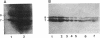Abstract
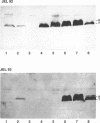
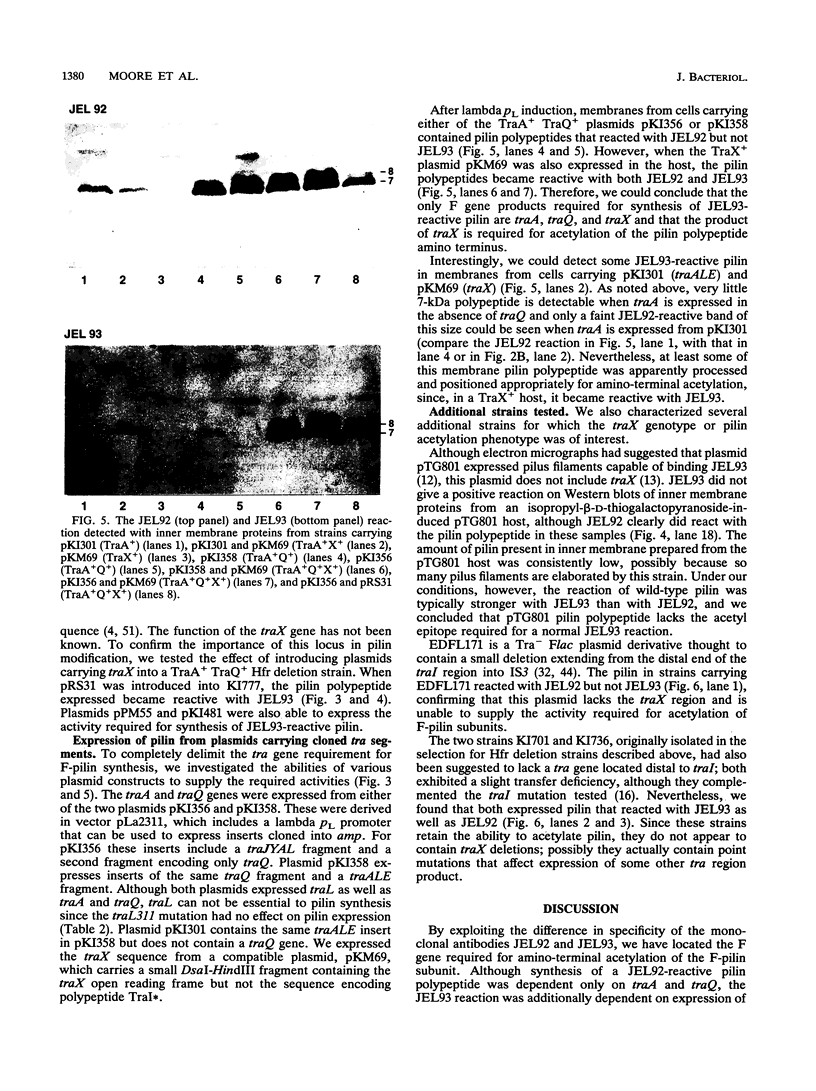
The Escherichia coli F plasmid gene required for amino-terminal acetylation of F-pilin subunits was identified. Using Western blots (immunoblots), we assayed the reaction of monoclonal antibodies with F-pilin polypeptides in inner membrane preparations from various F mutant strains. It was known that JEL92 recognizes an internal pilin epitope and JEL93 recognizes the acetylated amino-terminal sequence (L.S. Frost, J.S. Lee, D.G. Scraba, and W. Paranchych, J. Bacteriol. 168:192-198, 1986). As expected, neither antibody reacted with inner membranes from F- cells or Flac derivatives that do not synthesize pilin. Mutations that affected the individual activities of F tra genes traA, -B, -C, -D, -E, -F, -G, -H, -I, -J, -K, -L, -M, -N, -P, -R, -U, -V and -W or trb genes trbA, -B, -C, -D, -E, -G, -H, and -I did not prevent JEL92 or JEL93 recognition of membrane pilin. However, Hfr deletion mutants that lacked the most-distal transfer region genes did not express pilin that reacted with JEL93. Nevertheless, all strains that retained traA and traQ did express JEL92-reactive pilin polypeptides. Analysis of strains expressing cloned tra segments showed that traA and traQ suffice for synthesis of JEL92-reactive pilin, but synthesis of JEL93-reactive pilin is additionally dependent on traX. We concluded that the traX product is required for acetylation of F pilin. Interestingly, our data also showed that TraA+ TraQ+ cells synthesize two forms of pilin which migrate at approximately 7 and 8 kDa. In TraX+ cells, both become acetylated and react with JEL93. Preparations of wild-type F-pilus filaments contain both types of subunits.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Skurray R. A., Thompson R., Helmuth R., Hall S., Beutin L., Clark A. J. Assignment of tra cistrons to EcoRI fragments of F sex factor DNA. J Bacteriol. 1978 Mar;133(3):1383–1392. doi: 10.1128/jb.133.3.1383-1392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M., Galas D. J. Cointegrate formation mediated by Tn9. II. Activity of IS1 is modulated by external DNA sequences. J Mol Biol. 1983 Oct 15;170(1):61–91. doi: 10.1016/s0022-2836(83)80227-7. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cram D. S., Loh S. M., Cheah K. C., Skurray R. A. Sequence and conservation of genes at the distal end of the transfer region on plasmids F and R6-5. Gene. 1991 Jul 31;104(1):85–90. doi: 10.1016/0378-1119(91)90469-r. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Frost L. S., Paranchych W., Parker J. M., Hodges R. S. Major antigenic determinants of F and ColB2 pili. J Bacteriol. 1985 Jul;163(1):331–335. doi: 10.1128/jb.163.1.331-335.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth N., Skurray R. Characterization of the F plasmid bifunctional conjugation gene, traG. Mol Gen Genet. 1992 Mar;232(1):145–153. doi: 10.1007/BF00299147. [DOI] [PubMed] [Google Scholar]
- Frost L. S., Finlay B. B., Opgenorth A., Paranchych W., Lee J. S. Characterization and sequence analysis of pilin from F-like plasmids. J Bacteriol. 1985 Dec;164(3):1238–1247. doi: 10.1128/jb.164.3.1238-1247.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Lee J. S., Scraba D. G., Paranchych W. Two monoclonal antibodies specific for different epitopes within the amino-terminal region of F pilin. J Bacteriol. 1986 Oct;168(1):192–198. doi: 10.1128/jb.168.1.192-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost L. S., Paranchych W., Willetts N. S. DNA sequence of the F traALE region that includes the gene for F pilin. J Bacteriol. 1984 Oct;160(1):395–401. doi: 10.1128/jb.160.1.395-401.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman M. E., Adhya S., Das A. Transcription antitermination by bacteriophage lambda N gene product. J Mol Biol. 1980 Jun 15;140(1):57–75. doi: 10.1016/0022-2836(80)90356-3. [DOI] [PubMed] [Google Scholar]
- Grossman T. H., Frost L. S., Silverman P. M. Structure and function of conjugative pili: monoclonal antibodies as probes for structural variants of F pili. J Bacteriol. 1990 Mar;172(3):1174–1179. doi: 10.1128/jb.172.3.1174-1179.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman T. H., Silverman P. M. Structure and function of conjugative pili: inducible synthesis of functional F pili by Escherichia coli K-12 containing a lac-tra operon fusion. J Bacteriol. 1989 Feb;171(2):650–656. doi: 10.1128/jb.171.2.650-656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Reed R. R., Steitz J. A., Low K. B. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):135–140. doi: 10.1101/sqb.1981.045.01.022. [DOI] [PubMed] [Google Scholar]
- Ippen-Ihler K., Achtman M., Willetts N. Deletion map of the Escherichia coli K-12 sex factor F: the order of eleven transfer cistrons. J Bacteriol. 1972 Jun;110(3):857–863. doi: 10.1128/jb.110.3.857-863.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippen-Ihler K., Moore D., Laine S., Johnson D. A., Willetts N. S. Synthesis of F-pilin polypeptide in the absence of F traJ product. Plasmid. 1984 Mar;11(2):116–129. doi: 10.1016/0147-619x(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Ito K., Sato T., Yura T. Synthesis and assembly of the membrane proteins in E. coli. Cell. 1977 Jul;11(3):551–559. doi: 10.1016/0092-8674(77)90073-3. [DOI] [PubMed] [Google Scholar]
- Kathir P., Ippen-Ihler K. Construction and characterization of derivatives carrying insertion mutations in F plasmid transfer region genes, trbA, artA, traQ, and trbB. Plasmid. 1991 Jul;26(1):40–54. doi: 10.1016/0147-619x(91)90035-u. [DOI] [PubMed] [Google Scholar]
- Maneewannakul K., Maneewannakul S., Ippen-Ihler K. Sequence alterations affecting F plasmid transfer gene expression: a conjugation system dependent on transcription by the RNA polymerase of phage T7. Mol Microbiol. 1992 Oct;6(20):2961–2973. doi: 10.1111/j.1365-2958.1992.tb01755.x. [DOI] [PubMed] [Google Scholar]
- Maneewannakul S., Kathir P., Ippen-Ihler K. Characterization of the F plasmid mating aggregation gene traN and of a new F transfer region locus trbE. J Mol Biol. 1992 May 20;225(2):299–311. doi: 10.1016/0022-2836(92)90923-8. [DOI] [PubMed] [Google Scholar]
- Maneewannakul S., Maneewannakul K., Ippen-Ihler K. Characterization of trbC, a new F plasmid tra operon gene that is essential to conjugative transfer. J Bacteriol. 1991 Jun;173(12):3872–3878. doi: 10.1128/jb.173.12.3872-3878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneewannakul S., Maneewannakul K., Ippen-Ihler K. Characterization, localization, and sequence of F transfer region products: the pilus assembly gene product TraW and a new product, TrbI. J Bacteriol. 1992 Sep;174(17):5567–5574. doi: 10.1128/jb.174.17.5567-5574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Kusecek B., Morelli G., Fisseau C., Achtman M. Analysis of the promoter-distal region of the tra operon of the F sex factor of Escherichia coli K-12 encoded by EcoRI restriction fragments f17, f19, and f2. J Bacteriol. 1982 Apr;150(1):76–88. doi: 10.1128/jb.150.1.76-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Morelli G., Achtman M. traG protein of the F sex factor of Escherichia coli K-12 and its role in conjugation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7487–7491. doi: 10.1073/pnas.78.12.7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire S., Willetts N. Plasmid cointegrates of Flac and lambda prophage. J Bacteriol. 1978 Apr;134(1):184–192. doi: 10.1128/jb.134.1.184-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Horiuchi T., Willetts N. S. Identification and characterization of four new tra cistrons on the E. coli K12 sex factor F. Plasmid. 1978 Jun;1(3):316–323. doi: 10.1016/0147-619x(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Moore D., Maneewannakul K., Maneewannakul S., Wu J. H., Ippen-Ihler K., Bradley D. E. Characterization of the F-plasmid conjugative transfer gene traU. J Bacteriol. 1990 Aug;172(8):4263–4270. doi: 10.1128/jb.172.8.4263-4270.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. A new activity in the Ftra operon which is required for F-pilin synthesis. Mol Gen Genet. 1982;188(3):459–464. doi: 10.1007/BF00330049. [DOI] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. Location of an F-pilin pool in the inner membrane. J Bacteriol. 1981 Apr;146(1):251–259. doi: 10.1128/jb.146.1.251-259.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Sowa B. A., Ippen-Ihler K. The effect of tra mutations on the synthesis of the F-pilin membrane polypeptide. Mol Gen Genet. 1981;184(2):260–264. doi: 10.1007/BF00272914. [DOI] [PubMed] [Google Scholar]
- Paranchych W., Frost L. S. The physiology and biochemistry of pili. Adv Microb Physiol. 1988;29:53–114. doi: 10.1016/s0065-2911(08)60346-x. [DOI] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Plasmid vectors for high-efficiency expression controlled by the PL promoter of coliphage lambda. Gene. 1981 Oct;15(1):81–93. doi: 10.1016/0378-1119(81)90106-2. [DOI] [PubMed] [Google Scholar]
- Skurray R. A., Nagaishi H., Clark A. J. Molecular cloning of DNA from F sex factor of Escherichia coli K-12. Proc Natl Acad Sci U S A. 1976 Jan;73(1):64–68. doi: 10.1073/pnas.73.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Matsushita Y., Yoshikawa A., Isono K. Cloning and molecular characterization of the gene rimL which encodes an enzyme acetylating ribosomal protein L12 of Escherichia coli K12. Mol Gen Genet. 1989 Jun;217(2-3):289–293. doi: 10.1007/BF02464895. [DOI] [PubMed] [Google Scholar]
- Traxler B. A., Minkley E. G., Jr Revised genetic map of the distal end of the F transfer operon: implications for DNA helicase I, nicking at oriT, and conjugal DNA transport. J Bacteriol. 1987 Jul;169(7):3251–3259. doi: 10.1128/jb.169.7.3251-3259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Characterization of the F transfer cistron, traL. Genet Res. 1973 Apr;21(2):205–213. doi: 10.1017/s0016672300013379. [DOI] [PubMed] [Google Scholar]
- Willetts N., Achtman M. Genetic analysis of transfer by the Escherichia coli sex factor F, using P1 transductional complementation. J Bacteriol. 1972 Jun;110(3):843–851. doi: 10.1128/jb.110.3.843-851.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Ippen-Ihler K. Nucleotide sequence of traQ and adjacent loci in the Escherichia coli K-12 F-plasmid transfer operon. J Bacteriol. 1989 Jan;171(1):213–221. doi: 10.1128/jb.171.1.213-221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. H., Moore D., Lee T., Ippen-Ihler K. Analysis of Escherichia coli K12 F factor transfer genes: traQ, trbA, and trbB. Plasmid. 1987 Jul;18(1):54–69. doi: 10.1016/0147-619x(87)90078-3. [DOI] [PubMed] [Google Scholar]
- Yoshikawa A., Isono S., Sheback A., Isono K. Cloning and nucleotide sequencing of the genes rimI and rimJ which encode enzymes acetylating ribosomal proteins S18 and S5 of Escherichia coli K12. Mol Gen Genet. 1987 Oct;209(3):481–488. doi: 10.1007/BF00331153. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y., Fujita Y., Ohtsubo E. Nucleotide sequence of the promoter-distal region of the tra operon of plasmid R100, including traI (DNA helicase I) and traD genes. J Mol Biol. 1990 Jul 5;214(1):39–53. doi: 10.1016/0022-2836(90)90145-C. [DOI] [PubMed] [Google Scholar]