Abstract
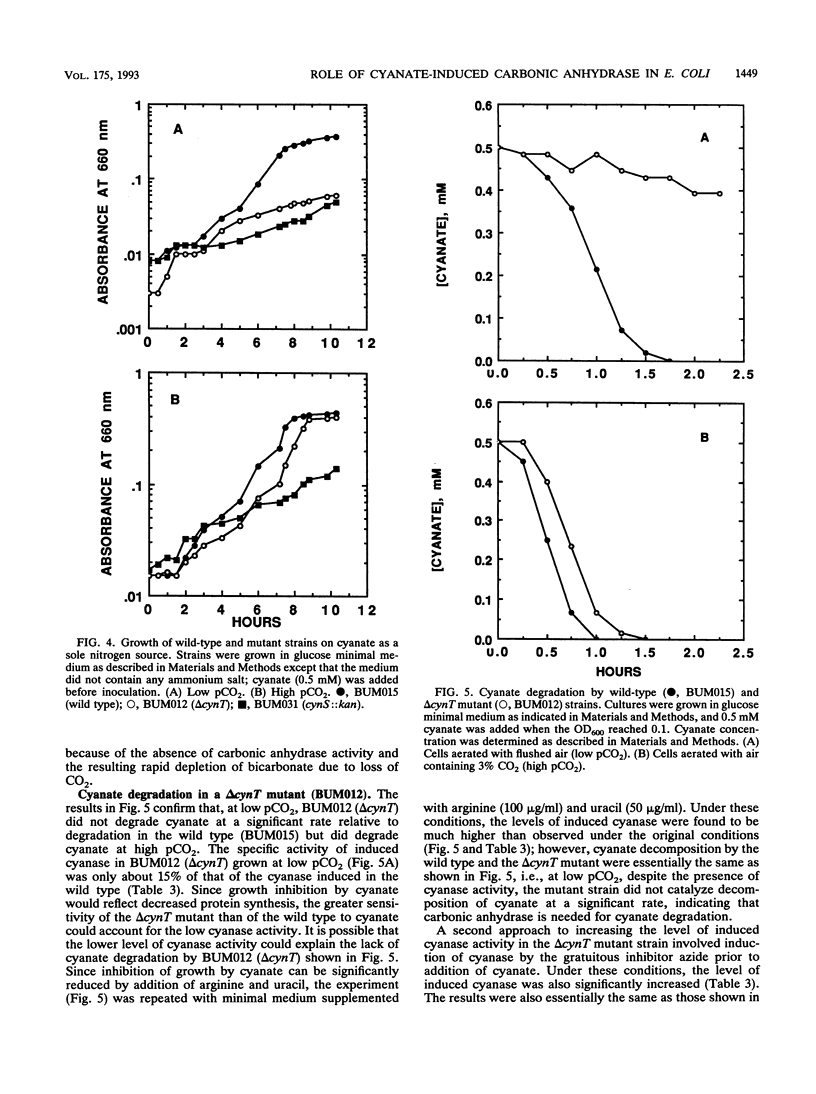
Cyanate induces expression of the cyn operon in Escherichia coli. The cyn operon includes the gene cynS, encoding cyanase, which catalyzes the reaction of cyanate with bicarbonate to give ammonia and carbon dioxide. A carbonic anhydrase activity was recently found to be encoded by the cynT gene, the first gene of the cyn operon; it was proposed that carbonic anhydrase prevents depletion of bicarbonate during cyanate decomposition due to loss of CO2 by diffusion out of the cell (M. B. Guilloton, J. J. Korte, A. F. Lamblin, J. A. Fuchs, and P. M. Anderson, J. Biol. Chem. 267:3731-3734, 1992). The function of the product of the third gene of this operon, cynX, is unknown. In the study reported here, the physiological roles of cynT and cynX were investigated by construction of chromosomal mutants in which each of the three genes was rendered inactive. The delta cynT chromosomal mutant expressed an active cyanase but no active carbonic anhydrase. In contrast to the wild-type strain, the growth of the delta cynT strain was inhibited by cyanate, and the mutant strain was unable to degrade cyanate and therefore could not use cyanate as the sole nitrogen source when grown at a partial CO2 pressures (pCO2) of 0.03% (air). At a high pCO2 (3%), however, the delta cynT strain behaved like the wild-type strain; it was significantly less sensitive to the toxic effects of cyanate and could degrade cyanate and use cyanate as the sole nitrogen source for growth. These results are consistent with the proposed function for carbonic anhydrase. The chromosomal mutant carrying cynS::kan expressed induced carbonic anhydrase activity but no active cyanase. The cynS::kan mutant was found to be much less sensitive to cyanate than the delta cynT mutant at a low pCO2, indicating that bicarbonate depletion due to the reaction of bicarbonate with cyanate catalyzed by cyanase is more deleterious to growth than direct inhibition by cyanate. Mutants carrying a nonfunctional cynX gene (cynX::kan and delta cynT cynX::kan) did not differ from the parental strains with respect to cyanate sensitivity, presence of carbonic anhydrase and cyanase, or degradation of cyanate by whole cells; the physiological role of the cynX product remains unknown.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Carlson J. D. Reversible reaction of cyanate with a reactive sulfhydryl group at the glutamine binding site of carbamyl phosphate synthetase. Biochemistry. 1975 Aug 12;14(16):3688–3694. doi: 10.1021/bi00687a027. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Johnson W. V., Endrizzi J. A., Little R. M., Korte J. J. Interaction of mono- and dianions with cyanase: evidence for apparent half-site binding. Biochemistry. 1987 Jun 30;26(13):3938–3943. doi: 10.1021/bi00387a029. [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Little R. M. Kinetic properties of cyanase. Biochemistry. 1986 Apr 8;25(7):1621–1626. doi: 10.1021/bi00355a026. [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Purification and properties of the inducible enzyme cyanase. Biochemistry. 1980 Jun 24;19(13):2882–2888. doi: 10.1021/bi00554a010. [DOI] [PubMed] [Google Scholar]
- Blum P., Holzschu D., Kwan H. S., Riggs D., Artz S. Gene replacement and retrieval with recombinant M13mp bacteriophages. J Bacteriol. 1989 Jan;171(1):538–546. doi: 10.1128/jb.171.1.538-546.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
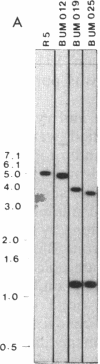
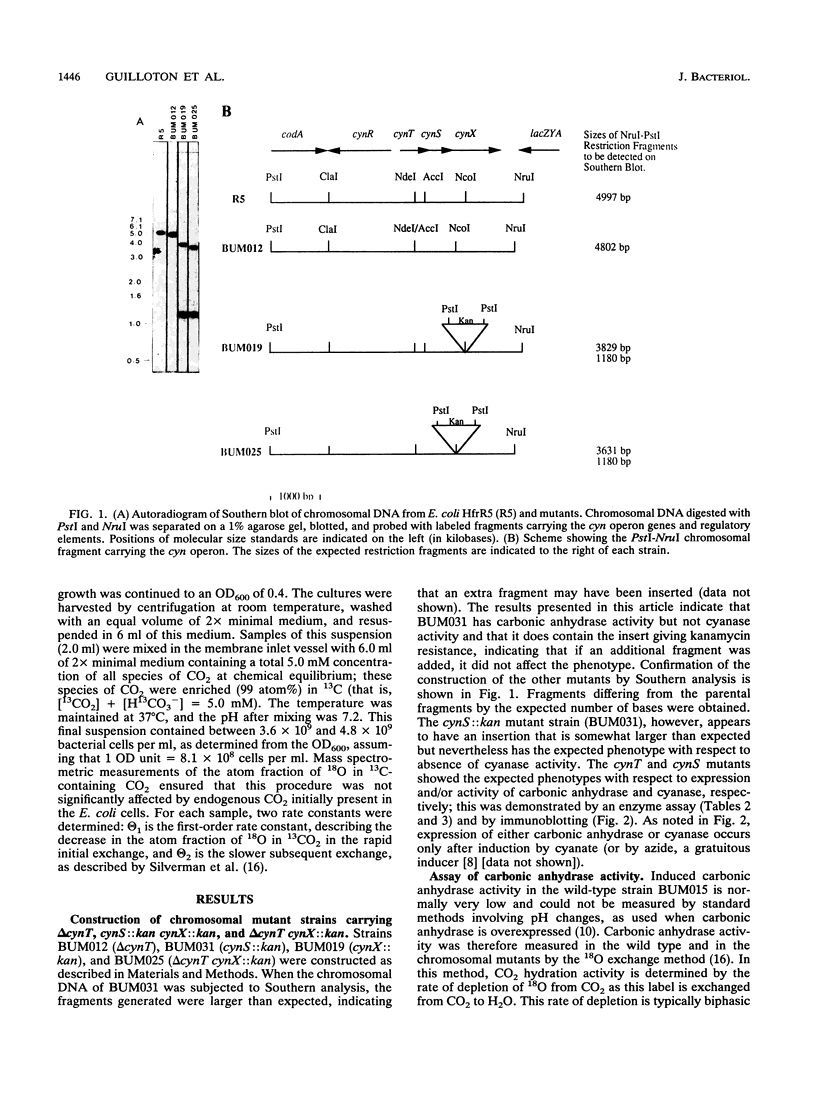
- Guilloton M. B., Korte J. J., Lamblin A. F., Fuchs J. A., Anderson P. M. Carbonic anhydrase in Escherichia coli. A product of the cyn operon. J Biol Chem. 1992 Feb 25;267(6):3731–3734. [PubMed] [Google Scholar]
- Guilloton M., Karst F. A spectrophotometric determination of cyanate using reaction with 2-aminobenzoic acid. Anal Biochem. 1985 Sep;149(2):291–295. doi: 10.1016/0003-2697(85)90572-x. [DOI] [PubMed] [Google Scholar]
- Guilloton M., Karst F. Cyanate specifically inhibits arginine biosynthesis in Escherichia coli K12: a case of by-product inhibition? J Gen Microbiol. 1987 Mar;133(3):655–665. doi: 10.1099/00221287-133-3-655. [DOI] [PubMed] [Google Scholar]
- Guilloton M., Karst F. Isolation and characterization of Escherichia coli mutants lacking inducible cyanase. J Gen Microbiol. 1987 Mar;133(3):645–653. doi: 10.1099/00221287-133-3-645. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repaske R., Clayton M. A. Control of Escherichia coli growth by CO2. J Bacteriol. 1978 Sep;135(3):1162–1164. doi: 10.1128/jb.135.3.1162-1164.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D. N., Tu C. K., Roessler N. Diffusion-limited exchange of 18O between CO2 and water in red cell suspensions. Respir Physiol. 1981 Jun;44(3):285–298. doi: 10.1016/0034-5687(81)90024-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sung Y. C., Anderson P. M., Fuchs J. A. Characterization of high-level expression and sequencing of the Escherichia coli K-12 cynS gene encoding cyanase. J Bacteriol. 1987 Nov;169(11):5224–5230. doi: 10.1128/jb.169.11.5224-5230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y. C., Fuchs J. A. Characterization of the cyn operon in Escherichia coli K12. J Biol Chem. 1988 Oct 15;263(29):14769–14775. [PubMed] [Google Scholar]
- Sung Y. C., Fuchs J. A. Identification and characterization of a cyanate permease in Escherichia coli K-12. J Bacteriol. 1989 Sep;171(9):4674–4678. doi: 10.1128/jb.171.9.4674-4678.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y. C., Fuchs J. A. The Escherichia coli K-12 cyn operon is positively regulated by a member of the lysR family. J Bacteriol. 1992 Jun;174(11):3645–3650. doi: 10.1128/jb.174.11.3645-3650.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Y. C., Parsell D., Anderson P. M., Fuchs J. A. Identification, mapping, and cloning of the gene encoding cyanase in Escherichia coli K-12. J Bacteriol. 1987 Jun;169(6):2639–2642. doi: 10.1128/jb.169.6.2639-2642.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L. A., Rose R. E. A correction in the nucleotide sequence of the Tn903 kanamycin resistance determinant in pUC4K. Nucleic Acids Res. 1988 Jan 11;16(1):358–358. doi: 10.1093/nar/16.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]