Abstract
Proton pump inhibitors (PPIs) have been identified as a risk factor for Clostridium difficile-associated diarrhea (CDAD), though the mechanism is unclear because gastric acid does not kill C. difficile spores. We hypothesized that the vegetative form of C. difficile, which is killed by acid, could contribute to disease pathogenesis if it survives in room air and in gastric contents with elevated pH. We compared the numbers of C. difficile spores and vegetative cells in stools of patients prior to and during the treatment of CDAD. We assessed the survival of vegetative cells on moist or dry surfaces in room air versus anaerobic conditions and in human gastric contents, in pH-adjusted gastric contents, and in gastric contents from individuals receiving PPI therapy. Stool samples obtained from patients prior to the initiation of antibiotic treatment for C. difficile contained ∼10-fold more vegetative cells than spores. On dry surfaces, vegetative C. difficile cells died rapidly, whereas they remained viable for up to 6 h on moist surfaces in room air. Vegetative C. difficile cells had only marginal survival in gastric contents at low pH; adjustment to a pH of >5 resulted in survival similar to that in the phosphate-buffered saline control. The survival of vegetative C. difficile in gastric contents obtained from patients receiving PPIs was also increased at a pH of >5. The ability of the vegetative form of C. difficile to survive on moist surfaces and in gastric contents with an elevated pH suggests a potential mechanism by which PPI therapy could increase the risk of acquiring C. difficile.
Clostridium difficile is the principal causative agent of nosocomial diarrhea, causing a clinical spectrum of disease ranging from mild diarrhea to life-threatening colitis (17), leading to an increased length of hospitalization and an estimated $1.1 billion in health care costs annually (12). Disease transmission occurs via the ingestion of Clostridium difficile by a susceptible host, followed by intestinal colonization and toxin-mediated diarrhea. This process is believed to be mediated by the spore form of the pathogen. In addition to established risk factors, such as exposure to antibiotics, age, and underlying disease severity (13), several recent studies have reported an association between proton pump inhibitors (PPIs) and nosocomial (1, 3, 4, 32) or community-acquired (5) Clostridium difficile-associated diarrhea (CDAD). Other recent studies dispute this association (14, 15, 20, 22). The mechanism by which PPIs contribute to an increased risk of CDAD is unclear, because gastric acid does not kill C. difficile spores (21).
One potential explanation for the association between CDAD and PPIs could be that the vegetative form of C. difficile, which is killed by acid, plays a role in pathogenesis. Vegetative forms, thought to die quickly in aerobic conditions, cause infection in animal models and are shed in feces along with spores (2, 8, 17). If the vegetative form survives on surfaces in room air, it could be ingested by patients. Suppression of gastric acid production in these individuals may facilitate the survival of the vegetative bacteria. Furthermore, Wilson et al. demonstrated that spores germinate in the small bowel of hamsters and suggested that the bile salts, normally found in the duodenum, stimulate the transition of spores to vegetative cells (31). Bile salts are also present in the gastric contents, particularly among patients with gastroesophogeal reflux disease (19). As has been proposed by Dial et al., bile salts within the stomach may stimulate C. difficile spore germination and the reduced gastric acidity among patients taking PPIs may facilitate the survival of the resultant vegetative bacteria (5). We examined shedding of vegetative C. difficile cells in the stools of patients with CDAD and survival of vegetative cells in room air and tested the hypothesis that the vegetative form may survive exposure to gastric contents with reduced acidity.
MATERIALS AND METHODS
Patients.
The experimental protocol was approved by the Cleveland Veterans Affairs Medical Center's institutional review board. Patients with positive stool cytotoxin assays were identified through the clinical microbiology laboratory, and information regarding antibiotic treatment for CDAD was obtained by medical record review. Samples of gastric contents were obtained from adult patients with a nasogastric tube placed as a part of their hospital care. Informed consent was obtained, and information regarding the use of PPIs was obtained by medical record review.
Quantification of vegetative C. difficile cells versus spores in stool samples.
We performed alcohol shock to quantify the number of C. difficile spores and vegetative cells in clinical specimens (29). A small quantity of the stool (∼200 mg) from samples stored aerobically at 4°C for ≤48 h was brought into an anaerobic chamber (Coy Laboratories, Grass Lake, MI) and mixed with prereduced phosphate-buffered saline (PBS, pH 7.4) to form an emulsion, a portion of which was subsequently diluted 1:1 with either PBS or 100% ethanol. Aliquots were removed after 1 h, diluted serially in PBS, and plated onto prereduced cefoxitin-cycloserine-fructose agar (7) containing 0.1% taurocholic acid (30) and 5 mg/liter of lysozyme (28) for the enumeration of C. difficile cells. The plates were incubated at 37°C in the anaerobic chamber for 24 to 48 h. The lower limit of detection was ∼2.0 log10 CFU/ml.
C. difficile strains.
Four Clostridium difficile isolates were studied. The isolates of three strains were cultured from patients with CDAD in Cleveland and were characterized by restriction enzyme analysis (REA) typing (courtesy of D. Gerding). Two isolates (VA 17 and CC 20) were REA BI-type strains, and another (VA 11) was an REA J-type strain. BI strains that have acquired increased fluoroquinolone resistance have been associated with recent epidemics in North America and Europe (14, 16), and J strains were associated with epidemics of clindamycin-resistant C. difficile in the 1980s and 1990s and remain endemic in many areas (9). The fourth isolate was American Type Culture Collection (ATCC) strain ATCC 9689. The isolates were grown overnight in prereduced cefoxitin-cycloserine-fructose broth to a final concentration of 107 to 108 CFU/ml. The concentration of spores was determined by the alcohol shock method to be less than 0.01% (data not shown). Spore stocks were made as described previously and stored at 4°C until use (18).
Survival of vegetative C. difficile in room air.
To test their viability in room air, approximately 106 CFU of C. difficile spores or vegetative forms were inoculated onto glass slides or plated onto both prereduced and nonreduced nutrient-free agar. The glass slides or plates were removed from the anaerobic chamber to room air at ambient temperature for a predetermined length of time before being returned to the anaerobic chamber. C. difficile was recovered by applying a cotton-tipped swab soaked in PBS with 0.1% taurocholic acid to the surfaces and then streaking onto prereduced blood agar (Becton Dickinson BBL, Sparks, MD). The plates were incubated at 37°C in the anaerobic chamber for 24 to 48 h, and the growth of C. difficile assessed by using a semiquantitative scale: 1+, fewer than 30 scattered colonies; 2+, 30 to 100 scattered colonies, few touching; 3+, >100 crowded but distinct colonies; and 4+, lawn. Using this method, the lower limit of detection is approximately 103 to 104 CFU/ml (data not shown).
Survival of vegetative C. difficile in gastric contents.
Samples of gastric contents were obtained from patients either taking no gastric-acid-suppressive agents (n = 8) or taking a PPI (n = 8) and used within 2 h of collection or stored at −20°C; preliminary experiments demonstrated similar results with fresh and frozen samples (data not shown). C. difficile was not detected in any of the gastric-content samples at baseline (data not shown). The survival of vegetative C. difficile in unmodified gastric contents was assessed. To assess the impact of pH on vegetative-cell survival, the pH of the gastric contents was adjusted by the addition of concentrated HCl or NaOH. No adjustments were made to gastric contents obtained from patients receiving PPIs. Approximately 105 CFU of vegetative C. difficile was suspended in the gastric contents and incubated at 37°C in the anaerobic chamber. Aliquots were removed after 1 h, serially diluted in PBS, and plated onto prereduced blood agar for the enumeration of surviving C. difficile cells. The plates were incubated at 37°C in the anaerobic chamber for 24 to 48 h. The lower limit of detection was 1.0 log10 CFU/ml. For comparison, spores were treated in a similar fashion. The pH rose to approximately 7.4 when aliquots were diluted in PBS for quantitative counts after incubation.
Statistical analysis.
Statistical comparisons were calculated using Student's t test for two experimental groups and using a one-way analysis of variance for three or more groups (Tools for Science—Statistics, http://www.physics.csbsju.edu; Collegeville, MN). P < 0.05 was considered significant.
RESULTS
Quantification of vegetative C. difficile cells versus spores in stool samples.
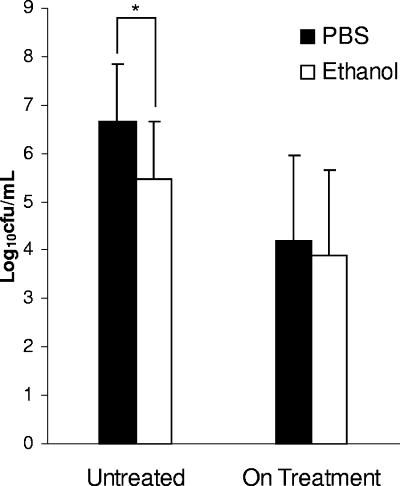
The relative quantities of C. difficile vegetative cells and spores in feces positive for cytotoxin were determined by comparing samples diluted in PBS (vegetative and spore forms) to those diluted in ethanol (spores only). Samples obtained from patients prior to the initiation of antibiotic treatment for C. difficile (n = 26) contained, on average, approximately 10-fold-more vegetative cells than spores (P < 0.001) (Fig. 1). Fecal samples from patients who had initiated treatment for CDAD (n = 16) yielded similar quantities of C. difficile forms from the PBS- and ethanol-treated samples (P = 0.63), indicating the presence of primarily spores.
FIG. 1.
Quantification of vegetative cells and spores (PBS) versus spores alone (ethanol) in cytotoxin-positive feces obtained from patients untreated (n = 26) or while on treatment (n = 16) for CDAD. Error bars indicate SDs. *, P < 0.001.
Survival of vegetative C. difficile in room air.
With data to suggest that vegetative C. difficile may be shed into the environment, we next addressed the viability of these obligate anaerobes in room air. Vegetative cells died within 15 min of inoculation onto glass slides in room air or under anaerobic conditions at 37°C, which corresponded with the drying of the aqueous suspension used as a vehicle for transferring the bacteria. To test the hypothesis that the rapid death was due to desiccation, similar experiments were performed on nutrient-free agar. Vegetative C. difficile routinely survived for up to 3 h in room air on both prereduced and nonreduced nutrient-free agar, with some sporadic survival detected even at 12 h (Table 1). When incubated on nutrient-free agar inside the anaerobic chamber, vegetative cells did not show a population decline for 12 to 24 h (data not shown). Spores survived in aerobic and anaerobic environments for 24 h on glass slides and nutrient-free agar.
TABLE 1.
Survival of vegetative C. difficile on nutrient-free agara
| Time (h:min) | ATCC 9689b
|
VA 11
|
VA 17
|
CC 20
|
||||
|---|---|---|---|---|---|---|---|---|
| Aerobicc | Anaerobicd | Aerobic | Anaerobic | Aerobic | Anaerobic | Aerobic | Anaerobic | |
| 0 | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ |
| 0:15 | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ |
| 0:30 | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 3+ | 4+ |
| 1:00 | 3+ | 4+ | 4+ | 4+ | 4+ | 4+ | 2+ | 4+ |
| 2:00 | 4+ | 4+ | 4+ | 4+ | 4+ | 4+ | 3+ | 4+ |
| 3:00 | 2+ | 4+ | 4+ | 4+ | 3+ | 4+ | 1+ | 4+ |
| 6:00 | 4+ | 1+ | 4+ | 1+ | 4+ | 4+ | ||
| 9:00 | 4+ | 4+ | 4+ | 4+ | ||||
1+, fewer than 30 scattered colonies; 2+, 30 to 100 scattered colonies, few touching; 3+, >100 crowded but distinct colonies; 4+, lawn.
Strain of C. difficile.
Room air at ambient temperature.
Anaerobic chamber at 37°C.
Survival of vegetative C. difficile in gastric contents.
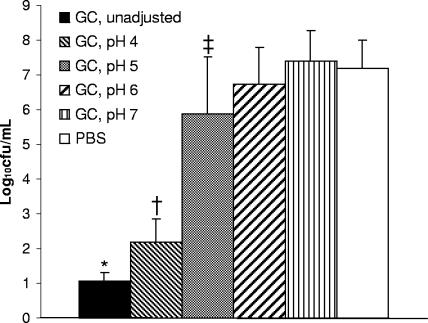
Vegetative C. difficile cells incubated in unadjusted gastric contents of patients not receiving acid-suppressive therapy (mean pH ± standard deviation [SD], 1.77 ± 0.66) had minimal rates of survival compared to that of those incubated in PBS (P < 0.0001) (Fig. 2). Reducing the acidity of gastric contents resulted in a modest increase in the rate of survival of vegetative C. difficile at pH 4 (P < 0.0001 versus rate of survival in unadjusted gastric contents) and a substantial rate of survival at pH 5 (P < 0.0001 versus rate of survival in gastric contents at pH 4). The survival rates of the pathogen at pH 6 and 7 were similar to that in PBS. C. difficile spores incubated in unadjusted gastric contents for 3 h showed no difference in rates of survival compared to the rate of survival in the PBS control (mean ± SD, 6.88 ± 0.96 and 6.17 ± 0.88 log10 CFU/ml, respectively).
FIG. 2.
Survival rates of vegetative C. difficile after 1 h of exposure to unadjusted gastric contents (GC), to gastric contents adjusted to pH 4 to 7, or to PBS. Pooled data from four strains of C. difficile are shown. Gastric contents that were grossly bilious were excluded. Error bars indicate SDs. *, P < 0.0001 versus survival rate in gastric contents at pH 4; †, P < 0.0001 versus survival rate in gastric contents at pH 5; ‡, P < 0.0001 versus survival rates in gastric contents at pH 6 and 7 and in PBS.
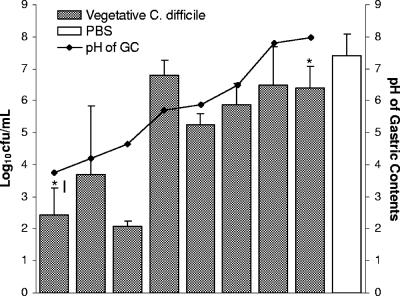
The survival of vegetative C. difficile in gastric contents obtained from patients receiving PPIs increased as the pH rose (Fig. 3). As was demonstrated in the experiments in which the pH of the gastric contents was adjusted, the rate of survival of vegetative cells in gastric contents with a pH of <5 (mean ± SD, 2.73 ± 1.41 log10 CFU/ml; n = 3) was significantly reduced in comparison to the rate of survival in gastric contents with a pH of >5 (mean ± SD, 6.16 ± 0.86 log10 CFU/ml; n = 5; P < 0.0001).
FIG. 3.
Survival rates of vegetative C. difficile after 1 h of exposure to gastric contents (GC) from individuals taking PPIs. Results for individual donors are arranged by increasing pH of gastric contents. Pooled data from four strains of C. difficile are shown. Error bars indicate SDs. An asterisk denotes a grossly bilious sample.
DISCUSSION
Spores are generally regarded as the major mode of transmission of C. difficile because of their ability to persist for months on environmental surfaces (11). The vegetative form of C. difficile has not been considered important, in part because previous work with a nonsporulating strain demonstrated death within 15 min of exposure to room air on a dry glass plate (2). Here, we show that four strains of vegetative C. difficile remained viable on moist, but not dry, surfaces for up to 3 h in room air, suggesting that the rapid death observed in the previous study was due to desiccation (2). Our data suggest that moist surfaces in hospitals may provide a suitable setting for vegetative C. difficile to persist for several hours. Such surfaces could include toilets and their immediate surroundings, sinks, food trays, beds, linens, moist dressings, and even skin. While most work has appropriately examined environmental contamination by spores, future studies to identify environmental contamination by vegetative C. difficile are indicated.
We detected significant quantities of the vegetative form of C. difficile shed in patient feces prior to the initiation of treatment for CDAD. A study by Freeman and Wilcox demonstrated that vegetative C. difficile remained viable for up to 56 days when inoculated into fecal emulsions buffered with PBS and stored aerobically at 4°C (6). The ability of the vegetative form to persist for several weeks in that study is unexplained but may be related to cooler storage conditions, the buffering of specimens with PBS, or an anaerobic microenvironment created in the stool by other bacteria. In contrast, Weese et al. found that the rate of recovery of C. difficile inoculated into unbuffered equine fecal samples was reduced significantly when the samples were stored aerobically versus anaerobically, presumably due to loss of vegetative cells (27). Freeman and Wilcox hypothesized that the buffering of the stool samples might account for the more prolonged survival of vegetative C. difficile in their study (6). In its vegetative form, C. difficile shed in the stools of untreated patients may persist for several hours on moist surfaces, long enough, potentially, to be transmitted.
Our finding that vegetative C. difficile survived exposure to gastric contents if the pH was greater than or equal to 5 suggests two potential mechanism by which PPIs could contribute to the pathogenesis of CDAD. First, individuals receiving acid-suppressive therapy would be particularly susceptible because reduced acidity would allow the organisms to survive exposure to stomach contents. A previous study examining gastric contents from a patient with pernicious anemia also demonstrated survival of vegetative C. difficile at a pH of ≥5 (8). Second, other investigators have proposed a mechanism by which PPIs could facilitate colonization by spores (3, 5, 32). Spores are highly resistant to acid, and it has been suggested that suppression of gastric acid is unlikely to impact upon the natural course of infection due to ingested C. difficile spores (20). However, if ingested spores germinate in the stomach, the newly emerged vegetative forms may survive in gastric contents with a higher pH, leading to increased numbers of actively dividing C. difficile cells reaching and colonizing the intestinal tracts of susceptible individuals. Our data demonstrate that suppression of gastric acid would permit the survival of germinated C. difficile cells. This finding is consistent with the results of other studies correlating reduced acidity (pH > 3) and increased bacterial growth in gastric contents from people taking PPIs (10, 23, 25, 26). Because conditions in the stomach are not as anaerobic or reduced as in the colon, the ability of vegetative C. difficile to survive under aerobic conditions could also facilitate the survival of germinated spores in the stomach.
Neither the timing of nor the signals triggering C. difficile spore germination, particularly after ingestion, are well understood. In their seminal work using a hamster model, Wilson et al. demonstrated that within 1 h of intragastric administration, nearly 80% of C. difficile spores had germinated, with the majority of organisms detected in the small intestine (31). They suggested that germination occurred in the small intestine of hamsters upon exposure to bile salts. It is notable that bile salts are commonly detected in the esophageal aspirates of patients with gastroesophogeal reflux disease (19), a common malady, with 4 to 7% of the adult patients experiencing daily symptoms (24). If bile salts within gastric contents stimulate germination, the resultant vegetative form of C. difficile will be more likely to survive and cause infection in individuals receiving PPI therapy for gastroesophogeal reflux disease. In this study, while we did not specifically assay for the presence of bile acid in gastric contents, two grossly bilious samples were among the eight samples collected from patients with nasogastric tubes taking PPIs. Notably, bile salts stimulate C. difficile germination in vitro, an interesting characteristic that we are currently investigating (30).
In summary, we found that the vegetative form of C. difficile was shed in feces in significant quantities prior to the initiation of treatment for CDAD and remained viable in room air on moist surfaces and in gastric contents with elevated pH. Future work is needed to examine the survival of vegetative C. difficile in the hospital environment and to assess the germination of spores in gastric contents, including the potential role of bile acids.
Acknowledgments
This work was supported by an advanced research career development award from the Department of Veterans Affairs (C.J.D.).
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Al-Tureihi, F. I., A. Hassoun, G. Wolf-Klein, and H. Isenberg. 2005. Albumin, length of stay, and proton pump inhibitors: key factors in Clostridium difficile-associated disease in nursing home patients. J. Am. Med. Dir. Assoc. 6:105-108. [DOI] [PubMed] [Google Scholar]
- 2.Buggy, B. P., K. H. Wilson, and R. Fekety. 1983. Comparison of methods for recovery of Clostridium difficile from an environmental surface. J. Clin. Microbiol. 18:348-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham, R., B. Dale, B. Undy, and N. Gaunt. 2003. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J. Hosp. Infect. 54:243. [DOI] [PubMed] [Google Scholar]
- 4.Dial, S., J. A. Delaney, V. Schneider, and S. Suissa. 2006. Proton pump inhibitor use and risk of community-acquired Clostridium difficile-associated disease defined by prescription for oral vancomycin therapy. Can. Med. Assoc. J. 175:745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dial, S., J. A. C. Delaney, A. N. Barkun, and S. Suissa. 2005. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA 294:2989-2995. [DOI] [PubMed] [Google Scholar]
- 6.Freeman, J., and M. H. Wilcox. 2003. The effects of storage conditions on viability of Clostridium difficile vegetative cells and spores and toxin activity in human faeces. J. Clin. Pathol. 56:126-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.George, W. L., V. L. Sutter, D. Citron, and S. M. Finegold. 1979. Selective and differential medium for isolation of Clostridium difficile. J. Clin. Microbiol. 9:214-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurian, L., T. T. Ward, and R. M. Katon. 1982. Possible foodborne transmission in a case of pseudomembranous colitis due to Clostridium difficile: influence of gastrointestinal secretions on Clostridium difficile infection. Gastroenterology 83:465-469. [PubMed] [Google Scholar]
- 9.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. I. Rood, P. DeGirolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 10.Karmeli, Y., R. Stalnikowitz, R. Eliakim, and G. Rahav. 1995. Conventional dose of omeprazole alters gastric flora. Dig. Dis. Sci. 40:2070-2073. [DOI] [PubMed] [Google Scholar]
- 11.Kim, K. H., R. Fekety, D. H. Batts, D. Brown, M. Cudmore, J. Silva, Jr., and D. Waters. 1981. Isolation of Clostridium difficile from the environment and contacts of patients with antibiotic-associated colitis. J. Infect. Dis. 143:42-50. [DOI] [PubMed] [Google Scholar]
- 12.Kyne, L., M. B. Hamel, R. Polavaram, and C. P. Kelly. 2002. Health care costs and mortality associated with nosocomial diarrhea due to Clostridium difficile. Clin. Infect. Dis. 34:346-353. [DOI] [PubMed] [Google Scholar]
- 13.Kyne, L., S. Sougioultzis, L. V. McFarland, and C. P. Kelly. 2002. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect. Control Hosp. Epidemiol. 23:653-659. [DOI] [PubMed] [Google Scholar]
- 14.Loo, V. G., L. Poirier, M. A. Miller, M. Oughton, M. D. Libman, S. Michaud, A. M. Bourgault, T. Nguyen, C. Frenette, M. Kelly, A. Vibien, P. Brassard, S. Fenn, K. Dewar, T. J. Hudson, R. Horn, P. Rene, Y. Monczak, and A. Dascal. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 15.Lowe, D. O., M. M. Mamdani, A. Kopp, D. E. Low, and D. N. Juurlink. 2006. Proton pump inhibitors and hospitalization for Clostridium difficile-associated disease: a population-based study. Clin. Infect. Dis. 43:1272-1276. [DOI] [PubMed] [Google Scholar]
- 16.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433-2441. [DOI] [PubMed] [Google Scholar]
- 17.McDonald, L. C., M. Owings, and D. B. Jernigan. 2006. Clostridium difficile infection in patients discharged from US short-stay hospitals, 1996-2003. Emerg. Infect. Dis. 12:409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merrigan, M. M., S. P. Sambol, S. Johnson, and D. N. Gerding. 2003. Prevention of fatal Clostridium difficile-associated disease during continuous administration of clindamycin in hamsters. J. Infect. Dis. 188:1922-1927. [DOI] [PubMed] [Google Scholar]
- 19.Nehra, D., P. Howell, C. P. Williams, J. K. Pye, and J. Beynon. 1999. Toxic bile acids in gastro-oesophageal reflux disease: influence of gastric acidity. Gut 44:598-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pepin, J., N. Saheb, M. A. Coulombe, M. E. Alary, M. P. Corriveau, S. Authier, M. Leblanc, G. Rivard, M. Bettez, V. Primeau, M. Nguyen, C. E. Jacob, and L. Lanthier. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 41:1254-1260. [DOI] [PubMed] [Google Scholar]
- 21.Rao, A., R. L. Jump, N. J. Pultz, M. J. Pultz, and C. J. Donskey. 2006. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob. Agents Chemother. 50:3901-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah, S., A. Lewis, D. Leopold, F. Dunstan, and K. Woodhouse. 2000. Gastric acid suppression does not promote clostridial diarrhoea in the elderly. Q. J. Med. 93:175-181. [DOI] [PubMed] [Google Scholar]
- 23.Sharma, B. K., I. A. Santana, E. C. Wood, R. P. Walt, M. Pereira, P. Noone, P. L. Smith, C. L. Walters, and R. E. Pounder. 1984. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br. Med. J. Clin. Res. 289:717-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonnenberg, A. 1994. Esophogeal disease, p. 299-355. In J. E. Everhart (ed.), Digestive diseases in the United States: epidemiology and impact—NIH publication no. 94-1447. National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Washington, DC.
- 25.Thorens, J., F. Froehlich, W. Schwizer, E. Saraga, J. Bille, K. Gyr, P. Duroux, M. Nicolet, B. Pignatelli, A. L. Blum, J. J. Gonvers, and M. Fried. 1996. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut 39:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdu, E., F. Viani, D. Armstrong, R. Fraser, H. H. Siegrist, B. Pignatelli, J. P. Idstrom, C. Cederberg, A. L. Blum, and M. Fried. 1994. Effect of omeprazole on intragastric bacterial counts, nitrates, nitrites, and N-nitroso compounds. Gut 35:455-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weese, J. S., H. R. Staempfli, and J. F. Prescott. 2000. Survival of Clostridium difficile and its toxins in equine feces: implications for diagnostic test selection and interpretation. J. Vet. Diagn. Investig. 12:332-336. [DOI] [PubMed] [Google Scholar]
- 28.Wilcox, M. H., W. N. Fawley, and P. Parnell. 2000. Value of lysozyme agar incorporation and alkaline thioglycollate exposure for the environmental recovery of Clostridium difficile. J. Hosp. Infect. 44:65-69. [DOI] [PubMed] [Google Scholar]
- 29.Willey, S. H., and J. G. Bartlett. 1979. Cultures for Clostridium difficile in stools containing a cytotoxin neutralized by Clostridium sordellii antitoxin. J. Clin. Microbiol. 10:880-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson, K. H. 1983. Efficiency of various bile salt preparations for stimulation of Clostridium difficile spore germination. J. Clin. Microbiol. 18:1017-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson, K. H., J. N. Sheagren, and R. Freter. 1985. Population dynamics of ingested Clostridium difficile in the gastrointestinal tract of the Syrian hamster. J. Infect. Dis. 151:355-361. [DOI] [PubMed] [Google Scholar]
- 32.Yearsley, K. A., L. J. Gilby, A. V. Ramadas, E. M. Kubiak, D. L. Fone, and M. C. Allison. 2006. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment. Pharmacol. Ther. 24:613-619. [DOI] [PubMed] [Google Scholar]