Abstract
Treatment regimens combining moxifloxacin and rifampin for drug-susceptible tuberculosis are being studied intensively. However, rifampin induces enzymes that transport and metabolize moxifloxacin. We evaluated the effect of rifampin and the human multidrug resistance gene (MDR1) C3435T polymorphisms (P-glycoprotein) on moxifloxacin pharmacokinetic parameters. This was a single-center, sequential design study with 16 volunteers in which sampling was performed after four daily oral doses of moxifloxacin (400 mg) and again after 10 days of combined rifampin (600 mg) and moxifloxacin. After daily coadministration of rifampin, the area under the concentration-time curve from 0 to 24 h (AUC0-24) for moxifloxacin decreased 27%. Average bioequivalence between moxifloxacin coadministered with rifampin and moxifloxacin alone was not demonstrated: the ratio of geometric means (RGM) of the moxifloxacin AUC0-24 was 73.3 (90% confidence intervals [CI], 64.3, 83.5) (total P value, 0.87 for two one-sided t tests). Peak moxifloxacin concentrations, however, were equivalent: the RGM of the maximum concentration of the drug in serum was 93.6 (90% CI, 80.2, 109.3) (total P value, 0.049). Concentrations of the sulfate conjugate metabolite of moxifloxacin were increased twofold following rifampin coadministration (AUC0-24, 1.29 versus 2.79 μg·h/ml). Concomitant rifampin administration resulted in a 27% decrease in the mean moxifloxacin AUC0-24 and a marked increase in the AUC0-24 of the microbiologically inactive M1 metabolite. Additional studies are required to understand the clinical significance of the moxifloxacin-rifampin interaction.
There is increasing interest in the possible role of newer fluoroquinolone antibiotics in the treatment of drug-susceptible tuberculosis. In a murine model of tuberculosis treatment, the substitution of moxifloxacin for isoniazid allows treatment to be shortened from 6 months to 4 months (10). Moxifloxacin-containing regimens are currently being evaluated in phase II treatment trials (1; Tuberculosis Trials Consortium Study 28). Moxifloxacin undergoes phase II biotransformation by two pathways: (i) sulfate conjugation, with the resultant metabolite (M1 conjugate) accounting for 38% of an oral dose of moxifloxacin, and (ii) glucuronide conjugation, with the resultant metabolite (M2 conjugate) accounting for 14% of an oral dose (16). The CYP450 system does not play a role in moxifloxacin metabolism. Although best known for its effects on the CYP450 system, rifampin can also up-regulate the phase II metabolic glucuronidation pathway (5).
P-glycoprotein is a protein found in the intestinal mucosa, liver, and kidney that plays an important role in the absorption, distribution, and elimination of xenobiotics (8). The multidrug resistance gene (MDR1) codes for P-glycoprotein, and polymorphisms in the MDR1 gene can lead to altered P-glycoprotein function. For example, subjects homozygous for the MDR1 C3435T polymorphism had lower intestinal P-glycoprotein concentrations and higher plasma digoxin concentrations (6, 7). P-glycoprotein is induced by rifampin (9, 12). This observation suggests another possible mechanism for a rifampin-induced drug-drug interaction. We evaluated the effects of rifampin and MDR1 C3435T polymorphisms on the pharmacokinetics of moxifloxacin.
The primary objective of the study was to compare the pharmacokinetics of daily moxifloxacin without and with coadministration of rifampin. A secondary objective was to characterize the effects of MDR1 C3435T polymorphisms on moxifloxacin pharmacokinetics.
(Some of the results of this study have been reported previously in abstract form [18]).
MATERIALS AND METHODS
Experimental design.
This was a single-center, two-period, open-label, sequential-design pharmacokinetic study. The first pharmacokinetic sampling was performed with the fourth daily dose of oral moxifloxacin (400 mg once daily). Rifampin (600 mg once-daily, given at the same time as moxifloxacin) was then added, and a second sampling was performed with the 10th dose of rifampin (the 14th dose of daily moxifloxacin). The study was performed with healthy adults (age, ≥18 years) without tuberculosis recruited from the VAMC San Antonio. Women of child-bearing potential who agreed to practice an adequate method of birth control (barrier or abstinence) were eligible. Exclusion criteria included current or planned therapy with drugs that have interactions with study drugs, a history of prolonged QTc syndrome, or concurrent treatment with drugs associated with prolongation of the QTc interval. Concomitant medications were not administered orally to study subjects during the 14 days of study drug administration, except for one subject who self-administered acetaminophen (1,500 mg) for headache on the day prior to the initial pharmacokinetic sampling. Institutional Review Boards of the CDC and of the University Texas approved the study. Written informed consent was obtained from all participants. Adverse events were classified as definitely, probably, possibly, or not associated with study drug therapy and graded with a severity toxicity scale adapted from the National Cancer Institute (1a).
Sample collection and gene analyses.
All study drugs were administered to subjects in a fasting state. For pharmacokinetic sampling, no food was allowed from 8 h before to 4 h after drug administration. Blood samples were collected just before observed doses of the study drug(s) and then 0.5, 1.0, 1.5, 2, 3, 4, 6, 8, 12, 16, and 24 h thereafter. MDR1 C3435T polymorphisms were detected using TaqMan real-time PCR (15).
Drug determinations. (i) Moxifloxacin and metabolites.
Moxifloxacin was analyzed using a reverse-phase high-performance liquid chromatography (HPLC) method with fluorescence detection (Bayer internal method report BAY 12-8039/HPLC/S/1.01). Sample preparation included protein precipitation using acidified acetonitrile followed by dilution with buffer. The calibration range of the procedure was 0.026 to 4.0 μg/ml. The interday precision, based on the quality control samples, during the analysis of samples ranged from 2.2 to 4.8%, and accuracy ranged from 102.8 to 105.7%.
Metabolite M1 (sulfate) was analyzed using reverse-phase HPLC with tandem mass spectrometry detection (Bayer internal method report BAY 12-8039/M/LCMS/P/YX/1.01). Sample preparation included protein precipitation using acidified acetonitrile. The calibration range for the method was 0.0156 to 0.525 μg/ml. The interday precision, based on quality control samples, during the analysis of samples ranged from 8.5 to 10.6%, and accuracy ranged from 98.1 to 101.6%. In general, if a subject had one sample in which the M1 metabolite was below the level of quantification, it was collected at the 24-h time point; if two, at 16 and 24 h; if three, at 12, 16, and 24 h. For moxifloxacin administration alone, 4 subjects had two samples, 2 subjects had one sample, and 10 subjects had no samples in which the M1 metabolite was below the level of quantification. For coadministration of moxifloxacin and rifampin, six subjects had three samples, five had two samples, four had one sample, and one had no samples in which the M1 metabolite was below the level of quantification.
Metabolite M2 (glucuronide) was analyzed by the same procedure that was used for moxifloxacin (Bayer internal method report BAY 12-8039/MJL/M/2.0E). Levels of metabolite M2 were determined after it had been converted to moxifloxacin, since no reference standard for metabolite M2 was available. This method included enzymatic hydrolysis of plasma to convert metabolite M2 to moxifloxacin. Samples were further processed using the same procedure that was carried out for the analysis of moxifloxacin. Metabolite M2 was measured by subtracting free-moxifloxacin levels from total-moxifloxacin levels. The interday precision, based on quality control samples, during the analysis of samples ranged from 3.9 to 6.2%, and accuracy ranged from 100.3 to 100.9%. The lower limit of quantitation for the M2 metabolite was 0.026 μg/ml.
(ii) Rifampin.
Standard techniques were used to determine plasma rifampin concentrations, as previously described (11), with the following modifications. The HPLC procedures for analysis were performed with the following equipment: a ThermoFinnigan (San Jose, CA) model P4000 pump, a model AS 3000 fixed-volume autosampler, and a model UV2000 detector. The standard curves for the concentration of rifampin in plasma covered a range from 0.5 to 50 μg/ml. The absolute recovery of rifampin from serum was 95.5%. The within-day precision of validation quality control samples was 2.4 to 4.6%, and the overall validation precision was 6.3 to 7.1%.
Statistical and pharmacokinetic analyses.
A sample size of 12 subjects was estimated to provide 80% power to detect a 10% change in the mean area under the concentration-time curve from 0 to 24 h (AUC0-24) for moxifloxacin, assuming that the standard deviation of the moxifloxacin AUC0-24 would be twice that observed in previous pharmacokinetic studies (Avelox package insert; Bayer Pharma). Additional subjects were enrolled to ensure that an adequate number of subjects would complete the study.
Analyses of AUC0-24 were performed using noncompartmental techniques (WinNonlin, version 4; Pharsight Corporation, Mountain View CA). M1 pharmacokinetic values are estimates, because M1 concentrations during the later times of both sampling periods approximated the lower limits of detection of the M1 assay. For binominal data, differences between groups were determined using the chi-square or Fisher exact test. Pharmacokinetic data were reported as arithmetic means (± standard deviations), geometric means, and ratios of the geometric means (RGM) (with 90% confidence intervals [CI]) for moxifloxacin plus rifampin to the geometric means for moxifloxacin alone. Data were transformed with natural logarithms to obtain geometric means, 90% CI, and the RGM. The data were then back-transformed to the original scale. Average bioequivalence of moxifloxacin with rifampin to moxifloxacin alone was demonstrated to have occurred if the 90% CI for the RGM of the moxifloxacin AUC0-24 fell entirely within the equivalence range of 80 to 125%. Equivalence and two one-sided t tests were performed for the moxifloxacin AUC0-24 and peak concentration using WinNonlin, version 4. Moxifloxacin pharmacokinetic parameters were examined for associations with covariates of age, sex, race, weight, and MDR1 C3435T genotypes (see Table 2) by univariate analysis. If independent variables were found to be possibly associated (with a P value of <0.2) with a pharmacokinetic dependent variable by univariate analysis, the variables were further examined by multivariate forward and backward stepwise regression analyses. Differences between groups were considered statistically significant at a P value of <0.05. Data analyses were performed using SAS software (Statview for Windows, version 5.0.1; SAS, Cary, NC).
TABLE 2.
Univariate and multivariate analyses of pharmacokinetic parameters of moxifloxacin grouped by MDR1 3435 CC genotype versus CT or TT genotype
| Drug parameter and time of pharmacokinetic sampling | Day 14 (moxifloxacin + rifampin)
|
Day 4 (moxifloxacin alone)
|
|||
|---|---|---|---|---|---|
| Geometric mean | Univariate P value (t test) | Multivariate P value | Geometric mean | Univariate P value (t test) | |
| AUC0-24 (μg·h/ml) | |||||
| CC genotype | 25.5 | 0.16 | 0.42 | 37.2 | 0.53 |
| CT or TT genotype | 30.3 | 39.7 | |||
| Peak concn (μg/ml) | |||||
| CC genotype | 2.94 | 0.08 | 0.14 | 3.53 | 0.51 |
| CT or TT genotype | 3.83 | 3.81 | |||
| Tmax (h) | |||||
| CC genotype | 1.98 | 0.03 | 0.06 | 1.47 | 0.84 |
| CT or TT genotype | 1.05 | 1.91 | |||
| Half-life (h) | |||||
| CC genotype | 6.51 | 0.92 | 10.90 | 0.29 | |
| CT or TT genotype | 6.46 | 9.77 | |||
RESULTS
Subjects.
Seventeen subjects enrolled in the study. One subject who did not adhere to protocol criteria was removed from the study prior to pharmacokinetic sampling. The median age (interquartile range) of the 16 remaining volunteers who underwent pharmacokinetic sampling was 35.5 (30.5 to 53.0) years, and the median (interquartile range) body weight was 83.5 (75.3 to 100.3) kg. Twelve subjects were male, and four were female. The race/ethnicity distribution was as follows: black (n = 4), white, non-Hispanic (n = 3), white, Hispanic (n = 8), and Asian (n = 1). Medical conditions reported by subjects included a history of migraines (n = 3), hypercholesterolemia (n = 2), degenerative joint disease (n = 2), osteoarthritis (n = 2), hepatitis C virus infection (n = 1), and chronic obstructive pulmonary disease (n = 1). The only nonstudy concomitant medication started during the study was a topical clotrimazole preparation for a woman who developed candidal vaginitis while on study medications. One subject self-administered acetaminophen (1,500 mg) for headache on the day prior to the initial pharmacokinetic sampling.
Interaction between moxifloxacin and rifampin.
The moxifloxacin AUC0-24 was 28.4 μg·h/ml after the administration of moxifloxacin plus rifampin compared to 38.7 μg·h/ml for moxifloxacin alone. Average bioequivalence was not demonstrated (RGM, 73.3 [90% CI, 64.3, 83.5]) (Table 1; Fig. 1), since the 90% CI for the RGM of the moxifloxacin AUC0-24 was outside the equivalence range of 80 to 125%. Peak concentrations of moxifloxacin were equivalent for the two time periods (RGM, 93.6 [90% CI, 80.2 to 109.3]). The moxifloxacin half-life was shorter after rifampin coadministration (6.5 versus 10.2 h).
TABLE 1.
Pharmacokinetic parameters of moxifloxacin, the M1 moxifloxacin metabolite, and rifampin for 16 healthy volunteers
| Drug parameter and time of pharmacokinetic sampling | Value for:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moxifloxacin
|
M1 metabolite (sulfate conjugate)
|
Rifampin
|
||||||||||||||||
| Arithmetic mean (± SD) | Geometric mean | RGM of day 14 to day 4 (90% CI) | P valuea for equivalenceb (<80%/>125%) | Arithmetic mean (± SD) | Geometric mean | RGM of day 14 to day 4 (90% CI) | Arithmetic mean (± SD) | Geometric mean | ||||||||||
| AUC0-24 (μg·h/ml) | ||||||||||||||||||
| Day 14 (moxifloxacin + rifampin) | 29.2 ± 7.5 | 28.4 | 73.3 (64.3-83.5) | 0.87/<0.0001 | 2.79 ± 1.28 | 2.58 | 238 (177-319) | 44.0 ± 18.6 | 40.9 | |||||||||
| Day 4 (moxifloxacin alone) | 39.4 ± 7.7 | 38.7 | 1.29 ± 0.63 | 1.08 | ||||||||||||||
| Peak concn (μg/ml) | ||||||||||||||||||
| Day 14 (moxifloxacin + rifampin) | 3.6 ± 1.1 | 3.5 | 93.6 (80.2-109.3) | 0.047/0.002 | 0.71 ± 0.24 | 0.67 | 403 (303-535) | 9.9 ± 3.6 | 9.2 | |||||||||
| Day 4 (moxifloxacin alone) | 3.8 ± 0.8 | 3.7 | 0.20 ± 0.14 | 0.17 | ||||||||||||||
| Half life (h) | ||||||||||||||||||
| Day 14 (moxifloxacin + rifampin) | 6.5 ± 0.9 | 6.5 | 3.18 ± 4.70 | 2.30 | 1.9 ± 0.3 | 2.0 | ||||||||||||
| Day 4 (moxifloxacin alone) | 10.4 ± 2.0 | 10.2 | 9.97 ± 5.07 | 8.80 | ||||||||||||||
| Tmax (h) | ||||||||||||||||||
| Day 14 (moxifloxacin + rifampin) | 1.56 ± 0.91 | 1.34 | 1.75 ± 1.03 | 1.54 | ||||||||||||||
| Day 4 (moxifloxacin alone) | 1.97 ± 0.97 | 1.73 | 1.84 ± 0.98 | 1.60 | ||||||||||||||
By two one-sided t tests.
Of moxifloxacin with rifampin to moxifloxacin alone.
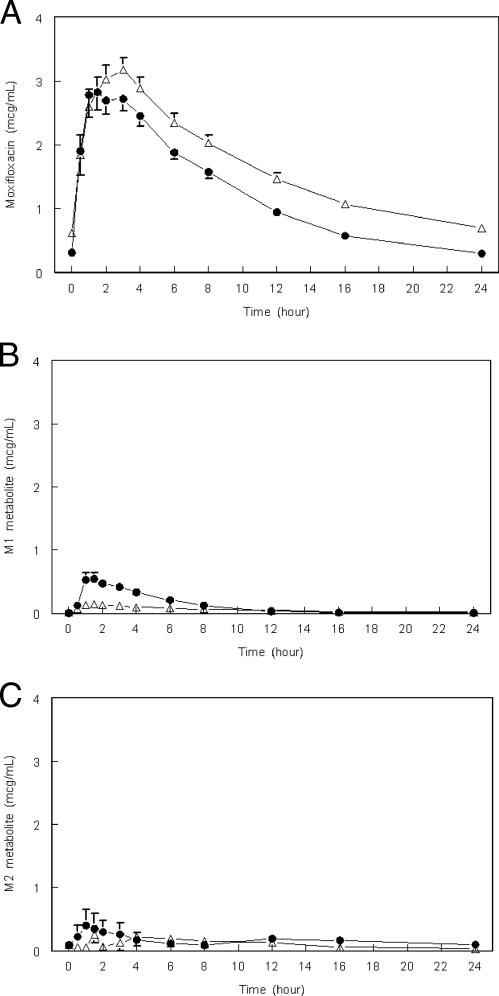
FIG. 1.
Mean plasma moxifloxacin (A), moxifloxacin metabolite M1 (B), and moxifloxacin metabolite M2 (C) concentrations versus time (hours) resulting from an oral dose of moxifloxacin (400 mg) without (▵) or with (•) coadministered rifampin (600 mg) at steady state (pharmacokinetic sampling at days 4 and 14 of moxifloxacin administered daily; n = 16). Values are arithmetic means; error bars, standard errors. The lower limit of quantitation was 0.0156 μg/ml for M1 and 0.026 μg/ml for M2.
The AUC0-24 and peak concentration of the M1 metabolite (the sulfate conjugate) were twofold and fourfold higher, respectively, with rifampin coadministration than with moxifloxacin alone, even though the half-life of this metabolite in serum was reduced (Table 1; Fig. 1B). Concentrations of the M2 metabolite (the glucuronide conjugate) in serum were detected only in a minority of patients and were somewhat higher following rifampin coadministration (Fig. 1C).
Moxifloxacin exposure and MDR1 polymorphisms.
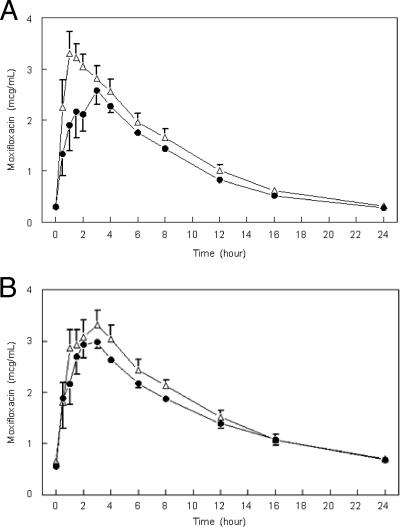
The MDR1 3435 CC genotype was detected in six study subjects, the CT genotype in eight, and the TT genotype in two. In univariate analyses of the pharmacogenetic data obtained with moxifloxacin plus rifampin, a significant increase in the time to the peak concentration of moxifloxacin (Tmax) (2 versus 1.1 h [P = 0.03]) was found for cases with the MDR1 3435 CC genotype compared to the other genotypes, but the differences in the mean peak concentration of moxifloxacin (a 23% lower geometric mean for the 3435 CC genotype [P = 0.08]) and the moxifloxacin AUC0-24 (a 16% lower geometric mean [P = 0.16]) were not significant (Fig. 2; Table 2). In stepwise multivariate regression analyses, no significant association was found between pharmacokinetic values and MDR1 3435 genotype groups when the MDR1 genotype was adjusted for weight and sex (for AUC0-24) or adjusted for weight (for the peak concentration of moxifloxacin and Tmax) (Table 2).
FIG. 2.
Mean plasma moxifloxacin concentration versus time (hours) resulting from an oral dose of moxifloxacin (400) mg with (A) or without (B) coadministered rifampin (600 mg) at steady state grouped by MDR1 3435 CC (•) versus other genotypes (▵). Values are arithmetic means; error bars, standard errors.
Tolerability of drug administrations.
Nine of 16 subjects reported no symptoms attributed to study drug administration. No serious adverse experiences occurred. Seven subjects reported a total of 13 grade 1 or 2 adverse experiences considered at least possibly study drug related. These adverse experiences were gastrointestinal (nausea [n = 2], anorexia [n = 2], diarrhea [n = 1], change in taste [n = 1], dry mouth [n = 1]), neurological (headache [n = 2] and abnormal vision [n = 1]), systemic (fatigue [n = 1] and pruritis [n = 1]), and urogenital (vaginitis [n = 1]). No association was found between the adverse experiences and moxifloxacin or M1 metabolite concentrations (data not shown).
DISCUSSION
Treatment regimens combining moxifloxacin and rifampin for drug-susceptible tuberculosis are being studied intensively. In this study, however, a significant drug interaction between moxifloxacin and rifampin was found among healthy volunteers. With coadministration of rifampin, the geometric mean AUC0-24 of moxifloxacin decreased 27% and the half-life decreased 36%, although no change in the peak concentration in serum was identified. This effect appeared to be mediated in part by increased activity of the sulfate conjugation pathway of moxifloxacin metabolism, because coadministration of rifampin resulted in marked increases in levels of the M-1 metabolite. Polymorphisms of the MDR1 gene had no effect on pharmacokinetics when moxifloxacin was administered alone. In pilot data for coadministration of moxifloxacin and rifampin among cases with the MDR1 3435 CC genotype, the concentrations of moxifloxacin were decreased in the first 3 h after drug administration, suggesting an effect on the absorption of moxifloxacin. However, no significant decrease in moxifloxacin exposure (AUC0-24) was found for six cases with the MDR1 3435 CC genotype compared to other genotypes.
The pharmacodynamics of moxifloxacin activity against Mycobacterium tuberculosis are not well understood. By using in vitro and in vivo models of tuberculosis treatment, lower concentrations and doses of moxifloxacin are associated with decreased activity (14). However, it is not known whether the concentrations of moxifloxacin achieved in humans are close to some threshold value for drug activity. Therefore, we are uncertain whether the decreases in moxifloxacin concentrations seen in this study would significantly affect its treatment efficacy. Additional studies on patients with tuberculosis will be required in order to determine whether the effect of rifampin on moxifloxacin is clinically relevant.
With rifampin coadministration, the increase in the M1 metabolite AUC0-24 and peak concentration are consistent with increased phase II sulfation of moxifloxacin. Rifampin induction of phase II sulfation of propafenone has been reported (3). Although rifampin induction of phase II glucuronidation has been described (5), only minimal concentrations of the M2 (glucuronide) conjugate were detected in this study (Fig. 1C). Both metabolites M1 and M2 are reported to be pharmacologically inactive (16).
P-glycoprotein is an ATP-binding membrane protein that exports unnecessary or harmful substances from cells. It is thought to play an important role in the absorption, distribution, and elimination of xenobiotics (8, 13), and quinolones are reported to be a substrate of the transporter (2, 17). P-glycoprotein located in the intestinal mucosa may limit absorption, and in the liver and kidney it may accelerate secretion into bile and urine, respectively (4, 13, 19). The frequency of MDR1 C3435T single-nucleotide polymorphisms differs among races. Also, single-nucleotide polymorphism effects may differ with drug, P-glycoprotein induction, and MDR1 haplotype analyses. In pilot pharmacogenetic data, no significant differences in moxifloxacin pharmacokinetic values among patients with different MDR1 3435 genotypes were identified. Additional studies with a larger study group are needed to evaluate an association between MDR1 genotype and the moxifloxacin-rifampin interaction.
This pharmacokinetic study has several limitations. First, this was a two-period, one-sequence design, and a period effect of the study cannot be excluded. Second, the study subjects were a diverse group of individuals enrolled in the Veterans Administration health care system. However, the medical conditions of subjects were not believed to influence the pharmacokinetics of moxifloxacin or rifampin, and concomitant medications were not administered systemically (except for a single dose of acetaminophen prior to pharmacokinetic sampling in one case). Patients with tuberculosis may have other reasons for altered moxifloxacin pharmacokinetics, and the rifampin interaction may differ from that found in this relatively healthy group of subjects. The sample size of the pharmacokinetic interaction study was powered to detect a 10% change in the moxifloxacin AUC0-24 but had only limited power in pilot secondary analyses of MDR1 gene polymorphisms. Further, residual confounding by measured or unmeasured variables is possible.
In summary, concomitant rifampin administration resulted in a 27% mean decrease in the moxifloxacin AUC0-24. Additional studies are required to understand the possible role of MDR1 gene polymorphisms and the clinical significance of the moxifloxacin-rifampin interaction.
Acknowledgments
This work was supported by the Centers for Disease Control and Prevention, the United States Public Health Service, and Bayer Pharma. The manufacturer provided moxifloxacin for the study and supported measurements of moxifloxacin and its metabolites. The Frederic C. Bartter General Clinical Research Center at the VAMC San Antonio (supported by NIH grant MO1-RR-01346) provided assistance in the evaluation of patients. This study was sponsored by the Tuberculosis Trials Consortium.
We are grateful to Elsa Villarino and Kenneth Castro for support and leadership within the CDC, to Lorna Bozeman for help with study logistics, and to Timothy Sterling, Heino Stass, and Nong Shang for review of the manuscript.
All authors report no potential conflict of interests, except for V.A., who is an employee of a contract research laboratory that provides services to Bayer Pharma.
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Burman, W. J., S. Goldberg, J. L. Johnson, G. Muzanye, M. Engle, A. W. Mosher, S. Choudhri, C. L. Daley, S. S. Munsiff, Z. Zhao, A. Vernon, R. E. Chaisson, and the Tuberculosis Trials Consortium. 2006. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174:331-338. [DOI] [PubMed] [Google Scholar]
- 1a.Cancer Therapy Evaluation Program, National Cancer Institute. 30 April 1999, posting date. Common toxicity criteria, version 2.0. http://ctep.cancer.gov/forms/CTCv20_4-30-992.pdf.
- 2.de Lange, E., S. Marchand, D. van den Berg, I. van der Sandt, A. de Boer, A. Delon, S. Bouquet, and W. Couet. 2000. In vitro and in vivo investigations on fluoroquinolones; effects of the P-glycoprotein efflux transporter on brain distribution of sparfloxacin. Eur. J. Pharm. Sci. 12:85-93. [DOI] [PubMed] [Google Scholar]
- 3.Dilger, K., B. Greiner, M. F. Fromm, U. Hofmann, H. K. Kroemer, and M. Eichelbaum. 1999. Consequences of rifampin treatment on propafenone disposition in extensive and poor metabolizers of CYP2D6. Pharmacogenetics 9:551-559. [PubMed] [Google Scholar]
- 4.Eichelbaum, M., M. Fromm, and M. Shwab. 2004. Clinical aspects of the MDR1 (ABCB1) gene polymorphism. Ther. Drug Monit. 26:180-185. [DOI] [PubMed] [Google Scholar]
- 5.Gallicano, K. D., J. Sahai, V. K. Shukla, I. Seguin, A. Pakuts, D. Kwok, B. C. Foster, and D. W. Cameron. 1999. Induction of zidovudine glucuronidation and amination pathways by rifampicin in HIV-infected patients. Br. J. Clin. Pharmacol. 48:168-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoffmeyer, S., O. Burk, O. von Richter, H. P. Arnold, J. Brockmoller, A. Johne, I. Cascorbi, T. Gerloff, I. Roots, M. Eichelbaum, and U. Brinkmann. 2000. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 97:3473-3478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johne, A., K. Kopke, T. Gerloff, I. Mai, S. Rietbrock, C. Meisel, S. Hoffmeyer, R. Kerb, M. F. Fromm, U. Brinkmann, M. Eichelbaum, J. Brockmoller, I. Cascorbi, and I. Roots. 2002. Modulation of steady-state kinetics of digoxin by haplotypes of the P-glycoprotein MDR1 gene. Clin. Pharmacol. Ther. 72:584-594. [DOI] [PubMed] [Google Scholar]
- 8.Kim, R. B. 2002. MDR1 single nucleotide polymorphisms: multiplicity of haplotypes and functional consequences. Pharmacogenetics 12:425-427. [DOI] [PubMed] [Google Scholar]
- 9.Mills, J. B., K. A. Rose, N. Sadagopan, J. Sahi, and S. M. de Morais. 2004. Induction of drug metabolism enzymes and MDR1 using a novel human hepatocyte cell line. J. Pharmacol. Exp. Ther. 309:303-330. [DOI] [PubMed] [Google Scholar]
- 10.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 11.Peloquin, C. A., R. Namdar, M. D. Singleton, and D. E. Nix. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12-18. [DOI] [PubMed] [Google Scholar]
- 12.Putnam, W. S., J. M. Woo, Y. Huang, and L. Z. Benet. 2005. Effect of the MDR1 C3435T variant and P-glycoprotein induction on dicloxacillin pharmacokinetics. J. Clin. Pharmacol. 45:411-421. [DOI] [PubMed] [Google Scholar]
- 13.Sakaeda, T., T. Nakamura, and K. Okumura. 2004. Pharmacogenetics of drug transporters and its impact on the pharmacotherapy. Curr. Top. Med. Chem. 4:1385-1398. [DOI] [PubMed] [Google Scholar]
- 14.Shandil, R. K., R. Jayaram, P. Kaur, S. Gaonkar, B. L. Suresh, B. N. Mahesh, R. Jayashree, V. Nandi, S. Bharath, and V. Balasubramanian. 2007. Moxifloxacin, ofloxacin, sparfloxacin, and ciprofloxacin against Mycobacterium tuberculosis: evaluation of in vitro and pharmacodynamic indices that best predict in vivo efficacy. Antimicrob. Agents Chemother. 51:576-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song, P., S. Li, B. Meibohm, A. O. Gaber, M. R. Honaker, M. Kotb, and C. R. Yates. 2002. Detection of MDR1 single nucleotide polymorphisms C3435T and G2677T using real-time polymerase chain reaction: MDR1 single nucleotide polymorphism genotyping assay. AAPS PharmSci 4:E29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stass, H., and D. Kubitza. 1999. Pharmacokinetics and elimination of moxifloxacin after oral and intravenous administration in man. J. Antimicrob. Chemother. 43(Suppl. B):83-90. [DOI] [PubMed] [Google Scholar]
- 17.Venkatesan, K. 1992. Pharmacokinetic drug interactions with rifampicin. Clin. Pharmacokinet. 22:47-65. [DOI] [PubMed] [Google Scholar]
- 18.Weiner, M., W. Burman, C. C. Luo, C. A. Peloquin, M. Engle, S. Goldberg, Z. Zhao, and A. Vernon for the Tuberculosis Trials Consortium. 2006. The effects of rifampin and human multidrug resistance gene polymorphism on serum concentrations of moxifloxacin. Proc. Am. Thorac. Soc. 3:A745. [Google Scholar]
- 19.Woodahl, E. L., and R. J. Ho. 2004. The role of MDR1 genetic polymorphisms in interindividual variability in P-glycoprotein expression and function. Curr. Drug Metab. 5:11-19. [DOI] [PubMed] [Google Scholar]