Abstract
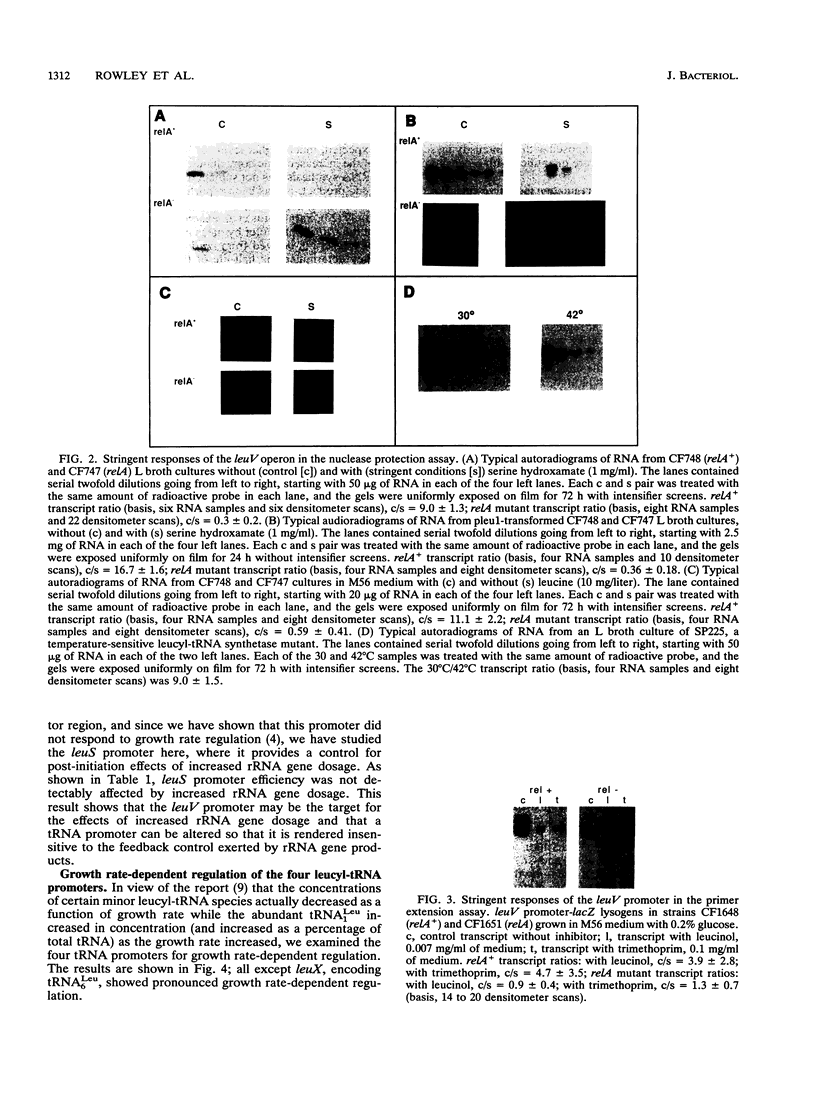
Four Escherichia coli operons, the leuV operon which encodes tRNA(1Leu), the leuX operon which encodes tRNA(6Leu), the metT operon which encodes tRNA(3Leu), and the argT operon which encodes tRNA(1Leu), were examined for the stringent response induced by serine hydroxamate and for growth rate-dependent regulation. In nuclease protection assays, the leuV operon displayed the stringent response in response to leucine starvation, analog inhibition, and growth of a temperature-sensitive leucyl-tRNA synthetase mutant at nonpermissive temperatures. The leuV operon also exhibited the stringent response in multicopy plasmids. The promoters of all four leucyl operons were fused to the gene for beta-galactosidase and inserted into the chromosome by using bacteriophage lambda. All except the leuX promoter displayed growth rate-dependent regulation, consistent with the recent report that the concentration of tRNA(6Leu) actually decreases as growth rate increases. The leuV promoter fused to the beta-galactosidase gene showed a decrease in efficiency in the presence of extrachromosomal copies of rRNA genes. All chromosomal tRNA genes examined showed decreased transcriptional activity following a stringent response, but the leuX gene responded to a lesser extent (3-fold versus 10-fold or more) than the others. Primer extension analysis of this promoter showed little if any response to serine hydroxamate treatment, suggesting that multiple levels of control may exist or that promoter context effects are important in regulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer B. F., Kar E. G., Elford R. M., Holmes W. M. Sequence determinants for promoter strength in the leuV operon of Escherichia coli. Gene. 1988;63(1):123–134. doi: 10.1016/0378-1119(88)90551-3. [DOI] [PubMed] [Google Scholar]
- Bertrand K. P., Postle K., Wray L. V., Jr, Reznikoff W. S. Construction of a single-copy promoter vector and its use in analysis of regulation of the transposon Tn10 tetracycline resistance determinant. J Bacteriol. 1984 Jun;158(3):910–919. doi: 10.1128/jb.158.3.910-919.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. R., Gaal T., deBoer H. A., deHaseth P. L., Gourse R. L. Identification of promoter mutants defective in growth-rate-dependent regulation of rRNA transcription in Escherichia coli. J Bacteriol. 1989 Sep;171(9):4862–4870. doi: 10.1128/jb.171.9.4862-4870.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duester G., Elford R. M., Holmes W. M. Fusion of the Escherichia coli tRNALeu1 promoter to the galK gene: analysis of sequences necessary for growth-rate-dependent regulation. Cell. 1982 Oct;30(3):855–864. doi: 10.1016/0092-8674(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Emilsson V., Kurland C. G. Growth rate dependence of transfer RNA abundance in Escherichia coli. EMBO J. 1990 Dec;9(13):4359–4366. doi: 10.1002/j.1460-2075.1990.tb07885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M. J., Ozeki H. Structure and organization of the transfer ribonucleic acid genes of Escherichia coli K-12. Microbiol Rev. 1985 Dec;49(4):379–397. doi: 10.1128/mr.49.4.379-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Barkei J., Dickson R. R., deBoer H. A., deHaseth P. L., Alavi H., Gourse R. L. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J Bacteriol. 1989 Sep;171(9):4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T., Gourse R. L. Guanosine 3'-diphosphate 5'-diphosphate is not required for growth rate-dependent control of rRNA synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5533–5537. doi: 10.1073/pnas.87.14.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourse R. L., de Boer H. A., Nomura M. DNA determinants of rRNA synthesis in E. coli: growth rate dependent regulation, feedback inhibition, upstream activation, antitermination. Cell. 1986 Jan 17;44(1):197–205. doi: 10.1016/0092-8674(86)90498-8. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Ikemura T., Dahlberg J. E. Small ribonucleic acids of Escherichia coli. II. Noncoordinate accumulation during stringent control. J Biol Chem. 1973 Jul 25;248(14):5033–5041. [PubMed] [Google Scholar]
- Jensen K. F., Pedersen S. Metabolic growth rate control in Escherichia coli may be a consequence of subsaturation of the macromolecular biosynthetic apparatus with substrates and catalytic components. Microbiol Rev. 1990 Jun;54(2):89–100. doi: 10.1128/mr.54.2.89-100.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks-Robertson S., Gourse R. L., Nomura M. Expression of rRNA and tRNA genes in Escherichia coli: evidence for feedback regulation by products of rRNA operons. Cell. 1983 Jul;33(3):865–876. doi: 10.1016/0092-8674(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Komine Y., Adachi T., Inokuchi H., Ozeki H. Genomic organization and physical mapping of the transfer RNA genes in Escherichia coli K12. J Mol Biol. 1990 Apr 20;212(4):579–598. doi: 10.1016/0022-2836(90)90224-A. [DOI] [PubMed] [Google Scholar]
- Miura A., Krueger J. H., Itoh S., de Boer H. A., Nomura M. Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell. 1981 Sep;25(3):773–782. doi: 10.1016/0092-8674(81)90185-9. [DOI] [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Reactions sequence of leucine activation catalysed by leucyl-RNA synthetase. 1. Kinetic studies. Eur J Biochem. 1968 Apr;4(3):305–309. doi: 10.1111/j.1432-1033.1968.tb00209.x. [DOI] [PubMed] [Google Scholar]
- Rouget P., Chapeville F. Reactions sequence of leucine activation catalysed by leucyl-RNA synthetase. 2. Formation of complexes between the enzyme and substrates. Eur J Biochem. 1968 Apr;4(3):310–314. doi: 10.1111/j.1432-1033.1968.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Smith R. J., Midgley J. E. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Regulation of ribonucleic acid synthesis by 'Magic Spot' nucleotides. Biochem J. 1973 Oct;136(2):249–257. doi: 10.1042/bj1360249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. J., Midgley J. E. The effect of trimethoprim on macromolecular synthesis in Escherichia coli. Biochem J. 1973 Oct;136(2):225–234. doi: 10.1042/bj1360225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A., Berman M. L. RNA polymerase interactions with the upstream region of the E. coli tyrT promoter. Cell. 1983 Nov;35(1):265–273. doi: 10.1016/0092-8674(83)90229-5. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A. PpGpp regulates the binding of two RNA polymerase molecules to the tyrT promoter. Nucleic Acids Res. 1982 Aug 25;10(16):5043–5057. doi: 10.1093/nar/10.16.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Weeks J. R. Alteration of the growth-rate-dependent regulation of Escherichia coli tyrT expression by promoter mutations. J Mol Biol. 1986 May 5;189(1):251–255. doi: 10.1016/0022-2836(86)90397-9. [DOI] [PubMed] [Google Scholar]
- Vogel U., Pedersen S., Jensen K. F. An unusual correlation between ppGpp pool size and rate of ribosome synthesis during partial pyrimidine starvation of Escherichia coli. J Bacteriol. 1991 Feb;173(3):1168–1174. doi: 10.1128/jb.173.3.1168-1174.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharias M., Göringer H. U., Wagner R. The signal for growth rate control and stringent sensitivity in E. coli is not restricted to a particular sequence motif within the promoter region. Nucleic Acids Res. 1990 Nov 11;18(21):6271–6275. doi: 10.1093/nar/18.21.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]






