Abstract
The microtubule-associated protein (MAP) tau is abnormally hyperphosphorylated in Alzheimer disease and accumulates in neurons undergoing neurofibrillary degeneration. In the present study, the associations of the Alzheimer-hyperphosphorylated tau (AD P-tau) with the high molecular weight MAPs (HMW-MAPs) MAP1 and MAP2 were investigated. The AD P-tau was found to aggregate with MAP1 and MAP2 in solution. The association of AD P-tau to the MAPs resulted in inhibition of MAP-promoted microtubule assembly. However, unlike the coaggregation of AD P-tau and normal tau, the association between AD P-tau and the HMW-MAPs did not result in the formation of filaments/tangles. The affinity of the tau–AD P-tau association was higher than that of HMW-MAPs–AD P-tau because normal tau inhibited the latter binding. The association between AD P-tau and the HMW-MAPs also appeared to occur in situ because these proteins cosedimented from the Alzheimer brain extracts, and, in the sediment, the levels of the HMW-MAPs correlated with the levels of AD P-tau. These studies suggested that the abnormally phosphorylated tau can sequester both normal tau and HMW-MAPs and disassemble microtubules but, under physiological conditions, can form tangles of filaments only from tau.
Keywords: microtubule assembly, paired helical filaments, cytoskeleton, neurofibillary tangles
Alzheimer disease (AD) is characterized by a specific type of neuronal degeneration (called “neurofibrillary degeneration”), in which the neuronal cytoskeleton is progressively disrupted and displaced by the appearance of bundles of paired helical filaments (PHF), the neurofibrillary tangles. Neurons with neurofibrillary tangles lack microtubules, and microtubule assembly from AD brain cytosol is not observed (1). PHF are comprised mainly of the microtubule-associated protein (MAP) tau in an abnormally hyperphosphorylated state (2, 3). In AD brain, in addition to PHF, there is a soluble pool of the abnormally hyperphosphorylated tau (AD P-tau) (4). Compared with normal tau, which contains two to three phosphate groups, the AD P-tau contains 5–9 mol of phosphate per mol of the protein (4).
Unlike normal tau, the abnormal tau does not promote the in vitro assembly of microtubules (5, 6). By enzymatic dephosphorylation, but not deglycosylation, the microtubule assembly-promoting activity of both the PHF–tau and the AD P-tau can be restored (7–9). The AD P-tau inhibits the normal tau-promoted microtubule assembly, which is probably due to interaction with normal tau, and the coaggregation of these proteins results in tangles of ≈3.3-nm straight filaments (10) structurally similar to those formed from the enzymatic deglycosylation of the PHF tangles (8). However, in this likely mechanism of the Alzheimer neurofibrillary degeneration, it is not understood why, in the absence of functional tau, microtubules are not maintained by the high molecular weight MAPs (HMW-MAPs) or whether HMW-MAPs are involved in the formation of the neurofibrillary tangles.
The assembly of microtubules is not promoted by the endogenous MAPs in AD brain cytosol (1), and the heat-stable fraction from AD brain cytosol does not promote the assembly of tubulin into microtubules (11). Like tau, the HMW-MAPs are also phosphoproteins, and hyperphosphorylation depresses their ability to promote microtubule assembly (12). But, to date, there are no data available on either the phosphorylation state or the microtubule assembly-promoting activity of MAP1 or MAP2 isolated from AD brain. In this paper, we show that the AD P-tau sequesters not only normal tau but also MAP1 and MAP2, inhibits the ability of these HMW-MAPs to maintain the structure of microtubules, and, under physiological conditions, forms tangles of filaments with tau but not MAP1 or MAP2.
MATERIALS AND METHODS
Tissue Source.
Histopathologically confirmed AD and, as a control, Huntington disease brains obtained between 3 and 5 h postmortem and stored at −75°C were used. The human tissue was obtained from the Brain Tissue Resource Center McLean Hospital, Belmont, MA.
Antibodies.
mAb Tau-1, ascites (13), and anti-MAP1 and anti-MAP2 (Boehringer Mannheim) were used at dilutions of 1:25,000, 1:200, and 1:500, respectively.
Preparation of Rat Brain Cytosol.
One-month-old rats were killed, and the brains were removed and kept at −20°C for 24 h. The tissue was then thawed and homogenized in 1 g/1.0 ml of 100 mM Mes buffer (pH 6.7) containing 2 mM EGTA and 0.5 mM MgCl2. The homogenate was centrifuged at 100,000 × g for 1 h, and the supernatant was referred as the cytosolic extract.
Isolation of AD P-Tau and Normal Tau.
AD P-tau was isolated by the method of Köpke et al. (4). Normal tau was isolated as described (5) for acid-soluble tau (tau isolated from a HClO4 brain extract).
Isolation of MAP1 and MAP2 from Rat Brain.
HMW-MAPs were isolated from fresh rat brains. They were isolated from taxol-stabilized microtubules according to the protocols of Vallee (14) and Fujii et al. (15). To protect proteins from the action of phosphatases and proteases, fresh rat brain tissue was chopped and immediately placed in buffer solution containing a mixture of the following protease and phosphatase inhibitors: 0.1 mg/ml N-p-tosyl-l-arginine methyl ester, 5 μg/ml each of leupeptin, pepstatin, and aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM chloroquine, 10 mM soybean trypsin inhibitor, 50 mM sodium fluoride, 1 mM sodium vanadate, and 10 mM β-glycerophosphate. The tissue was homogenized, and, from the 180,000 × g supernatant, taxol-stabilized microtubules were prepared at 10 μM taxol and 1 mM GTP concentrations according to Fujii et al. (15). MAP1 was dissociated from the 100,000 × g microtubule pellet with 10 μg/ml poly-l-aspartic acid (47 kDa; Sigma) and 1 mM GTP according to Fujii et al. (15). MAP2 was then dissociated from microtubules devoid of MAP1 with 10 μM taxol and 350 mM KCl according to Vallee (14). Preparations of both MAPs were dialyzed against 10 mM Mes buffer (pH 6.7) containing 0.2 mM EGTA and 0.05 mM MgCl2 for 18 h with three changes. Then, the samples were lyophilized and stored at −75°C until used.
Binding of AD P-Tau to MAPs in Solution.
MAP1 or MAP2 (70 μg) was mixed with AD P-tau (8 μg) in a final volume of 100 μl of 100 mM Mes buffer (pH 6.7) containing 2 mM EGTA, 2% BSA, 0.5 mM MgCl2, 1 μM aprotinin, and 1 μM leupeptin (binding buffer). The mixture was allowed to interact for 30 min at 37°C and then was overlaid on 100 μl of 8% sucrose in the binding buffer and centrifuged for 1 h at 100,000 × g. After the centrifugation, 150 μl of the upper portion was separated, and the lower portion either was mixed by a Vortex mixer for negative stain electron microscopy or was sonicated for 1 h in a bath sonicator for determination of the levels of MAPs and AD P-tau by radioimmuno-slot blot assay (see below).
Protein Determination, Immunoblots, Radioimmuno-Slot Blot, Dephosphorylation, and Electron Microscopy.
Protein concentrations were estimated by the method of Bensadoun and Weinstein (16). Sample preparations and immunoblots were carried out as described (17). The levels of normal and AD P-tau MAP1 and MAP2 were determined by the radioimmuno-slot blot method of Khatoon et al. (18).
Isolation of Tubulin.
Rat brain tubulin was isolated through two temperature-dependent cycles of microtubule polymerization–depolymerization (19) followed by phosphocellulose ion exchange column chromatography (20).
Microtubule Assembly Assay.
MAP1 or MAP2 (1 mg/ml) was mixed at 4°C with purified rat brain tubulin (2 mg/ml) and 1 mM GTP, all in polymerization buffer (200 mM Mes, pH 6.7/1 mM EGTA/1 mM MgCl2). The assembly was carried out as described (5). The state of assembly of microtubules was confirmed by negative stain electron microscopy (21).
RESULTS
MAP1 and MAP2 Bind to AD P-Tau.
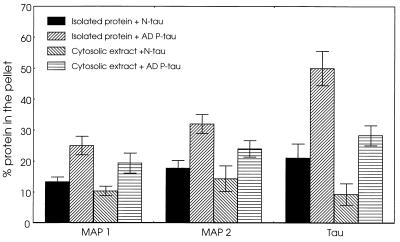
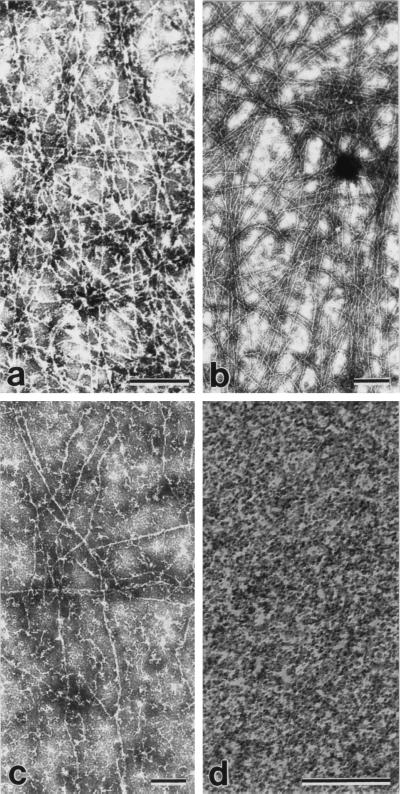
Our observation of the sequestration of normal tau by the AD P-tau (5, 10) led us to examine whether the abnormal tau also interacted with the HMW-MAPs and could in this way be responsible for the breakdown of the microtubule network in the affected neurons in AD. We determined the association of the increasing amounts of HMW-MAPs to a fixed amount of AD P-tau in solution under near physiological conditions, as described (10). The levels of the MAPs and AD P-tau were determined in the sedimentable phase. The sedimentation of the proteins alone was deducted from that of the proteins mixed at each concentration. We found that isolated MAP1 and MAP2 bound to AD P-tau (Fig. 1). Because the isolation procedure could have modified the MAPs and affected their interaction with AD P-tau, the binding of AD P-tau also was carried out with the cytosolic extract of the rat brain. To avoid polymerization of tubulin during the incubation, the rat brain was kept at −20°C for 24 h before it was used for the preparation of the cytosolic extract. When the cytosol was used, binding of AD P-tau to MAP1, MAP2, and normal tau was seen (Fig. 1), and no aggregation of tubulin was observed in the sediment (data not shown). However, unlike association of the AD P-tau with normal tau, the coaggregation of HMW-MAPs and AD P-tau did not form tangles or long filaments (Fig. 2). These results suggest that AD P-tau binds not only normal tau but also MAP1 and MAP2 but that the latter binding does not result in the formation of long filaments or tangles.
Figure 1.
Binding of MAP1 and MAP2 to AD P-tau. The binding of MAP1 and MAP2 to AD P-tau was determined as described using a fixed amount of AD P-tau or normal tau (8 μg in 100 μl) and MAP1 and MAP2 (70 μg/100 μl). The proteins were mixed in 100 mM Mes buffer (pH 6.7) containing 2 mM EGTA, 0.5 mM MgCl2, 2% BSA, 1 μM aprotinin, and 20 μM leupeptin (binding buffer) and were incubated for 30 min at 37°C. Similar incubations were carried out using rat brain cytosol (100 μg protein/100 μl) as the source of tau, MAP1, and MAP2. The incubated samples were overlaid a 100-μl cushion of 8% sucrose in the binding buffer and centrifuged at 100,000 × g for 60 min. As a control, MAP1, MAP2, and brain cytosol mixed with normal tau (8 μg) were processed identically to the mixtures of these proteins with AD P-tau. The amounts of tau, MAP1, MAP2, and tubulin (in the case of cytosolic extract) in the pellet and the supernatant fractions were assayed by radioimmuno-slot blot. AD P-tau bound to all of the MAPs when using both purified proteins and proteins in brain extract. The levels of binding of AD P-tau were within tau > MAP2 > MAP1.
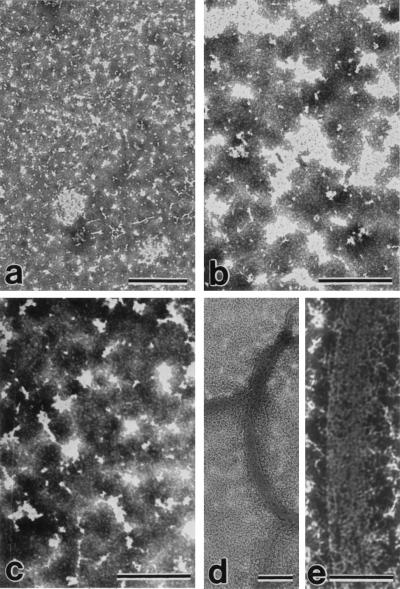
Figure 2.
Electron micrographs showing the products of association of AD P-tau with tau, MAP1, and MAP2 negatively stained with phosphotungstic acid. MAP1– and MAP2–AD P-tau aggregates were induced as described in Fig. 1. Under identical conditions, 150 μg of normal tau and 8 μg of AD P-tau/100 μl also were incubated. Aliquots of the incubated mixture of MAP1 and AD P-tau (a), MAP1 alone (b), MAP2 and AD P-tau (c), or tau and AD P-tau (d and e) were taken after centrifugation and were examined by negative stain electron microscopy. Association of AD P-tau with only tau and not MAP1 or MAP2 resulted in the formation of filaments/tangles. (Bars = 1 μm for a and d; 0.5 μm for b, c, and e.)
Normal Tau Competes with MAP1 and MAP2 for Binding with AD P-Tau.
Because normal tau also binds to AD P-tau (5, 10) and because, in the affected neurons of patients with AD, the PHF are mainly comprised of tau (2, 3), we investigated whether normal tau could compete with MAP1 and MAP2 for the binding to AD P-tau. For this investigation, the binding of AD P-tau to MAP1 and MAP2 was carried out with or without the addition of normal tau. The presence of normal tau almost abolished the binding of AD P-tau to the HMW-MAPs, and the amount of normal tau bound to AD P-tau was higher than the amounts of MAP1 and MAP2 (Table 1). These results suggested that the affinity of the association of normal tau to AD P-tau is higher than that between the HMW-MAPs and the AD P-tau.
Table 1.
Effect of normal tau on the binding of MAP1 and MAP2 to AD P-tau
| Addition of | % bound protein
|
||
|---|---|---|---|
| MAP1, 70 μg | MAP2, 70 μg | Tau, 70 μg | |
| Normal tau (8 μg) | 12.3 ± 3.2 | 18.0 ± 3.6 | 14.3 ± 3.5 |
| AD P-tau (8 μg) | 24.0 ± 3.6 | 30.7 ± 2.4 | 45.7 ± 2.1 |
| Normal tau (50 μg) plus AD P-tau (8 μg) | 14.3 ± 3.5 | 19.7 ± 3.0 | 42.0 ± 2.7 |
The binding and the quantitation of the bound proteins were carried out in solution as described. Results represents the mean ± SD of six values. Addition of normal tau abolished the binding of MAP1 and MAP2 to AD P-tau.
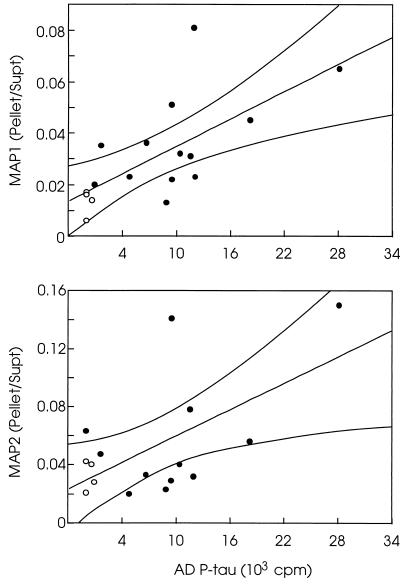
To examine whether the association between the AD P-tau and the HMW-MAPs also occurred in situ, we determined the levels of these proteins in the 27,000 × g to 200,000 × g extracts of AD and control brains; AD P-tau is known to isolate in the 27,000 × g to 200,000 × g brain extracts whereas normal tau and HMW-MAPs stay in the 200,000 × g supernatant (4). The levels of MAP1 and MAP2 showed a direct correlation with the levels of AD P-tau in the 27,000 to 200,000 × g pellet whereas the levels of the HMW-MAPs in the 200,000 × g supernatant had an inverse correlation. Therefore, the ratio of the HMW-MAPs in the pellet to these proteins in the supernatant directly correlated with the amount of AD P-tau in the 27,000 to 200,000 × g fraction (Fig. 3). The control brains did not contain any detectable levels of the abnormally phosphorylated tau and had only background levels of MAP1 and MAP2 in the 27,000 × g to 200,000 × g fraction. Furthermore, no tubulin aggregation was detected in either AD or control brain extracts. These findings suggested that AD P-tau associated with HMW-MAPs not only in vitro but probably also in situ.
Figure 3.
Relationship of the ratio of HMW-MAPs in the pellet/supernatant to the levels of AD P-tau in the pellet. The levels of MAP1 or MAP2 were determined in the 200,000 × g supernatant (Supt) and the 27,000 to 200,000 × g pellet (Pellet) from brain homogenates of 12 (MAP2) to 13 (MAP1) AD cases (•) and four Huntington disease cases (○). The levels of AD P-tau also were determined in the 27,000 × g to 200,000 × g fraction from the same brains by radioimmuno-slot blot using Tau-1 as the primary antibody (18). The AD P-tau values are expressed as cpm of the 125I secondary antibody used. The Pellet/Supt ratios of the HMW-MAPs were obtained from the means of triplicate assays determined at three different concentrations. The levels of the MAPs correlate directly with the levels of AD P-tau in the 27,000 × g to 200,000 × g pellet, and levels of the HMW-MAPs in the 200,000 × g supernatant correlate inversely with the AD P-tau in the pellet. The Pellet/Supt ratios of the HMW-MAPs show a significant direct correlation with the AD P-tau levels (p < 0.05).
AD P-Tau Inhibits MAP1- and MAP2-Promoted Microtubule Assembly.
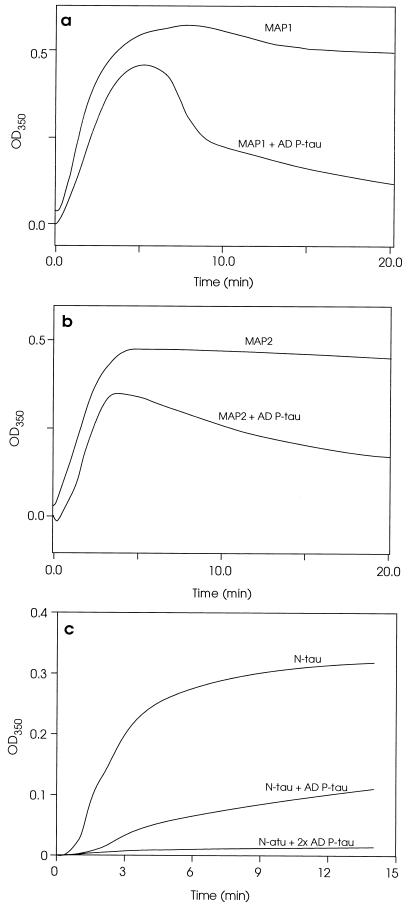
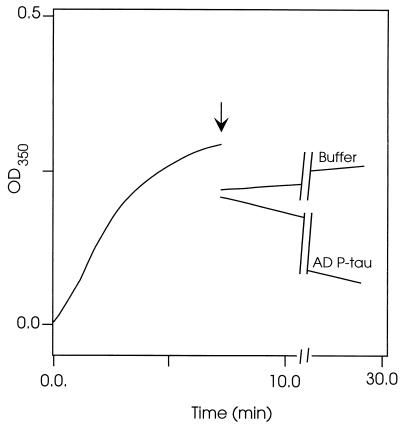
Like tau, MAP1 and MAP2 promote the assembly of tubulin into microtubules and help maintain the microtubule structure. Because of the association of MAP1 and MAP2 with AD P-tau, we investigated, in an in vitro microtubule assembly assay, whether this association competed with the interaction of the HMW-MAPs with tubulin and microtubules. The microtubule assembly assay was carried out as described. AD P-tau was added to MAP1 or MAP2 before it was mixed with tubulin, and the microtubule assembly was determined. AD P-tau inhibited the microtubule assembly (Fig. 4 a and b). However, unlike the inhibition of the tau-promoted microtubule assembly (Fig. 4c), which appears to be due to a higher affinity of normal tau to associate to AD P-tau than to tubulin, the HMW-MAPs-promoted assembly appears to proceed for ≈5 min, followed by disassembly. These turbidimetric changes of the microtubule assembly reaction, which were confirmed by negative stain electron microscopy (Fig. 5), suggested that, in the case of the HMW-MAPs, the AD P-tau mainly competes with microtubules in binding to MAP1 and MAP2. This scenario was further confirmed by studies in which microtubules first preformed with tubulin and the HMW-MAPs were rapidly depolymerized by addition of AD P-tau to the reaction mixture (Fig. 6). Negative stain electron microscopy showed formation of a mass of microtubules from tubulin and the HMW-MAPs (Fig. 5 a and b). A large number of microtubules also were observed during the first 5 min of the HMW-MAP-promoted microtubule assembly reaction in the presence of AD P-tau (Fig. 5c), but no microtubules were detected after 20 min of incubation (Fig. 5d). Similarly, by negative stain electron microscopy, a practically complete disassembly of microtubules was observed 20 min after the addition of AD P-tau to the assembled microtubules (data not shown).
Figure 4.
Inhibition of the MAP1- and MAP2-promoted microtubule assembly by AD P-tau. Polymerization of tubulin was determined as described. The assembly reaction was carried out using (i) 1.0 mg/ml MAP1 or a mixture of MAP1 and 2 mg/ml AD P-tau (a) or (ii) 1.0 mg/ml MAP2 or MAP2 plus 2 mg/ml AD P-tau (b). For comparison, the inhibition of tau-promoted microtubule assembly is reproduced (c) from our previous report (5).
Figure 5.
Electron micrographs showing the products of microtubule assembly with MAP1 and MAP2 and the effect of AD P-tau on the assembly. Microtubule assembly was carried out from rat brain tubulin by the addition of MAP1 (a), MAP2 (b), or MAP2 and AD P-tau (c and d, respectively) as in Fig. 4. Aliquots of the reaction mixture were negatively stained with phosphotungstic acid after 4 (c) or 30 (a, b, and d) min of incubation. Only an occasional microtubule was seen with tubulin alone (data not shown) and with AD P-tau after 30 min of incubation (d), and a large number of microtubules was observed in the assembly with MAP1 (a), MAP2 (b), and MAP2 with AD P-tau after 4 min of incubation (c). (Bars = 1 μm for a-c and 0.5 μm for d.)
Figure 6.
Disruption of the MAP2-promoted microtubules by AD P-tau. The assembly of microtubules was determined turbidimetrically as described. The arrow indicates the time of addition of the AD P-tau (2 mg/ml final concentration) or the same volume of buffer to MAP2-preassembled microtubules. Samples of the reaction mixture were examined by negatively stained electron microscopy. Only an occasional microtubule was seen after 20 min of AD P-tau addition (not shown).
The possibility that a direct action of AD P-tau on microtubules could be the cause of their disassembly (5) was investigated (although it was unlikely because AD P-tau does not interact with tubulin). Addition of AD P-tau to microtubules assembled from MAP-free tubulin and protamine sulfate (0.1 mg/ml) or taxol (20 μM) did not cause any detectable disassembly (data not shown), suggesting that AD P-tau does not disrupt microtubules directly but most likely disrupts only by sequestering MAPs from microbutules.
DISCUSSION
Independent of etiology, whether genetic or nongenetic, one of the most characteristic brain lesions of AD (which appears to be required for the clinical expression of the disease) is the neurons with neurofibrillary changes undergoing degeneration (22, 23). Understanding of the mechanism of this neurofibrillary degeneration is critical to devising a rational, therapeutic treatment of AD. We have shown that the AD P-tau competes with tubulin in binding to normal tau and inhibits the assembly of microtubules. The association of AD P-tau with normal tau results in tangles of ≈3.3-nm filaments structurally similar to AD neurofibrillary tangles, in which the PHF had been untwisted by deglycosylation with endoglycosidase F/N glycosidase F (8, 10). In addition to tau, neurons contain MAP1 and MAP2, which also promote microtubule assembly and maintain the structure of microtubules. A major missing link in the understanding of the role of the abnormal phosphorylation of tau in neurofibrillary degeneration has been the nature of the involvement of the HMW-MAPs.
In this study, we investigated whether, in the presence of the abnormally hyperphosphorylated tau, the HMW-MAPs could maintain the structure of microtubules. We have found that (i) the high molecular weight MAP1 and MAP2 associate to AD P-tau, (ii) the affinity of the binding between the AD P-tau and normal tau is higher than that between AD P-tau and the HMW-MAPs, and (iii) the AD P-tau sequesters the HMW-MAPs from microtubules, resulting in the disassembly of microtubules. However, although the association between the AD P-tau and the normal tau results in the formation of tangles of thin (≈3.3 nm), long filaments reminiscent of Alzheimer neurofibrillary tangles, the binding of AD P-tau to MAP1 or MAP2 does not result in the formation of tangles or individual long filaments.
Previously, it was shown that microtubule assembly was not achieved from cytosolic extracts from AD brains and that this impairment was not due to tubulin because microtubules were formed in the presence of DEAE–dextran (1). Because of the abnormal phosphorylation of tau and its lack of activity, it was postulated to be responsible for the lack of microtubule assembly. Nevertheless, nothing was known as to why the other MAPs, MAP1 and MAP2, did not compensate. The present study shows that the AD P-tau is capable of disrupting the neuronal microtubule network by sequestering not only normal tau but also MAP1 and MAP2. The association between the AD P-tau and the HMW-MAPs occurs when using isolated proteins and probably also occurs in situ, as demonstrated from the findings that (i) in the 27,000 × g to 200,000 × g AD brain extract, the HMW-MAPs cosedimented with the AD P-tau and (ii) the levels of the HMW-MAPs sedimenting in this fraction correlated directly with the levels of AD P-tau in the same fraction and with a reduction of the levels of MAP1 and MAP2 in the 200,000 × g supernatant. These results suggest that AD P-tau also sequesters HMW-MAPs, making them sedimentable. However, the composition of this sediment is unknown, and we cannot rule out the possibility that MAPs in this fraction also are aggregated with other proteins or membranous components.
In the present study, we have been able to induce the association of AD P-tau with HMW-MAPs under near physiological conditions. When the nature of these aggregated proteins was analyzed by electron microscopy, we detected the presence of protein aggregates. These findings were different from the bundles of long, thin filaments that we reported for the association of tau to AD P-tau (10). In fact, HMW-MAPs have not been observed in isolated PHF (2) except by the immunocytochemical labeling of Alzheimer neurofibrillary tangles with an antibody to MAP2 (24) and antibodies to a fragment of MAP1 (25).
A decrease in the tau phosphatase activity in AD brains has been shown (26, 27), suggesting that the tau is abnormally phosphorylated in AD brains, probably due to an imbalance in the protein phosphorylation/dephosphorylation system. The nature of the signal that causes the decrease in tau phosphatase activity of tau is presently not known. Nevertheless, the present study along with our other recent reports (5, 8, 10) is consistent with a mechanism of neurofibrillary degeneration in which the abnormally hyperphosphorylated tau competes with tubulin in associating with normal tau, MAP1, and MAP2 and inhibits microtubule assembly. The sequestration of the MAPs (microtubule-stabilizing proteins) from microtubules causes their disruption. The aggregates of the AD P-tau and normal tau work as “seeds” for the polymerization of tau into neurofibrillary tangles in which PHF are formed by glycosylation. The breakdown of the microtubule network leads to a compromised axonal transport and consequently loss of synapses and retrograde degeneration. The present study and our previous studies (1, 5, 6, 10) taken together demonstrate that the abnormally phosphorylated tau is not only inactive in promoting microtubule assembly but also causes disassembly of microtubules assembled with tau, MAP1, and MAP2. Thus, neurofibrillary degeneration might be prevented by inhibiting abnormal hyperphosphorylation of tau.
Acknowledgments
We thank Dr. L. I. Binder for a generous supply of mAb Tau-1, Ms. Tanweer Zaidi for her help in the isolation of tau, the Biomedical Photography Unit for the preparation of the figures, and Joanne Lopez and Diane Cocozza for secretarial assistance. Autopsied brain specimens were provided by the Brain Tissue Resource Center (Public Health Service Grant MH/NS 31862), McLean Hospital, Belmont, MA. These studies were supported in part by the New York State Office of Mental Retardation and Developmental Disabilities; National Institutes of Health Grants TW00507, AG05892, AG08076, and NS18105; the Zenith Award (to K.I.) from the Alzheimer Association, Chicago; and Consejo de Investigación de Córdoba and Secretaría de Ciemcia y Technología–Universidad Nacional de Córdoba grants from Cordoba, Argentina.
Footnotes
Abbreviations: AD, Alzheimer disease; PHF, paired helical filaments; MAP, microtubule-associated protein; HMW-MAP, high molecular weight MAP; AD P-tau, AD-hyperphosphorylated tau.
References
- 1.Iqbal, K., Grundke-Iqbal, I., Zaidi, T., Merz, P. A., Wen, G. Y., Shaikh, S. S., Wisniewski, H. M., Alafuzoff, I. & Wimblad, B. (1986) Lancet ii, 421–426. [DOI] [PubMed]
- 2.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi M S, Wisniewski H M. J Biol Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 3.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski H M, Binder L. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köpke E, Tung Y-C, Shaikh S, Alonso A del C, Iqbal K, Grundke-Iqbal I. J Biol Chem. 1993;268:24374–24384. [PubMed] [Google Scholar]
- 5.Alonso A del C, Zaidi T, Grundke-Iqbal I, Iqbal K. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal K, Zaidi T, Bancher C, Grundke-Iqbal I. FEBS Lett. 1994;349:104–108. doi: 10.1016/0014-5793(94)00650-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang J-Z, Gong C-X, Zaidi T, Grundke-Iqbal I, Iqbal K. J Biol Chem. 1995;270:4854–4860. doi: 10.1074/jbc.270.9.4854. [DOI] [PubMed] [Google Scholar]
- 8.Wang J-Z, Grundke-Iqbal I, Iqbal K. Nat Med. 1996;2:871–875. doi: 10.1038/nm0896-871. [DOI] [PubMed] [Google Scholar]
- 9.Wang J-Z, Grundke-Iqbal I, Iqbal K. Mol Brain Res. 1996;38:200–208. doi: 10.1016/0169-328x(95)00316-k. [DOI] [PubMed] [Google Scholar]
- 10.Alonso A del C, Grundke-Iqbal I, Iqbal K. Nat Med. 1996;2:783–787. doi: 10.1038/nm0796-783. [DOI] [PubMed] [Google Scholar]
- 11.Nieto A, DeGarcini E M, Correas I, Avila J. Neuroscience. 1990;37:163–170. doi: 10.1016/0306-4522(90)90201-e. [DOI] [PubMed] [Google Scholar]
- 12.Avila J, Domínguez J, Díaz-Nido J. Int J Dev Biol. 1994;38:13–25. [PubMed] [Google Scholar]
- 13.Binder L I, Frankfurter A, Rebhun L I. J Cell Biol. 1985;101:1371–1378. doi: 10.1083/jcb.101.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vallee R B. Methods Enzymol. 1986;134:89–114. doi: 10.1016/0076-6879(86)34078-3. [DOI] [PubMed] [Google Scholar]
- 15.Fujii T, Nakamura A, Ogoma Y, Kondo Y, Arai T. Anal Biochem. 1990;184:268–273. doi: 10.1016/0003-2697(90)90679-4. [DOI] [PubMed] [Google Scholar]
- 16.Bensadoun A, Weinstein D. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 17.Grundke-Iqbal I, Iqbal K, Tung Y-C, Wang G P, Wisniewski H M. Acta Neuropathol. 1984;62:259–267. doi: 10.1007/BF00687607. [DOI] [PubMed] [Google Scholar]
- 18.Khatoon S, Grundke-Iqbal I, Iqbal K. FEBS Lett. 1994;351:80–84. doi: 10.1016/0014-5793(94)00829-9. [DOI] [PubMed] [Google Scholar]
- 19.Shelanski M L, Gaskin F, Cantor C R. Proc Natl Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloboda R D, Rosenbaum J L. Biochemistry. 1979;18:48–55. doi: 10.1021/bi00568a008. [DOI] [PubMed] [Google Scholar]
- 21.Wisniewski H M, Merz P A, Iqbal K. J Neuropathol Exp Neurol. 1984;43:643–656. doi: 10.1097/00005072-198411000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Tomlinson B E, Blessed G, Roth M. J Neurol Sci. 1970;11:205–242. doi: 10.1016/0022-510x(70)90063-8. [DOI] [PubMed] [Google Scholar]
- 23.Alafuzoff I, Iqbal K, Friden H, Adolfsson R, Winblad B. Acta Neuropathol. 1987;74:209–225. doi: 10.1007/BF00688184. [DOI] [PubMed] [Google Scholar]
- 24.Kosik K S, Duffy L K, Dowling M M, Abraham C, McCluskey A, Selkoe D J. Proc Natl Acad Sci USA. 1984;81:7941–7945. doi: 10.1073/pnas.81.24.7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasegawa M, Arai T, Ihara Y. Neuron. 1990;4:909–918. doi: 10.1016/0896-6273(90)90144-5. [DOI] [PubMed] [Google Scholar]
- 26.Gong C-X, Singh T J, Grundke-Iqbal I, Iqbal K. J Neurochem. 1993;61:921–927. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 27.Gong C-X, Shaikh S, Wang J-Z, Zaidi T, Grundke-Iqbal I, Iqbal K. J Neurochem. 1995;65:732–738. doi: 10.1046/j.1471-4159.1995.65020732.x. [DOI] [PubMed] [Google Scholar]