Abstract
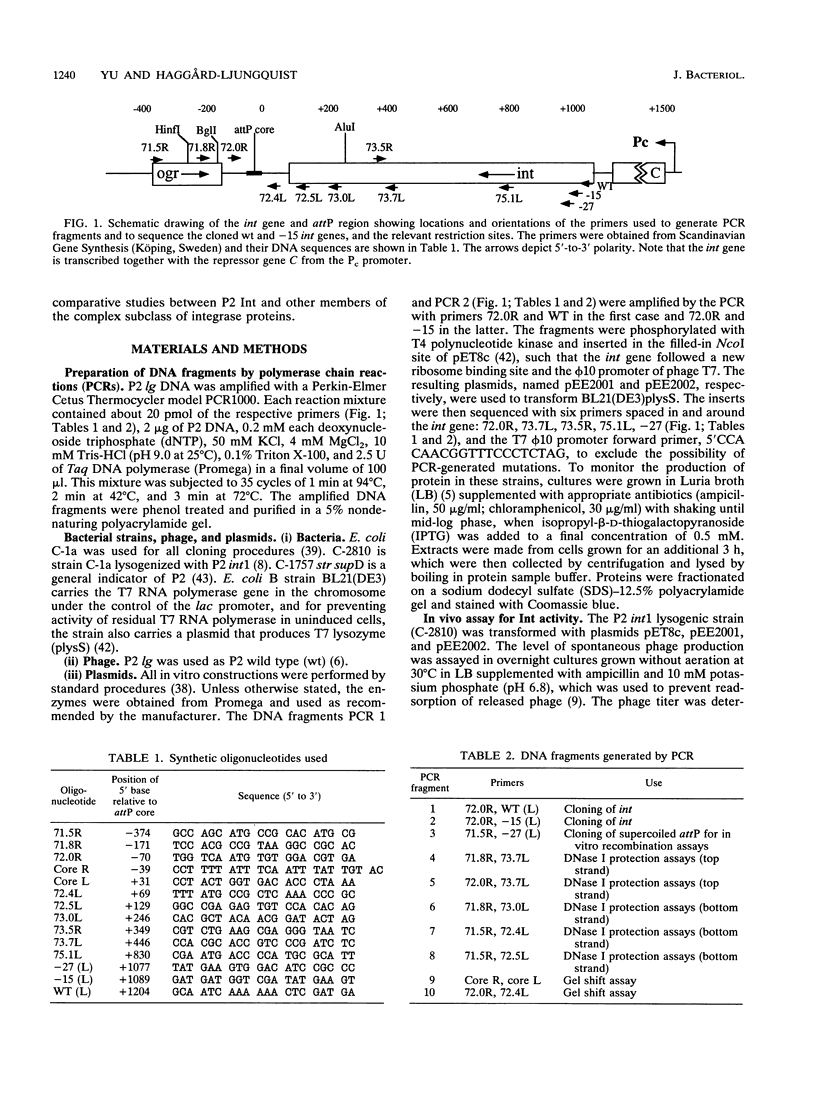
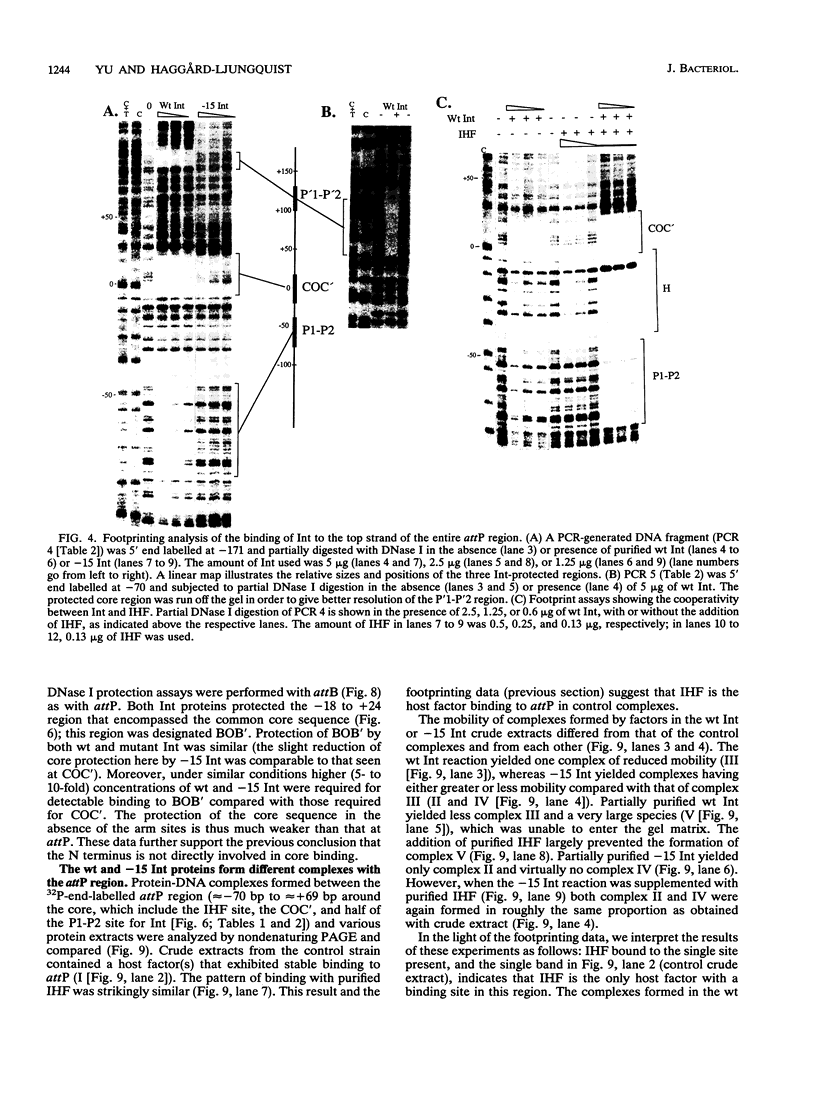
Integration of the bacteriophage P2 genome into the Escherichia coli host chromosome occurs by site-specific recombination between the phage attP and E. coli attB sites. The phage-encoded 38-kDa protein, integrase, is known to be necessary for both phage integration as well as excision. In order to begin the molecular characterization of this recombination event, we have cloned the int gene and overproduced and partially purified the Int protein and an N-terminal truncated form of Int. Both the wild-type Int protein and the integration host factor (IHF) of E. coli were required to mediate integrative recombination in vitro between a supercoiled attP plasmid and a linear attB substrate. Footprint experiments revealed one Int-protected region on both of the attP arms, each containing direct repeats of the consensus sequence TGTGGACA. The common core sequences at attP and attB were also protected by Int from nuclease digestion, and these contained a different consensus sequence, AA T/A T/A C/A T/G CCC, arranged as inverted repeats at each core. A single IHF-protected site was located on the P (left) arm, placed between the core- and P arm-binding site for Int. Cooperative binding by Int and IHF to the attP region was demonstrated with band-shift assays and footprinting studies. Our data support the existence of two DNA-binding domains on Int, having unrelated sequence specificities. We propose that P2 Int, IHF, attP, and attB assemble in a higher-order complex, or intasome, prior to site-specific integrative recombination analogous to that formed during lambda integration.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abremski K. E., Hoess R. H. Evidence for a second conserved arginine residue in the integrase family of recombination proteins. Protein Eng. 1992 Jan;5(1):87–91. doi: 10.1093/protein/5.1.87. [DOI] [PubMed] [Google Scholar]
- Andrews B. J., Proteau G. A., Beatty L. G., Sadowski P. D. The FLP recombinase of the 2 micron circle DNA of yeast: interaction with its target sequences. Cell. 1985 Apr;40(4):795–803. doi: 10.1016/0092-8674(85)90339-3. [DOI] [PubMed] [Google Scholar]
- Argos P., Landy A., Abremski K., Egan J. B., Haggard-Ljungquist E., Hoess R. H., Kahn M. L., Kalionis B., Narayana S. V., Pierson L. S., 3rd The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986 Feb;5(2):433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreiro V., Haggård-Ljungquist E. Attachment sites for bacteriophage P2 on the Escherichia coli chromosome: DNA sequences, localization on the physical map, and detection of a P2-like remnant in E. coli K-12 derivatives. J Bacteriol. 1992 Jun;174(12):4086–4093. doi: 10.1128/jb.174.12.4086-4093.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani G., Ljungquist E., Jagusztyn-Krynicka K., Jupp S. Defective particle assembly in wild type P2 bacteriophage and its correction by the lg mutation. J Gen Virol. 1978 Feb;38(2):251–261. doi: 10.1099/0022-1317-38-2-251. [DOI] [PubMed] [Google Scholar]
- Bertani L. E., Bertani G. Preparation and characterization of temperate, non-inducible bacteriophage P2 (host: Escherichia coli). J Gen Virol. 1970 Feb;6(2):201–212. doi: 10.1099/0022-1317-6-2-201. [DOI] [PubMed] [Google Scholar]
- Bertani L. E. Genetic interaction between the nip1 mutation and genes affecting integration and excision in phage P2. Mol Gen Genet. 1980 Apr;178(1):91–99. doi: 10.1007/BF00267217. [DOI] [PubMed] [Google Scholar]
- Better M., Lu C., Williams R. C., Echols H. Site-specific DNA condensation and pairing mediated by the int protein of bacteriophage lambda. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5837–5841. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Better M., Wickner S., Auerbach J., Echols H. Role of the Xis protein of bacteriophage lambda in a specific reactive complex at the attR prophage attachment site. Cell. 1983 Jan;32(1):161–168. doi: 10.1016/0092-8674(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Echols H. Integrative and excisive recombination by bacteriophage lambda: evidence for an excision-specific recombination protein. J Mol Biol. 1970 Feb 14;47(3):575–583. doi: 10.1016/0022-2836(70)90324-4. [DOI] [PubMed] [Google Scholar]
- Friedman D. I. Integration host factor: a protein for all reasons. Cell. 1988 Nov 18;55(4):545–554. doi: 10.1016/0092-8674(88)90213-9. [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Abremski K. Interaction of the bacteriophage P1 recombinase Cre with the recombining site loxP. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1026–1029. doi: 10.1073/pnas.81.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. L., Ross W., Landy A. The lambda phage att site: functional limits and interaction with Int protein. Nature. 1980 May 8;285(5760):85–91. doi: 10.1038/285085a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Moitoso de Vargas L., Nunes-Düby S. E., Landy A. Mapping of a higher order protein-DNA complex: two kinds of long-range interactions in lambda attL. Cell. 1990 Nov 16;63(4):773–781. doi: 10.1016/0092-8674(90)90143-3. [DOI] [PubMed] [Google Scholar]
- Landy A. Dynamic, structural, and regulatory aspects of lambda site-specific recombination. Annu Rev Biochem. 1989;58:913–949. doi: 10.1146/annurev.bi.58.070189.004405. [DOI] [PubMed] [Google Scholar]
- Leong J. M., Nunes-Düby S., Lesser C. F., Youderian P., Susskind M. M., Landy A. The phi 80 and P22 attachment sites. Primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985 Apr 10;260(7):4468–4477. [PubMed] [Google Scholar]
- Lindahl G. Multiple recombination mechanisms in bacteriophage P2. Virology. 1969 Dec;39(4):861–866. doi: 10.1016/0042-6822(69)90022-1. [DOI] [PubMed] [Google Scholar]
- Lindahl G., Sunshine M. Excision-deficient mutants of bacteriophage P2. Virology. 1972 Jul;49(1):180–187. doi: 10.1016/s0042-6822(72)80019-9. [DOI] [PubMed] [Google Scholar]
- Link C. D., Reiner A. M. Genotypic exclusion: a novel relationship between the ribitol-arabitol and galactitol genes of E. coli. Mol Gen Genet. 1983;189(2):337–339. doi: 10.1007/BF00337827. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mizuuchi M., Mizuuchi K. Integrative recombination of bacteriophage lambda: extent of the DNA sequence involved in attachment site function. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3220–3224. doi: 10.1073/pnas.77.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuuchi M., Mizuuchi K. The extent of DNA sequence required for a functional bacterial attachment site of phage lambda. Nucleic Acids Res. 1985 Feb 25;13(4):1193–1208. doi: 10.1093/nar/13.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moitoso de Vargas L., Pargellis C. A., Hasan N. M., Bushman E. W., Landy A. Autonomous DNA binding domains of lambda integrase recognize two different sequence families. Cell. 1988 Sep 23;54(7):923–929. doi: 10.1016/0092-8674(88)90107-9. [DOI] [PubMed] [Google Scholar]
- Nash H. A., Robertson C. A., Flamm E., Weisberg R. A., Miller H. I. Overproduction of Escherichia coli integration host factor, a protein with nonidentical subunits. J Bacteriol. 1987 Sep;169(9):4124–4127. doi: 10.1128/jb.169.9.4124-4127.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numrych T. E., Gumport R. I., Gardner J. F. A comparison of the effects of single-base and triple-base changes in the integrase arm-type binding sites on the site-specific recombination of bacteriophage lambda. Nucleic Acids Res. 1990 Jul 11;18(13):3953–3959. doi: 10.1093/nar/18.13.3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pargellis C. A., Nunes-Düby S. E., de Vargas L. M., Landy A. Suicide recombination substrates yield covalent lambda integrase-DNA complexes and lead to identification of the active site tyrosine. J Biol Chem. 1988 Jun 5;263(16):7678–7685. [PubMed] [Google Scholar]
- Prentki P., Chandler M., Galas D. J. Escherichia coli integration host factor bends the DNA at the ends of IS1 and in an insertion hotspot with multiple IHF binding sites. EMBO J. 1987 Aug;6(8):2479–2487. doi: 10.1002/j.1460-2075.1987.tb02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richet E., Abcarian P., Nash H. A. Synapsis of attachment sites during lambda integrative recombination involves capture of a naked DNA by a protein-DNA complex. Cell. 1988 Jan 15;52(1):9–17. doi: 10.1016/0092-8674(88)90526-0. [DOI] [PubMed] [Google Scholar]
- Robertson C. A., Nash H. A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988 Mar 15;263(8):3554–3557. [PubMed] [Google Scholar]
- Saha S., Haggård-Ljungquist E., Nordström K. Integration host factor is necessary for lysogenization of Escherichia coli by bacteriophage P2. Mol Microbiol. 1990 Jan;4(1):3–11. doi: 10.1111/j.1365-2958.1990.tb02009.x. [DOI] [PubMed] [Google Scholar]
- Saha S., Haggård-Ljungquist E., Nordström K. The cox protein of bacteriophage P2 inhibits the formation of the repressor protein and autoregulates the early operon. EMBO J. 1987 Oct;6(10):3191–3199. doi: 10.1002/j.1460-2075.1987.tb02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Somerville R. L., Shieh T. L., Hagewood B., Cui J. S. Gene expression from multicopy T7 promoter vectors proceeds at single copy rates in the absence of T7 RNA polymerase. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1056–1062. doi: 10.1016/0006-291x(91)92044-k. [DOI] [PubMed] [Google Scholar]
- Stenzel T. T., Patel P., Bastia D. The integration host factor of Escherichia coli binds to bent DNA at the origin of replication of the plasmid pSC101. Cell. 1987 Jun 5;49(5):709–717. doi: 10.1016/0092-8674(87)90547-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sunshine M. G., Kelly B. Extent of host deletions associated with bacteriophage P2-mediated eduction. J Bacteriol. 1971 Nov;108(2):695–704. doi: 10.1128/jb.108.2.695-704.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]