Abstract
Photodynamic therapy is a rapidly developing antimicrobial technology which combines a nontoxic photoactivatable dye or photosensitizer with harmless visible light of the correct wavelength to excite the dye to its reactive triplet state to generate reactive oxygen species toxic to cells. In this report we present evidence that the fungal pathogen Cryptococcus neoformans is susceptible to photodynamic inactivation by use of a polycationic conjugate of polyethyleneimine and the photosensitizer chlorin(e6). A C. neoformans rom2 mutant, with a mutation involving a putative Rho1 guanyl nucleotide exchange factor that is part of the protein kinase C-cell wall integrity pathway, demonstrated a compromised cell wall and less (1,3)β-d glucan than the wild-type strain and increased accumulation of PEI-ce6 as assessed by fluorescence uptake and confocal microscopy. Interestingly, C. neoformans rom2 was hypersusceptible to photodynamic inactivation and coincubation of wild-type C. neoformans strain KN99α with caspofungin-enhanced photoinactivation. These studies demonstrated that C. neoformans is sensitive to photodynamic therapy and illustrated the significance of cell wall integrity in microbial susceptibility to antimicrobial photodynamic inactivation.
Cryptococcus neoformans is a significant cause of morbidity and mortality. Cryptococcal disease most commonly involves the lungs, central nervous system, skin, skeletal system, and prostate gland (reviewed in references 8 and 13). Drug therapies to treat the fungal infection have included amphotericin B, fluconazole, and flucytosine or combinations of these drugs (37). Although there has been some success treating C. neoformans with these drugs, they can require prolonged treatment, with potential toxic effects (12, 13).
Photodynamic therapy (PDT) employs a nontoxic dye termed a photosensitizer (PS) and low-intensity visible light; in the presence of oxygen, these produce cytotoxic reactive oxygen species (5-7). PDT has the advantage of dual selectivity in that the PS can be targeted to its destination cell or tissue, and in addition the illumination can be spatially directed to the lesion. PDT was originally discovered by its effect on microorganisms (31) but since then has been principally developed as a treatment for cancer (14) and age-related macular degeneration (3).
PDT has attracted attention as a possible treatment for localized infections (10, 19, 45). It is known that gram-negative bacteria are resistant to PDT mediated by many commonly used photosensitizers that readily lead to phototoxicity for gram-positive species (28) and that photosensitizers bearing a cationic charge (30) or the use of agents that increase the permeability of the outer membrane increases the efficacy of killing of gram-negative organisms (28). The ideal PS for killing bacteria should possess an overall cationic charge and preferably multiple cationic charges (43). It was previously shown that covalent conjugates between poly-l-lysine and chlorin(e6) were efficient photosensitizers for pathogenic bacteria (21), and members of a novel set of polycationic conjugates of chlorin(e6) and polyethyleneimine (PEI) were capable of killing a panel of pathogenic bacteria and the yeast Candida albicans after exposure to low levels of red light (23). Photoactivated dyes produce either singlet oxygen or other reactive oxygen species such as superoxide and hydroxyl radicals, but in either case it is thought that these reactive oxygen species primarily react with the microbial cell wall, leading to membrane permeabilization and cell death. The fact that the skin is a common site of cryptococcal infections implies that PDT could be considered an alternative therapy for localized cryptococcal infections because of its advantage of killing fungal cells more rapidly by several log values. Selectivity for microbial cells over host tissue cells is provided by the rapid uptake of macromolecular photosensitizers such as PEI-ce6 by microbes and the slow uptake by mammalian cells (20, 44).
The fungal cell wall provides structure to the cell and protects the cell from the environment. It is primarily composed of α- and β-glucans (the principal polysaccharides of the bilayer structured cell wall), N-acetylglucosamine, and mannoproteins. It has been established using the model yeast Saccharomyces cerevisiae that the Rho1-protein kinase C pathway controls cell wall integrity. In this pathway, Rom2p receives signals from the extracellular stress sensors Wsc1p and Mid2p and is a guanyl nucleotide exchange factor for Rho1 (34, 35). Rho1 interacts with Fks1p, which encodes the (1,3)β-D glucan synthase for the production of (1,3)β-d glucan, a major cell wall component (15, 29, 36), and activates Pkc1p (25, 32). Activation of the protein kinase C pathway results in cell wall synthesis (22, 24, 27, 40).
ROM2 of C. neoformans was identified through an in silico search as an S. cerevisiae ROM2 homologue (18) and through a screen of insertional mutants in a Caenorhabditis elegans model of infection (42) and has been described as a C. neoformans virulence factor (42). Here we show that C. neoformans ROM2 also plays a role in cell wall integrity. The rom2 mutant was hypersensitive to Congo red and sodium dodecyl sulfate (SDS), indicating cell wall defects. Indeed, there was a deficiency in (1,3)β-d glucan in the rom2 mutant compared to wild-type cells. In this report we show that cell wall defects allow the rom2 mutant to be hypersusceptible to antimicrobial photoinactivation with the PS polyethyleneimine chlorin(e6) conjugate (PEI-ce6) and that this hypersusceptibility can be reversed in the ROM2 reconstituted strain.
MATERIALS AND METHODS
Strains and media.
C. neoformans strains used in this study included wild-type KN99α, a KN99α rom2 mutant, and the reconstituted strain KN99α rom2 + ROM2 (42). The KN99α rom2 strain lacked all but the last 18 codons of the ROM2 locus (42). The reconstituted strain KN99α rom2 + ROM2 incorporated a wild-type copy of the ROM2 gene cloned into a plasmid conferring resistance to neomycin and was introduced into the rom2 mutant strain through Agrobacterium-mediated transformation (42). All strains grew in 1% yeast extract-2% peptone-2% dextrose (YPD) media and were stored in glycerol at −80°C. Cultures grew at 30°C unless otherwise specified.
TEM.
We grew C. neoformans cells at 37°C and collected the cells with centrifugation. Following centrifugation, cells were suspended in 2% glutaraldehyde-0.1 M sodium cacodylate buffer (pH 7.4) for 60 min at room temperature. Cells were fixed overnight at 4°C in glutaraldehyde. Fixed cells were collected with centrifugation and washed three times with cacodylate buffer and then suspended in 1% osmium tetroxide in cacodylate buffer for 60 min at room temperature. Cells were washed with cacodylate buffer followed by double-distilled water and then suspended in 2% uranyl acetate for 60 min at room temperature and rinsed with double-distilled water. Pelleted cells suspended in 2% agarose were dehydrated through a graded ethanol series and then infiltrated overnight in a 1:1 mixture of Epon (Ted Pella, Inc.):100% ethanol with agitation. Further infiltration occurred by embedding cells in 100% fresh Epon overnight at 60°C. Poststaining of thin sections with 2% uranyl acetate and lead citrate provided contrast for viewing cells with a JEOL 1011 transmission electron microscope (TEM) at 80 kV.
(1,3)β-d glucan quantification.
We compared the cellular amounts of (1,3)β-d glucan by use of the dye aniline blue (Sigma) as described previously (38, 46). In brief, cells grew overnight at 37°C and 2.5 × 106 cells were used for quantification for each strain. Cells collected with centrifugation were washed twice with Tris-EDTA buffer. Cells suspended in 500 μl of Tris-EDTA received NaOH to achieve a final concentration of 1 M. Cells were incubated at 80°C for 30 min to solubilize (1,3)β-d glucan followed by the addition of 2.1 ml of aniline blue mixture (0.03% aniline blue, 0.18 M HCl, 0.49 M glycine/NaOH [pH 9.5]). Aniline blue interacts with linear (1,3)β-d glucan (39). Cells were mixed through agitation and then incubated at 50°C for 30 min followed by a 30-min incubation at room temperature. We examined each strain in triplicate. Fluorescence of (1,3)β-d glucan was measured with a fluorescent plate reader (SpectraMax Gemini; Molecular Probes). An excitation wavelength of 400 nm/slit (width, 3 nm) and an emission wavelength of 460 nm (width, 3 nm) with a cutoff of 455 nm were used to measure fluorescence.
Cell wall integrity.
Evaluation of cell wall integrity compared strain KN99α rom2 to KN99α and KN99α rom2 + ROM2. Quantities of a 10-fold serial dilution of 104 to 101 cells were plated in a volume of 5 μl on YPD medium supplemented with 0.5% Congo red for cells grown under 30°C growth conditions. Cell wall integrity was also evaluated with 0.03% SDS. Cells grew under 25°C, 30°C, and 37°C conditions until colonies appeared.
Photodynamic inactivation (PDI) studies and CFU determination.
C. neoformans cells grew overnight at 30°C or 37°C. Cells collected through centrifugation for 5 min were then suspended in phosphate-buffered saline (PBS). The preparation and characterization of the covalent conjugate between chlorin(e6) and branched polyethyleneimine of an average molecular weight of 10,000 (abbreviated as PEI-ce6) were described in detail by Tegos and colleagues (44). A cell suspension consisting of 3.5 ml at 107 cells ml−1 for each strain was incubated with a 10 μM ce6 equivalent of PEI-ce6 for 10 min at room temperature in the dark. Cells were collected with centrifugation for 5 min and suspended in PBS. Aliquots (500 μl) were transferred to a 48-well plate and illuminated at room temperature with a 665-nm, 1-W diode laser (BWF-665-1; B&W Tek, Newark, DE) coupled to a 1-mm optical fiber. Red light was delivered at an irradiance of 100 mW cm−2, with fluence ranging from 0 to 16 J cm−2.
In drug combination experiments, we added 8 μg ml−1 caspofungin (Merck) or 0.25 μg ml−1 fluconazole to the cells in combination with 10 μM PEI-ce6. All other aspects remained the same. Cells were incubated with caspofungin or fluconazole for 0, 8, and 24 h before PEI-ce6 exposure for 30 min. Cells were collected and illuminated as described above.
At intervals during the illumination after delivery of requisite fluence levels, aliquots (100 μl) were taken from each well to determine CFU. Tenfold aliquots serially diluted in PBS generated dilutions of 10−1 to 10−6 times the original concentrations, and 10 μl aliquots of each of the dilutions were streaked horizontally on square YPD plates by the method of Jett and colleagues (23). We streaked the plates in triplicate and incubated for 48 h at 30°C or 37°C in the dark to allow colony formation. Control groups included cells that were not treated with a PS or light, cells incubated with a PS but kept in 48-well plates covered with aluminum foil at room temperature for the duration of the illumination, and cells treated with light but not with a PS. Survival fractions (SF) were routinely expressed as ratios of CFU of microbial cells treated with light and a PS to CFU of microbes treated with neither. The survival fraction at 0 J/cm2 gives a measure of the toxicity of the conjugate in the dark.
Uptake of PEI-ce6 by microbial cells.
Suspensions of C. neoformans cells received 10 μM PEI-ce6 in the dark at room temperature. A 3-ml aliquot was centrifuged, and the pellet was suspended in 6 ml of 0.1 M NaOH and 1% SDS for 24 h to dissolve the cells. The fluorescence was measured (FluoroMax3; SPEX Industries, Edison, NJ) at an excitation wavelength of 400 nm and an emission wavelength of 580 nm to 700 nm. Uptake values were calculated by dividing the number of nanomoles of photosensitizer in the dissolved pellet by the number of CFU obtained by a serial dilution, and the number of PS molecules per cell was calculated using Avagadro's number.
We also examined cells for fluorescence accumulation with fluorescent-activated cell sorting (FACS) of strains grown overnight at 37°C. Cells (3.5 × 107) collected with centrifugation and washed with 100 μl of PBS were incubated with 1 μM PEI-ce6 for 10 min in the dark at room temperature. Cells collected with centrifugation for 5 min and washed twice with 100 μl of PBS were then suspended in 500 μl of PBS and fixed with paraformaldehyde according to the method of Kopeckà and colleagues (26). In brief, cells received 100 μl of fixative (5% paraformaldehyde in wash buffer) and were incubated at room temperature for 90 min and then washed three times with wash buffer (0.1 M KH2PO4, 1.25 mM EGTA, 1.25 mM MgCl2, pH 6.9) for 5 min. Cells were washed and suspended in 500 μl PBS. A BD (Becton Dickinson) FACS apparatus was used to measure fluorescence differences of 10,000 cells.
Statistical analysis.
Statistical values represent the means of three separate experiments, and bars presented in the graphs represent standard errors of the means. Differences between means values were tested for significance by an unpaired two-tailed Student t test, assuming equal or unequal variations as appropriate. A P value of less than 0.05 indicated significance.
RESULTS AND DISCUSSION
We sought to determine the susceptibility of the fungal pathogen C. neoformans to PDT. The wild-type strain KN99α was grown at 30°C and 37°C and incubated with PEI-ce6 and subsequently irradiated, with fluence ranging from 0 to 16 J/cm2. Antimicrobial inactivation with PEI-ce6 resulted in killing of C. neoformans cells at both growth temperatures at a log10 of 2 (Fig. 1). We evaluated C. neoformans for PEI-ce6 resistance but did not see resistance after five repetitive cycles of PEI-ce6 exposure, indicating that under these brief testing conditions there was no evidence for resistance (data not shown). This is the first report of antimicrobial photoinactivation of C. neoformans. Antimicrobial PDT has been extensively used for C. albicans with a panel of photosensitizers including PEI-ce6, methylene blue, toluidine blue, rose Bengal, and Photofrin (2, 11, 44, 47). Interestingly, treatment of C. albicans required 10 times more PEI-ce6 than treatment of bacterial cells; this result may have had to do with the larger size of the fungal cells (44). C. albicans susceptibility to PDT was affected by growth conditions and cell morphology (2). Our results also showed a slight difference in killing levels based on growth conditions. There was a slight increase in killing at 37°C compared to the results seen with cells grown at 30°C, but the amount of killing at 30°C was not significantly different from the amount of killing at 37°C.
FIG. 1.
Wild-type C. neoformans strain KN99α is susceptible to photoinactivation. Phototoxicity of 10 μM ce6-equivalent PEI-ce6 was measured after incubation at 30°C (closed circles) and 37°C (open circles) of C. neoformans. KN99α cells were incubated with the PS for 30 min. Cells were then illuminated with 100 mW cm−2 and the survival fractions determined, as described in Materials and Methods.
Because wild-type C. neoformans was more resistant to PDI (under similar conditions we noted killing of C. neoformans at 2 log10 [Fig. 1] compared to killing of C. albicans at 4 to 6 log10 [44]), we examined C. neoformans mutant strains to identify mutants that were more susceptible to PDI with PEI-ce6. The C. neoformans mutants sod1 (superoxide dismutase 1) (9), cna1 (calcineurin) (33), and rom2 (putative Rho1 guanyl nucleotide exchange factor) (42) were among the mutants evaluated for increased PEI-ce6 efficacy; all were found to exhibit increased killing compared to wild-type C. neoformans results (unpublished data). The most significant amount of photoinactivation with PEI-ce6 was found with C. neoformans rom2 mutant cells; we therefore focused our study on the rom2 mutant. We recently identified ROM2 as a virulence factor for C. neoformans and demonstrated that the rom2 mutant was hypersensitive to caffeine and sodium chloride, potentially indicating defects in cell wall integrity (42). We sought to determine how cell wall defects would affect the efficiency of photodynamic inactivation. We evaluated the susceptibility of the C. neoformans rom2 mutant to photoinactivation and found that this mutant is hypersusceptible to PEI-ce6 photoinactivation compared to the wild-type and the reconstituted strains (Fig. 2A and B). Since differences in fungal susceptibility to photosensitizers have been associated with various growth conditions (2), cells were grown at 30°C (Fig. 2A) and at 37°C (Fig. 2B). Increased cell death for strain KN99α rom2 compared to the wild-type and reconstituted strains was demonstrated under both sets of growth conditions but was more significant at 37°C. Notably, reconstitution of the Rom2 phenotype (by integrating the wild-type ROM2 gene into the rom2 mutant strain as described in reference 34) restored resistance to PDI completely (at 30°C; Fig. 2A) or almost completely (at 37°C; Fig. 2B).
FIG. 2.
The C. neoformans rom2 mutant is hypersusceptible to photoinactivation with PEI-ce6. Phototoxicity of 10 μM ce6-equivalent PEI-ce6 after incubation at 30°C (A) and 37°C (B) for strains KN99α (closed circles), KN99α rom2 (open circles), and KN99α rom2 +ROM2 (closed triangles). Incubation with the PS was 30 min. Cells were then illuminated at 100 mW cm−2 and the survival fractions determined, as described in Materials and Methods. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
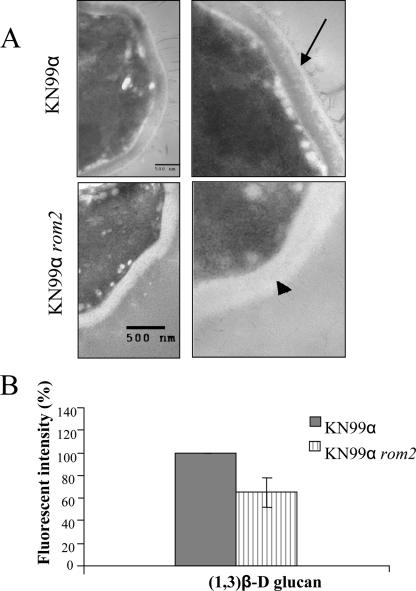
Since the role of ROM2 in cell wall integrity has not been completely elucidated (42), we further evaluated the integrity of the cell wall in the rom2 mutant. We evaluated whether the increased susceptibility was related to cell wall defects in the C. neoformans rom2 mutant. Initial visualization of the cell wall for C. neoformans rom2 mutant with TEM showed that the cell wall was intact; however, there were visible differences in the cell wall of KN99α rom2 compared to that of KN99α. More specifically, the rom2 mutant cell wall was consistently less electron dense than the wild-type KN99α strain cell wall (Fig. 3A). A potential cause for the visible difference was a change in the composition of the cell wall, which is primarily comprised of α- and β-glucans. Strain KN99α rom2 was evaluated for changes in the cell wall component (1,3)β-d glucan, because ROM2p in S. cerevisiae is a guanyl nucleotide exchange factor for Rho1p, which activates the glucan synthase Fks1p for (1,3)β-d glucan (15, 29, 36). The amount of (1,3)β-d glucan in strain KN99α rom2 was compared to that in strain KN99α by use of aniline blue (Sigma) (46). Interestingly, we found there was 35% (P < 0.05) less (1,3)β-d glucan available in KN99α rom2 cells compared to wild-type cell results (Fig. 3B). The reconstituted strain KN99α rom2 + ROM2 was also evaluated for (1,3)β-d glucan levels by use of the dye aniline blue. A slightly lower amount of (1,3)β-d glucan was found for KN99α rom2 + ROM2 cells at 85% of wild-type (1,3)β-d glucan levels, but the amount was not significantly (P = 0.17) different from that seen with wild-type C. neoformans cells (data not shown).
FIG. 3.
Cell wall defect in the C. neoformans rom2 mutant. (A) Strain KN99α and KN99α rom2 cells were compared visually for cell wall differences by use of TEM (×25,000). Scale bars, 500 nm. The arrow indicates the KN99α cell wall, and the arrowhead indicates the KN99α rom2 cell wall. (B) The cellular amounts of (1,3)β-d glucan was compared between KN99α and KN99α rom2 by use of the dye aniline blue. KN99α rom2 had a lower level of (1,3)β-d glucan available in the cell compared to KN99α under 37°C growth conditions.
We investigated whether the difference in strain KN99α rom2 cell wall composition affected cell wall integrity. Cell wall integrity was assessed with SDS, which disrupts the plasma membrane, and with Congo red, which interacts with β-d glucan (48) (Fig. 4A to C). Cells with a defective cell wall were sensitive to the presence of either chemical. We found KN99α rom2 was sensitive to 0.03% SDS at 25°C, 30°C, and 37°C (Fig. 4B). Wild-type C. neoformans and the ROM2 reconstituted strain were not sensitive to 0.03% SDS under these conditions. Gerik and colleagues previously tested KN99α rom2 sensitivity to 0.5% Congo red and showed that KN99α rom2 was slightly sensitive to Congo red at 30°C (18). We found that KN99α rom2 was significantly hypersensitive to 0.5% Congo red under high-temperature growth conditions (Fig. 4C).
FIG. 4.
Cell wall integrity of the strain KN99α rom2 mutant compared to the wild-type strain, KN99α, and the reconstituted strain KN99α rom2 + ROM2. Tenfold serial dilutions of cells were plated from overnight cultures. The plated cultures were maintained at 25°C, 30°C, and 37°C until colonies were visible. Panels show YPD medium (A) compared to YPD supplemented with 0.03% SDS (B) or with 0.5% Congo red (C) to detect lysis from cells with weakened cell wall integrity.
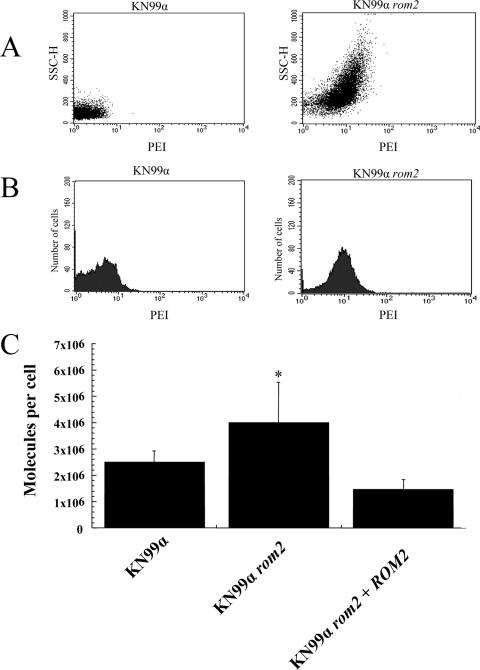
In order to investigate the hypothesis that the cell wall integrity defect of the rom2 mutant is directly associated with the hypersusceptible phenotype with respect to photodynamic inactivation, we studied whether weakened cell wall integrity is associated with increased accumulation of PEI-ce6 by KN99α rom2 compared to KN99α. The association with increased accumulation of PEI-ce6 was indicated by FACS results that showed increased fluorescence of KN99α rom2 cells in the presence of PEI-ce6 compared to KN99α cells in the presence of PEI-ce6 (Fig. 5A and B). KN99α rom2 cells were shown to exhibit an increased amount of fluorescence in a greater number of cells compared to KN99α cells treated with PEI-ce6 by use of a scatter plot; this result was also shown in a shift in the histogram plot (Fig. 5A and B). The increased accumulation of PEI-ce6 was also assessed with fluorometry, showing that more PEI-ce6 was associated with KN99α rom2 compared to KN99α or KN99α rom2 + ROM2 results (Fig. 5C). Both FACS and fluorometry results indicated a greater association of PEI-ce6 with the rom2 mutant compared to wild-type C. neoformans.
FIG. 5.
Uptake of PEI-ce6 by strains KN99α and KN99α rom2. (A and B) KN99α and KN99α rom2 cells were exposed to 1 μM PEI-ce6. Cells were incubated with the photosensitizer for 30 min, washed, and fixed with paraformaldehyde as described in Materials and Methods. (A) FACS results indicated increased side scatter height for 10,000 cells of KN99α rom2 compared to KN99α. (B) Histogram plot of 10,000 cells from FACS. (C) Fluorometer-measured uptake of PEI-ce6 in terms of molecules per cell for KN99α, KN99α rom2, and KN99α rom2 + ROM2. Values represent the means of three separate determinations; bars represent standard errors of the means. *, P < 0.05.
The results from the FACS and fluorometry analyses indicated an increase in fluorescence that could have been due either to increased amounts of PEI-ce6 attached to the strain KN99α rom2 cell wall or to the KN99α rom2 cell being permeable to PEI-ce6 because of the defects on the cell wall. In order to investigate the location of increased association of PEI-ce6 to the rom2 mutant, we visually evaluated cells exposed to PEI-ce6 with confocal microscopy. Indeed, the KN99α rom2 strain was more permeable to PEI-ce6 than was KN99α. Microscopy of the C. neoformans cells after a 10 min incubation with PEI-ce6 showed that the dye was predominately located on the exterior of KN99α cells. For KN99α rom2 cells, PEI-ce6 was located both on the cell exterior and on the cell interior (Fig. 6). This demonstrates that the rom2 mutant cells are leaky with respect to PEI-ce6 and correlates with the increase in cell death and the increased association with PEI-ce6 observed with FACS and fluorescent quantification.
FIG. 6.
Visualization of cell permeability. Cells were grown at 37°C, exposed to PEI-ce6 for 10 min, and then washed with PBS. Cells were observed for PEI-ce6 localization with a confocal laser spectrophotometer (TCS; NT Leica). Arrows indicate PEI-ce6 localization to the cell wall. Arrowheads indicate localization of PEI-ce6 to the cell interior. PEI-ce6 appears red.
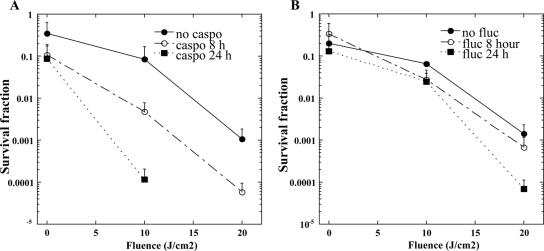
Increased cell permeability of the strain KN99α rom2 mutant compared to the wild-type KN99α strain indicates that the PEI-ce6 PS is more effective when it penetrates the C. neoformans cell. Accordingly, we examined the effect of coincubating cells with the echinocandin caspofungin and PEI-ce6. Caspofungin is an antifungal drug that targets the (1,3)β-d glucan synthase Fks1p. While it has been shown to be effective at treating fungal pathogens such as Candida spp., caspofungin is ineffective at killing C. neoformans both in vitro and in vivo (1, 16). Although caspofungin treatment of C. neoformans does not kill the cells, it does affect the cell wall by reducing the amount of (1,3)β-d glucan found in the cell (17). We were interested to determine whether PDI was effective against wild-type C. neoformans treated with caspofungin in combination with PEI-ce6. Indeed, wild-type KN99α treated with a subinhibitory amount of caspofungin at 8 μg ml−1 and PEI-ce6 for either 8 or 24 h showed increased photoinactivation compared to the results seen with PEI-ce6 treatment alone (Fig. 7A). Strain KN99α treated with caspofungin showed significantly more killing with PEI-ce6 after 8 h of caspofungin treatment (P < 0.01) compared to the results seen in the absence of treatment with caspofungin. Further, 24 h of treatment with caspofungin increased killing to 4 log10 (P < 0.001) compared to the results seen with KN99α cells that were not treated with caspofungin and that demonstrated killing at a log10 of 2. This suggests that caspofungin is capable of damaging the cell wall and increasing the efficacy of PEI-ce6, likely by allowing more PEI-ce6 to penetrate the cell.
FIG. 7.
Coincubation of wild-type C. neoformans strain KN99α with caspofungin- or fluconazole-enhanced photoinactivation. Phototoxicity of 10 μM ce6-equivalent PEI-ce6 was measured after incubation at 30°C with a subinhibitory concentration of caspofungin (8 μg/ml) (A) or fluconazole (0.25 μg/ml) (B). C. neoformans KN99α cells were exposed to the PS for 30 min after incubation with the corresponding antifungal agent for 0 (closed circles), 8 (open circles), and 24 (closed squares) h. Cells were then illuminated with 100 mW cm−2 and the survival fractions determined, as described in the Materials and Methods.
We further examined a drug combination effect by use of the azole fluconazole with PEI-ce6. Fluconazole targets P450 to reduce the amount of the cell wall sterol ergosterol. A subinhibitory concentration of 0.25 μg ml−1 did not exhibit a significant synergistic effect with PEI-ce6 (Fig. 7B).
This evidence suggests potential clinical use of PDT with cutaneous C. neoformans infections in the future, but more studies are necessary. Thus far, the use of PDI is feasible with members of the Ascomycetes such as C. albicans and with Basidiomycetes, as indicated by this work with C. neoformans, and its potential use extends to dermatophytes (see references 2 and 44 and reviews in reference 4). Growth inhibition was achieved with thiophenes 2.2′:5,2″-terthienyl and 5-(4-OH-1-butinyl)2,2′-bithienyl for dermatophytes such as Trichophyton mentagrophytes, T. rubrum, T. tonsurans, Microsporum cookei, M. canis, M. gypseum, Epidermophyton floccosum, and Nannizia cajetani (4). Exposure of T. rubrum suspension cultures to Sylsen B [5,10,15-tris(4-methylpyridinium)-20-phenyl-[21H,23H]-porphine trichloride] or DP mme (deuteroporphyrin monomethylester) resulted in complete killing (41). Our findings show that PEI-ce6 can be used for photoinactivation of C. neoformans. Importantly, the efficacy of cell killing is affected by the amount of PEI-ce6 that penetrates the cell. By introducing cell wall defects with caspofungin, photodynamic inactivation increased.
In conclusion, this is the first report showing that C. neoformans is sensitive to PDI and we provide further evidence on the role of ROM2 in C. neoformans cell wall integrity. The susceptibility of C. neoformans to the photosensitizer PEI-ce6 is associated with the cell wall. The cell wall defects found in the rom2 mutant were associated with increased accumulation and cell permeation by the photosensitizer dye, leading to increased photoinactivation after light exposure. An increase in photoinactivation was also achieved by exposing wild-type C. neoformans cells to caspofungin as a means to weaken the cell wall and promote increased penetration of PEI-ce6. PDT deserves further study as a potential treatment for C. neoformans fungal infections, especially when it is coupled with disruption of the cell wall integrity.
Acknowledgments
This work was supported by K08 award AI63084 from the NIH and a New Scholar award in Global Infectious Diseases from the Ellison Medical Foundation to E.M. Support was also provided by NIH grant R01AI050875 to M.R.H. Inflammatory Bowel Disease grant DK43351 and Boston Area Diabetes and Endocrinology Research Center award DK57521 support the electron microscopy core facility.
We thank Qurat Naqvi for assistance with antimicrobial PDI studies. We also thank Mary McKee for assistance with microscopy images.
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliss, J. M., C. E. Bigelow, T. H. Foster, and C. G. Haidaris. 2004. Susceptibility of Candida species to photodynamic effects of Photofrin. Antimicrob. Agents Chemother. 48:2000-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown, S. B., and K. J. Mellish. 2001. Verteporfin: a milestone in opthalmology [sic] and photodynamic therapy. Expert. Opin. Pharmacother. 2:351-361. [DOI] [PubMed] [Google Scholar]
- 4.Calzavara-Pinton, P. G., M. Venturini, and R. Sala. 2005. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J. Photochem. Photobiol. B 78:1-6. [DOI] [PubMed] [Google Scholar]
- 5.Castano, A. P., T. N. Demidova, and M. R. Hamblin. 2004. Mechanisms in photodynamic therapy: part one—photosensitizers, photochemistry and cellular localization. Photodiagn. Photodynam. Ther. 1:279-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castano, A. P., T. N. Demidova, and M. R. Hamblin. 2005. Mechanisms in photodynamic therapy: part three—photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagn. Photodynam. Ther. 2:91-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castano, A. P., T. N. Demidova, and M. R. Hamblin. 2005. Mechanisms in photodynamic therapy: part two—cellular signaling, cell metabolism and modes of cell death. Photodiagn. Photodynam. Ther. 2:1-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chayakulkeeree, M., and J. R. Perfect. 2006. Cryptococcosis. Infect. Dis. Clin. N. Am. 20:507-544. [DOI] [PubMed] [Google Scholar]
- 9.Cox, G. M., T. S. Harrison, H. C. McDade, C. P. Taborda, G. Heinrich, A. Casadevall, and J. R. Perfect. 2003. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 71:173-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demidova, T. N., and M. R. Hamblin. 2004. Photodynamic therapy targeted to pathogens. Int. J. Immunopathol. Pharmacol. 17:245-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demidova, T. N., and M. R. Hamblin. 2005. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 49:2329-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 13.Dismukes, W. E. 1993. Management of cryptococcosis. Clin. Infect. Dis. 17(Suppl. 2):S507-S512. [DOI] [PubMed] [Google Scholar]
- 14.Dolmans, D. E., D. Fukumura, and R. K. Jain. 2003. Photodynamic therapy for cancer. Nat. Rev. Cancer 3:380-387. [DOI] [PubMed] [Google Scholar]
- 15.Drgonova, J., T. Drgon, K. Tanaka, R. Kollar, G. C. Chen, R. A. Ford, C. S. Chan, Y. Takai, and E. Cabib. 1996. Rho1p, a yeast protein at the interface between cell polarization and morphogenesis. Science 272:277-279. [DOI] [PubMed] [Google Scholar]
- 16.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feldmesser, M., Y. Kress, A. Mednick, and A. Casadevall. 2000. The effect of the echinocandin analogue caspofungin on cell wall glucan synthesis by Cryptococcus neoformans. J. Infect. Dis. 182:1791-1795. [DOI] [PubMed] [Google Scholar]
- 18.Gerik, K. J., M. J. Donlin, C. E. Soto, A. M. Banks, I. R. Banks, M. A. Maligie, C. P. Selitrennikoff, and J. K. Lodge. 2005. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol. Microbiol. 58:393-408. [DOI] [PubMed] [Google Scholar]
- 19.Hamblin, M. R., and T. Hasan. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem. Photobiol. Sci. 3:436-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamblin, M. R., J. L. Miller, I. Rizvi, H. G. Loew, and T. Hasan. 2003. Pegylation of charged polymer-photosensitiser conjugates: effects on photodynamic efficacy. Br. J. Cancer 89:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamblin, M. R., D. A. O'Donnell, N. Murthy, K. Rajagopalan, N. Michaud, M. E. Sherwood, and T. Hasan. 2002. Polycationic photosensitizer conjugates: effects of chain length and Gram classification on the photodynamic inactivation of bacteria. J. Antimicrob. Chemother. 49:941-951. [DOI] [PubMed] [Google Scholar]
- 22.Igual, J. C., A. L. Johnson, and L. H. Johnston. 1996. Coordinated regulation of gene expression by the cell cycle transcription factor Swi4 and the protein kinase C MAP kinase pathway for yeast cell integrity. EMBO J. 15:5001-5013. [PMC free article] [PubMed] [Google Scholar]
- 23.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 24.Jiang, B., A. F. Ram, J. Sheraton, F. M. Klis, and H. Bussey. 1995. Regulation of cell wall beta-glucan assembly: PTC1 negatively affects PBS2 action in a pathway that includes modulation of EXG1 transcription. Mol. Gen. Genet. 248:260-269. [DOI] [PubMed] [Google Scholar]
- 25.Kamada, Y., H. Qadota, C. P. Python, Y. Anraku, Y. Ohya, and D. E. Levin. 1996. Activation of yeast protein kinase C by Rho1 GTPase. J. Biol. Chem. 271:9193-9196. [DOI] [PubMed] [Google Scholar]
- 26.Kopeckà, M., M. Gabriel, K. Takeo, M. Yamaguchi, A. Svoboda, M. Ohkusu, K. Hata, and S. Yoshida. 2001. Microtubules and actin cytoskeleton in Cryptococcus neoformans compared with ascomycetous budding and fission yeasts. Eur. J. Cell Biol. 80:303-311. [DOI] [PubMed] [Google Scholar]
- 27.Madden, K., Y. J. Sheu, K. Baetz, B. Andrews, and M. Snyder. 1997. SBF cell cycle regulator as a target of the yeast PKC-MAP kinase pathway. Science 275:1781-1784. [DOI] [PubMed] [Google Scholar]
- 28.Malik, Z., H. Ladan, and Y. Nitzan. 1992. Photodynamic inactivation of Gram-negative bacteria: problems and possible solutions. J. Photochem. Photobiol. B 14:262-266. [DOI] [PubMed] [Google Scholar]
- 29.Mazur, P., and W. Baginsky. 1996. In vitro activity of 1,3-beta-d-glucan synthase requires the GTP-binding protein Rho1. J. Biol. Chem. 271:14604-14609. [DOI] [PubMed] [Google Scholar]
- 30.Merchat, M., G. Bertolini, P. Giacomini, A. Villanueva, and G. Jori. 1996. Meso-substituted cationic porphyrins as efficient photosensitizers of gram-positive and gram-negative bacteria. J. Photochem. Photobiol. B 32:153-157. [DOI] [PubMed] [Google Scholar]
- 31.Moan, J., and Q. Peng. 2003. An outline of the hundred-year history of PDT. Anticancer Res. 23:3591-3600. [PubMed] [Google Scholar]
- 32.Nonaka, H., K. Tanaka, H. Hirano, T. Fujiwara, H. Kohno, M. Umikawa, A. Mino, and Y. Takai. 1995. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 14:5931-5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 16:2576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozaki, K., K. Tanaka, H. Imamura, T. Hihara, T. Kameyama, H. Nonaka, H. Hirano, Y. Matsuura, and Y. Takai. 1996. Rom1p and Rom2p are GDP/GTP exchange proteins (GEPs) for the Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15:2196-2207. [PMC free article] [PubMed] [Google Scholar]
- 35.Philip, B., and D. E. Levin. 2001. Wsc1 and Mid2 are cell surface sensors for cell wall integrity signaling that act through Rom2, a guanine nucleotide exchange factor for Rho1. Mol. Cell. Biol. 21:271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qadota, H., C. P. Python, S. B. Inoue, M. Arisawa, Y. Anraku, Y. Zheng, T. Watanabe, D. E. Levin, and Y. Ohya. 1996. Identification of yeast Rho1p GTPase as a regulatory subunit of 1,3-beta-glucan synthase. Science 272:279-281. [DOI] [PubMed] [Google Scholar]
- 37.Saag, M. S., R. J. Graybill, R. A. Larsen, P. G. Pappas, J. R. Perfect, W. G. Powderly, J. D. Sobel, and W. E. Dismukes. 2000. Practice guidelines for the management of cryptococcal disease. Clin. Infect. Dis. 30:710-718. [DOI] [PubMed] [Google Scholar]
- 38.Sekiya-Kawasaki, M., M. Abe, A. Saka, D. Watanabe, K. Kono, M. Minemura-Asakawa, S. Ishihara, T. Watanabe, and Y. Ohya. 2002. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics 162:663-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shedletzky, E., C. Unger, and D. P. Delmer. 1997. A microtiter-based fluorescence assay for (1,3)-beta-glucan synthases. Anal. Biochem. 249:88-93. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu, J., K. Yoda, and M. Yamasaki. 1994. The hypo-osmolarity-sensitive phenotype of the Saccharomyces cerevisiae hpo2 mutant is due to a mutation in PKC1, which regulates expression of beta-glucanase. Mol. Gen. Genet. 242:641-648. [DOI] [PubMed] [Google Scholar]
- 41.Smijs, T. G., and H. J. Schuitmaker. 2003. Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem. Photobiol. 77:556-560. [DOI] [PubMed] [Google Scholar]
- 42.Tang, R. J., J. Breger, A. Idnurm, K. J. Gerik, J. K. Lodge, J. Heitman, S. B. Calderwood, and E. Mylonakis. 2005. Cryptococcus neoformans gene involved in mammalian pathogenesis identified by a Caenorhabditis elegans progeny-based approach. Infect. Immun. 73:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tegos, G. P., T. N. Demidova, D. Arcila-Lopez, H. Lee, T. Wharton, H. Gali, and M. R. Hamblin. 2005. Cationic fullerenes are effective and selective antimicrobial photosensitizers. Chem. Biol. 12:1127-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tegos, G. P., M. Anbe, C. Yang, T. N. Demidova, M. Satti, P. Mroz, S. Janjua, F. Gad, and M. R. Hamblin. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe, D., M. Abe, and Y. Ohya. 2001. Yeast Lrg1p acts as a specialized RhoGAP regulating 1,3-beta-glucan synthesis. Yeast 18:943-951. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, M., and N. Mia. 1993. Sensitisation of Candida albicans to killing by low-power laser light. J. Oral Pathol. Med. 22:354-357. [DOI] [PubMed] [Google Scholar]
- 48.Wood, P. J., and R. G. Fulcher. 1983. Dye interactions. A basis for specific detection and histochemistry of polysaccharides. J. Histochem. Cytochem. 31:823-826. [DOI] [PubMed] [Google Scholar]