Abstract
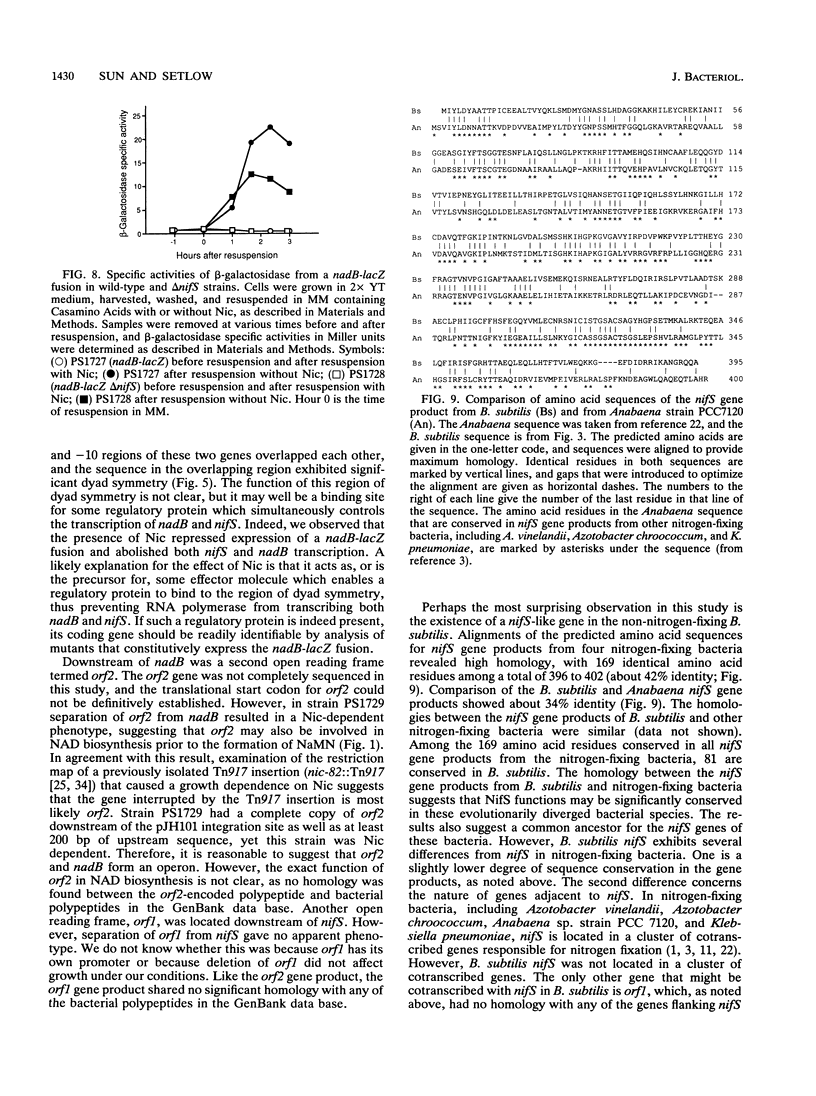
A number of Bacillus subtilis genes involved in NAD biosynthesis have been cloned and sequenced. One of the genes encodes a polypeptide homologous to Escherichia coli L-aspartate oxidase, and its mutation resulted in a nicotinic acid (Nic)-dependent phenotype; this gene was termed nadB. A second open reading frame (orf2) was found downstream of nadB, and an insertional plasmid separating orf2 and nadB also gave a Nic-dependent phenotype. This result suggests that orf2 may also be involved in NAD biosynthesis and that nadB and orf2 are in the same operon. Upstream of nadB was a third gene, transcribed in the opposite direction to that of nadB-orf2. The amino acid sequence derived from the third gene was quite similar to those derived from nifS genes of various nitrogen-fixing bacteria; therefore, the third gene was termed nifS. As with nadB and orf2, mutations in nifS also resulted in a Nic-dependent phenotype. The promoter regions of nadB and nifS overlapped each other and both contained -10 and -35 sequences which resemble those of E sigma A-type promoters. Transcription from both the nifS and nadB promoters, as well as expression of a nadB-lacZ fusion, was repressed by Nic. However, nadB transcription and nadB-lacZ expression were decreased, at most, only slightly by a deletion in nifS. The possible role of the nifS gene product in NAD biosynthesis is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold W., Rump A., Klipp W., Priefer U. B., Pühler A. Nucleotide sequence of a 24,206-base-pair DNA fragment carrying the entire nitrogen fixation gene cluster of Klebsiella pneumoniae. J Mol Biol. 1988 Oct 5;203(3):715–738. doi: 10.1016/0022-2836(88)90205-7. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Smith I. Transformation and transduction in Bacillus subtilis: evidence for separate modes of recombinant formation. J Mol Biol. 1969 Oct 28;45(2):155–179. doi: 10.1016/0022-2836(69)90097-7. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jones R., Woodley P. R., Wilborn J. R., Robson R. L. Nucleotide sequence and genetic analysis of the Azotobacter chroococcum nifUSVWZM gene cluster, including a new gene (nifP) which encodes a serine acetyltransferase. J Bacteriol. 1991 Sep;173(17):5457–5469. doi: 10.1128/jb.173.17.5457-5469.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari F. A., Nguyen A., Lang D., Hoch J. A. Construction and properties of an integrable plasmid for Bacillus subtilis. J Bacteriol. 1983 Jun;154(3):1513–1515. doi: 10.1128/jb.154.3.1513-1515.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachmann R., Kunz N., Seifert J., Gütlich M., Wientjes F. J., Läufer A., Gassen H. G. Molecular biology of pyridine nucleotide biosynthesis in Escherichia coli. Cloning and characterization of quinolinate synthesis genes nadA and nadB. Eur J Biochem. 1988 Aug 1;175(2):221–228. doi: 10.1111/j.1432-1033.1988.tb14187.x. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster J. W., Park Y. K., Penfound T., Fenger T., Spector M. P. Regulation of NAD metabolism in Salmonella typhimurium: molecular sequence analysis of the bifunctional nadR regulator and the nadA-pnuC operon. J Bacteriol. 1990 Aug;172(8):4187–4196. doi: 10.1128/jb.172.8.4187-4196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govezensky D., Greener T., Segal G., Zamir A. Involvement of GroEL in nif gene regulation and nitrogenase assembly. J Bacteriol. 1991 Oct;173(20):6339–6346. doi: 10.1128/jb.173.20.6339-6346.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Anagnostopoulos C. Chromosomal location and properties of radiation sensitivity mutations in Bacillus subtilis. J Bacteriol. 1970 Aug;103(2):295–301. doi: 10.1128/jb.103.2.295-301.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Brigle K. E., Bennett L. T., Setterquist R. A., Wilson M. S., Cash V. L., Beynon J., Newton W. E., Dean D. R. Physical and genetic map of the major nif gene cluster from Azotobacter vinelandii. J Bacteriol. 1989 Feb;171(2):1017–1027. doi: 10.1128/jb.171.2.1017-1027.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. R., Cash V. L., Weiss M. C., Laird N. F., Newton W. E., Dean D. R. Biochemical and genetic analysis of the nifUSVWZM cluster from Azotobacter vinelandii. Mol Gen Genet. 1989 Oct;219(1-2):49–57. doi: 10.1007/BF00261156. [DOI] [PubMed] [Google Scholar]
- Kelly M. S. Physical and mapping properties of distant linkages between genetic markers in transformation of Bacillus subtilis. Mol Gen Genet. 1967;99(4):333–349. doi: 10.1007/BF00330909. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Dean D. The nifU, nifS and nifV gene products are required for activity of all three nitrogenases of Azotobacter vinelandii. Mol Gen Genet. 1992 Feb;231(3):494–498. doi: 10.1007/BF00292722. [DOI] [PubMed] [Google Scholar]
- Kolman C., Söll D. SPL1-1, a Saccharomyces cerevisiae mutation affecting tRNA splicing. J Bacteriol. 1993 Mar;175(5):1433–1442. doi: 10.1128/jb.175.5.1433-1442.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont I. L., Mandelstam J. Identification of a new sporulation locus, spoIIIF, in Bacillus subtilis. J Gen Microbiol. 1984 May;130(5):1253–1261. doi: 10.1099/00221287-130-5-1253. [DOI] [PubMed] [Google Scholar]
- Loshon C. A., Fliss E. R., Setlow B., Foerster H. F., Setlow P. Cloning and nucleotide sequencing of genes for small, acid-soluble spore proteins of Bacillus cereus, Bacillus stearothermophilus, and "Thermoactinomyces thalpophilus". J Bacteriol. 1986 Jul;167(1):168–173. doi: 10.1128/jb.167.1.168-173.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Fajardo-Cavazos P., Setlow P. Levels of mRNAs which code for small, acid-soluble spore proteins and their LacZ gene fusions in sporulating cells of Bacillus subtilis. Nucleic Acids Res. 1988 Jul 25;16(14A):6567–6583. doi: 10.1093/nar/16.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., LeGrice S. F., Lee G., Stephens M., Sonenshein A. L., Pero J., Losick R. Nucleotide sequences that signal the initiation of transcription and translation in Bacillus subtilis. Mol Gen Genet. 1982;186(3):339–346. doi: 10.1007/BF00729452. [DOI] [PubMed] [Google Scholar]
- Mulligan M. E., Haselkorn R. Nitrogen fixation (nif) genes of the cyanobacterium Anabaena species strain PCC 7120. The nifB-fdxN-nifS-nifU operon. J Biol Chem. 1989 Nov 15;264(32):19200–19207. [PubMed] [Google Scholar]
- NISHIZUKA Y., HAYAISHI O. STUDIES ON THE BIOSYNTHESIS OF NICOTINAMIDE ADENINE DINUCLEOTIDE. I. ENZYMIC SYNTHESIS OF NIACIN RIBONUCLEOTIDES FROM 3-HYDROXYANTHRANILIC ACID IN MAMMALIAN TISSUES. J Biol Chem. 1963 Oct;238:3369–3377. [PubMed] [Google Scholar]
- Oliver S. G., van der Aart Q. J., Agostoni-Carbone M. L., Aigle M., Alberghina L., Alexandraki D., Antoine G., Anwar R., Ballesta J. P., Benit P. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- Popham D. L., Stragier P. Cloning, characterization, and expression of the spoVB gene of Bacillus subtilis. J Bacteriol. 1991 Dec;173(24):7942–7949. doi: 10.1128/jb.173.24.7942-7949.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Mendelson N. H., Coyne S. I., Hallock L. L., Cole R. M. Minicells of Bacillus subtilis. J Bacteriol. 1973 May;114(2):860–873. doi: 10.1128/jb.114.2.860-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton R. E., Rocha V., Rosser R. J., Andreoli A. J., Shimoyama M., Kosaka A., Chandler J. L., Gholson R. K. A comparative study of the regulation of nicotinamide-adenine dinucleotide biosynthesis. Biochim Biophys Acta. 1968 Feb 1;156(1):77–84. doi: 10.1016/0304-4165(68)90106-2. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. X., Cabrera-Martinez R. M., Setlow P. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor sigma G. J Bacteriol. 1991 May;173(9):2977–2984. doi: 10.1128/jb.173.9.2977-2984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trach K., Hoch J. A. The Bacillus subtilis spo0B stage 0 sporulation operon encodes an essential GTP-binding protein. J Bacteriol. 1989 Mar;171(3):1362–1371. doi: 10.1128/jb.171.3.1362-1371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeyar M. A., Zahler S. A. Chromosomal insertions of Tn917 in Bacillus subtilis. J Bacteriol. 1986 Aug;167(2):530–534. doi: 10.1128/jb.167.2.530-534.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]