Abstract
Synaptotagmin that contains two repeats of C2 regulatory domains is considered to be involved in neurotransmitter release. To reveal the roles of synaptotagmin in the regulation of exocytosis, we examined the effects of antibodies against C2A and C2B domains on Ca2+-evoked catecholamine (CA) release from digitonin-permeabilized adrenal chromaffin cells, resolving the Ca2+-evoked release into ATP-dependent priming and ATP-independent Ca2+-triggered steps. Anti-C2A antibody clearly reduced the ATP-independent release, suggesting that the C2A domain directly facilitate or promote Ca2+-triggered step, vesicular fusion. In contrast, anti-C2B antibody did not affect Ca2+-evoked release by itself, but significantly increased the spontaneous Ca2+-independent release. In addition, inositol high-polyphosphate series (IHPS) that bind the C2B domain inhibited both the ATP-independent Ca2+-evoked release and the spontaneous release in a dose-dependent manner. The inhibition by IHPS was totally reversed by anti-C2B antibody and significantly reversed by high concentration of Ca2+. These results suggest that IHPS binding to C2B domain arrests membrane fusion by presumably preventing interaction of synaptotagmin with phospholipids or with proteins of plasma membrane. Thus, IHPS binding to the C2B domain might keep the docked or primed vesicles away from spontaneous fusion at resting level of intracellular Ca2+. Binding of the increased intracellular Ca2+ to the C2A domain may facilitate or trigger the vesicular fusion by releasing this suppression by IHPS.
Keywords: inositol high-polyphosphate series, catecholamine release, Ca2+-triggered fusion
Recent studies have indicated that synaptotagmin (Syt), an integral synaptic vesicle protein in nerve cells and some endocrine cells (1), may function as a Ca2+ sensor in synaptic vesicle exocytosis (2, 3). The cytoplasmic carboxy terminus of Syt has two repeats, known as the C2A and C2B domains, that are homologous to the C2 regulatory domain of protein kinase C (4). Possible involvement of the C2 domain of Syt in Ca2+-regulated exocytosis has been shown by microinjection studies. Microinjection of peptides from the central region of both C2 domains into squid giant presynaptic terminals inhibits neurotransmitter release (5). A soluble recombinant protein of the C2A domain inhibited Ca2+-regulated exocytosis in PC12 cells (6). Recently, it has been shown that the C2A domain of Syt binds phospholipid in a Ca2+-dependent manner, but the C2B domain binds phospholipids irrespective of Ca2+ and binds also inositol high-polyphosphate series (IHPS) (7). Microinjection of IHPS (8) or anti-C2A antibody into the squid giant presynaptic terminal (9) inhibited the transmitter release. Injection of anti-C2B antibody with inositol 1,3,4,5-tetrakisphosphate (IP4) prevented the inhibitory action of IP4, though the antibody itself had no effect (10). While these studies suggested a bidirectional role of Syt in fast transmitter release in neuron, the role of Syt in slow release in secretory cells as well as the functional relationship between the C2A and C2B domains remain obscure.
To address these questions in this study, we examined the effects of antibodies against C2A and C2B domains and IHPS on the release of catecholamines (CA) in digitonin (DG)-permeabilized chromaffin cells.
MATERIALS AND METHODS
Primary Cultures.
Adrenal chromaffin cells were isolated from fresh bovine adrenal glands by collagenase digestion and maintained in monolayer cultures as described (11). All the experiments were performed with the cells in culture for 4–10 days.
Preparation of Anti-Syt Antibodies.
Polyclonal antibodies against the C2A and C2B domains of the mouse Syt II (referred to as anti-C2A antibody and anti-C2B antibody, respectively) were prepared and characterized as described (12). These antibodies selectively reacted with Syt I and II (but not with III and IV) expressed in COS-7 cells (data not shown). These antibodies little cross-reacted with Syt V (13, 14) when examined with glutathione S-transferase (GST) fusion protein GST-Syt V (data not shown). A rabbit normal IgG was used as control IgG.
Immunoblot analysis was carried out with these antibodies as follows: 20 μg of total homogenate of bovine chromaffin cells was subjected to 10% SDS/PAGE and transferred to nitrocellulose membranes (Hybond-ECL; Amersham), blocked with 0.5% skim milk, 0.1% Tween 20 in PBS, incubated with anti-C2A antibody or anti-C2B antibody, and incubated with peroxidase-labeled anti-rabbit goat IgG. Immunoreactive bands were visualized using the enhanced chemiluminescence detection system (ECL kit; Amersham).
CA Release from Permeabilized Cells.
After washing with Ca2+-free Locke’s solution, the cells were permeabilized by incubating for 4 min at 37°C with digitonin buffer (DG buffer) containing 140 mM Na-glutamate, 20 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (Pipes), 5 mM glucose, 5 mM EGTA, and 0.02 mM DG (Calbiochem) (pH to 6.80 with NaOH) (15). At the end of permeabilization, the cells were stimulated for various time at 37°C with Ca2+-buffer containing 140 mM Na-glutamate, 20 mM Pipes, 5 mM glucose, 5 mM MgSO4, and 5 mM EGTA without or with CaCl2 (pH to 6.80 with NaOH) in the presence or absence of 5 mM MgATP. Ca2+-evoked release was normally examined under the permeabilization for 4 min and stimulation with Ca2+ buffer (10 μM) for 6 min in the presence of MgATP, except for step-specific assays (see below). Ca2+ concentration in the Ca2+ buffer was calculated according to Iino (16), computed by solving multiequilibrium equations using binding constants compiled by Martell and Smith (17). Instead of EGTA, HEEDTA (N-hydroxyethylethylenediamine-N,N′,N′-triacetic acid) was used to extend the range of buffered Ca2+ concentrations (over 100 μM) in the Ca2+ buffer (18). When the effects of the antibodies against C2A and C2B domains were examined, permeabilization and stimulation were done in the presence of the antibodies. Released CA and cellular CA were extracted with 0.4 M perchloric acid and assayed by HPLC (11). Released CA is expressed as a percentage of total cellular CA content.
Step-Specific Assay for ATP-Dependent Priming Step and for ATP-Independent Ca2+-Triggered Step in Ca2+-Evoked CA Release.
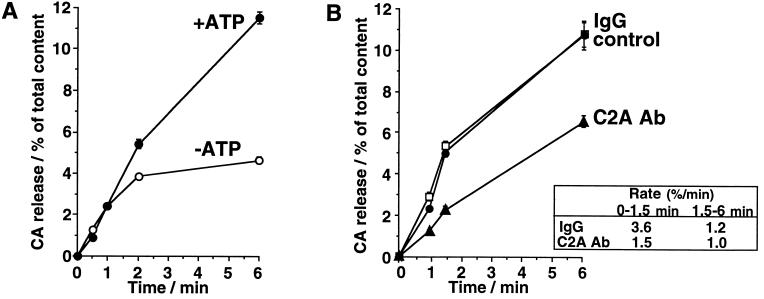
Ca2+-evoked CA release from the permeabilized chromaffin cells has been resolved into at least two distinct steps: ATP-dependent priming step and ATP-independent Ca2+-triggered step (19). The release through ATP-independent Ca2+-triggered step was distinguished as the release during the initial 1–1.5 min upon a stimulation with 10 μM Ca2+ termed as the initial release, or as the release evoked by 100 μM Ca2+ in the absence of MgATP termed as ATP-independent release. The second phase of the time course of the release evoked by 10 μM Ca2+ in the presence of MgATP reflects the release sustained by ATP-dependent priming, because MgATP is required for the release to continue after the initial 1.5 min (see Fig. 3A), and was termed as ATP-dependent release.
Figure 3.
Time course of CA release evoked by 10 μM Ca2+ from permeabilized cells (A) and effects of anti-C2A antibody on the time course of CA release (B). (A) Cells were permeabilized with DG buffer for 4 min and were stimulated for an additional 0–6 min with Ca2+ (0 or 10 μM) buffer in the presence (•) or absence (○) of 5 mM MgATP. (B) Cells were permeabilized with DG buffer for 4 min and were stimulated for an additional 0–6 min with Ca2+ (0 or 10 μM) buffer in the presence of 5 mM MgATP. Anti-C2A antibody (40 μg/ml) (▴) or control IgG (40 μg/ml) (□) was added during the permeabilization and the subsequent stimulation. Control (•) was the data from the cells without IgG. The amounts of released CA are expressed as the percentage of total cellular content. Amount of CA released in the absence of Ca2+ was measured for each group, and the mean value for each group was subtracted from each evoked release. Data are the mean ± SEM of at least eight determinations from individual wells.
IHPS.
IP4 was obtained from Dojindo Laboratories (Kumamoto, Japan), and inositol 1,3,4,5,6-pentakisphosphate (IP5) and inositol 1,2,3,4,5,6-hexakisphosphate (IP6) were from Calbiochem. When the effects of the IHPS on Ca2+-evoked release were examined, IHPS were added during the incubation after permeabilization and the subsequent stimulation.
RESULTS
To examine the distinct function of the C2A and C2B domains, we used anti-C2A antibody and anti-C2B antibody of the mouse Syt I and II. Immumoblotting analysis of total homogenate of bovine adrenal chromaffin cells using the anti-C2A and anti-C2B antibodies indicated that both antibodies recognized Syt I and/or II in bovine adrenal chromaffin cells (Fig. 1). The 65-kDa immunoreactive band most probably corresponds Syt I because Northern blotting experiments (20) and in situ hybridization study (21) indicated that only Syt I seems to be dominantly present in adrenal chromaffin cells. In addition, these antibodies weakly recognized another immunoreactive band of 47–50 kDa, which is similar to that of a novel isoform, Syt V (50 kDa) (13, 14). However, because these antibodies do not cross-react with GST-Syt V as described in Materials and Methods, the 47- to 50-kDa band in Fig. 1 probably is a degradation product derived from Syt I (22).
Figure 1.
Syt in bovine adrenal chromaffin cells. Total homogenate of bovine adrenal chromaffin cells was subjected to SDS/PAGE followed by immunoblot analysis using anti-C2A or anti-C2B antibodies. Strong immunoreactive band of 65 kDa indicates bovine Syt (I and/or II). Positions of molecular weight markers (×10−3) are indicated.
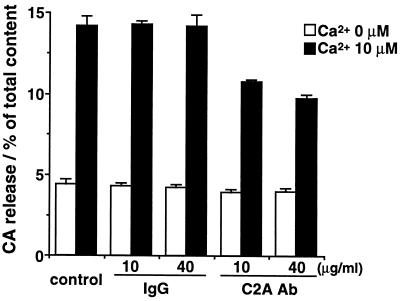
The anti-C2A antibody at 10 and 40 μg/ml inhibited Ca2+-evoked CA release from DG-permeabilized adrenal chromaffin cells to about 70% and 60% of that in the presence of control IgG. The anti-C2A antibody did not affect the spontaneous Ca2+-independent release (Fig. 2). To reveal whether the inhibitory effect of the anti-C2A antibody is on the ATP-dependent priming step or on the ATP-independent Ca2+-triggered step, we examined the effects of the anti-C2A antibody on the time course of CA release. Anti-C2A antibody clearly reduced the rate of initial release for 1–1.5 min from 3.6%/min to 1.5%/min, while the rate of the release in the second phase was less affected (Fig. 3B). The anti-C2A antibody at 40 μg/ml also reduced the release evoked by 100 μM Ca2+ in the absence of MgATP to 40% of that in control or in the presence of control IgG (data not shown). The same anti-C2A antibody inhibited Ca2+/phospholipid binding to the C2A domain (12). Therefore, the present results suggest that C2A domain has a role to directly facilitate or promote Ca2+-triggered step, vesicular fusion, rather than priming or vesicular docking.
Figure 2.
Effects of anti-C2A antibody on Ca2+-evoked CA release from DG-permeabilized adrenal chromaffin cells. The cells were permeabilized with DG buffer for 4 min and then stimulated with Ca2+ (0 or 10 μM) buffer for 6 min in the presence of 5 mM MgATP; anti-C2A antibody (C2A Ab) or control IgG (10 or 40 μg/ml) was added during permeabilization and stimulation. The amounts of released CA are expressed as the percentage of total cellular CA content. Data are the mean ± SEM of at least eight determinations from individual wells.
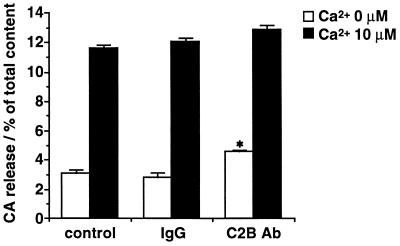
In contrast, anti-C2B antibody did not affect Ca2+-evoked release by itself even at the higher concentration (100 μg/ml) (Fig. 4). But a significant augmentation of the spontaneous Ca2+-independent release was consistently produced by the anti-C2B antibody. The spontaneous release was increased by anti-C2B antibody at 100 μg/ml to about 150% of that in control or in the presence of control IgG.
Figure 4.
Effects of anti-C2B antibody (100 μg/ml) on Ca2+-evoked CA release from DG-permeabilized adrenal chromaffin cells. The cells were permeabilized with DG buffer for 4 min, and were stimulated for an additional 6 min with Ca2+ (0 or 10 μM) buffer in the presence of 5 mM MgATP. Anti-C2B antibody (C2B Ab) or control IgG (100 μg/ml) was added during permeabilization and subsequent stimulation. The amounts of released CA are expressed as the percentage of total cellular content. Spontaneous CA release (CA released in the absence of Ca2+) in anti-C2B antibody-treated cells was significantly different (∗, P < 0.001) from that in control cells (control IgG-treated cells) when tested by Student’s t test. Data are the mean ± SEM of at least eight determinations from individual wells.
On the other hand, IHPS (IP4, IP5, and IP6) reduced the initial rate of CA release evoked by 10 μM Ca2+ (Fig. 5). The initial release was almost completely suppressed by IHPS at the concentration over 10−5 M, and the apparent IC50 was about 10−6 M. The inhibition by IHPS was more prominent during the initial 1.5 min than in the second phase of ATP-dependent release (data not shown). Moreover, IHPS reduced the spontaneous release during initial phase (Fig. 5). Both the spontaneous release and the release evoked by 100 μM Ca2+ in the absence of MgATP were also reduced by IHPS (data not shown). These results suggest that IHPS suppress both the spontaneous and evoked release by preventing vesicular fusion with little effect on ATP-dependent priming.
Figure 5.
Dose-response curve for IHPS inhibition of the evoked and spontaneous CA release from DG-permeabilized cells during the initial phase. The cells were permeabilized with DG buffer for 4 min, incubated two times for 2 min with the buffer without DG, and then stimulated with Ca2+ (0 or 10 μM) buffer for 1.5 min. IHPS (IP4, IP5, or IP6) was added during the incubation for the last 2 min and the subsequent stimulation. The amounts of released CA are expressed as the percentage of total cellular content. Data are the mean ± SEM of at least eight experiments.
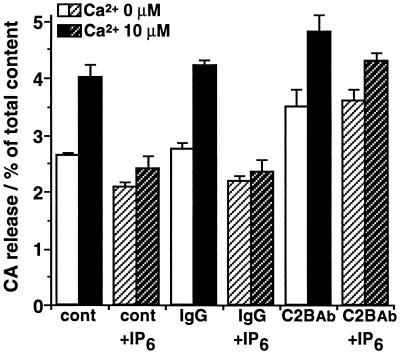
The inhibitory action of IHPS was overcome by anti-C2B antibody. The Ca2+-evoked release during the initial 1.5 min was reduced to 10–20% of control by 3 × 10−5 M IP6, and was reversed to 50% of control by anti-C2B antibody (Fig. 6). In addition, the inhibitory action of IP6 on the spontaneous Ca2+-independent release was also reversed by anti-C2B antibody (Fig. 6). Taken together with the facts that anti-C2B antibody inhibits IHPS binding to the C2B domain (12), these results suggest that IHPS binding to C2B domain of Syt prevents its interaction with phospholipids or with proteins of plasma membrane, and thus inhibits Ca2+-triggered vesicular fusion. The binding of IHPS to the C2B domain might be important to keep primed or docked vesicles away from spontaneous fusion at resting level of intracellular Ca2+.
Figure 6.
Effects of anti-C2B antibody on IP6-induced inhibition of the initial CA release from DG-permeabilized cells. The cells were permeabilized with DG buffer for 4 min, incubated two times for 2 min with the buffer without DG, and then stimulated with Ca2+ (0 or 10 μM) buffer for 1.5 min. IP6 (3 × 10−5 M) was added during the incubation for the last 2 min and the subsequent stimulation. Anti-C2B antibody (C2B Ab) or control IgG (60 μg/ml) was added during the permeabilization, the incubation for the last 2 min, and the subsequent stimulation. The amounts of released CA are expressed as the percentage of total cellular content. Data are the mean ± SEM of at least eight experiments.
In Fig. 7 we examined the relationship between the inhibitory effects of IP6 and the different concentration of Ca2+ that triggered the release in the absence of MgATP. IP6 at 10−4 M strongly inhibited the ATP-independent release evoked by Ca2+ below 10 μM, but the inhibitory action of IP6 was much less when the release was evoked by Ca2+ above 50 μM. Moreover, when the release was evoked by Ca2+ over 200 μM, IP6 could not produce any significant inhibition. These results suggest that the inhibitory action of IHPS bound to the C2B domain can be overcome by Ca2+ at this high concentration.
Figure 7.
Effects of Ca2+ on the inhibition by IP6 of the ATP-independent release from DG-permeabilized cells. The cells were permeabilized for 4 min with DG buffer, incubated two times for 2 min with the buffer without DG, and then stimulated with Ca2+ (0–500 μM) buffer (Ca2+-HEEDTA buffer) for 1.5 min in the absence of MgATP. MgATP (5 mM) was only added during the permeabilization and the incubation for the first 2 min. IP6 (10−4 M) was added during the incubation for the last 2 min and the subsequent stimulation. The amounts of released CA are expressed as the percentage of total cellular content. Amount of CA released in the absence of Ca2+ was measured for each group, and the mean value for each group was subtracted from each evoked release. Data are the mean ± SEM of at least eight determinations from individual wells. ∗, P < 0.01; ∗∗, P < 0.001, when compared with control.
DISCUSSION
Our results suggested that C2A and C2B domains of Syt have distinct roles in the regulation of exocytosis of secretory vesicles. Anti-C2A antibody clearly reduced the ATP-independent Ca2+-triggered release, suggesting that the C2A domain may function as a Ca2+ sensor that directly facilitate vesicular fusion. On the other hand, the anti-C2B antibody did not affect Ca2+-evoked release by itself, however significantly increased the spontaneous Ca2+-independent release. In addition, IHPS inhibited both the spontaneous and the ATP-independent Ca2+-triggered release. The inhibition by IHPS was totally reversed by anti-C2B antibody. The binding of IHPS to the C2B domain has been clarified recently (7). These results suggest that the C2B domain may have a role as a negative regulator through IHPS binding. In particular, IHPS binding to the C2B domain may clamp vesicles to suppress spontaneous release at the resting level of Ca2+. IHPS binding to C2B domain may arrest membrane fusion by preventing interaction of Syt with phospholipids or with proteins of plasma membrane.
The secretory process has been clearly dissolved into ATP-dependent priming and Ca2+-triggered fusion steps in chromaffin and PC12 cells, but not in synaptic terminals. Present results clearly suggest that Ca2+-triggered fusion of the docked or primed vesicles is under the clamp by Syt through IHPS binding to C2B domain. And this will explain a part of the mechanisms by which the secretory cells can keep a certain number of the docked/primed vesicles ready for fusion until the last moment of the signal arrival. Microinjection of the antibodies against C2A and C2B domains of the squid Syt into the squid giant presynaptic terminal was also examined recently (9, 10). Injection of the anti-C2A antibody blocked transmitter release by preventing vesicular fusion (9). Injection of the anti-C2B antibody abolished the inhibition by IP4 of the transmitter release, but did not affect the transmitter release by itself (10). Microinjection of IHPS into the presynaptic neurons of superior cervical ganglion neurons in culture also blocked acetylcholine release, and the block by IHPS was prevented by anti-C2B antibody (23). Although these studies demonstrated inhibition by IHPS through binding to C2B domain, such a physiological significance of the inhibition was difficult to interpret, as it was impossible to dissolve the transmitter release into priming step and fusion step with these systems.
The inhibitory effects of Syt in spontaneous release were also reported in other systems. The electrophysiological recordings from Drosophila deficient in Syt revealed an increase in spontaneous transmitter release (24–26). Expression of Syt I in CHO fibroblasts reduced spontaneous quantal acetylcholine release (27). The anti-C2B antibody by itself affected the spontaneous release in adrenal chromaffin cells, but not in the presynaptic neurons. This discrepancy may be due to the different concentration of the endogenous IHPS in the squid presynaptic neurons and bovine adrenal chromaffin cells. No such change was observed in the Syt I knockout mouse (28); however, these data do not exclude the possibility of masking effect of other isoforms.
Another physiological significance of the inhibitory action of IHPS that we clarified in this study was the release of IHPS-regulated clamp by Ca2+, which was not possible to demonstrate in the previous microinjection studies because of the difficulty in manipulating the intracellular concentration of Ca2+. The inhibitory action of IHPS bound to the C2B domain was competed by Ca2+ at the concentration above 50 μM (Fig. 7). These observations may allow us to assume that the liberation of IHPS from the C2B domain of Syt following Ca2+ binding to the C2A domain facilitates Ca2+-triggered vesicular fusion. Syt I was recently shown to bind syntaxin I in a Ca2+-dependent manner (29, 30). The binding of Syt I to syntaxin, which required more than 200 μM Ca2+ for half maximal binding (29), might be attributable to the relief of IHPS from C2B domain by this high concentration of Ca2+. In adrenal chromaffin cells, rapid accumulation of IP5 upon a depolarizing stimulation has been reported where the accumulation reaches the maximum level at 15 s and decline to the basal level at 2 min (31). Such a rapid stimulus-induced accumulation of IHPS has been demonstrated also in N1E-115 neuroblastoma cells and in cultured rat cerebellar granule cells (32, 33). In either case, IHPS seem to be derived from other than IP3. These accumulation might be partly due to the liberation of IHPS from the C2B domain of Syt upon the stimulation.
Acknowledgments
We are grateful to Prof. M. Iino and Dr. K. Hirose for the computer program to calculate Ca2+-EGTA buffer and Ca2+-HEEDTA buffer. This work is supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (M.O.-I., K.M., and K.K.), Nissan Science Foundation, Hayashi Memorial Foundation for Female Natural Scientists (M.O.-I.), and by the Japan Society for the Promotion of Science (M.F.).
Footnotes
Abbreviations: CA, catecholamine(s); IHPS, inositol high-polyphosphate series; IP4, inositol 1,3,4,5-tetrakisphosphate; IP5, inositol 1,3,4,5,6-pentakisphosphate; IP6, inositol 1,2,3,4,5,6-hexakisphosphate; Syt, synaptotagmin(s); GST, glutathione S-transferase; DG, digitonin; HEEDTA, N-hydroxyethylethylenediamine-N,N′,N′-triacetic acid.
References
- 1.Matthew W D, Tsavaler L, Reichardt L F. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheller R H. Neuron. 1995;14:893–897. doi: 10.1016/0896-6273(95)90328-3. [DOI] [PubMed] [Google Scholar]
- 3.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 4.Perin M S, Fried V A, Mignery G A, Jahn R, Südhof T C. Nature (London) 1990;345:260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- 5.Bommert K, Charlton M P, DeBello W M, Chin G J, Betz H, Augustine G J. Nature (London) 1993;363:163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- 6.Elferink L A, Peterson M R, Scheller R H. Cell. 1993;72:153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Aruga J, Niinobe M, Aimoto S, Mikoshiba K. J Biol Chem. 1994;269:29206–29211. [PubMed] [Google Scholar]
- 8.Llinás R, Sugimori M, Lang E J, Morita M, Fukuda M, Niinobe M, Mikoshiba K. Proc Natl Acad Sci USA. 1994;91:12990–12993. doi: 10.1073/pnas.91.26.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikoshiba K, Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Llinás R. Proc Natl Acad Sci USA. 1995;92:10703–10707. doi: 10.1073/pnas.92.23.10703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukuda M, Moreira J E, Lewis F M T, Sugimori M, Niinobe M, Mikoshiba K, Llinás R. Proc Natl Acad Sci USA. 1995;92:10708–10712. doi: 10.1073/pnas.92.23.10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara-Imaizumi M, Kameyama K, Kawae N, Takeda K, Muramatsu S, Kumakura K. J Neurochem. 1992;58:2275–2284. doi: 10.1111/j.1471-4159.1992.tb10974.x. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda M, Kojima T, Aruga J, Niinobe M, Mikoshiba K. J Biol Chem. 1995;270:26523–26527. doi: 10.1074/jbc.270.44.26523. [DOI] [PubMed] [Google Scholar]
- 13.Hudson A W, Birnbaum M J. Proc Natl Acad Sci USA. 1995;92:5895–5899. doi: 10.1073/pnas.92.13.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craxton M, Goedert M. FEBS Lett. 1995;361:196–200. doi: 10.1016/0014-5793(95)00176-a. [DOI] [PubMed] [Google Scholar]
- 15.Kumakura K, Sasaki S, Sakurai T, Ohara-Imaizumi M, Misonou H, Nakamura S, Matsuda Y, Nonomura Y. J Neurosci. 1994;14:7695–7703. doi: 10.1523/JNEUROSCI.14-12-07695.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iino M J. Gen Physiol. 1989;94:363–383. doi: 10.1085/jgp.94.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martell A E, Smith R E. Critical Stability Constants. New York: Plenum; 1974–1982. [Google Scholar]
- 18.Tsukioka M, Iino M, Endo M. J Physiol (London) 1994;475:369–375. doi: 10.1113/jphysiol.1994.sp020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bittner M A, Holz R W. J Biol Chem. 1992;267:16219–16225. [PubMed] [Google Scholar]
- 20.Geppert M, Archer B T, III, Südhof T C. J Biol Chem. 1991;266:13548–13552. [PubMed] [Google Scholar]
- 21.Marquèze B, Boudier J A, Mizuta M, Inagaki N, Seino S, Seagar M. J Neurosci. 1995;15:4906–4917. doi: 10.1523/JNEUROSCI.15-07-04906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perin M S, Brose N, Jahn R, Südhof T C. J Biol Chem. 1991;266:623–629. [PubMed] [Google Scholar]
- 23.Mochida, S., Fukuda, M., Niinobe, M., Kobayashi, H. & Mikoshiba, K. (1997) Neuroscience, in press. [DOI] [PubMed]
- 24.DiAntonio A, Parfitt K D, Schwarz T L. Cell. 1993;73:1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- 25.Littleton J T, Stern M, Schulze K, Perin M, Bellen H J. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 26.DiAntonio A, Schwarz T L. Neuron. 1994;12:909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto T, Popov S, Buckley K M, Poo M-M. Neuron. 1995;15:689–696. doi: 10.1016/0896-6273(95)90156-6. [DOI] [PubMed] [Google Scholar]
- 28.Geppert M, Goda Y, Hammer R E, Li C, Rosahl T W, Srevens C F, Südhof T C. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Ullrich B, Zhang J Z, Anderson R G W, Brose N, Südhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 30.Chapman E R, Hanson P I, An S, Jahn R. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 31.Sasakawa N, Nakaki T, Kato R. J Biol Chem. 1990;265:17700–17705. [PubMed] [Google Scholar]
- 32.Sasakawa N, Nakaki T, Kashima R, Kanba S, Kato R. J Neurochem. 1992;58:2116–2123. doi: 10.1111/j.1471-4159.1992.tb10953.x. [DOI] [PubMed] [Google Scholar]
- 33.Sasakawa N, Nakaki T, Kakinuma E, Kato R. Brain Res. 1993;623:155–160. doi: 10.1016/0006-8993(93)90023-g. [DOI] [PubMed] [Google Scholar]