Abstract
At the present time we do not know when the circadian timing system of human infants becomes responsive to light. Because of human study limitations, it is not currently possible to address this issue in clinical studies. Therefore, to provide insights into when the circadian system of humans becomes responsive to light, baboons were studied. We first assessed if the biological clock located in suprachiasmatic nuclei (SCN) is responsive to light at birth. When term newborn infants were exposed to bright light at night (5000 lux), SCN metabolic activity and c-fos mRNA expression increased, indicating the presence of photic responsiveness. When photic entrainment of developing rhythmicity was examined in infants, low intensity (200 lux) cycled lighting was sufficient to entrain circadian phase. However, low intensity lighting was not sufficient to induce changes in SCN metabolic activity or c-fos mRNA expression. Phase–response studies indicated that light exposure (200 lux) before the onset of activity most effectively shifted circadian phase. These data provide direct evidence that the SCN are responsive to visually mediated light information in a primate at birth. Further consideration of lighting conditions that infants are exposed to is therefore warranted.
Circadian rhythms profoundly influence human physiology, behavior, and illness (1–3). In mammals, a biological clock that generates circadian rhythms is located in the hypothalamic suprachiasmatic nuclei (SCN) (3, 4). The SCN exhibit endogenous rhythmicity and have a period of oscillation of about 24 hr (3). Studies in rodents have convincingly shown that the SCN manifest day–night rhythms in metabolic and electrical activity as well as patterns in gene expression (3). Studies of nonhuman primates show complete abolition of circadian rhythmicity when the SCN are lesioned, showing that the SCN are the site of a circadian pacemaker (5).
Input pathways relay photic information from the retina to the SCN to synchronize or entrain the oscillations of the clock to the 24-hr light–dark (LD) cycle (6). A direct pathway from the retina to the SCN, the retinohypothalamic tract (RHT), has been shown to be both necessary and sufficient for photic entrainment in several species (6). In human and nonhuman primates, the RHT has been identified (7–9), and light has been shown to regulate the circadian clock (10–14).
Based on rodent studies, we know that the SCN form prenatally (15) and begin oscillating in utero (16, 17). In primates, available evidence suggests that primate SCN develop and begin oscillating in utero (18). Using [125I]2-iodo-melatonin to identify the SCN, the nuclei can be detected at gestational day (GD) 120 in humans (19). In squirrel monkeys, day–night differences in SCN metabolic activity have been detected in fetuses at the end of gestation (20).
Currently, our understanding of the developing primate circadian system is at very early stages (18). Although the RHT has been identified in a 36-week gestation human newborn (21), we do not know when the SCN of primates become functionally responsive to light. Considering that more than 200,000 infants per year are exposed to artificial environments of hospital nurseries in the United States (22), determining if the SCN of infants are responsive to light may be of considerable clinical importance.
Because of human study limitations, it is not possible to determine if human infants are functionally responsive to light at birth. Therefore, to provide insights into the developing human clock, baboons were used. Baboons are phylogenetically more closely related to humans than other Old World (e.g., macaques) and New World monkeys (e.g., squirrel monkeys) (23, 24). Baboons are diurnal animals that have robust day–night rhythms in locomotor activity and hormone secretion (23, 25). Baboon gestation also is the longest of any monkey species (185 days) (23). Monkey visual system development is also very similar to that of humans (24, 26).
Studying term baboon infants, we now provide direct evidence that the SCN of term primate infants are functionally innervated by the retina. We show that the postnatal development of expressed rhythmicity in baboons is very similar to that observed in human infants. Finally, we demonstrate that the circadian phase of newborn baboons is entrained by low intensity lighting.
MATERIALS AND METHODS
Animals.
Acute studies of term baboon infants (Papio sp.) were performed at the Southwest Foundation for Biomedical Research (San Antonio) or at the University of Illinois (Chicago). After matings, pregnant dams were maintained indoors in automated diurnal LD cycles. The LD cycle consisted of 12 hr of light per day (lights on from 0700 to 1900 hr; 1000 lux). On the morning after birth, dams were anesthetized with ketamine (5–10 mg/kg) and newborn infants were removed. Infants were then placed in individual newborn incubators (Ohio, Inc.; Madison, WI) that were kept at 32°C. Infants were fed Similac with iron (Mead Johnson).
Incubators were kept in rooms in which the LD cycle consisted of 12 hr of light per day (lights on from 0700 to 1900 hr; 1000 lux). During the light portion of the LD cycle, illumination was provided by Phillips Cool White fluorescent lights (Sommerset, NJ). During darkness, illumination was provided by Sigma spot lamps with 15-W bulbs and Kodak 2B red-light filters. For acute light-at-night studies, illumination was provided by 500-W Halogen lamps. Light intensity was measured using a SPER Scientific (Itaska, IL) light meter that was placed near the animal.
For long-term behavioral studies, infants were kept in temperature-controlled newborn incubators for the first 4–6 weeks after birth. Thereafter, animals were transferred to 1-m3 cages. Over the first week of life, animals were bottle-fed every 4 hr. During week 2, the animals were fed every 6 hr. From weeks 3 to 6, animals were fed every 8 hr. Thereafter, bottles were placed in the cage at randomly determined times during the day, and the animals fed ad libitum. Incubator and cage bedding was changed at a randomly selected time each day. Unless specified otherwise, animals were kept in constant dim light (1 lux) while being monitored. When animals were placed into light–dark cycles, cycled illumination was provided by 500-W Halogen lamps alternating with red light.
To monitor activity, telemetry units that monitored temperature and activity [Minimitter (Sunriver OR) or Data Sciences (St. Paul)] were used. Transmitters were either implanted subcutaneously while the animals were briefly anesthetized (at 1100 hr), or were placed in nylon jackets worn by the animals. Telemetry signals were acquired by a receiver placed on top of the incubators or within the cages. Activity and temperature data were collected and stored using dataquest (Data Sciences) software on an IBM computer. dataquest software was used to generate actograms.
The phase of activity onset and offset was determined using tau software (Minimitter). Circadian phase was determined by computer-generated lines of activity onset and offset. To assess circadian phase before the onset of clear day–night activity patterns, computer-generated lines of activity onset and offset from 15 consecutive days were extended backwards to the day of birth.
Deoxyglucose (DG) Studies.
DG studies were performed as described (27, 28). On the day of study, animals were weighed and intravenous catheters were placed in the saphenous vein. At specified times (see below), individual animals were injected with 100 μCi/kg i.v. with 2-deoxy-d-[14C]glucose (DG; Amersham; specific activity, 60 Ci/mmol; 1 Ci = 37 GBq). Animals were killed 40 min after injections with an overdose of pentobarbital (100 mg/kg; i.v.). Blood was then obtained by cardiac puncture and the brains were removed. The hypothalamus was dissected, frozen in chilled 2-methylbutane (−20°C), and stored at −80°C. Brains were cut in a cryostat (20 μm). Slide-mounted tissue sections were then exposed to Kodak SB5 radiography film. SCN metabolic activity was assessed by determining the relative optical density (OD) of the SCN (OD of SCN/OD of adjacent hypothalamus).
For each specimen, the OD of three consecutive sections in the midportion of the SCN were determined. To capture images a Leaf Lumina (New Bedford, MA) camera was used. OD values were computed using image software (National Institutes of Health) with Kodak autoradiographic standards.
Receptor-Labeling Autoradiography.
Autoradiography was performed as previously described to confirm the location of the SCN (29). Tissue sections were thawed at room temperature and incubated in PBS with 5 mM MgCl2 (binding buffer) that contained 1% formaldehyde (3 min, 21°C). Slides were then washed and incubated in binding buffer (30 min, 21°C). Sections were next incubated in binding buffer containing [125I]2-iodomelatonin ([125I]MEL; DuPont/New England Nuclear; specific activity, 2000 Ci/mmol; 60 min, 21°C; 100 pM). Nonspecific binding was assessed by incubating adjacent sections in buffer that also contained 1 μM melatonin. Sections were then washed in binding buffer (2 times for 15 min at 0°C), air dried, and exposed to Kodak SB5 film.
In Situ Hybridization.
Tissue sections adjacent to those used for DG studies were used. In situ hybridization for c-fos mRNA was performed similar to as described (30). [35S]α-Thio-UTP (DuPont/New England Nuclear) labeled antisense, and sense cRNA probes were generated using the Gemini System (Promega). A 400-bp fragment of the baboon c-fos gene isolated from baboon striatum by PCR was used as the template for cRNA probe generation. For prehybridization, sections were treated with HCl and acetylated. Sections were then hybridized for 18 hr at 58°C. The next day, slides were washed in 2× standard saline citrate (SSC), treated with RNase A (5 μg/ml; Sigma; 37°C, 45 min), washed in 0.1× SSC (58°C, 60 min), and dehydrated through ascending concentrations of ethanol containing 6 M ammonium hydroxide. Slides were apposed to Kodak SB5 film to generate autoradiographic images.
Retinohypothalamic Tract Tracing.
Retinal pathways to the hypothalamus were examined using modifications (31) of the anterograde tract-tracing method of Mesulam and Mufsson (32). Baboons were anesthetized with ketamine (5 mg/kg) and injected intraocularly with cholera toxin conjugated to horseradish peroxidase (CT-HRP; List Biological Laboratories; Campbell, CA; 100 μg in 200 μl) in sterile water with 2% dimethyl sulfoxide at 1200 hr. At 48–60 hr after injections, DG studies were performed. To assess CT-HRP labeling, slide-mounted cryostat-cut tissue sections were warmed to room temperature and placed in 1% formalin (2 min) and washed in distilled water (3 washes; 2 min each). Slide-mounted sections were then processed for labeling using tetramethylbenzidine as the chromogen.
Melatonin Assay.
Melatonin concentrations were determined in serum by John Nurnberger, Jr. (Indiana University School of Medicine), as described (33).
RESULTS
Photic Responsiveness of the SCN in Term Newborns.
We do not know when the primate clock begins to respond directly to light. To begin to address this issue, we used the DG method to test for photic responsiveness in term baboon infants and examined changes in SCN c-fos mRNA levels. Developmental studies in rodents and other species have revealed that if the SCN are functionally innervated by the retina, light at night induces robust increases in SCN metabolic activity and c-fos gene expression (34, 35).
Baboon infants, born after gestation lengths of 180–184 days, were studied 2 days after their natural birth. On the day of birth, animals were separated from the dams and placed in individual incubators. Animals were maintained in a LD cycle until the day of study when the animals were placed in constant darkness. Two animals were studied at 2300 hr in darkness (night). Three animals were exposed to light at night (5000 lux) for 5 min, injected with DG, and light exposure was continued until the animals were killed 40 min later. A total of 5000 lux of light was initially used based on studies showing that bright light affects the human circadian system (11, 13, 36). One animal also was injected with DG at 1200 hr in darkness (dim red light; midday) to determine if there is increased SCN metabolic activity during the daytime.
In the animals studied in darkness at night, DG autoradiographic images of the SCN were not greater than that of the adjacent hypothalamus (Figs. 1 and 2). However, in each animal exposed to light-at-night, autoradiographic images of the SCN were clearly apparent indicating that light exposure increased SCN metabolic activity. Increased DG uptake was seen rostral when tissue was first present over the optic chiasm and extended caudally for about 800 μM. In the animal examined in darkness during midday, SCN DG uptake was also observed (Fig. 2).
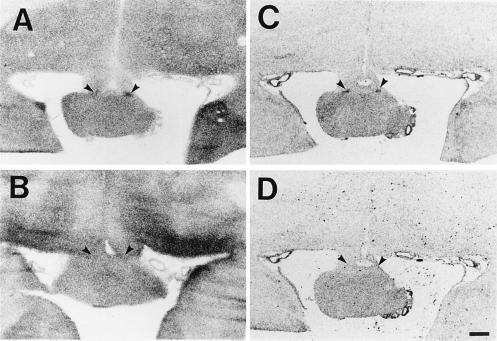
Figure 1.
Images generated from studies of DG uptake and c-fos mRNA expression. (A) DG autoradiographic image at level of mid-SCN generated from newborn baboon exposed to 5000 lux of light at night. (B) DG image generated from an animal studied at night in darkness. (C) In situ hybridization image generated using antisense probe showing c-fos mRNA expression in animal exposed to light-at-night. (D) In situ hybridization image generated from adjacent tissue section using sense c-fos probe. (Scale bar = 5 mm.)
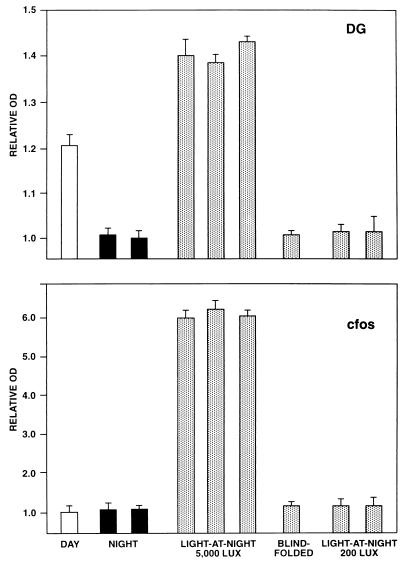
Figure 2.
Relative optical density (OD) values of individual animals generated from DG uptakes studies (Upper) and c-fos mRNA expression studies (Lower). Animals were studied in darkness at midday (open bars), in darkness at night (filled bars), or after 5000 or 200 lux of light exposure at night (stippled bars). One animal exposed to light-at-night was blindfolded before light exposure. Relative OD values are means ± SEM of three determinations at mid-SCN level per animal.
In addition to examining changes in SCN metabolic activity, we also tested for changes in SCN c-fos mRNA expression using riboprobes generated from a 400-bp baboon c-fos cDNA fragment that was 97% identical with human c-fos (37). Autoradiographs revealed that c-fos mRNA expression was induced in the light-exposed animals in the SCN. In contrast, a hybridization signal was not apparent over the SCN in the animals that remained in darkness or were studied at midday in darkness.
At the end of the DG studies, blood was obtained to assess circulating melatonin concentrations to determine if day–night differences in melatonin were apparent, as described in humans, baboons, and other monkeys (36, 38, 39). In all animals, we found that levels were very low (0–1.4 pg/ml) including those animals studied in darkness at night. Thus, a day–night rhythm in melatonin production was not detectable.
Light Activation of the SCN Is Retina-Mediated.
The above studies showed that the SCN of newborn baboons were responsive to light at birth. We next tested if the photic responsiveness was retina-mediated. In mammals, the RHT is both necessary and sufficient for photic entrainment (6, 7). Using anterograde tract tracing methods, we tested for the presence of the RHT.
Two newborn baboons received unilateral intraocular injections of CT-HRP at 6 hr after birth. The baboons were then maintained in a LD cycle for 2 days, and light-at-night studies were then performed. One animal had both eyes covered with light-occlusive cloth during exposure to 5000 lux of light. The other animal was exposed to light without being blindfolded.
Analysis of the distribution of CT-HRP labeling in each animal revealed bilateral labeling of the SCN following unilateral eye injections (Fig. 3). Labeled fibers were present at heaviest concentrations in the lateral regions of the SCN. The intensities of labeling were similar ipsilateral and contralateral to the side of injection.
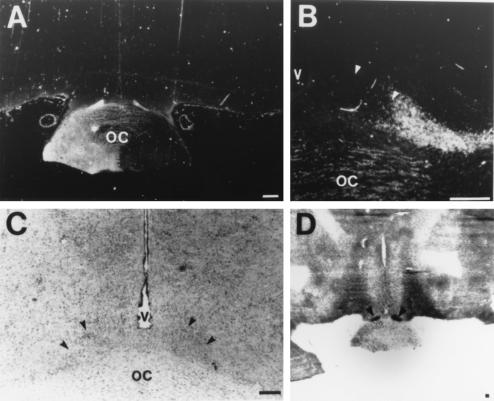
Figure 3.
Innervation of the SCN by the retinohypothalamic tract. (A) Low-power image of CT-HRP labeling of the optic chiasm. (B) Adjacent tissue section stained for Nissl substance. (C) High power image of CT-HRP labeling of SCN contralateral to injection. (D) Autoradiographic image generated from DG study. Arrows identify the SCN. (Bars = 1 mm in A and D; 50 μm in B and C.)
Autoradiographs generated from DG and c-fos in situ hybridization studies showed there was robust uptake of DG and increased c-fos mRNA expression in the nonblindfolded animal exposed to light-at-night. In contrast, DG uptake and c-fos mRNA expression was not induced in the blindfolded animal (Fig. 2). Thus, the RHT is present at birth and photic activation of the SCN is mediated by the eyes.
Photic Entrainment of Expressed Rhythmicity.
After demonstrating that the SCN are responsive to light at birth, we next assessed if there was photic entrainment of newborn circadian phase. Because little is known about the development of expressed rhythmicity in baboons, we examined the development of activity–rest patterns in newborn baboons reared under constant conditions in dim light (1 lux).
Double-plotted actograms revealed that consolidated periods of activity and rest were not clearly apparent until between 15 and 20 days of age. (Fig. 4). After 20 days of age, distinct periods of activity and rest could be seen and period lengths (tau) were 23.3, 24.3, and 24.1 hr for infants A, B and C, respectively. When circadian phase was extrapolated back to birth, the phase of animals B and C was synchronous with the light portion of the LD cycle that the dam was in before birth. However, the circadian phase at birth of animal A was out of phase with the dam’s lighting cycle.
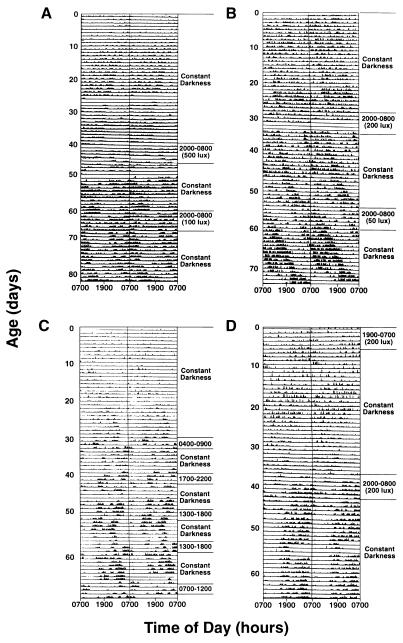
Figure 4.
Development and entrainment of rest–activity patterns in newborn baboons. Double-plotted actograms are shown with dark bars representing periods of activity. Animals were maintained in either constant darkness or exposed to 24-hr LD cycles. Periods of light exposure and lighting intensities are noted for each animal. In studies of infant C, all lighting intensities were 200 lux.
After distinguishing the circadian phase of rest–activity patterns, we next tested for photic entrainment. Animals were placed in 12-hr light/12-hr dark reversed LD cycles for 3 or 5 days with the light portion of the LD cycle generally corresponding with the period of inactivity of the infants (500 lux with infant A; 200 lux with infants B and C). Animals were then placed in constant dim light for 2 weeks and circadian phase was reassessed. In all animals studied, the circadian phase assumed that of the reversed lighting cycle (Fig. 4) showing that there was entrainment of circadian phase.
We next tested if circadian phase could be established by exposure to cycled lighting immediately after birth. Animal D was therefore placed in a 200 lux reversed LD cycle for 5 days after birth. During cycled light exposure, the animal was more active during light exposure than during darkness (Fig. 4). After the animal was placed in constant dim light (1 lux), clear day–night differences in activity–rest patterns were not apparent until after 15 days of age. Thereafter, free-running activity patterns were seen with a period length of 23.4 hr. To assess the animal’s phase before the onset of day–night differences in activity, computer-fitted lines of activity onset and offset from days 15 to 30 were extended backwards. The data suggested that the animal had been entrained to the lighting cycle, and the circadian phase of the animal free-ran from the phase of the LD cycle at day 5.
To provide insights into the phase shifting properties of light, we next defined phase–response relationships for animals C and D after exposure to 200 lux of light at different times of the circadian cycle. For these studies, paradigms used to define phase–response relationships in humans were used (11, 14).
Animals were maintained in dim light (1 lux) and circadian phase was assessed by monitoring activity patterns. The animals were then placed in a LD cycle for 3 consecutive days in which the duration of light exposure was 5 hr (5-hr light/19-hr dark). Animals were then placed back into dim light and circadian phase was reassessed. Phase shifts were then calculated relative to the midpoint of the light exposure period (14).
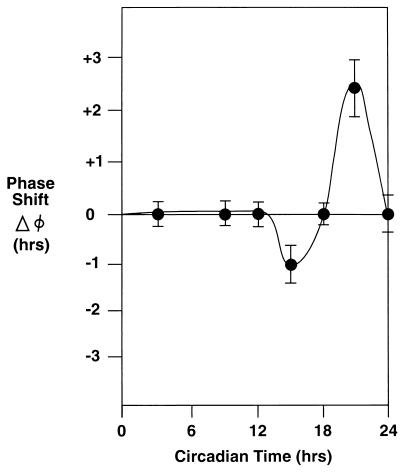
These studies revealed that 200-lux light exposure during subjective-day (period of activity) did not alter circadian phase. (Fig. 5). In contrast, light exposure during early subjective night induced phase advances, whereas phase advances were seen following light exposure during late subjective-night. The magnitude of phase advances following early subjective morning light exposure were greater than the magnitude of phase delays associated with early evening light exposure.
Figure 5.
Phase-shifting effects of 3 cycles of 5 hr of light exposure (5-hr light/19-hr dark; 200 lux) on the phase of rest-activity patterns of baboons C and D. Baboon C was studied beginning at 30 days of age; baboon D was studied beginning at 90 days of age. A phase-response curve is shown in which circadian time 0 corresponds with the onset of activity. The magnitude of phase advances and delays are shown on the ordinate. Mean ± SEMs of three or more values are shown at times corresponding with the center of light exposure periods.
Influence of Low Intensity Lighting on SCN Activity.
Because 200 lux of lighting was sufficient to entrain the neonatal baboons and 5000 lux had been used in our initial DG studies, we next examined DG uptake and c-fos mRNA expression following exposure to 200 lux of light at night. One baboon was studied 1 day after birth. Baboon infant A was studied at 120 days of age after being maintained in a 12-light (200 lux)/12-hr dark LD cycle for 4 weeks. In both animals, increased DG uptake in the SCN was not induced by the light at night (Fig. 2). SCN c-fos mRNA expression also was not induced in either baboon. Thus, although 200 lux of lighting is sufficient for photic entrainment, it does not induce acute changes in SCN metabolic activity or c-fos mRNA expression.
DISCUSSION
Previous studies of the onset of photic responsiveness have revealed notable differences among species. In rats, retina-mediated photic responsiveness is not present at birth, but develops by the end of the first postnatal week (40, 41). In contrast, in sheep the retina innervate the SCN by midgestation (43). In a single report, projections from the retina to the anterior hypothalamus have been detected in a 36-week-old human gestation (21). However, it is not possible to discern if the SCN are functionally innervated by the retina from tract tracing studies.
In human infants, studies of entrainment mechanisms have been limited by the absence of clear outputs of the circadian system in the neonatal period. Although it may be possible to identify circadian phase in some infants from activity patterns (18, 44), clear day–night differences in activity and rest are not usually apparent until after 1 month of age (18, 44). Circadian rhythms in cortisol and melatonin secretion are not apparent until after 3–6 months of age (45–47). A nonhuman primate model of the human infant is therefore needed to determine if primate newborns can respond to light at birth.
Because baboons have been used as models of human development (48), this species was selected for our studies. Supporting the concept that baboons are favorable models for human circadian system development, actograms generated from baboons A, B, and C were similar to the actograms of human newborns (44, 49–51).
When activity patterns in the baboon infants were analyzed, maternal and infant circadian phase was synchronous at birth for baboons B and C, as the phase of infant activity corresponded with the light portion of the lighting cycle the mother was in before giving birth. The results of DG studies also suggested that the newborns were entrained to the LD cycle before or at birth. The SCN were more metabolically active than the adjacent hypothalamus during the daytime, and the SCN were not more metabolically active than the adjacent hypothalamus at night in darkness or low intensity lighting. However, in contrast to the above observations, the circadian phase of baboon A at birth was out of phase with that of the dam, as activity corresponded with the dark portion of the lighting cycle the mother was in before giving birth. Thus, whereas maternal and newborn circadian phase is generally similar in baboons, the behavioral observations of infant A suggest that this may not always occur. Support for the concept that maternal–infant synchronization of circadian phase does not always occur in primates is also contained in the data of Parmelee (49), showing that the circadian phase of one human infant at birth was 180 degrees out of phase with that of the mother, similar to baboon A.
Because it had been previously suggested that bright light was needed to suppress nocturnal melatonin production or alter circadian phase in humans (11, 36), 5000-lux light exposure was used in our initial DG studies. Following 5000 lux of light-at-night, robust DG uptake and c-fos mRNA expression was seen in the SCN in all animals, indicating photic responsiveness. SCN metabolic activity at night did not increase in a blindfolded animal, but increased in the visually intact animals, showing that photic responsiveness was mediated by the eyes. When tract tracing studies were performed, CT-HRP labeling was observed at the lateral margins of the SCN which is consistent with that seen in other primates (7, 52).
After showing that the baboons were responsive to light at birth, we next assessed if the infants could be entrained by light by exposing the animals to reversed LD cycles while monitoring patterns of rest and activity. Temperature was also monitored in two animals (A and B) with subcutaneous implants. However, the temperature data were not informative. Externally applied transmitters were therefore used in subsequent studies. In each of the animals studied, we found that relatively low intensities of lighting (200 or 500 lux), altered circadian phase at the earliest ages studied.
One animal was also placed in reversed lighting cycles for 5 days after birth (infant D). When expressed rhythmicity developed in this animal, it was in phase with that of the reversed lighting cycle, raising the possibility that although there may not be overtly expressed circadian rhythmicity in the newborn period, photic entrainment of clock function may occur. However, it is important to note that animal A also had a reversed phase relationship in the absence of light treatment. Thus, we cannot be certain that the phase reversal of this animal D was due to the LD cycle alone. We also do not know if exposure to 1000 lux of light during the daytime on the day of birth influenced the circadian phase of this infant.
Challenging previous concepts (11, 36), recent observations in human adults show that low intensities of lighting can regulate circadian phase (14). Our observations of entrainment at low intensity lighting in baboons are consistent with these findings. Furthermore, the magnitude of phase delays observed after early morning light exposure to 200 lux was similar to that recently reported for humans [baboons, 2.2 + 0.5 hr; humans, 1.8 + 0.3 hr (14)].
Interestingly, although entrainment occurred at low intensity lighting, 200 lux was not sufficient to acutely influence SCN DG uptake or c-fos mRNA expression under the conditions used. Increasing evidence suggests that c-Fos-independent mechanisms can influence circadian clock function (53). Thus, primate photic entrainment by low intensity lighting may not involve induction of c-Fos expression or acute changes in SCN metabolic activity.
Presently, is not possible to determine when functional innervation of the SCN begins in human infants. Thus, non-human primate studies are the most practical means for providing insights into the factors that regulate the developing human circadian system. Our observation that the newborn baboon circadian system can be entrained by low intensity lighting suggests that further consideration of the lighting cycles that human newborns are exposed to is needed.
Acknowledgments
We are indebted to Dr. Dee Carey (Southwest Foundation for Biomedical Research, San Antonio) and Dr. Judy Lee (University of Illinois, Chicago) for their assistance in baboon studies, and to Dr. John Nurnberger, Jr. for performing melatonin assays. Mark Bender, Rebecca McClain, Mark Lasbury, and Curt Matlock are thanked for technical assistance. We are grateful to Dr. Richard E. Kronauer for useful discussions about study designs. S.A.R. is indebted to Steven M. Reppert and David R. Weaver for their past and continuing guidance and assistance. This work was supported by the Riley Memorial Association and National Institutes of Health Grant R01NS3994.
Footnotes
Abbreviations: SCN, suprachiasmatic nuclei; RHT, retinohypothalamic tract; LD, light–dark; DG, deoxyglucose; CT-HRP, cholera toxin-conjugated horseradish peroxidase.
References
- 1.Moore-Ede M C, Czeisler C A, Richardson G S. N Engl J Med. 1983;309:530–536. doi: 10.1056/NEJM198309013090905. [DOI] [PubMed] [Google Scholar]
- 2.Moore-Ede M C, Czeisler C A, Richardson G S. N Engl J Med. 1983;309:469–476. doi: 10.1056/NEJM198308253090806. [DOI] [PubMed] [Google Scholar]
- 3.Klein D C, Moore R Y, Reppert S M. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 4.Moore, R. Y. (1993) J. Biol. Rhythm 8, Suppl, S3–S9. [PubMed]
- 5.Edgar D M, Fuller C A. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morin L P. Brain Res Rev. 1994;19:102–127. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 7.Moore R Y. Brain Res. 1973;49:403–409. doi: 10.1016/0006-8993(73)90431-9. [DOI] [PubMed] [Google Scholar]
- 8.Sadun A A, Schaechter J D, Smith L E. Brain Res. 1984;302:371–377. doi: 10.1016/0006-8993(84)90252-x. [DOI] [PubMed] [Google Scholar]
- 9.Friedman D I, Johnson J K, Chorsky R L, Stopa E G. Brain Res. 1991;560:297–302. doi: 10.1016/0006-8993(91)91246-w. [DOI] [PubMed] [Google Scholar]
- 10.Hoban T M, Sulzman F M. Am J Physiol. 1985;249:R274–R280. doi: 10.1152/ajpregu.1985.249.2.R274. [DOI] [PubMed] [Google Scholar]
- 11.Czeisler C A, Allan J S, Strogatz S H, Ronda J M, Sanchez R, Rios C D, Freitag W O, Richardson G S, Kronauer R E. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 12.Czeisler C A, Kronauer R E, Allan J S, Duffy J F, Jewett M E, Brown E N, Ronda J M. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 13.Shanahan T L, Czeisler C A. J Clin Endocrinol Metab. 1991;73:227–235. doi: 10.1210/jcem-73-2-227. [DOI] [PubMed] [Google Scholar]
- 14.Boivin D B, Duffy J F, Kronauer R E, Czeisler C A. Nature (London) 1996;379:540–542. doi: 10.1038/379540a0. [DOI] [PubMed] [Google Scholar]
- 15.Reppert S M. Prog Brain Res. 1992;93:119–131. doi: 10.1016/s0079-6123(08)64568-9. [DOI] [PubMed] [Google Scholar]
- 16.Reppert S M, Schwartz W J. J Neurosci. 1984;4:1677–1682. doi: 10.1523/JNEUROSCI.04-07-01677.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reppert S M, Uhl G R. Endocrinology. 1987;120:2483–2487. doi: 10.1210/endo-120-6-2483. [DOI] [PubMed] [Google Scholar]
- 18.Rivkees, S. A. & Reppert, S. M. (1992) Horm. Res. 37, Suppl. 3, 99–104. [DOI] [PubMed]
- 19.Reppert S M, Weaver D R, Rivkees S A, Stopa E G. Science. 1988;242:78–81. doi: 10.1126/science.2845576. [DOI] [PubMed] [Google Scholar]
- 20.Reppert S M, Schwartz W J. Neurosci Lett. 1984;46:145–149. doi: 10.1016/0304-3940(84)90432-4. [DOI] [PubMed] [Google Scholar]
- 21.Glotzbach S T, Sollars P, Paragano R L, Pickard G H. Soc Neurosci Abstr. 1992;18:857. [Google Scholar]
- 22.Graven S N, Bowen F W J, Brooten D, Eaton A, Graven M N, et al. J Perinatol. 1992;12:164–172. [PubMed] [Google Scholar]
- 23.Hendrickx A G. Embryology of the Baboon. London: Univ. Chicago Press; 1971. [Google Scholar]
- 24.King F A, Anderson D C. Science. 1989;240:1475–1482. doi: 10.1126/science.3287624. [DOI] [PubMed] [Google Scholar]
- 25.Ducsay C A, Hess D L, McClellan M C, Novy M J. J Clin Endocrinol Metab. 1991;73:385–395. doi: 10.1210/jcem-73-2-385. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois J P, Rakic P. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwartz W J, Reppert S M, Eagan S M, Moore-Ede M C. Brain Res. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz W J, Gainer H. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 29.Rivkees, S. A. & Lachowicz, J. E. (1996) Synapse, in press. [DOI] [PubMed]
- 30.Rivkees S A. Endocrinology. 1994;136:2307–2313. doi: 10.1210/endo.135.6.7988413. [DOI] [PubMed] [Google Scholar]
- 31.Rivkees S A, Cassone V M, Weaver D R, Reppert S M. Endocrinology. 1989;125:363–368. doi: 10.1210/endo-125-1-363. [DOI] [PubMed] [Google Scholar]
- 32.Mesulam M M, Mufsson E J. Neuroscience. 1980;5:1277–1287. doi: 10.1016/0306-4522(80)90200-6. [DOI] [PubMed] [Google Scholar]
- 33.Lahiri D K, Davis D, Adkins M, Nurnberger J I., Jr Biochem Med Metab Biol. 1993;49:36–50. doi: 10.1006/bmmb.1993.1004. [DOI] [PubMed] [Google Scholar]
- 34.Weaver D R, Reppert S M. Dev Brain Res. 1989;47:151–155. doi: 10.1016/0165-3806(89)90119-3. [DOI] [PubMed] [Google Scholar]
- 35.Rivkees S A, Reppert S M. Am J Physiol. 1990;259:E384–E388. doi: 10.1152/ajpendo.1990.259.3.E384. [DOI] [PubMed] [Google Scholar]
- 36.Lewy A J, Wehr T A, Goodwin F K, Newsome D A, Markey S P. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 37.Curran T, MacConnell W P, van Straaten F, Verma I M. Mol Cell Biol. 1983;3:914–921. doi: 10.1128/mcb.3.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer A C, Nieuwenhuis J J, Kociszewska V J, Joubert W S, Meyer B J. Life Sci. 1986;39:1563–1569. doi: 10.1016/0024-3205(86)90388-7. [DOI] [PubMed] [Google Scholar]
- 39.Perlow M J, Reppert S M, Tamarkin L, Wyatt R J, Klein D C. Brain Res. 1980;182:211–216. doi: 10.1016/0006-8993(80)90848-3. [DOI] [PubMed] [Google Scholar]
- 40.Speh J C, Moore R Y. Dev Brain Res. 1993;76:171–181. doi: 10.1016/0165-3806(93)90205-o. [DOI] [PubMed] [Google Scholar]
- 41.Duncan M J, Banister M J, Reppert S M. Brain Res. 1986;369:326–330. doi: 10.1016/0006-8993(86)90544-5. [DOI] [PubMed] [Google Scholar]
- 42.Weaver D R, Reppert S M. Mol Brain Res. 1995;33:136–148. doi: 10.1016/0169-328x(95)00117-b. [DOI] [PubMed] [Google Scholar]
- 43.Torrealba F, Parraguez V H, Reyes T, Valenzuela G, Seron-Ferre M. J Comp Neurol. 1993;338:304–316. doi: 10.1002/cne.903380212. [DOI] [PubMed] [Google Scholar]
- 44.Kleitman J, Engelman J Appl Physiol. 1953;6:269–282. doi: 10.1152/jappl.1953.6.5.269. [DOI] [PubMed] [Google Scholar]
- 45.Attanasio A, Borrelli P, Gupta D. J Clin Endocrinol Metab. 1985;61:388–390. doi: 10.1210/jcem-61-2-388. [DOI] [PubMed] [Google Scholar]
- 46.Kennaway D J, Stamp G E, Goble F C. J Clin Endocrinol Metab. 1992;75:367–369. doi: 10.1210/jcem.75.2.1639937. [DOI] [PubMed] [Google Scholar]
- 47.Onishi S, Miyazawa G, Nishimura Y. Pediatrics. 1983;72:399–404. [PubMed] [Google Scholar]
- 48.Coalson J J, Winter V T, Gerstmann D R, Idell S, King R J, deLemos R A. Am Rev Respir Dis. 1992;145:872–881. doi: 10.1164/ajrccm/145.4_Pt_1.872. [DOI] [PubMed] [Google Scholar]
- 49.Parmelee A H. Acta Paediatr Scand. 1961;50:160–170. doi: 10.1111/j.1651-2227.1961.tb08035.x. [DOI] [PubMed] [Google Scholar]
- 50.Meier-Koll A, Hall U, Hellwig U, Kott G, Meier-Koll V. Chronobiologia. 1978;5:425–440. [PubMed] [Google Scholar]
- 51.Margraf R R, Zlomanczuk P, Liskin L A, Lynch G R. Brain Res. 1991;544:42–48. doi: 10.1016/0006-8993(91)90883-w. [DOI] [PubMed] [Google Scholar]
- 52.Murakami D M, Fuller C A. Brain Behav Evol. 1990;35:302–312. doi: 10.1159/000115876. [DOI] [PubMed] [Google Scholar]
- 53.Rea, M. A., Michel, A. M. & Lutton, L. M. (1993) J. Biol. Rhythm 8, Suppl., S59–S64. [PubMed]