Abstract
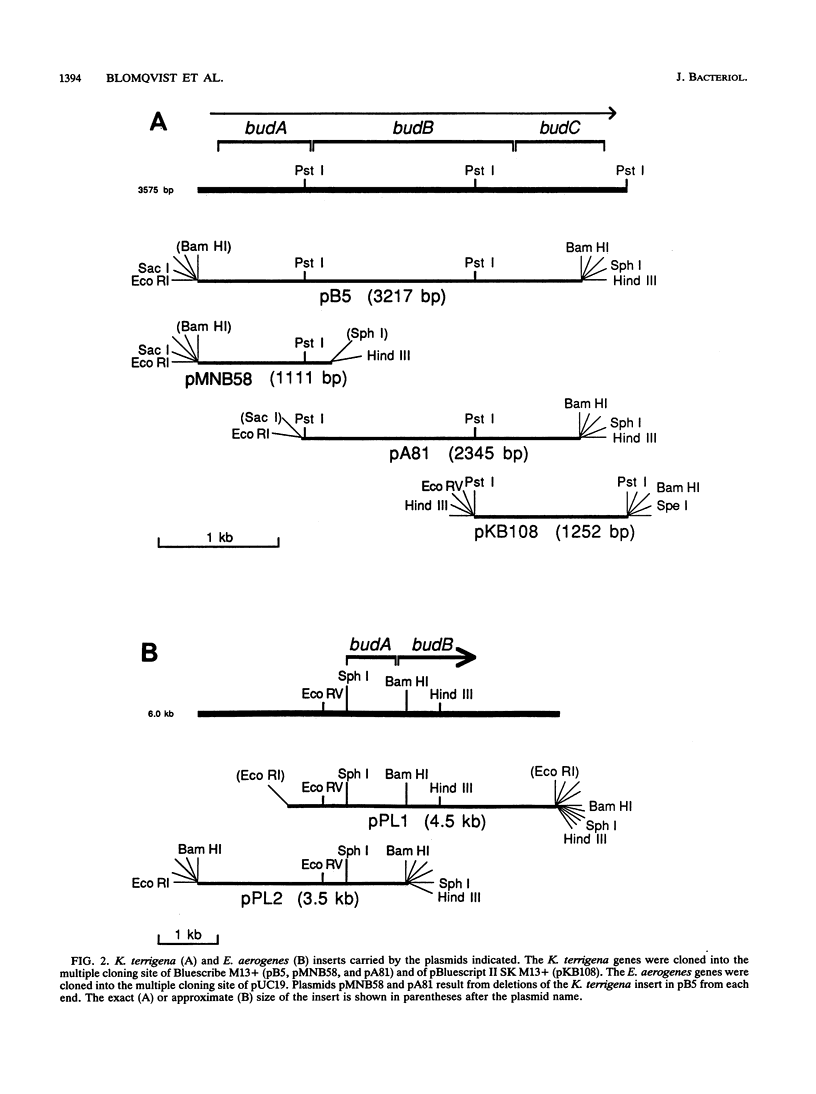
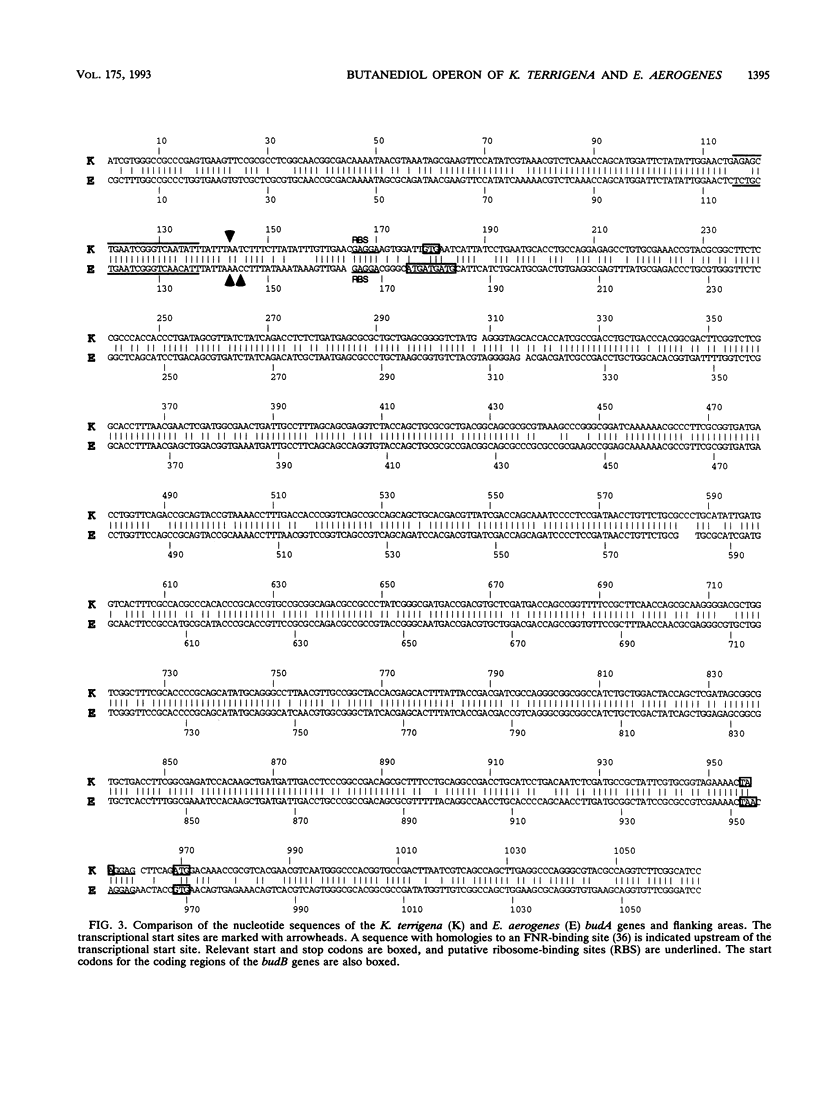
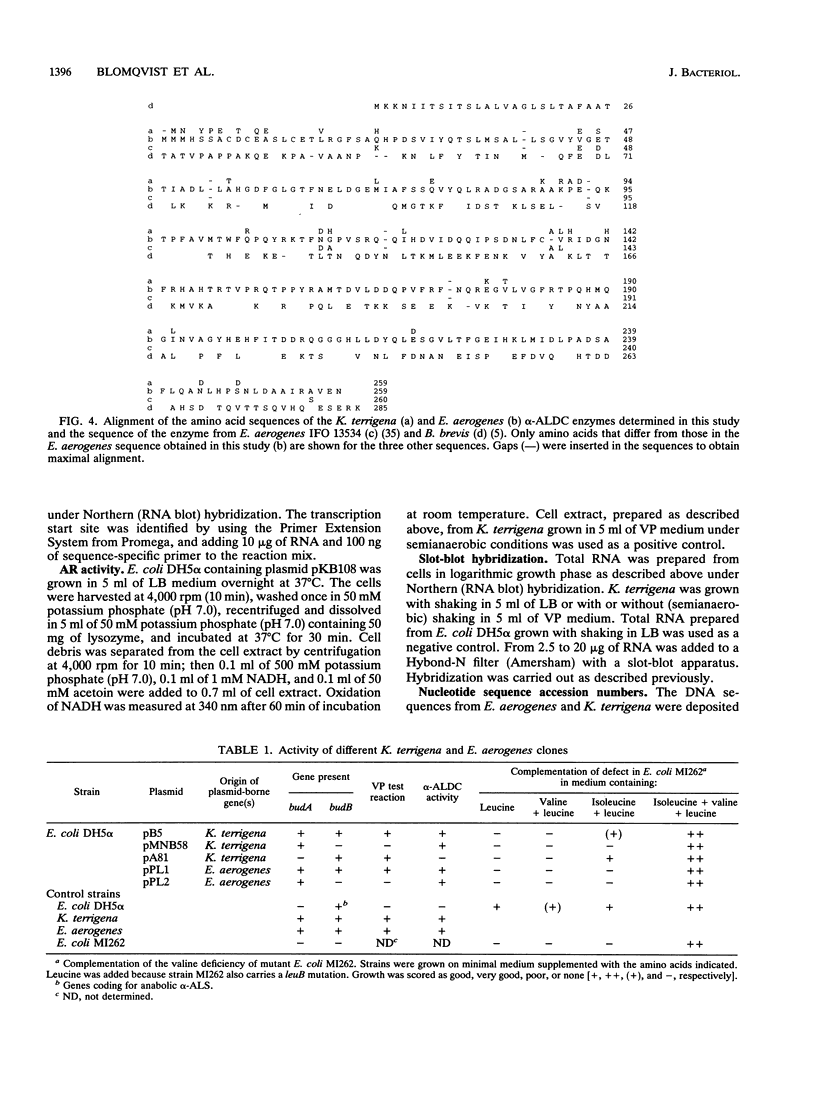
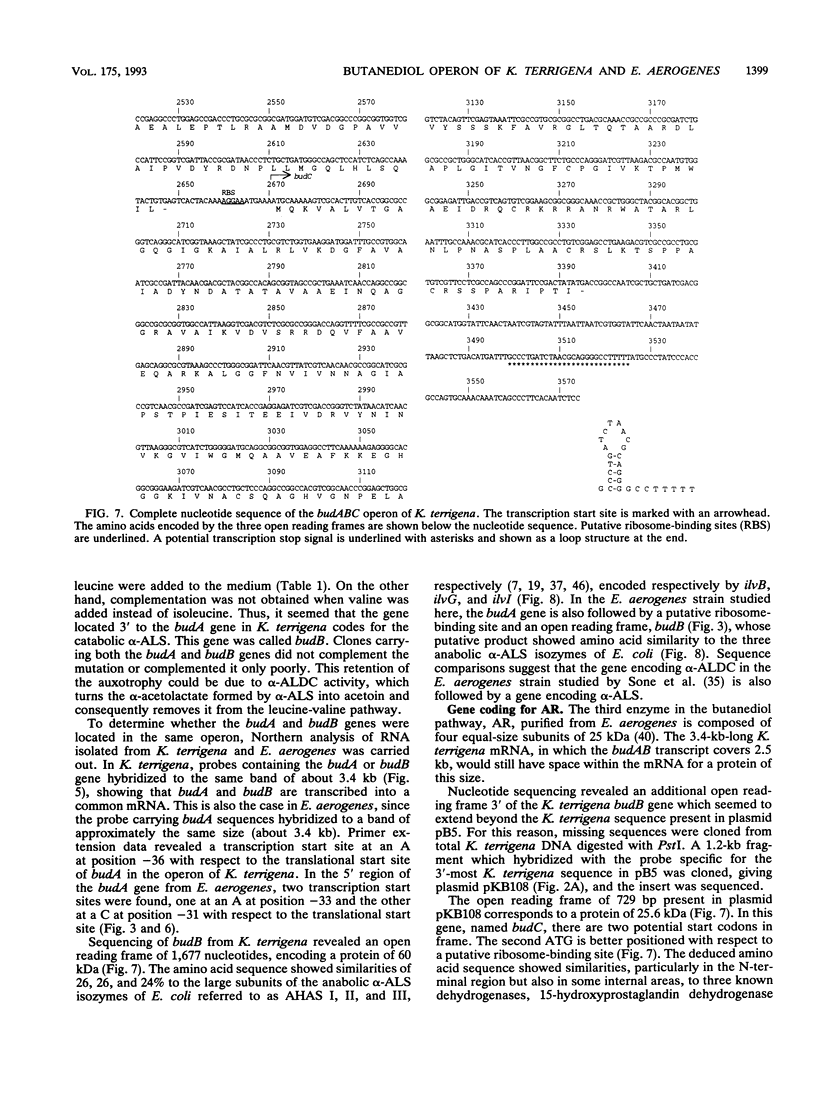
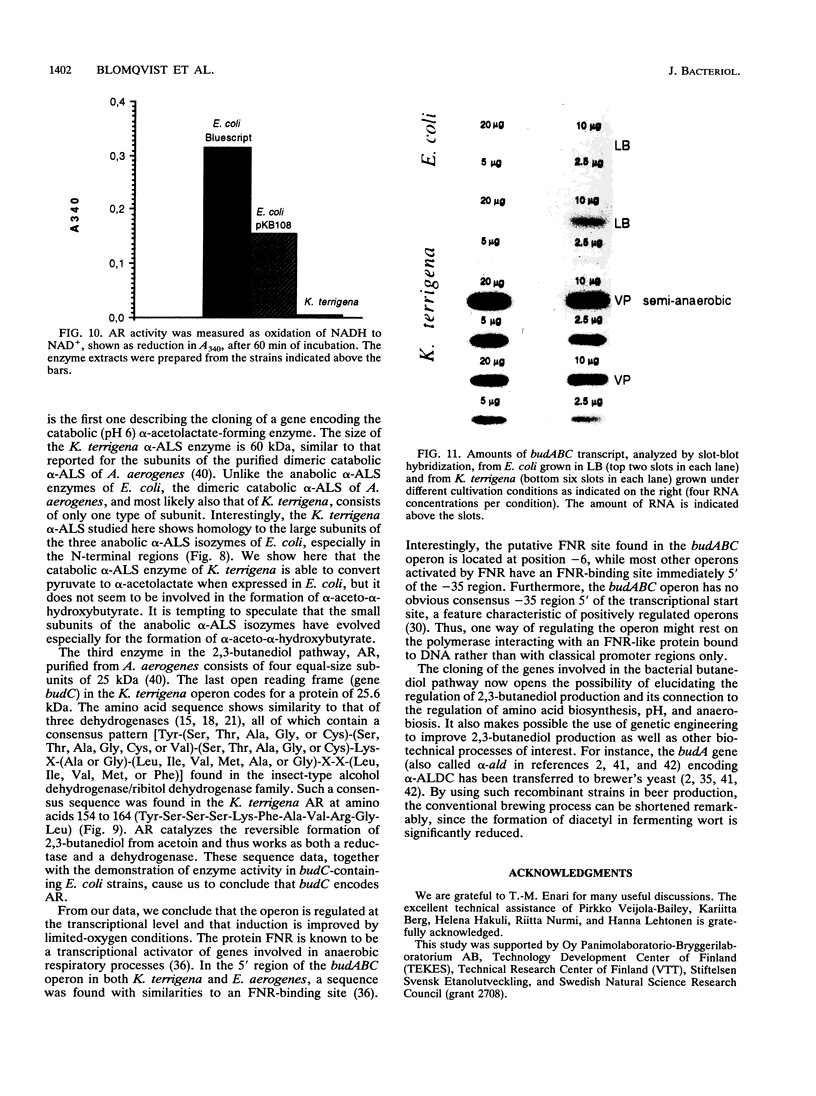
The genes involved in the 2,3-butanediol pathway coding for alpha-acetolactate decarboxylase, alpha-acetolactate synthase (alpha-ALS), and acetoin (diacetyl) reductase were isolated from Klebsiella terrigena and shown to be located in one operon. This operon was also shown to exist in Enterobacter aerogenes. The budA gene, coding for alpha-acetolactate decarboxylase, gives in both organisms a protein of 259 amino acids. The amino acid similarity between these proteins is 87%. The K. terrigena genes budB and budC, coding for alpha-ALS and acetoin reductase, respectively, were sequenced. The 559-amino-acid-long alpha-ALS enzyme shows similarities to the large subunits of the Escherichia coli anabolic alpha-ALS enzymes encoded by the genes ilvB, ilvG, and ilvI. The K. terrigena alpha-ALS is also shown to complement an anabolic alpha-ALS-deficient E. coli strain for valine synthesis. The 243-amino-acid-long acetoin reductase has the consensus amino acid sequence for the insect-type alcohol dehydrogenase/ribitol dehydrogenase family and has extensive similarities with the N-terminal and internal regions of three known dehydrogenases and one oxidoreductase.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amundesn S. K., Neville M. E. Comparison of three procedures for isolating DNA from bacteria. Microbios. 1979;24(95):29–39. [PubMed] [Google Scholar]
- Blomqvist K., Suihko M. L., Knowles J., Penttilä M. Chromosomal Integration and Expression of Two Bacterial alpha-Acetolactate Decarboxylase Genes in Brewer's Yeast. Appl Environ Microbiol. 1991 Oct;57(10):2796–2803. doi: 10.1128/aem.57.10.2796-2803.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth I. R. Regulation of cytoplasmic pH in bacteria. Microbiol Rev. 1985 Dec;49(4):359–378. doi: 10.1128/mr.49.4.359-378.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. D., Pereira C. R., Stormer F. C. Studies of the acetate kinase-phosphotransacetylase and the butanediol-forming systems in Aerobacter aerogenes. J Bacteriol. 1972 Dec;112(3):1106–1111. doi: 10.1128/jb.112.3.1106-1111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diderichsen B., Wedsted U., Hedegaard L., Jensen B. R., Sjøholm C. Cloning of aldB, which encodes alpha-acetolactate decarboxylase, an exoenzyme from Bacillus brevis. J Bacteriol. 1990 Aug;172(8):4315–4321. doi: 10.1128/jb.172.8.4315-4321.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDDY B. P. The Voges-Proskauer reaction and its significance: a review. J Appl Bacteriol. 1961 Apr;24:27–41. doi: 10.1111/j.1365-2672.1961.tb00230.x. [DOI] [PubMed] [Google Scholar]
- Friden P., Donegan J., Mullen J., Tsui P., Freundlich M., Eoyang L., Weber R., Silverman P. M. The ilvB locus of Escherichia coli K-12 is an operon encoding both subunits of acetohydroxyacid synthase I. Nucleic Acids Res. 1985 Jun 11;13(11):3979–3993. doi: 10.1093/nar/13.11.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelling D., Stahl U. Cloning and expression of an alpha-acetolactate decarboxylase gene from Streptococcus lactis subsp. diacetylactis in Escherichia coli. Appl Environ Microbiol. 1988 Jul;54(7):1889–1891. doi: 10.1128/aem.54.7.1889-1891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola J., De Felice M., Iaccarino M. Mutant of Escherichia coli K-12 missing acetolactate synthase activity. J Bacteriol. 1974 Oct;120(1):536–538. doi: 10.1128/jb.120.1.536-538.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Evidence for two distinct enzyme systems forming acetolactate in Aerobacter aerogenes. J Biol Chem. 1959 Dec;234:3067–3071. [PubMed] [Google Scholar]
- Harms E., Hsu J. H., Subrahmanyam C. S., Umbarger H. E. Comparison of the regulatory regions of ilvGEDA operons from several enteric organisms. J Bacteriol. 1985 Oct;164(1):207–216. doi: 10.1128/jb.164.1.207-216.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie S., Doi S., Yorifuji T., Takagi M., Yano K. Nucleotide sequencing and characterization of the genes encoding benzene oxidation enzymes of Pseudomonas putida. J Bacteriol. 1987 Nov;169(11):5174–5179. doi: 10.1128/jb.169.11.5174-5179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of formation of acetoin by bacteria. J Biol Chem. 1952 Apr;195(2):715–726. [PubMed] [Google Scholar]
- Johansen L., Bryn K., Stormer F. C. Physiological and biochemical role of the butanediol pathway in Aerobacter (Enterobacter) aerogenes. J Bacteriol. 1975 Sep;123(3):1124–1130. doi: 10.1128/jb.123.3.1124-1130.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krook M., Marekov L., Jörnvall H. Purification and structural characterization of placental NAD(+)-linked 15-hydroxyprostaglandin dehydrogenase. The primary structure reveals the enzyme to belong to the short-chain alcohol dehydrogenase family. Biochemistry. 1990 Jan 23;29(3):738–743. doi: 10.1021/bi00455a021. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken J. P., Stormer F. C. Acetolactate decarboxylase from Aerobacter aerogenes. Purification and properties. Eur J Biochem. 1970 May 1;14(1):133–137. doi: 10.1111/j.1432-1033.1970.tb00270.x. [DOI] [PubMed] [Google Scholar]
- Loviny T., Norton P. M., Hartley B. S. Ribitol dehydrogenase of Klebsiella aerogenes. Sequence of the structural gene. Biochem J. 1985 Sep 15;230(3):579–585. doi: 10.1042/bj2300579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navre M., Ringold G. M. A growth factor-repressible gene associated with protein kinase C-mediated inhibition of adipocyte differentiation. J Cell Biol. 1988 Jul;107(1):279–286. doi: 10.1083/jcb.107.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Berg C. M., Sobol T. J. Salmonella typhimurium mutants defective in acetohydroxy acid synthases I and II. J Bacteriol. 1980 Mar;141(3):1258–1263. doi: 10.1128/jb.141.3.1258-1263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Boyer H. W., Betlach M., Falkow S. Molecular cloning of an Escherichia coli plasmid determinant than encodes for the production of heat-stable enterotoxin. J Bacteriol. 1976 Oct;128(1):463–472. doi: 10.1128/jb.128.1.463-472.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone H., Fujii T., Kondo K., Shimizu F., Tanaka J., Inoue T. Nucleotide sequence and expression of the Enterobacter aerogenes alpha-acetolactate decarboxylase gene in brewer's yeast. Appl Environ Microbiol. 1988 Jan;54(1):38–42. doi: 10.1128/aem.54.1.38-42.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. FNR and its role in oxygen-regulated gene expression in Escherichia coli. FEMS Microbiol Rev. 1990 Aug;6(4):399–428. doi: 10.1111/j.1574-6968.1990.tb04109.x. [DOI] [PubMed] [Google Scholar]
- Squires C. H., De Felice M., Devereux J., Calvo J. M. Molecular structure of ilvIH and its evolutionary relationship to ilvG in Escherichia coli K12. Nucleic Acids Res. 1983 Aug 11;11(15):5299–5313. doi: 10.1093/nar/11.15.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stormer F. C. 2,3-Butanediol biosynthetic system in Aerobacter aerogenes. Methods Enzymol. 1975;41:518–532. doi: 10.1016/s0076-6879(75)41108-9. [DOI] [PubMed] [Google Scholar]
- Stormer F. C. Evidence for induction of the 2,3-butanediol-forming enzymes in Aerobacter aerogenes. FEBS Lett. 1968 Nov;2(1):36–38. doi: 10.1016/0014-5793(68)80094-8. [DOI] [PubMed] [Google Scholar]
- Suihko M. L., Blomqvist K., Penttilä M., Gisler R., Knowles J. Recombinant brewer's yeast strains suitable for accelerated brewing. J Biotechnol. 1990 Jun;14(3-4):285–300. doi: 10.1016/0168-1656(90)90113-p. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Wek R. C., Hauser C. A., Hatfield G. W. The nucleotide sequence of the ilvBN operon of Escherichia coli: sequence homologies of the acetohydroxy acid synthase isozymes. Nucleic Acids Res. 1985 Jun 11;13(11):3995–4010. doi: 10.1093/nar/13.11.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]