Abstract
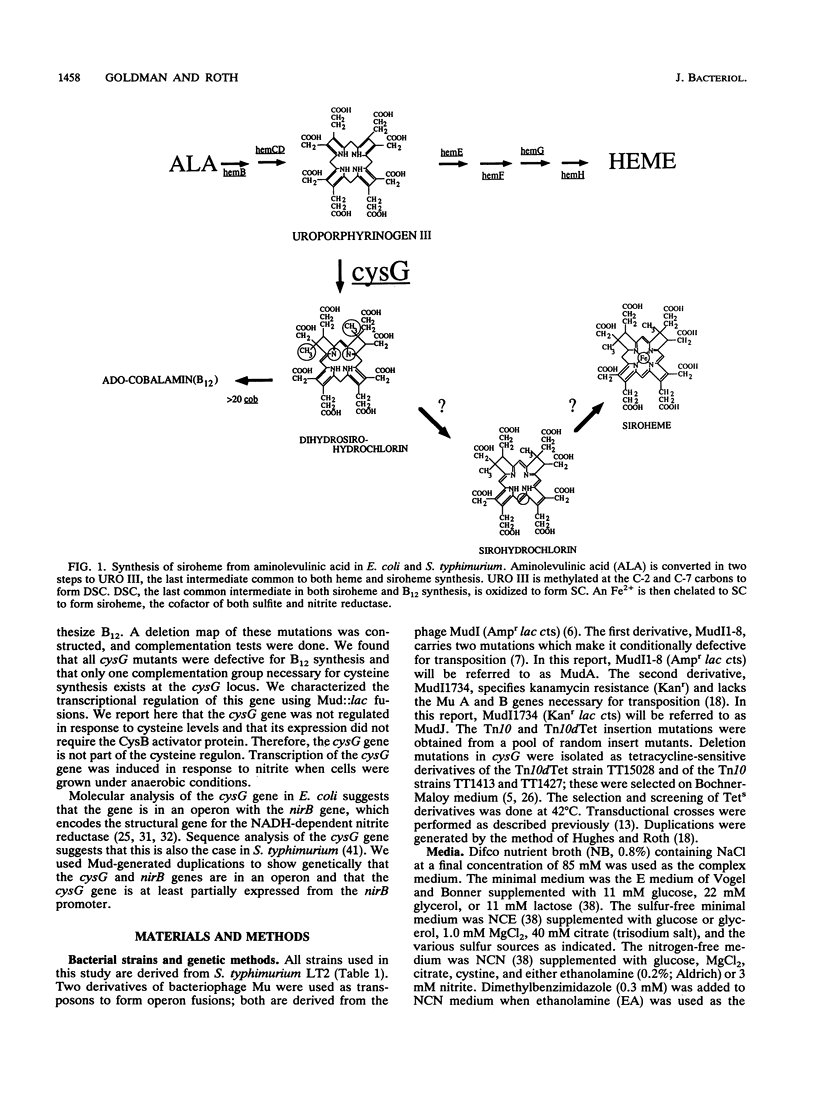
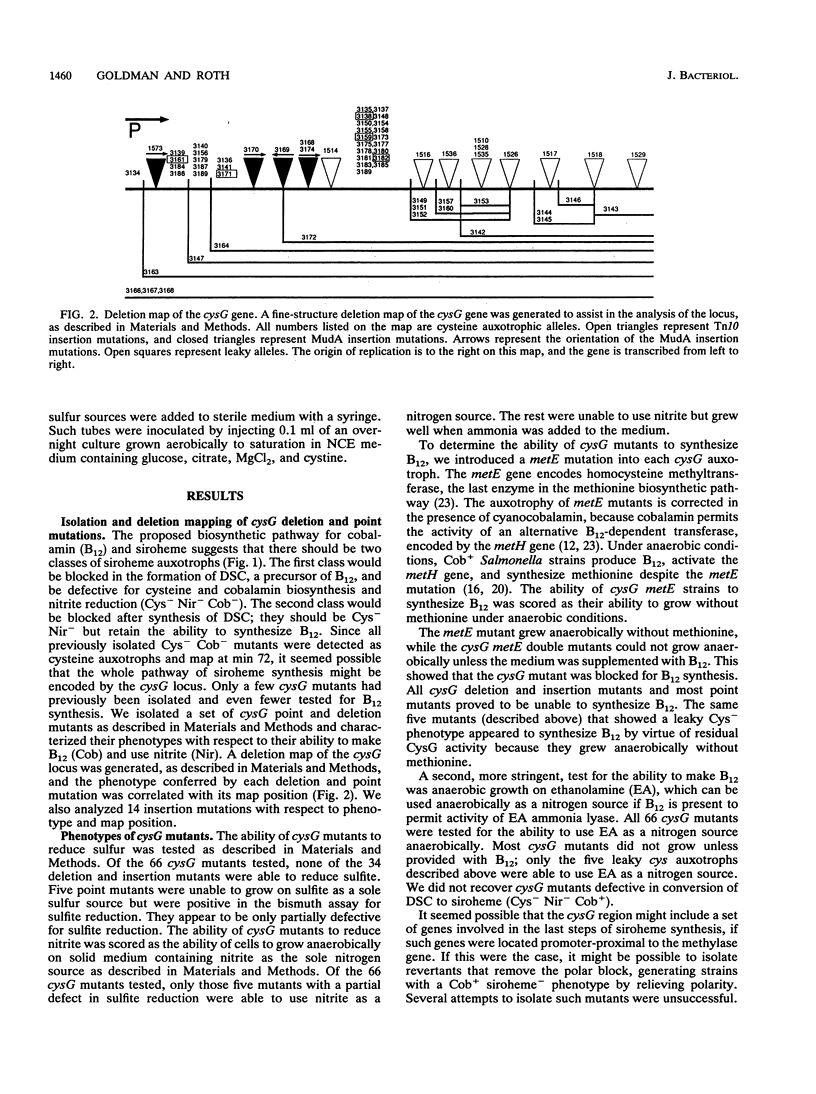
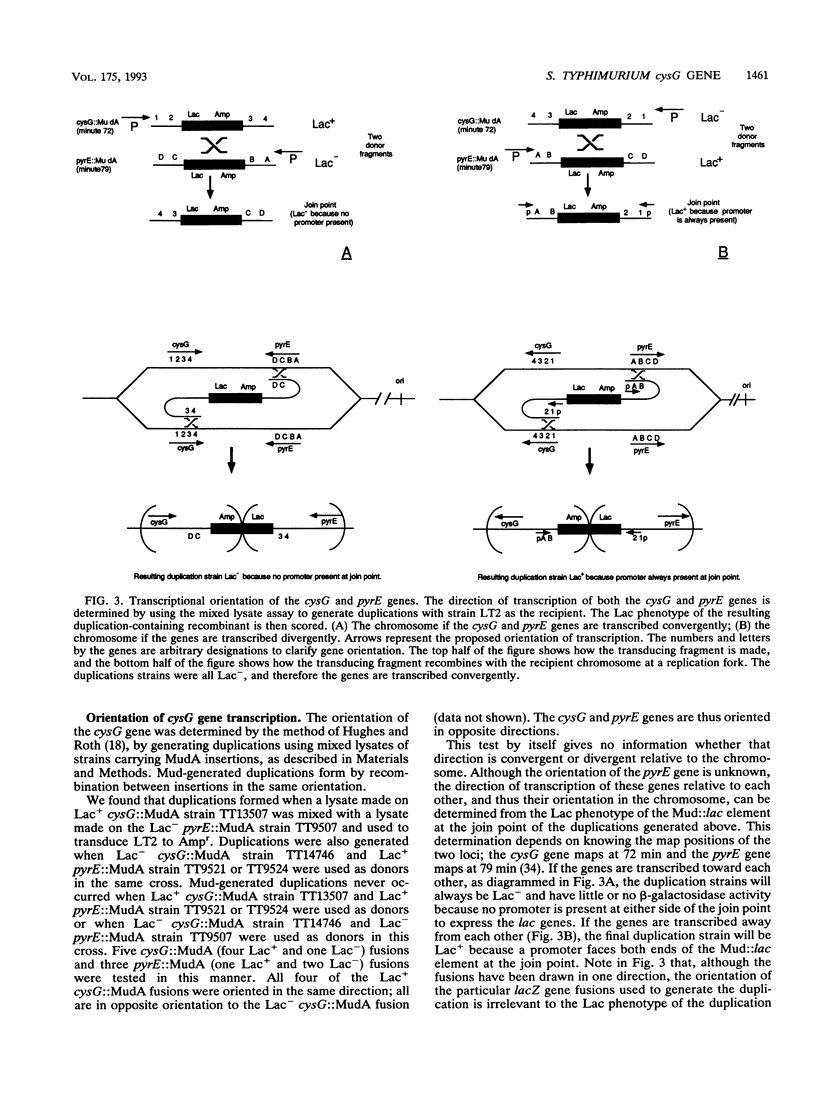
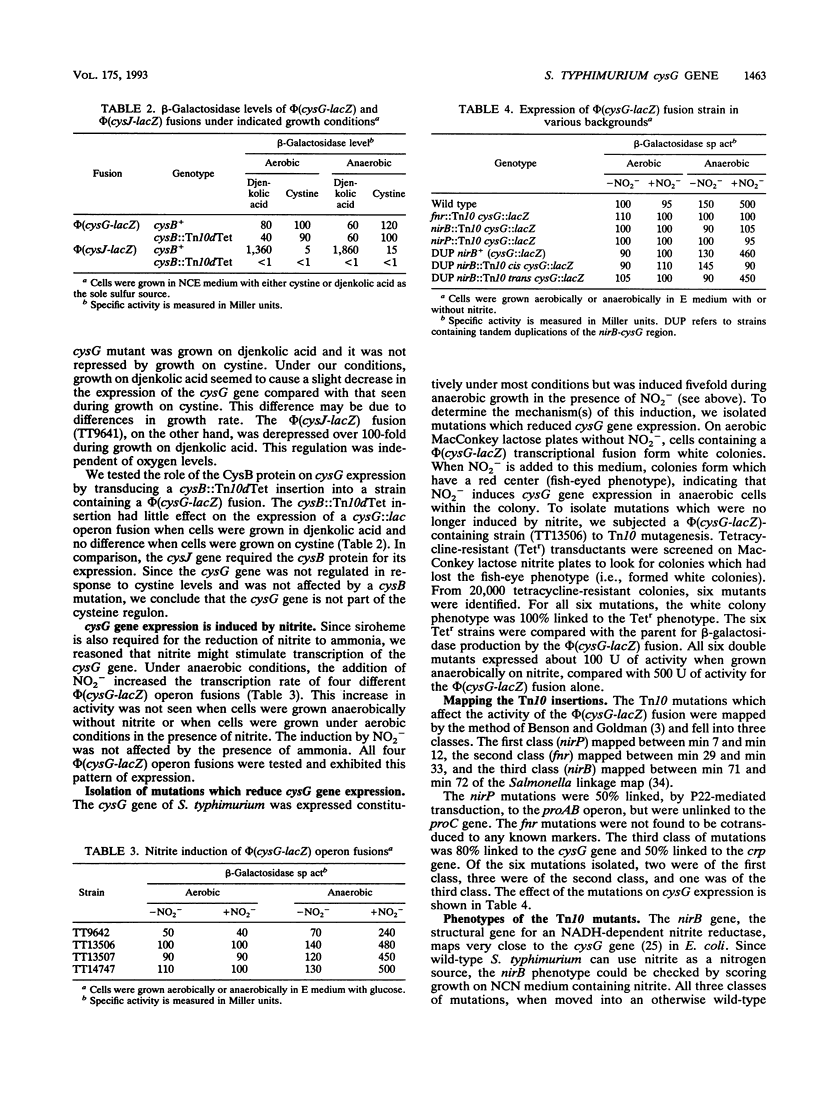
Siroheme, a cofactor of both sulfite and nitrite reductase in Salmonella typhimurium, requires the cysG gene for its synthesis. Three steps are required to synthesize siroheme from uroporphyrinogen III, the last common intermediate in the heme and siroheme pathways. All previously characterized cysG mutants were shown to be defective for the synthesis of cobalamin (B12), which shares a common precursor with siroheme. Since few cysG auxotrophs had been previously analyzed and since there is no evidence of siroheme mutants outside of the cysG region, we sought to expand the analysis of the region by isolating more mutations and studying the transcriptional regulation of the cysG gene using lacZ fusions. We isolated and analyzed 66 cysG auxotrophs. All were defective for both siroheme and cobalamin synthesis. Five exceptional mutants were partially defective for the synthesis of both and appear to be leaky. Complementation tests with tandem duplications suggest that the mutations causing the Cys auxotrophy affect only one cistron. The cysG gene is transcribed in a clockwise direction; this was demonstrated by a method that permits determining the orientation of two genes of unknown orientation provided their relative map order is known. The cysG gene was not part of the cysteine regulon, but had a substantial basal level of expression which was induced fivefold when cells were grown anaerobically on nitrite. Finally, we used Mud-generated duplications to genetically determine the organization of the cysG and nirB genes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson N. R., Goldman B. S. Rapid mapping in Salmonella typhimurium with Mud-P22 prophages. J Bacteriol. 1992 Mar;174(5):1673–1681. doi: 10.1128/jb.174.5.1673-1681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanche F., Debussche L., Thibaut D., Crouzet J., Cameron B. Purification and characterization of S-adenosyl-L-methionine: uroporphyrinogen III methyltransferase from Pseudomonas denitrificans. J Bacteriol. 1989 Aug;171(8):4222–4231. doi: 10.1128/jb.171.8.4222-4231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Huang H. C., Schieven G. L., Ames B. N. Positive selection for loss of tetracycline resistance. J Bacteriol. 1980 Aug;143(2):926–933. doi: 10.1128/jb.143.2.926-933.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. A., Coleman K. J., Compton B. E., Kavanagh B. M., Keevil C. W. Nitrite and ammonia assimilation by anaerobic continuous cultures of Escherichia coli. J Gen Microbiol. 1974 Nov;85(1):11–22. doi: 10.1099/00221287-85-1-11. [DOI] [PubMed] [Google Scholar]
- Cole J. A., Newman B. M., White P. Biochemical and genetic characterization of nirB mutants of Escherichia coli K 12 pleiotropically defective in nitrite and sulphite reduction. J Gen Microbiol. 1980 Oct;120(2):475–483. doi: 10.1099/00221287-120-2-475. [DOI] [PubMed] [Google Scholar]
- Coleman K. J., Cornish-Bowden A., Cole J. A. Purification and properties of nitrite reductase from Escherichia coli K12. Biochem J. 1978 Nov 1;175(2):483–493. doi: 10.1042/bj1750483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R., Charles H. P. Porphyrin-accumulating mutants of Escherichia coli. J Bacteriol. 1973 Jan;113(1):122–132. doi: 10.1128/jb.113.1.122-132.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMEREC M., GILLESPIE D. H., MIZOBUCHI K. GENETIC STRUCTURE OF THE CYST REGION OF THE SALMONELLA GENOME. Genetics. 1963 Aug;48:997–1009. doi: 10.1093/genetics/48.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena J. C., Roth J. R. Regulation of cobalamin biosynthetic operons in Salmonella typhimurium. J Bacteriol. 1987 May;169(5):2251–2258. doi: 10.1128/jb.169.5.2251-2258.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. T., Roth J. R. Directed formation of deletions and duplications using Mud(Ap, lac). Genetics. 1985 Feb;109(2):263–282. doi: 10.1093/genetics/109.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P. S., Peakman T. C., Busby S. J., Quincey R. V., Cole J. A. Location and sequence of the promoter of the gene for the NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the Fnr protein and nitrite. J Mol Biol. 1987 Aug 20;196(4):781–788. doi: 10.1016/0022-2836(87)90404-9. [DOI] [PubMed] [Google Scholar]
- Jeter R. M., Olivera B. M., Roth J. R. Salmonella typhimurium synthesizes cobalamin (vitamin B12) de novo under anaerobic growth conditions. J Bacteriol. 1984 Jul;159(1):206–213. doi: 10.1128/jb.159.1.206-213.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kredich N. M. Regulation of L-cysteine biosynthesis in Salmonella typhimurium. I. Effects of growth of varying sulfur sources and O-acetyl-L-serine on gene expression. J Biol Chem. 1971 Jun 10;246(11):3474–3484. [PubMed] [Google Scholar]
- Kung H. F., Spears C., Greene R. C., Weissbach H. Regulation of the terminal reactions in methionine biosynthesis by vitamin B 12 and methionine. Arch Biochem Biophys. 1972 May;150(1):23–31. doi: 10.1016/0003-9861(72)90005-7. [DOI] [PubMed] [Google Scholar]
- MacDonald H., Pope N. R., Cole J. A. Isolation, characterization and complementation analysis of nirB mutants of Escherichia coli deficient only in NADH-dependent nitrite reductase activity. J Gen Microbiol. 1985 Oct;131(10):2771–2782. doi: 10.1099/00221287-131-10-2771. [DOI] [PubMed] [Google Scholar]
- Macdonald H., Cole J. Molecular cloning and functional analysis of the cysG and nirB genes of Escherichia coli K12, two closely-linked genes required for NADH-dependent nitrite reductase activity. Mol Gen Genet. 1985;200(2):328–334. doi: 10.1007/BF00425444. [DOI] [PubMed] [Google Scholar]
- Maloy S. R., Nunn W. D. Selection for loss of tetracycline resistance by Escherichia coli. J Bacteriol. 1981 Feb;145(2):1110–1111. doi: 10.1128/jb.145.2.1110-1111.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Kamin H., Rosenthal D. Reduced nicotinamide adenine dinucleotide phosphate-sulfite reductase of enterobacteria. II. Identification of a new class of heme prosthetic group: an iron-tetrahydroporphyrin (isobacteriochlorin type) with eight carboxylic acid groups. J Biol Chem. 1973 Apr 25;248(8):2801–2814. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M. Siroheme and sirohydrochlorin. The basis for a new type of porphyrin-related prosthetic group common to both assimilatory and dissimilatory sulfite reductases. J Biol Chem. 1973 Oct 10;248(19):6911–6919. [PubMed] [Google Scholar]
- Murphy M. J., Siegel L. M., Tove S. R., Kamin H. Siroheme: a new prosthetic group participating in six-electron reduction reactions catalyzed by both sulfite and nitrite reductases. Proc Natl Acad Sci U S A. 1974 Mar;71(3):612–616. doi: 10.1073/pnas.71.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota N., Galsworthy P. R., Pardee A. B. Genetics of sulfate transport by Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1053–1062. doi: 10.1128/jb.105.3.1053-1062.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakman T., Busby S., Cole J. Transcriptional control of the cysG gene of Escherichia coli K-12 during aerobic and anaerobic growth. Eur J Biochem. 1990 Jul 31;191(2):325–331. doi: 10.1111/j.1432-1033.1990.tb19126.x. [DOI] [PubMed] [Google Scholar]
- Peakman T., Crouzet J., Mayaux J. F., Busby S., Mohan S., Harborne N., Wootton J., Nicolson R., Cole J. Nucleotide sequence, organisation and structural analysis of the products of genes in the nirB-cysG region of the Escherichia coli K-12 chromosome. Eur J Biochem. 1990 Jul 31;191(2):315–323. doi: 10.1111/j.1432-1033.1990.tb19125.x. [DOI] [PubMed] [Google Scholar]
- Powell K. A., Cox R., McConville M., Charles H. P. Mutations affecting porphyrin biosynthesis in Escherichia coli. Enzyme. 1973;16(1):65–73. doi: 10.1159/000459363. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E., Roth J. R. Linkage map of Salmonella typhimurium, edition VII. Microbiol Rev. 1988 Dec;52(4):485–532. doi: 10.1128/mr.52.4.485-532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart V. Nitrate respiration in relation to facultative metabolism in enterobacteria. Microbiol Rev. 1988 Jun;52(2):190–232. doi: 10.1128/mr.52.2.190-232.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch K. L., Lenk J. B., Gamble B. L., Miller C. G. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985 Feb;161(2):673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Warren M. J., Roessner C. A., Santander P. J., Scott A. I. The Escherichia coli cysG gene encodes S-adenosylmethionine-dependent uroporphyrinogen III methylase. Biochem J. 1990 Feb 1;265(3):725–729. doi: 10.1042/bj2650725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren M. J., Stolowich N. J., Santander P. J., Roessner C. A., Sowa B. A., Scott A. I. Enzymatic synthesis of dihydrosirohydrochlorin (precorrin-2) and of a novel pyrrocorphin by uroporphyrinogen III methylase. FEBS Lett. 1990 Feb 12;261(1):76–80. doi: 10.1016/0014-5793(90)80640-5. [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Siegel L. M., Kredich N. M. High-level expression of Escherichia coli NADPH-sulfite reductase: requirement for a cloned cysG plasmid to overcome limiting siroheme cofactor. J Bacteriol. 1991 Jan;173(1):325–333. doi: 10.1128/jb.173.1.325-333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K., Delling J., Elliott T. The genes required for heme synthesis in Salmonella typhimurium include those encoding alternative functions for aerobic and anaerobic coproporphyrinogen oxidation. J Bacteriol. 1992 Jun;174(12):3953–3963. doi: 10.1128/jb.174.12.3953-3963.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



