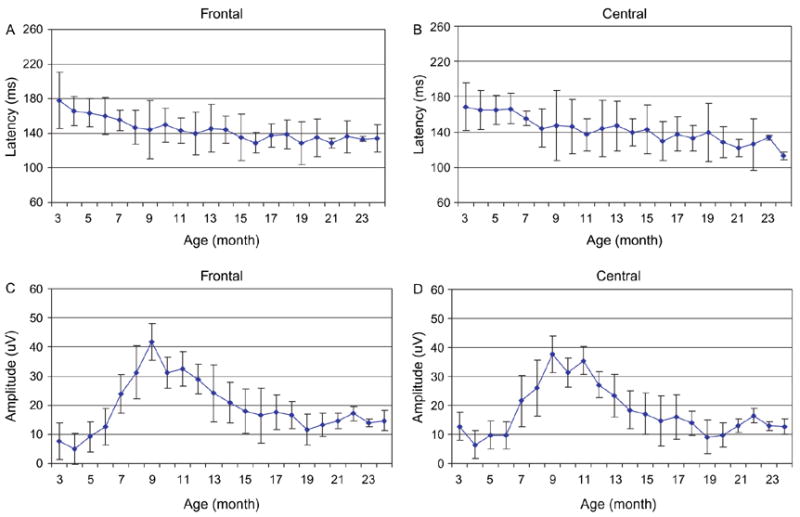
Comparisons of P150 latencies to the standard stimuli in the frontal (A) and central (B) areas and amplitudes in the frontal (C) and central (D) areas (five subjects, shown as mean ± SD). The amplitude was calculated as differences between the N250 and P350 amplitudes.
The main effect of age was significant for the P150 amplitude (F=52.3, P<0.01). The highest amplitude was measured at 10–11 months of age. The amplitude significantly varied among brain areas (F=43.5, P<0.01), with a maximal distribution over the fronto-central area. The P150 amplitude was higher in the left frontal area than in the right side at 6 months. This hemispheric predominance was not always seen across the development. After 6 months of age, relatively stronger responses were recorded from the right frontal area. This difference, however, did not reach significance (F=1.62, P=0.2).
3.2. The N250 component
Significant effect was detected on the N250 latencies for age (F=50.6, P<0.01) indicating that the N250 latency changes as a function of age. The N250 latency also differed among brain areas (F=7.78, P<0.01). For example, the latency measured in the frontal area decreased by 33 ms from 3 to 24 months (P<0.01). The latencies in the centro-parietal area were shorter than those in the frontal area (P<0.05). These trends were observed at all ages. On the other hand, the N250 latency to the deviant tones was relatively shorter than that to the standards at all ages. For instance, at 3 months the mean difference in latencies was 78 ms in the frontal area (P<0.01). No hemispheric difference was found on the N250 latency (F=0.74, P>0.3).
Significant effects were indicated on the N250 amplitude for the factors of age (F=8.40, P<0.01) and brain area (F=40.7, P<0.01) revealing that the amplitude varies as a function of age and brain area. The N250 amplitude induced by the deviant tones was significantly higher than that induced by the standards (F=116, P<0.01). This difference was measured from all brain areas at each age. Moreover, significant interaction effect was detected between age and stimulus type (F=6.70, P<0.01). The post hoc test showed that the N250 amplitude to the standard stimuli was similar across age; however, a complex changing pattern was observed on the amplitude to the deviant stimuli—the amplitude gradually increased and reached the maximum at 9 months of age, and then gradually decreased with increasing age. Hemispheric differences were not detected (F=0.104, P>0.7).
3.3. The P350 component
The P350 latency significantly varied across age (F= 3.00, P<0.05). Comparisons of the measures showed that the P350 latency was shorter at two age periods: 3–4 and 17–21 months. The P350 latency to the deviant stimuli was apparently shorter than that to the standards (F=3895, P<0.01). The interaction between age and stimulus type was significant (F=22.2, P<0.01). The post hoc test showed that the latency to the deviant stimuli was 117 ms shorter than that to the standard stimuli at 12 months of age (P<0.01). This difference in latency decreased to 91 ms at 24 months (P<0.01). No hemispheric effect was detected on the latency (F=0.06, P>0.8).
For the P350 amplitude, significant effects were detected for the factors of age (F=87.0, P<0.01) and brain region (F=23.1, P<0.01). Again, the P350 amplitude gradually increased and reached the maximum at 9 months of age, and then gradually decreased with increasing age, regardless of stimulus types. For example, the values of mean amplitudes to the standard stimuli in the frontal area were 2.06, 2.70, 2.59, 2.19, and 1.18 at the ages of 6, 9, 12, 16, and 24 months, respectively. Dynamical changes in amplitudes are demonstrated in Fig. 3C and D.
3.4. The N450 component
The N450 latency significantly varied among different ages (F=10.6, P<0.01) showing that the N450 latency changes as a function of age. Stimulus type significantly interacted with the brain area (F=2.51, P<0.05). The post hoc test revealed that the N450 component to the standard stimuli appeared earlier in the frontal area than in the central and parietal areas (by 11 and 9 ms, respectively, P<0.05), whereas the presence of N450 component to the deviant stimuli was earlier in the central and parietal areas than in the frontal area by 6 and 8 ms, respectively. Similar changes in the mean N450 latency were observed at all ages. Laterality effect was not significant (F=0.03, P>0.8).
The N450 amplitude varied as a function of age (F=47.8, P<0.01) and stimulus type (F=17.8, P<0.01). For example, in the frontal area, the highest N450 amplitude to the standard stimuli was measured at 16 months of age, whereas the highest amplitude to the deviant stimuli was recorded at 9 months.
3.5. The mismatch responses
The MMN latency was significantly affected by age (F= 5.05, P<0.01, see Fig. 4) and brain area (F=2.48, P< 0.05). MMNs were present earlier in the frontal area in both hemispheres at 6, 12, and 24 months, but at 9 and 16 months, the MMNs appeared earlier in the central area than other brain areas. The main hemispheric effect was not detected on the MMN latency (F=0.33, P>0.05).
Fig. 4.
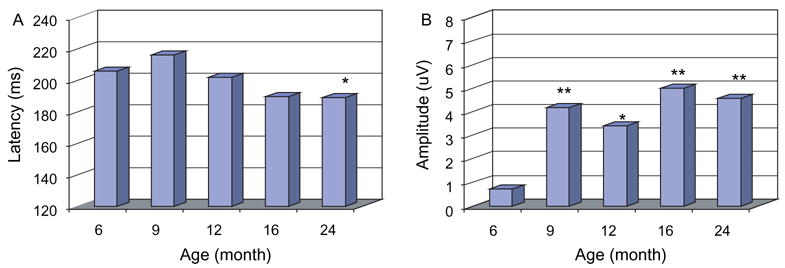
Latencies (A) and amplitudes (absolute values, B) of mismatch negativity components (from right mid-temporal areas, all subjects). All significant between-age differences are marked. *P<0.05, **P<0.01 (for the latencies: between 6 and 24 months; for the amplitudes, between 6 months and other ages).
Significant effects on MMN amplitudes were detected for age (F=18.9, P<0.01, see Fig. 4) and brain region (F= 5.59, P<0.01). The interaction between age and brain area was also significant (F=2.01, P< 0.01). The post hoc test revealed that the MMN recorded between 9 and 12 months was more negative than that recorded at other ages, and at 6 months of age, the highest MMN amplitude was seen over the parietal area; however, this maximal distribution gradually shifted anteriorly, showing a maximal fronto-central distribution after 6 months of age. The MMN component was more negative in the left parieto-occipital areas than in the right side between 6 and 9 months of age, where the MMN response was stronger in the right fronto-central areas than that in the left side between 12 and 24 months. These differences, however, did not reach significance (F=2.90, P=0.09).
The peak latency of the late positivity was significantly affected by the factors of age (F=16.9, P<0.01) and brain area (F=5.97, P<0.01). The interaction between age and brain area was also significant (F=1.84, P<0.05). The post hoc test revealed that the longest latencies were measured in the parietal area at all ages. At 16 months of age, the late positivity appeared relatively earlier in the frontal area, while at 6, 9, 12, and 24 months, the late positivity presented earlier in the central area. Hemispheric differences were not significant (F=0.04, P>0.05).
The amplitude of late positivity changed as a function of age (F=62.1, P<0.01), and significantly varied among brain areas (F=33.6, P<0.01). The post hoc test revealed a maximal amplitude distribution in the frontal areas. No significant hemispheric effect was observed on the amplitude (F=0.16, P>0.6, Fig. 5).
Fig. 5.
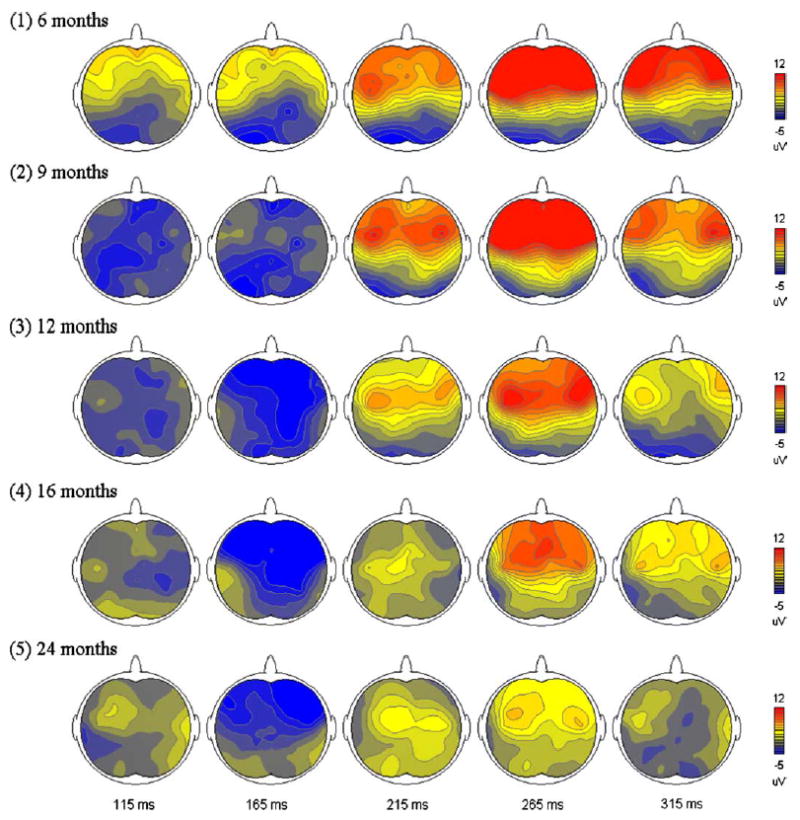
Amplitude scalp distributions of mismatch responses. Brain areas of higher amplitudes are shown in red, whereas those in lower amplitudes are in blue. The data were calculated from all subjects. (For interpretation of the reference to color in this legend, the reader is referred to the web version of this article.)
4. Discussion
Developmental trajectories of auditory ERP waveforms were investigated in the same infants between 3 and 24 months of age. Major findings of this study are the followings. (1) Important morphological changes in ERP components were observed at 6–7 months of age. (2) Latencies of ERP components gradually decreased with increasing age. (3) Biphasic changes in amplitudes of N250 and P350 components as well as mismatch responses were observed. With gradually increasing amplitude, these ERP components reached the maximal strength at 9 months of age and then gradually attenuated. (4) Positive mismatch responses were elicited at all ages, while robust adult-like MMN responses were induced after 6 months of age. The maximal amplitude distribution of MMN gradually moved to the frontal areas at 24 months of age.
4.1. Morphological changes of ERP components
With monthly acquired ERP records, several developmental changes were found from the ERP waveforms. One positive and one negative (N450) components were elicited by the deviant stimuli at 3 months. It is difficult to unambiguously link this positive wave to the P350 component at this age due to morphological differences between these two positive waves. At 4 months of age, a negative trough started to emerge in the front part of this positive wave, splitting the positive wave into two components (i.e. P150 and P350). The P150 and N250 components started to emerge at 5–6 months of age and gradually became higher in amplitude, and the appearance of P350 and N450 components was slightly postponed. All these four ERP components were robustly observed after 6 months of age. These morphological changes are consistent with previous findings [2,14]. The amplitudes of N250 and P350 components gradually increased between 3 and 9 months. This result is congruous with the one reported by Kushnerenko et al. [3]. Additional developmental changes were also revealed in this study—a biphasic changing pattern was detected on the amplitudes of N250 and P350 components. After reaching the maximum of strength at 9 months, the amplitude of the two components started to decrease.
Amplitude distributions of the P150 component dynamically changed across the development. The P150 component was predominant in the left frontal area at 6 months. The maximal amplitude distribution gradually shifted to the right frontal area after 6 months of age. This left hemispheric predominance is also observed at 2 and 4 months of age in studies of infants by Dehaene-Lambertz and associates [10,19,20]. In addition, sources underlying the occipital areas are also indicated in those studies. These occipital sources, however, were not present in our present study. One of the reasons causing the differences may be due to different referential methods used. EEG signals were transformed relative to the mean values of all electrodes in their studies, which might lead to the presence of large waveforms that otherwise were smaller in a link-mastoid montage [21]. This referential influence is demonstrated in a study, where the polarity of frontal P300 component is reversed when an average reference is used [22].
Regarding these developmental changes, it seems that neural networks consisting of multiple dynamically changing sources might have contributed to the P150, N250, P350, N450 components. Both strengths and orientations of these sources may change across age. At 3 months of age, activations of the P150 and P350 sources are predominant and overlap, resulting in a single positive waveform at this age. At 4–6 months, the N250 response gradually becomes stronger, leading to a robust N250 wave and the separation of P150 and P350 waves. The peak latency of P150 wave is decreased because of the growth of N250 activation. This effect also causes the postponement of the peak of P350 wave. From 6 months of age, activation of the N450 source becomes stronger. Activities of the N250 and N450 sources overlap with those of the P350 source, leading to a short N450 latency and attenuated P350 waveform. Variations in dipole orientations may contribute to the biphasically changing ERP amplitudes. These changes may be due to the consequence of different maturation rate of cerebral layers and connectivity [23]. The amplitude variations may also be caused by changes in the planum temporale secondary to the growth of frontal lobes [10]. These continuous changes in dipole orientations result in the decrease in signal strength at the scalp. This neural mechanism is parallel to that proposed by Kushnerenko et al. [3].
There are several possible mechanisms underlying those changes. First, axons and dendrites of neurons rapidly develop during the first several months after birth. The growth of dendritic trees and spines occurs in all layers of cortex, and the length of prefrontal dendrites may increase 5–10 times during the first 6 months of postnatal life [24]. Second, improvement in synaptic efficacy may also result in changes in ERP latencies and amplitudes. For example, Huttenlocher and de Courten [25] found that the greatest increases in synaptogenesis in the occipital cortex occur between 2.5 and 8 postnatal months; however, this process is relatively slower in the frontal cortex where synapse formation does not reach its maximum density until after 15 postnatal months. Third, influences of myelin development on the morphology of waveforms have been demonstrated in animal studies [26], showing that brain responses can be impaired if the brain is exposed to carbon monoxide during the developmental stage of myelination [27]. Moreover, the number of neurons involved in the sensory processes may vary at different ages. In a histological study of Vasil’eva and Shumeiko [28], structural organizations of human neurons in the pre-central, temporal, parietal and occipital cortex were examined. Continuously changing morphology accompanied by varying spatial distributions and cell numbers were found in these neurons within the first year of life.
4.2. Morphological changes of mismatch responses
The MMN latencies remarkably differed for the first 24 months of life. It appears that the most obvious decreases in MMN latency occurred within the first year. This result is parallel to previous studies [14]. Ejiri et al. [29] also observed a progressive decrease in MMN latency in subjects within 7 years of age. According to our study, the MMN was not apparent at 3 months of age in all the subjects. In fact, one individual showed a slightly negative wave prior to the large positive component, but it was difficult to determine whether it was a real MMN component. Robust MMN was observed after 6–7 months of age. The absence of MMN at this young age has also been documented by Morr et al. [15] and Kushnerenko et al. [4]. In their studies, MMN could be elicited in some infants at 6 months of age, but not at 3 months. The absence of MMN might be caused by the mask of other components, such as P3b [4]. On the other hand, the late positivity is robust at each of the ages. This brain response has been associated with the perceptual saliency [5,30]. Latencies of both MMN and late positivity decreased with increasing age. These findings are parallel to those of earlier studies [13,31–32]. The changes in amplitudes of mismatch responses found in the present study are consistent with the previous observations [4].
Topographic distributions of mismatch responses dynamically change across development. At 6 months of age, the MMN is predominant in the parietal areas. The maximal amplitude distribution gradually moves to the frontal areas at 24 months. In addition, the MMN responses are more prominent in the right frontal area than in the left side. These results are consistent with those reported by Dehaene-Lambertz [10] and Martin et al. [9]. There are several possibilities causing these changes. First, a single dipole source in the superior temporal gyrus (STG) might be the contributor of the MMN component [33]. It is obvious that frontward dipoles located in both temporal lobes will generate a maximal amplitude distribution in the fronto-central areas, and changes in dipole directions or locations (e.g. anterior or posterior to the STG) will alter the patterns of topographical distributions. Second, multiple MMN sources have been suggested in several neuroimaging studies [6,34], in which neural sources in both temporal and frontal lobes may contribute to the generation of MMN. Therefore, it might be possible that before 6 months of age, activation of the frontal sources is relatively weaker, resulting in the maximal parieto-occipital distribution. With the increase of age, the frontal sources become more active, leading to higher amplitudes in the fronto-central areas. This neural mechanism is compatible with the ones reported previously [35–36], and is also supported by the studies that decreased mismatch responses were recorded in patients who suffered from dorso-lateral prefrontal cortex lesions [37]. Third, as we stated above, changes of neural source numbers may also play a role in our findings. However, due to the facts that infant brains developed rapidly and their anatomical and physiological features vary monthly, dipole estimations using conventional methods will be difficult and yielded results should be explained carefully.
We note that activations seen in the difference waves may reflect neural mechanisms involving the generation processes of both N1 and MMN. In a recent study, Cranford et al. [38] demonstrated that MMN waveforms could be modified by inter-stimulus intervals, indicating that the negative mismatch responses can be contaminated by neural refractory effects. However, in the present study, the N250 latency to deviant stimuli was significantly shorter than the MMN latency, revealing that the negative mismatch responses recorded here cannot be completely explained by the obligation effect. It has also been demonstrated that the N250 latency to deviant stimuli is highly correlated to the N250 latency to standard stimuli, not to MMN latency [14], suggesting a neural mechanism differing from the sensory processing underlying the negative responses [39].
In summary, the maturation of auditory perceptual and discrimination processing were investigated in this longitudinal study of infants. The N250 component and MMN are not obvious at 3 months of age. Robust ERP components are seen after 6 months. Latencies of the ERP components gradually decrease, while the N250 and P350 components as well as mismatch responses are enhanced with increasing age, reaching the maximal strength at 9 months of age. The maximal amplitude distributions dynamically change across development. Two important developmental periods—one around 6 months and the other around 9 months of age—are suggested in the maturation of auditory central system.
References
- 1.Johnson MH, de Haan M, Oliver A, Smith W, Hatzakis H, Tucker LA, et al. Recording and analyzing high-density event-related potentials with infants. Using the Geodesic sensor net. Dev Neuropsychol. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- 2.Kurtzberg D, Hilpert PL, Kreuzer JA, Vaughan HG., Jr Differential maturation of cortical auditory evoked potentials to speech sounds in normal fullterm and very low-birthweight infants. Dev Med Child Neurol. 1984;26:466–75. doi: 10.1111/j.1469-8749.1984.tb04473.x. [DOI] [PubMed] [Google Scholar]
- 3.Kushnerenko E, Ceponiene R, Balan F, Fellman V, Huotilaine M, Näätänen R. Maturation of the auditory event-related potentials during the first year of life. Neuroreport. 2002;13:47–51. doi: 10.1097/00001756-200201210-00014. [DOI] [PubMed] [Google Scholar]
- 4.Kushnerenko E, Ceponiene R, Balan P, Fellman V, Näätänen R. Maturation of the auditory change detection response in infants: a longitudinal ERP study. Neuroreport. 2002;13:1843–8. doi: 10.1097/00001756-200210280-00002. [DOI] [PubMed] [Google Scholar]
- 5.Friederici AD, Friedrich M, Weber C. Neural manifestation of cognitive and precognitive mismatch detection in early infancy. Neuroreport. 2002;13:1251–4. doi: 10.1097/00001756-200207190-00006. [DOI] [PubMed] [Google Scholar]
- 6.Doeller CF, Opitz B, Mecklinger A, Krick C, Reith W, Schroger E. Prefrontal cortex involvement in preattentive auditory deviance detection: neuroimaging and electrophysiological evidence. Neuro-image. 2003;20:1270–82. doi: 10.1016/S1053-8119(03)00389-6. [DOI] [PubMed] [Google Scholar]
- 7.Molfese DL, Burger-Judisch LM. Dynamical temporal-spatial allocation of resources in the human brain. An alternative to the static view of hemispheric differences. In: Kitterle FL, editor. Cerebral laterality: theory and research. Hillsdale, NJ: Erlbaum; 1991. pp. 71–102. [Google Scholar]
- 8.Levanen S, Ahonen A, Han R, McEvoy L, Sams M. Deviant auditory stimuli activate human left and right auditory cortex differently. Cereb Cortex. 1996;6:288–96. doi: 10.1093/cercor/6.2.288. [DOI] [PubMed] [Google Scholar]
- 9.Martin BA, Shafer VL, Morr ML, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity: a scalp current density analysis. Ear Hear. 2003;24:463–71. doi: 10.1097/01.AUD.0000100306.20188.0E. [DOI] [PubMed] [Google Scholar]
- 10.Dehaene-Lambertz G. Cerebral specialization for speech and non-speech stimuli in infants. J Cogn Neurosci. 2000;12:449–60. doi: 10.1162/089892900562264. [DOI] [PubMed] [Google Scholar]
- 11.Muller BW, Juptner M, Jentzen W, Muller SP. Cortical activation to auditory mismatch elicited by frequency deviant and complex novel sounds: a PET study. Neuroimage. 2002;17:231–9. doi: 10.1006/nimg.2002.1176. [DOI] [PubMed] [Google Scholar]
- 12.Näätänen R, Paavilainen P, Alho K, Reinikainen K, Sams M. Do event-related potentials reveal the mechanism of the auditory sensory memory in the human brain? Neurosci Lett. 1989;98:217–21. doi: 10.1016/0304-3940(89)90513-2. [DOI] [PubMed] [Google Scholar]
- 13.Cheour M, Alho K, Ceponiene R, Reinikainen K, Sainio K, Pohjavuori M, et al. Maturation of mismatch negativity in infants. Int J Psychophysiol. 1998;29:217–26. doi: 10.1016/s0167-8760(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 14.Shafer VL, Morr ML, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in school-age children. Ear Hear. 2000;21:242–51. doi: 10.1097/00003446-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Morr ML, Shafer VL, Kreuzer JA, Kurtzberg D. Maturation of mismatch negativity in typically developing infants and preschool children. Ear Hear. 2002;23:118–36. doi: 10.1097/00003446-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Cheour M, Ceponiene R, Lehtokoski A, Luuk A, Allik J, Alho K, et al. Development of language-specific phoneme representations in the infant brain. Nat Neurosci. 1998;1:351–3. doi: 10.1038/1561. [DOI] [PubMed] [Google Scholar]
- 17.Benasich AA, Tallal P. Infant discrimination of rapid auditory cues predicts later language impairment. Behav Brain Res. 2002;136:31–49. doi: 10.1016/s0166-4328(02)00098-0. [DOI] [PubMed] [Google Scholar]
- 18.Jing H, Flax J, Roesler C, Thomas J, Choudhury N, Leevers H, et al. Brain event-related potentials as a tool for evaluation of auditory processing in children with Holoprosencephaly. J Cogn Neurosci. 2002;(Suppl):39. [Google Scholar]
- 19.Dehaene-Lambertz G, Dehaene S. Speed and cerebral correlates of syllable discrimination in infants. Nature. 1994;370:292–5. doi: 10.1038/370292a0. [DOI] [PubMed] [Google Scholar]
- 20.Dehaene-Lambertz G, Baillet S. A phonological representation in the infant brain. Neuroreport. 1998;9:1885–8. doi: 10.1097/00001756-199806010-00040. [DOI] [PubMed] [Google Scholar]
- 21.Faux SF, Shenton ME, McCarley RW, Nestor PG, Marcy B, Ludwig A. Preservation of P300 event-related potential topographic asymmetries in schizophrenia with use of either linked-ear or nose reference sites. Electroencephalogr Clin Neurophysiol. 1990;75:378–91. doi: 10.1016/0013-4694(90)90083-v. [DOI] [PubMed] [Google Scholar]
- 22.Onofrj M, Fulgente T, Thomas A, Locatelli T, Comi G. P300 asymmetries in focal brain lesions are reference dependent. Electroencephalogr Clin Neurophysiol. 1995;94:432–9. doi: 10.1016/0013-4694(94)00332-f. [DOI] [PubMed] [Google Scholar]
- 23.Novak GP, Kurtzberg D, Kreuzer JA, Vaughan HG., Jr Cortical responses to speech sounds and their formants in normal infants: maturational sequence and spatiotemporal analysis. Electroencephalogr Clin Neurophysiol. 1989;73:295–305. doi: 10.1016/0013-4694(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 24.Michel AE, Garey LJ. The development of dendritic spines in the human visual cortex. Hum Neurobiol. 1984;3:223–7. [PubMed] [Google Scholar]
- 25.Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- 26.Roncagliolo M, Benitez J, Eguibar JR. Progressive deterioration of central components of auditory brainstem responses during postnatal development of the myelin mutant taiep rat. Audiol Neurootol. 2000;5:267–75. doi: 10.1159/000013891. [DOI] [PubMed] [Google Scholar]
- 27.Stockard-Sullivan JE, Korsak RA, Webber DS, Edmond J. Mild carbon monoxide exposure and auditory function in the developing rat. J Neurosci Res. 2003;74:644–54. doi: 10.1002/jnr.10808. [DOI] [PubMed] [Google Scholar]
- 28.Vasil’eva VA, Shumeiko NS. Features of the structural organization of neuron groups in functionally different zones of the human cerebral cortex from birth to the age of 20 years. Neurosci Behav Physiol. 2001;31:609–12. doi: 10.1023/a:1012321127838. [DOI] [PubMed] [Google Scholar]
- 29.Ejiri K, Okubo O, Okuni M. The study of mismatch negativity. No To Hattatsu. 1992;24:565–70. in Japanese. [PubMed] [Google Scholar]
- 30.Leppanen PH, Pihko E, Eklund KM, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: II. Group effects. Neuroreport. 1999;10:969–73. doi: 10.1097/00001756-199904060-00014. [DOI] [PubMed] [Google Scholar]
- 31.Nittono H, Momose D, Hori T. The vanishing point of the mismatch negativity at sleep onset. Clin Neurophysiol. 2001;112:732–9. doi: 10.1016/s1388-2457(01)00491-6. [DOI] [PubMed] [Google Scholar]
- 32.Pihko E, Leppanen PH, Eklund KM, Cheour M, Guttorm TK, Lyytinen H. Cortical responses of infants with and without a genetic risk for dyslexia: I. Age effects. Neuroreport. 1999;10:901–5. doi: 10.1097/00001756-199904060-00002. [DOI] [PubMed] [Google Scholar]
- 33.Scherg M, Vajsar J, Picton TW. A source analysis of the late human auditory evoked potentials. J Cogn Neurosci. 1989;1:336–55. doi: 10.1162/jocn.1989.1.4.336. [DOI] [PubMed] [Google Scholar]
- 34.Jemel B, Oades RD, Oknina L, Achenbach C, Ropcke B. Frontal and temporal lobe sources for a marker of controlled auditory attention; the negative difference (Nd) event-related potential. Brain Topogr. 2003;15:249–62. doi: 10.1023/a:1023915730566. [DOI] [PubMed] [Google Scholar]
- 35.Jaaskelainen IP, Pekkonen E, Hirvonen J, Sillanaukee P, Näätänen R. Mismatch negativity subcomponents and ethyl alcohol. Biol Psychol. 1996;43:13–25. doi: 10.1016/0301-0511(95)05174-0. [DOI] [PubMed] [Google Scholar]
- 36.Baldeweg T, Klugman A, Gruzelier JH, Hirsch SR. Impairment in frontal but not temporal components of mismatch negativity in schizophrenia. Int J Psychophysiol. 2002;43:111–22. doi: 10.1016/s0167-8760(01)00183-0. [DOI] [PubMed] [Google Scholar]
- 37.Alho K, Woods DL, Algazi A, Knight RT, Näätänen R. Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalogr Clin Neurophysiol. 1994;91:353–62. doi: 10.1016/0013-4694(94)00173-1. [DOI] [PubMed] [Google Scholar]
- 38.Cranford JL, Walker LU, Stuart A, Elangovan S, Pravica D. Potential contamination effects of neuronal refractoriness on the speech-evoked mismatch negativity response. J Am Acad Audiol. 2003;14:251–9. [PubMed] [Google Scholar]
- 39.Picton TW, Alain C, Otten L, Ritter W, Achim A. Mismatch negativity: different water in the same river. Audiol Neurootol. 2000;5:111–39. doi: 10.1159/000013875. [DOI] [PubMed] [Google Scholar]


