Abstract
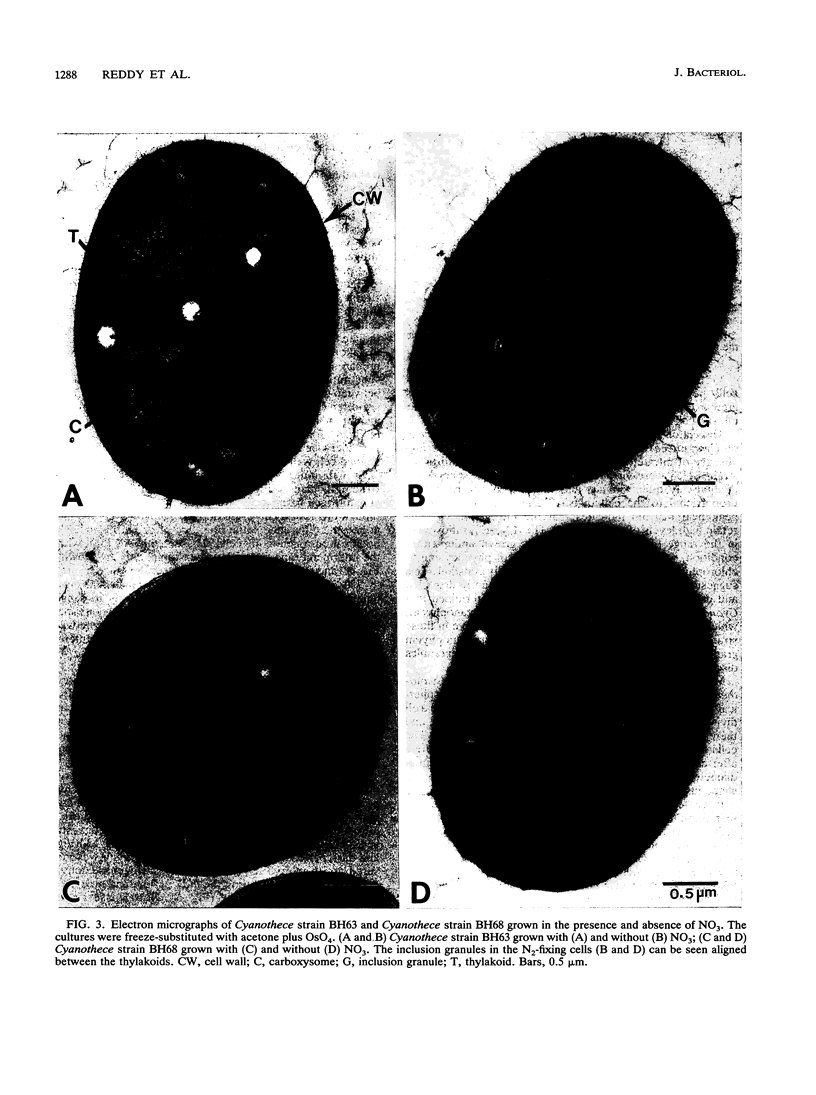
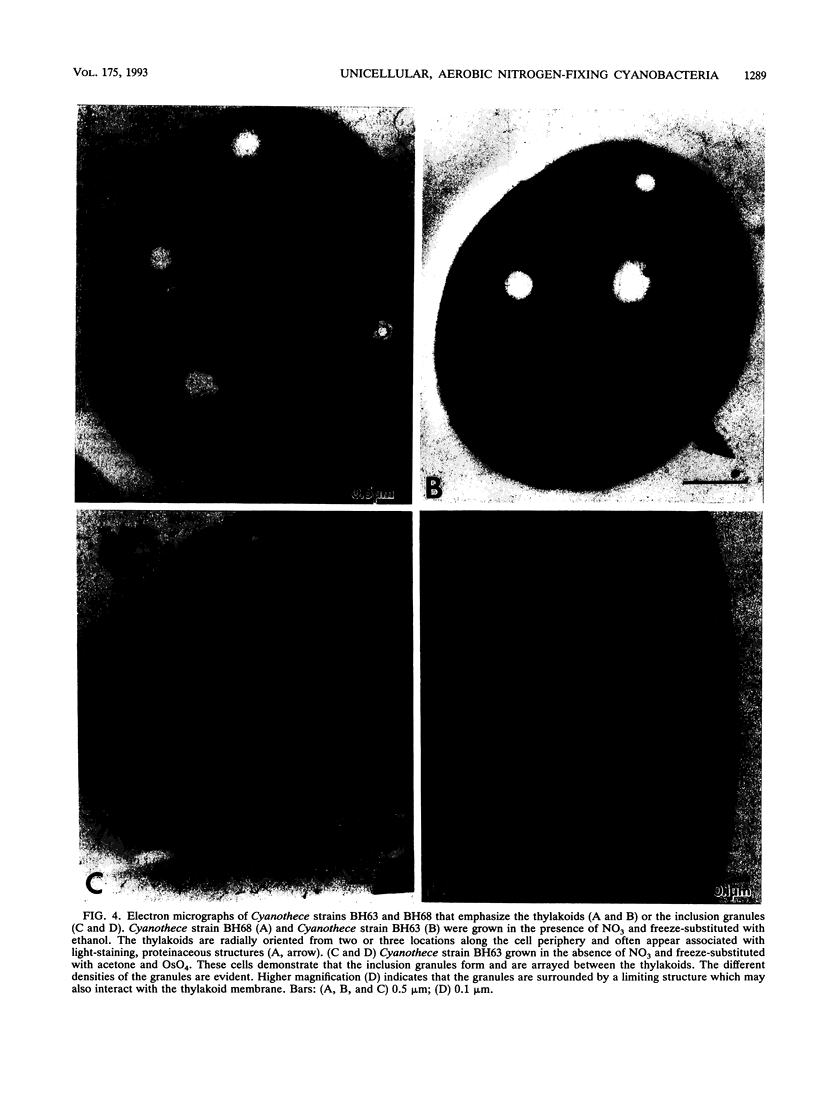
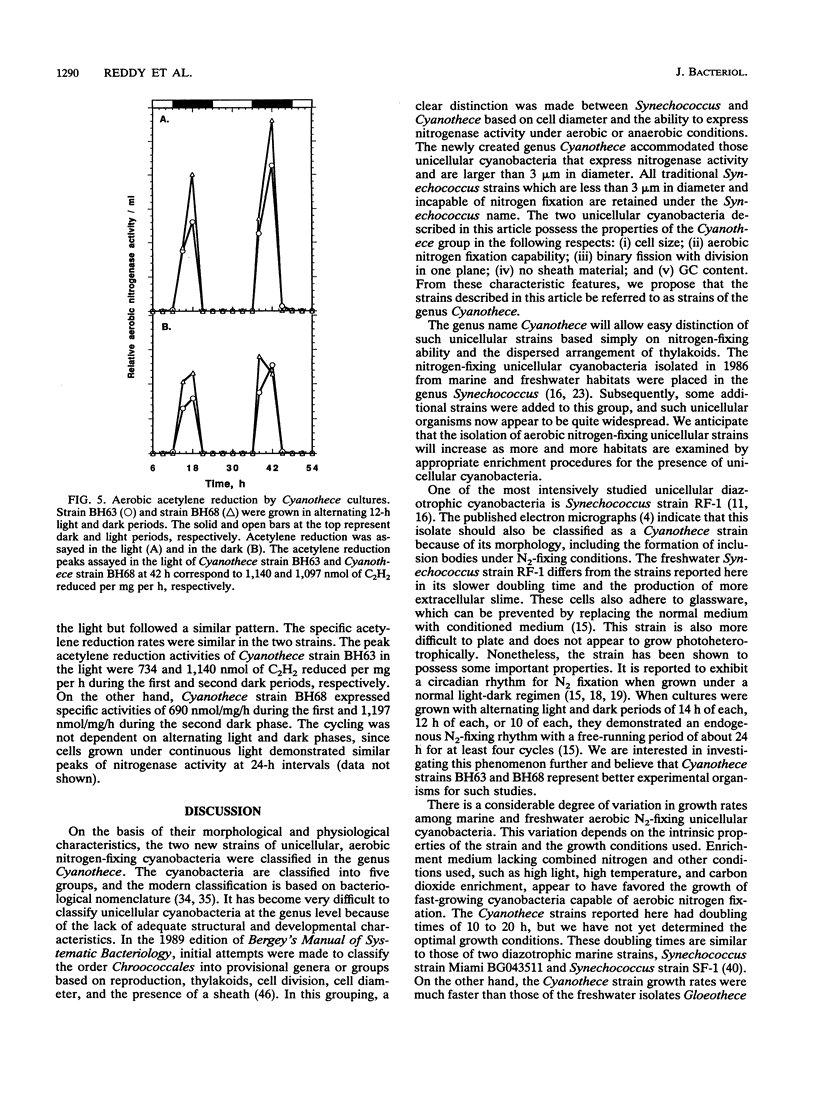
Two marine, unicellular aerobic nitrogen-fixing cyanobacteria, Cyanothece strain BH63 and Cyanothece strain BH68, were isolated from the intertidal sands of the Texas Gulf coast in enrichment conditions designed to favor rapid growth. By cell morphology, ultrastructure, a GC content of 40%, and aerobic nitrogen fixation ability, these strains were assigned to the genus Cyanothece. These strains can use molecular nitrogen as the sole nitrogen source and are capable of photoheterotrophic growth in the presence of 3-(3,4-dichlorophenyl)-1,1-dimethylurea and glycerol. The strains demonstrated a doubling time of 10 to 14 h in the presence of nitrate and 16 to 20 h under nitrogen-fixing conditions. Rapid growth of nitrogen-fixing cultures can be obtained in continuous light even when the cultures are continuously shaken or bubbled with air. Under 12-h alternating light and dark cycles, the aerobic nitrogenase activity was confined to the dark phase. The typical rates of aerobic nitrogenase activity in Cyanothece strains BH63 and BH68 were 1,140 and 1,097 nmol of C2H2 reduced per mg (dry weight) per h, respectively, and nitrogenase activity was stimulated twofold by light. Ultrastructural observations revealed that numerous inclusion granules formed between the photosynthetic membranes in cells grown under nitrogen-fixing conditions. These Cyanothece strains posses many characteristics that make them particularly attractive for a detailed analysis of the interaction of nitrogen fixation and photosynthesis in an aerobic diazotroph.
Full text
PDF



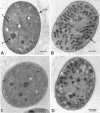
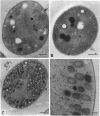
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burris R. H. Nitrogenases. J Biol Chem. 1991 May 25;266(15):9339–9342. [PubMed] [Google Scholar]
- Carpenter E. J., Price C. C. Marine oscillatoria (Trichodesmium): explanation for aerobic nitrogen fixation without heterocysts. Science. 1976 Mar 26;191(4233):1278–1280. doi: 10.1126/science.1257749. [DOI] [PubMed] [Google Scholar]
- Fay P. Oxygen relations of nitrogen fixation in cyanobacteria. Microbiol Rev. 1992 Jun;56(2):340–373. doi: 10.1128/mr.56.2.340-373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. C., Chou W. M. Setting of the Circadian N(2)-Fixing Rhythm of the Prokaryotic Synechococcus sp. RF-1 while Its nif Gene Is Repressed. Plant Physiol. 1991 May;96(1):324–326. doi: 10.1104/pp.96.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. C., Tu J., Chow T. J., Chen T. H. Circadian Rhythm of the Prokaryote Synechococcus sp. RF-1. Plant Physiol. 1990 Feb;92(2):531–533. doi: 10.1104/pp.92.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert D. H., Stevens S. E., Jr Photoheterotrophic growth of Agmenellum quadruplicatum PR-6. J Bacteriol. 1986 Feb;165(2):654–656. doi: 10.1128/jb.165.2.654-656.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROVASOLI L., MCLAUGHLIN J. J., DROOP M. R. The development of artificial media for marine algae. Arch Mikrobiol. 1957;25(4):392–428. doi: 10.1007/BF00446694. [DOI] [PubMed] [Google Scholar]
- Rippka R., Cohen-Bazire G. The cyanobacteriales: a legitimate order based on the type strain Cyanobacterium stanieri? Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):21–36. doi: 10.1016/s0769-2609(83)80094-5. [DOI] [PubMed] [Google Scholar]
- Spiller H., Shanmugam K. T. Physiological conditions for nitrogen fixation in a unicellular marine cyanobacterium, Synechococcus sp. strain SF1. J Bacteriol. 1987 Dec;169(12):5379–5384. doi: 10.1128/jb.169.12.5379-5384.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt J. T., Silvey J. K. Nitrogen fixation by gloeocapsa. Science. 1969 Aug 29;165(3896):908–909. doi: 10.1126/science.165.3896.908. [DOI] [PubMed] [Google Scholar]