Abstract
Lipopolysaccharide (LPS), or endotoxin, a structural component of gram-negative bacterial outer membranes, plays a key role in the pathogenesis of septic shock, a syndrome of severe systemic inflammation which leads to multiple-system organ failure. Despite advances in antimicrobial chemotherapy, sepsis continues to be the commonest cause of death in the critically ill patient. This is attributable to the lack of therapeutic options that aim at limiting the exposure to the toxin and the prevention of subsequent downstream inflammatory processes. Polymyxin B (PMB), a peptide antibiotic, is a prototype small molecule that binds and neutralizes LPS toxicity. However, the antibiotic is too toxic for systemic use as an LPS sequestrant. Based on a nuclear magnetic resonance-derived model of polymyxin B-LPS complex, we had earlier identified the pharmacophore necessary for optimal recognition and neutralization of the toxin. Iterative cycles of pharmacophore-based ligand design and evaluation have yielded a synthetically easily accessible N1,mono-alkyl-mono-homologated spermine derivative, DS-96. We have found that DS-96 binds LPS and neutralizes its toxicity with a potency indistinguishable from that of PMB in a wide range of in vitro assays, affords complete protection in a murine model of LPS-induced lethality, and is apparently nontoxic in vertebrate animal models.
Endotoxin, or lipopolysaccharide (LPS), a structural component of the outer membrane of most gram-negative bacteria (31), plays a pivotal role in septic shock, a syndrome of systemic toxicity which occurs frequently as a sequel to serious systemic gram-negative infections (23). The activation by LPS of the innate immune response, mediated via toll-like receptor 4 (TLR4) (39), leads to a dysregulated production of numerous inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-6 (11), gamma interferon (IFN-γ), and IL-12, which appears to be inadequately compensated for by the production of anti-inflammatory cytokines, such as IL-10 and transforming growth factor β (6). The resultant systemic inflammatory response progresses to the frequently fatal syndrome of multiple-system organ failure (3). Despite continuing advances in antimicrobial chemotherapy, the incidence of sepsis has risen almost threefold from 1979 through 2000 (25), emphasizing an urgent, unmet need to develop therapeutic options specifically targeting the pathophysiology of sepsis.
The toxicity of LPS resides in its structurally highly conserved glycolipid component called lipid A (22), which is composed of a hydrophilic, bis-phosphorylated diglucosamine backbone, and a hydrophobic domain comprised of acyl chains in amide and ester linkages (14). Polymyxin B (PMB) is a membrane-active peptide antibiotic (37) known to sequester LPS and abrogate its toxicity (12, 16). The oto- and nephrotoxicity of PMB limit its systemic use and have led to the development of an extracorporeal hemoperfusion cartridge based on PMB covalently immobilized on a polystyrene-based fiber (Toraymyxin; Toray Industries Inc., Tokyo, Japan) (40). Approved for clinical use in Japan in late 2000, Toraymyxin provides a clinically validated proof-of-concept of the therapeutic potential of sequestering circulating LPS.
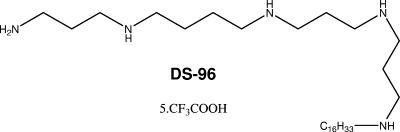
Based on a nuclear magnetic resonance-derived model of PMB-lipid A complex (2), we had earlier identified the pharmacophore necessary for optimal recognition and neutralization of lipid A (reviewed in reference 8). Iterative cycles of pharmacophore-based ligand design have yielded an N1,mono-alkyl-mono-homologated spermine derivative, DS-96 (Fig. 1), which binds lipid A and neutralizes its toxicity with a potency indistinguishable from that of PMB, affords complete protection in a murine model of LPS-induced lethality, and is nontoxic for vertebrate animal models.
FIG. 1.
The chemical structure of DS-96 [N1-(3-aminopropyl)-N4-(3-(3-(hexadecylamino)-propylamino)propyl)butane-1,4-diamine; pentatrifluoroacetate salt].
MATERIALS AND METHODS
Reagents.
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. LPS from Escherichia coli O111:B4 and O55:B5 (Ra chemotypes) and E. coli k12 D31m4 (Re chemotype) was purchased from List Biological Laboratories (Campbell, CA). LPS from Salmonella enterica serovar Abortus equi, Vibrio cholerae, and Pseudomonas aeruginosa were procured from Sigma Chemicals (St. Louis, MO). The fluorescent probe, BODIPY-TR-cadaverine (5-(((4-(4,4-difluoro-5-(2-thienyl)-4-bora-3a,4a-diaza-s-indacene-3-yl) phenoxy)acetyl)amino)pentylamine hydrochloride) (BC), was obtained from Molecular Probes/Invitrogen (Carlsbad, CA).
Binding affinity measurements.
The BC displacement assay for quantifying binding affinity has been described previously (5, 43, 44). Briefly, to the first column (16 wells) of a Corning Nonbinding Surface 384-well flat-bottom black fluorescence microplate 80-μl aliquots of 1 mM stock solutions of DS-96 or PMB (reference compound) were added in quadruplicate and were serially diluted twofold across the remaining 23 columns in 50 mM Tris buffer, pH 7.4, using a Precision 2000 automated microplate pipetting system, achieving a final dilution of 0.112 nM in a volume of 40 μl. Forty-microliter aliquots of a mixture of 50 μg/ml of LPS and 5 μM BC in buffer were added to each well of the plate using the Precision 2000 instrument. Fluorescence measurements were made at 25°C on a SpectraMax M2 multifunction plate reader (Molecular Devices, Sunnyvale, CA) with excitation and emission wavelengths at 580 and 620 nm, respectively. Relative binding affinities were measured as the effective displacement of 50% of bound probe (ED50) using standard four-parameter logistic curve fitting subroutines in Origin version 7.0 (OriginLab, Northampton, MA). E. coli O111:B4 smooth LPS was used as the ligand in the BC displacement experiment and as stimuli in all in vitro activity assays that follow, except in experiments designed to test whether DS-96 neutralized LPS isolated from diverse, non-E. coli gram-negative bacteria (described in relation to NF-κB assay, below).
Measurement of NO release in murine macrophages.
Nitric oxide (NO) was measured as total nitrite in murine macrophage J774.A1 cells using the Griess reagent system (17, 18) as described previously (5, 26). J774.A1 cells were grown in RPMI-1640 cell culture medium containing l-glutamine and sodium bicarbonate and supplemented with 10% fetal bovine serum, 1% l-glutamine-penicillin-streptomycin solution, and 200 μg/ml l-arginine at 37°C in a 5%-CO2 atmosphere, plated at ∼105 cells/ml in a volume of 80 μl/well in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluence, and subsequently stimulated with 10 ng/ml LPS. Concurrent with LPS stimulation, serially diluted concentrations of test compounds were added to the cell medium and left to incubate overnight for 16 h. PMB was used as the reference compound in each plate. Positive (LPS stimulation only) and negative controls (J774.A1 medium only) were included in each experiment. Nitrite concentrations were measured, adding 50 μl of supernatant to equal volumes of Griess reagents (50 μl/well; 0.1% NED solution in double-distilled water and 1% sulfanilamide-5% phosphoric acid solution in double-distilled water) and incubating for 15 min at room temperature in the dark. Absorbance at 535 nm was measured using a Molecular Devices Spectramax M2 multifunction plate reader. Nitrite concentrations were interpolated from standard curves obtained from serially diluted sodium nitrite standards.
Multiplexed cytokine assay ex vivo with human blood and with in vivo murine blood.
One-hundred-microliter aliquots of fresh whole blood, anticoagulated with EDTA, obtained by venipuncture from healthy human volunteers with informed consent and as per guidelines approved by the Human Subjects Experimentation Committee, was exposed to an equal volume of 20 ng/ml of E. coli 0111:B4 LPS, with graded concentrations of test compounds diluted in saline for 4 h in a 96-well microtiter plate as described previously (5, 26). The effect of the compounds on modulating cytokine production was examined using a FACSArray multiplexed flow-cytometric bead array (CBA) system (Becton-Dickinson-Pharmingen, San Jose, CA). The system uses a sandwich enzyme-linked immunosorbent assay-on-a-bead principle (7, 15) and is comprised of six populations of microbeads that are spectrally unique in terms of their intrinsic fluorescence emission intensities (detected in the FL3 channel of a standard flow cytometer). Each bead population is coated with a distinct capture antibody to detect six different cytokines concurrently from biological samples (the human inflammation CBA kit includes the following analytes: TNF-α, IL-1β, IL-6, IL-8, IL-10, and IL-12p70). The beads are incubated with 30 μl of sample, and the cytokines of interest are first captured on the bead. After the beads are washed, a mixture of optimally paired second antibodies conjugated to phycoerythrin is added, which then forms a fluorescent ternary complex with the immobilized cytokine, the intensity (measured in the FL2 channel) of which is proportional to the cytokine concentration on the bead. The assay was performed according to protocols provided by the vendor. Standard curves were generated using recombinant cytokines provided in the kit. The data were analyzed using the CBA software suite that is integral to the FACSArray system. The CBA multiplexed assay was also used to quantify cytokine production in mouse blood samples (see below) using the mouse inflammation CBA kit, which includes the following analytes: TNF-α, IL-6, IL-10, macrophage chemotactic protein 1, IFN-γ, and IL-12p70.
Inhibition of LPS-induced NF-κB induction.
The inhibition of induction of NF-κB (a key transcriptional activator of the innate immune system) was quantified using human embryonic kidney 293 cells cotransfected with TLR4 (LPS receptor) and CD14 and MD2 (coreceptors), available from InvivoGen, Inc. (HEK-Blue; San Diego, CA), as described elsewhere (24). Stable expression of secreted alkaline phosphatase (seAP) under control of the NF-κB/AP-1 promoters is inducible by LPS, and extracellular seAP in the supernatant is proportional to NF-κB induction. HEK-4 cells were incubated at a density of ∼105 cells/ml in a volume of 80 μl/well in 384-well, flat-bottomed, cell culture-treated microtiter plates until confluence was achieved and subsequently stimulated with 10 ng/ml LPS. Concurrent with LPS stimulation, serially diluted concentrations of test compounds were added to the cell medium using a rapid-throughput, automated protocol employing a Bio-Tek P2000 liquid handler as described above and left to incubate overnight. PMB was used as a reference compound in each plate. Positive (LPS stimulation only) and negative controls (HEK detection medium only) were included in each experiment. seAP was assayed spectrophotometrically using an alkaline phosphatase-specific chromogen (present in HEK detection medium as supplied by the vendor) at 620 nm.
Phosflow flow cytometric assay for p38 MAPK.
One-milliliter aliquots of fresh whole blood, anticoagulated with heparin (obtained by venipuncture from healthy human volunteers with informed consent and as per guidelines approved by the Human Subjects Experimentation Committee), were incubated with 25 μl of a mix of 8 μg/ml of E. coli 0111:B4 LPS and graded concentrations of test compounds diluted in saline (typically serially diluted from 80 μM) for 15 min at 37°C. This resulted in final concentrations of 100 ng/ml of LPS and 1 nM of compound (at the lowest dilution). Positive (LPS alone) and negative (saline) controls were included in each experiment. Erythrocytes were lysed and leukocytes were fixed in one step by mixing 200 μl of the samples in 4 ml prewarmed Whole Blood Lyse/Fix buffer (Becton-Dickinson Biosciences, San Jose, CA). After the cells were washed at 500 × g for 8 min in CBA buffer, the cells were permeabilized in ice-cold methanol for 30 min, washed twice in CBA buffer, transferred to a Millipore MultiScreen BV 1.2μ filter plate, and stained with either phycoerythrin (PE)-conjugated mouse anti-p38 mitogen-activated protein kinase (MAPK) (pT180/pY182; BD Biosciences) monoclonal antibody or a matched PE-labeled mouse immunoglobulin G1 κ isotype control monoclonal antibody for 60 min. The cells were washed twice in the plate by aspiration as per protocols supplied by the vendor. Cytometry was performed using a BD FACSArray instrument in the single-color mode for PE acquisition on 20,000 gated events. Postacquisition analyses were performed using FlowJo v 7.0 software (Treestar, Ashland, OR).
Murine in vivo experiments.
Dose-response and time course experiments with a d-galactosamine-sensitized mouse model of endotoxic shock were performed as described elsewhere (5, 26) using a supralethal dose (twice the 100% lethal dose (LD100), i.e., 200 ng/mouse) of LPS. Female, outbred, 9- to 11-week-old CF-1 mice (Charles River, Wilmington, MA) weighing 22 to 28 g were used in all studies. In some experiments, animals were bled by terminal cardiac puncture under anesthesia at various times following LPS/compound administration for cytokine analyses using the CBA assay (Mouse Inflammation CBA kit; BD Biosciences) as described above. For toxicity studies, mice received DS-96 (40 mg/kg of body weight) dissolved in 0.2 ml isotonic saline containing human serum albumin at 66 mg/ml subcutaneously in the lower flank in a volume of 0.2 ml, each day for 5 days (200 mg/kg DS-96, total dose), and were monitored for abnormal external characteristics. Blood was harvested on day 6 via cardiac puncture under anesthesia into heparin tubes and centrifuged at 4,000 rpm for 10 min. Plasma samples were submitted for testing to University of Missouri Research Animal Diagnostic Laboratory (Columbia, MO) and were also assayed for polyamine levels. The statistical significance of lethality data was analyzed by using the χ2 test.
Assay for plasma polyamine levels in mice exposed to high-dose DS-96.
One-hundred-microliter aliquots of either control plasma or DS-96-treated plasma were placed in separate Eppendorf tubes to which an equal volume of ice-cold 10% trichloroacetic acid was added to precipitate proteins. After incubation at 4°C for 10 min, the tubes were centrifuged at 4°C at 13,000 rpm for 25 min and then dried in a Speed-Vac concentrator (Thermo Electron Corporation) for 1 h at 43°C. The precipitate was resuspended in 100 μl of a solution of 13.8 mM fluorescamine (38) in methanol and alkalinized by adding 20 μl of 1 M NaOH. A 1:10 dilution in methanol was injected into an Agilent Stable Bond C18 column (3.0 mm by 150 mm) on a Shimadzu LC-10 HPLC instrument. Peaks were detected using an SPD-M10 VP diode array detector (λmax, 380 nm) and an RF-10Axl fluorescence detector (λex = 380 nm; λem = 464 nm). Initial column conditions were 0.1% trifluoroacetic acid in 10% acetonitrile (CH3CN) in 0.1% trifluoroacetic acid-H2O, increasing to 100% CH3CN over 30 min. The retention times of fluorescamine-labeled standards (spermine, spermidine, and putrescine) were 14.292 min, 16.30 min, and 11.267 min, respectively.
RESULTS AND DISCUSSION
Design rationale and synthesis of DS-96.
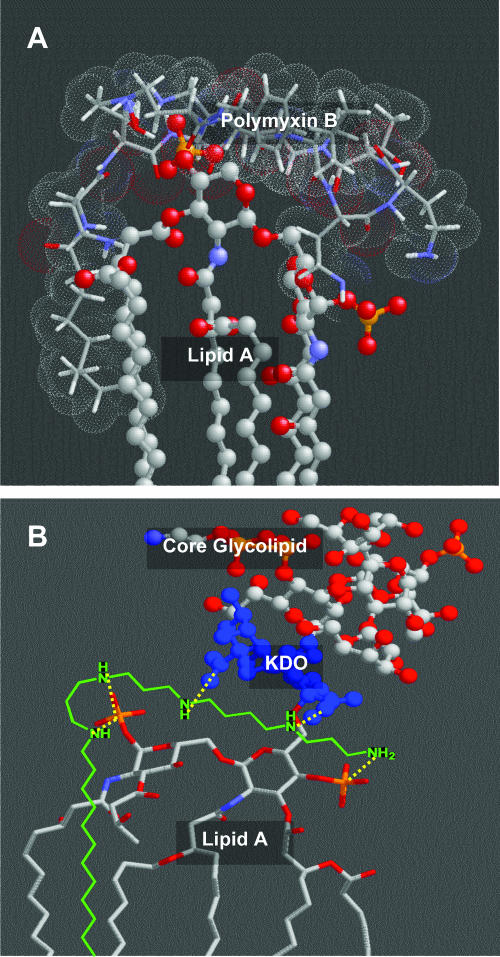
Our identification of the lipopolyamines as potential endotoxin-sequestering molecules was based on two simple heuristics which have been experimentally tested and validated (26): (i) an optimal distance of ≈14 Å is necessary between protonatable functions in linear bis-cationic molecules for simultaneous ionic interactions with the anionic phosphates on lipid A (Fig. 2A), and (ii) additional, appropriately positioned hydrophobic group(s) are obligatory for the interaction to manifest in neutralization of endotoxicity. Leads obtained from high-throughput screening on focused libraries (4, 44, 45) and from molecular modeling studies and in silico docking studies (19) suggested that extension of the spermine backbone and the introduction of an additional, appropriately positioned amine on the scaffold would allow favorable salt bridges with the lipid A phosphate groups, analogous to that observed in complexes of lipid A and PMB (2), as well as additional H-bond interactions with the 3-deoxy-d-manno-octulosonic acid (KDO) sugars (30) in the inner-core region of LPS (Fig. 2B). In recent structure-activity relationship studies, we had determined that C16 hydrophobic groups are optimal for effective LPS sequestration (5).
FIG. 2.
(A) A nuclear magnetic resonance-derived model of the PMB (stick representation with van der Waals surface)-lipid A (ball-and-stick) complex (2). Bidentate salt bridges (dotted lines) are postulated to occur between each phosphate group on lipid A and two pairs of the γ-NH2 groups of Dab residues (2). The poly-acyl domain of lipid A is shown truncated. (B) Molecular-modeling-derived geometry of the complex between LPS and DS-96. The atomic coordinates of LPS were derived from its crystal structure (14). The mono-homologated spermine backbone is predicted to form salt bridges with both phosphate groups on lipid A, as well as participating in additional ionic H bonds with the inner core KDO sugars.
Binding affinity and in vitro neutralization activity.
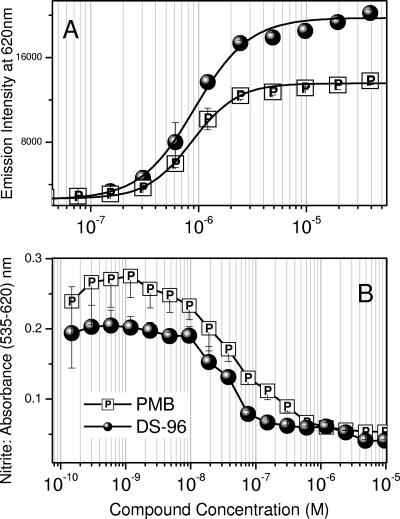
We first examined quantitatively the binding affinities of DS-96 and PMB to LPS with an automated fluorescence displacement assay using BC (43). The ED50 values for DS-96 and PMB were found to be virtually identical: 1.2 ± 0.16 μM and 1.31 ± 0.12 μM, respectively (Fig. 3A). Murine but not human monocytes produce measurable quantities of NO, an important surrogate marker of immune activation by bacterial products (42). DS-96 and PMB inhibited NO production in a concentration-dependent manner in murine J774A.1 cells stimulated with 10 ng/ml LPS, with the 50% inhibitory concentrations (IC50s) for DS-96 and PMB being 21 ± 3 nM and 27 ± 2 nM, respectively (Fig. 3B).
FIG. 3.
(A) Binding affinities of DS-96 and PMB (reference compound) to E. coli O111:B4 LPS determined by BODIPY-cadaverine displacement assay. The ED50s for the two compounds are 1.2 ± 0.16 μM and 1.31 ± 0.12 μM, respectively. (B) Inhibitory activity of NO production by DS-96 and PMB in LPS-stimulated murine J774 macrophage cells. The IC50s for DS-96 and PMB are, respectively, 21 ± 3 nM and 27 ± 2 nM.
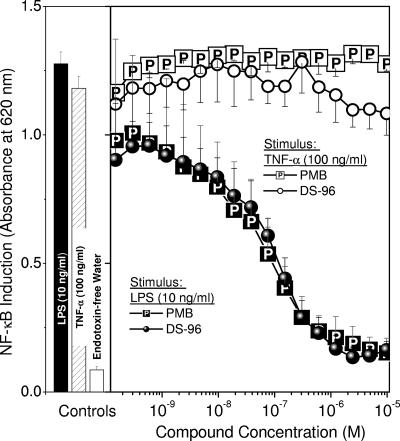
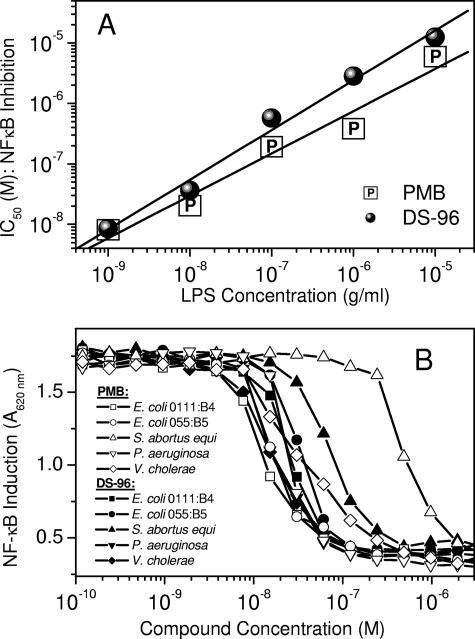
The nuclear translocation of NF-κB is a key transcriptional activation event that occurs in response to noxious stimuli. NF-κB induction is upstream of a plethora of signaling cascades, which include the production of cytokines and other proinflammatory molecules. True sequestration of LPS should therefore result in the inhibition not only of NF-κB induction but also of downstream cellular responses. Furthermore, a compound that specifically antagonizes the effects of LPS should cause inhibition of NF-κB induction and cognate signaling events as a response to LPS but not other stimuli. We used HEK-Blue (Invivogen, San Diego, CA) cells (human embryonic kidney 293 cells stably transfected with a seAP reporter gene under the control of NF-κB/AP-1 promoters along with the LPS receptor [TLR4] and coreceptors [CD-14 and MD-2]) (24). DS-96 and PMB inhibited LPS-induced NF-κB induction with virtually indistinguishable IC50s (32 ± 2 nM) but showed no inhibitory effect on TNF-α-stimulated cells (Fig. 4). Specificity of action for both PMB and DS-96 could also be demonstrated by showing that the IC50s were inversely related to the stimulus (LPS) concentration in a Schild-type plot (Fig. 5A). Both compounds were also without effect on a range of non-LPS stimuli, such as TNF-α (Fig. 4), phorbol esters, ionomycin, and IL-1β (data not shown), demonstrating that the mechanism of action of PMB and DS-96 was by sequestration of LPS.
FIG. 4.
Inhibition of NF-κB reporter gene induction in HEK-293 cells stably transfected with TLR4/CD-14/MD-2/NF-κB-SEAP construct. Cells were stimulated with either LPS (10 ng/ml) or recombinant human TNF-α (100 ng/ml) and exposed to graded concentrations of test compounds. Both DS-96 and PMB inhibit LPS-stimulated NF-κB induction with identical potencies (IC50, 32 ± 2 nM) but show no effect on TNF-α-stimulated cells, showing specificity of action. Shown on the left are negative and positive (LPS alone and TNF-α alone) controls.
FIG. 5.
(A) Schild-type analysis of dependence of IC50 (NF-κB induction) of DS-96 and PMB on the dose of LPS used. HEK-4 cells were stimulated with escalating (1 ng/ml to 10 μg/ml) doses of E. coli O111:B4 LPS, preincubated with graded doses of either PMB or DS-96. IC50s for either compound were determined at each LPS stimulus dose. (B) Inhibition of NF-κB reporter gene induction by DS-96 and PMB in HEK-293 cells stimulated with 10 ng/ml LPS isolated from a wide variety of gram-negative bacteria. A stimulus of 10 ng/ml LPS was used. Note that the IC50s for both PMB and DS-96 are very similar, irrespective of the source of LPS. With serovar Abortus equi LPS as stimulus, DS-96 was observed to be more potent (IC50, 82 nM) than PMB (IC50, 562 nM).
An ideal LPS sequestrant of potential clinical utility should be one which binds to and neutralizes a wide range of endotoxins from different gram-negative bacteria. Although the lipid A portion of LPS is structurally highly conserved (32), substoichiometric substitutions on the phosphate group with aminoarabinose or phosphoethanolamine (27), as seen, for example, in Salmonella enterica serovar Typhimurium, can significantly alter the binding of cationic antimicrobials, such as PMB (21). We therefore examined if DS-96 would effectively neutralize a broad range of LPS (E. coli 0111:B4, E. coli 055:B5, serovar Abortus equi, P. aeruginosa, and V. cholerae) in the NF-κB assay. We found that this was indeed the case (Fig. 5B), with the IC50s being very similar for most LPS species (∼30 nM). Interestingly, we also observed that DS-96 was approximately eightfold more potent than PMB in inhibiting serovar Abortus LPS (Fig. 5B).
Ex vivo neutralization activity in human blood.
We sought to verify that the endotoxin-sequestering activity of DS-96 would be manifested in the milieu of whole human blood, characterized not only by its high ionic strength (∼300 mosmol), which attenuates electrostatic interactions (10), but also by near-millimolar concentrations of albumin. Albumin has been shown to bind both LPS (9) and DS-96 (unpublished data). Also present in human serum are a number of high-affinity LPS-binding proteins, such as soluble CD-14 (13) and LPS-binding protein (1). Furthermore, given the amphipathic nature of both LPS and DS-96, it is conceivable that substantial partitioning of both the target and the ligand into the lipoprotein constituents could occur, as has been observed with E5564, a lipid A receptor antagonist currently undergoing clinical trials (33). For these reasons and prior to initiating in vivo evaluation, we compared the effects of DS-96 and PMB in two ex vivo assays using whole human blood.
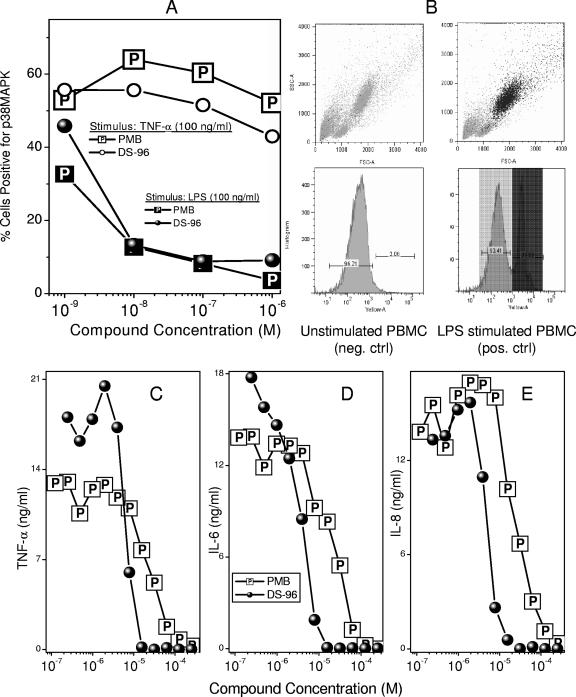
In the first, we examined the specificity and potency of DS-96 relative to those of PMB in inhibiting the phosphorylation of p38 MAPK, a key component of a phosphorylation cascade that is upstream of NF-κB (20). Exposure of whole blood obtained from healthy human volunteers to 100 ng/ml LPS results in a marked elevation in T180/Y182 dually phosphorylated p38 MAPK as probed by flow cytometry using a PE-labeled anti-phospho-p38 MAPK (T180/Y182) antibody (Fig. 6A). The activation seems to occur chiefly in polymorphonuclear (PMN) cells, since the positive population maps directly to the subset of cells adjudged to be PMN based on forward and side-scatter characteristics (Fig. 6B). The addition of either PMB or DS-96 concomitant with LPS exposure results in a concentration-dependent attenuation of p38 MAPK phosphorylation, with the potencies of both compounds being very similar. Notably, p38 MAPK phosphorylation induced by TNF-α is affected by neither compound, emphasizing again the specificity of action against LPS (Fig. 6A).
FIG. 6.
(A) Inhibition of phosphorylation of p38 MAP kinase in neutrophils in whole human blood (ex vivo), stimulated with either LPS (100 ng/ml) or human TNF-α (100 ng/ml) for 15 min in the presence of graded concentrations of DS-96 or PMB. Quantification of p38 MAPK was performed using flow cytometry. (B) Forward-scatter/side-scatter profile and the gating for p38 MAPK-negative and -positive gates obtained on unstimulated cells (negative control), respectively. Back gating on the p38 MAPK-positive cells (dark-shaded peak) maps to the polymorphonuclear population. (C to E) Inhibition of LPS-induced proinflammatory cytokine production in human blood. Whole human blood was stimulated with 100 ng/ml LPS preincubated with graded concentrations of either DS-96 or PMB. Cytokine levels were quantified using a multiplexed flow-cytometric bead array system (CBA). Only TNF-α, IL-6, and IL-8 levels were quantifiable; IL-10 and IL-12p70 levels were below detection limits, and signal-to-noise ratios for IL-1β were unacceptably high.
We next evaluated the efficacy of DS-96 in inhibiting proinflammatory cytokine release in whole human blood, using a multiplexed cytokine detection system (26). DS-96 inhibits TNF-α, IL-6, and IL-8 production with an IC50 of 3 to 5 μM (an LPS stimulus of 100 ng/ml was used), which is considerably lower than that of PMB (11 to 13 μM) (Fig. 6C to E). As controls, we used phorbol myristate acetate (100 ng/ml) plus ionomycin (1 μM) or phorbol myristate acetate plus phytohemagglutinin (2 μg/ml) as non-LPS stimuli. Neither compound inhibited TNF-α, IL-6, or IL-8 appreciably up to concentrations of 40 μM, verifying that the inhibition observed was specific for LPS (data not shown). It should be noted that detectable levels of neither IFN-γ nor IL-10 were observed in the plasma samples with the 4-h LPS stimulation regime that was employed in our studies. It would appear that the early phase of LPS-induced stimulation is characterized predominantly by a proinflammatory profile, which is effectively suppressed by LPS sequestrants, such as PMB and DS-96.
Efficacy in murine models of septic shock.
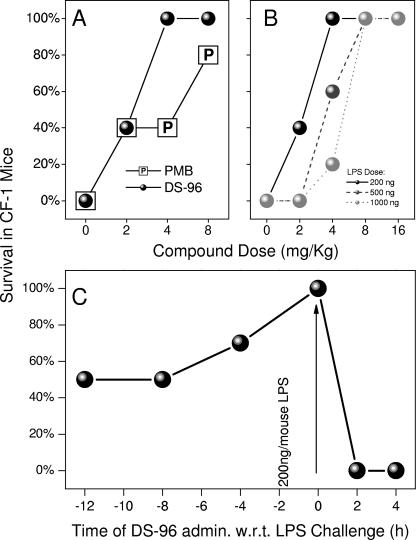
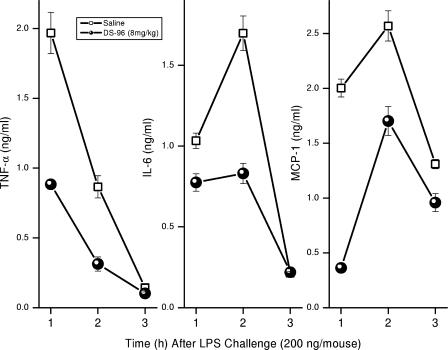
A well-established murine model of lethal septic shock (5, 24, 26) was employed to compare the potencies of DS-96 and PMB. Pilot experiments clearly showed virtually superimposable profiles of dose-dependent survival when LPS was precomplexed first with graded concentrations of either DS-96 or PMB and then administered as a single intraperitoneal (i.p.) injection to d-galactosamine-primed mice (data not shown). However, it was important to verify that complexation of circulatory LPS could occur under in vivo conditions (i.e., the compound would sequester systemic LPS when given parenterally via another route). We therefore employed an alternate model. Cohorts of 10 CF-1 mice per group, sensitized to the lethal effects of LPS with d-galactosamine, were challenged with a supralethal dose of LPS (2× LD100 = 200 ng/animal) administered i.p. This was preceded by a subcutaneous (s.c.) injection of graded doses of test compounds given 1 h prior to LPS challenge. Although similar patterns of dose-dependent increases in survival were observed for the two compounds, DS-96 afforded significantly better protection than PMB at 4 mg/kg (Fig. 7A). We confirmed that the protection afforded by DS-96 was attributable to attenuated LPS-induced cytokine production (Fig. 8). Furthermore, DS-96 was without any effect on lethality induced by 100 ng/animal of recombinant murine TNF-α (data not shown). As mentioned earlier, pharmacokinetic experiments aimed at quantifying the half-lives of test compounds have on occasion led to apparent incongruencies with pharmacodynamics data due to unusual partitioning and/or protein-binding behavior, as has been observed with E5564, for instance (33). We elected therefore to first characterize the pharmacodynamics of DS-96. Mice were administered DS-96 at a dose of 8 mg/kg s.c. at various time points before and after a supralethal LPS challenge. As depicted in Fig. 7C, DS-96 is maximally effective when it is administered concurrently with LPS administration; the data further suggest that sufficiently high plasma concentrations apparently persist even up to 8 h so as to provide partial protection against LPS-induced lethality (Fig. 7C). It should be noted that DS-96 is bereft of any protective effect if administered following LPS challenge, suggesting that once the innate immune system has responded to the presence of circulatory LPS, anti-endotoxin agents are unlikely to be of any benefit. This is crucial since this defines the subset of patients in whom a compound such as DS-96 is likely to be of benefit.
FIG. 7.
(A) Comparison of in vivo potencies of PMB and DS-96: dose-dependent increase in survival in mice challenged with a supralethal dose of LPS (200 ng/animal). (B) Schild-type response in vivo: dose dependence of survival in mice challenged with escalating supralethal doses of LPS (200, 500, or 1,000 ng/animal). The LD100 of LPS was determined to be 100 ng/mouse. (C) Time course (pharmacodynamics) of protection conferred by 8 mg/kg of DS-96 administered subcutaneously at various times prior to and following supralethal (200 ng/mouse) LPS challenge.
FIG. 8.
Time course of cytokine levels in mice receiving 4 mg/kg DS-96 and challenged with a 200-ng/ml dose of LPS. Cohorts of five animals each received either 4 mg/kg DS-96 or saline s.c. at t = −1 h, followed by d-galactosamine-LPS given i.p. at t = 0 h. At t = 1, 2, or 3 h, animals were bled by terminal cardiac puncture, and cytokines were assayed in plasma by cytometric bead array assays. It should be noted that only TNF-α, IL-6, and macrophage chemotactic protein 1 levels were quantifiable; IL-10, IL-12p70, and IFN-γ levels were below detection limits.
Toxicity.
DS-96 is a cationic amphipath and, as such, is expected to be surface active, with the possible consequence of nonspecific cytotoxicity. Gratifyingly, we found that our apprehension was unwarranted: the hemolytic activity of DS-96 was virtually abrogated in the presence of physiological concentrations of albumin. In whole human blood, significant hemolytic activity occurred only at millimolar concentrations (data not shown). Daily s.c. administration of DS-96 to mice at a dose of 40 mg/kg per day for 5 days (note that full protection against LPS-induced lethality was conferred with a single dose of 4 mg/kg) resulted in no clinically observable signs of apparent toxicity. We paid particular attention to the development of CNS toxicity in light of the fact that compounds such as N1-dansylspermine and N1-(n-octanesulfonyl)spermine have been reported to be potent N-methyl d-aspartate receptor antagonists (35, 36), and we have ourselves observed the rapid onset of tonic-clonic seizures upon the systemic administration of long-chain polyamine sulfonamides that we had evaluated (unpublished data). Importantly, at the end of the experiment (day 6), there were no demonstrable signs of nephro- or hepatotoxicity as assessed by clinical chemistry (unpublished data). Several polyamine derivatives have been shown to cause derangement of polyamine metabolism as a consequence of polyamine oxidase inhibition (41), (34), and we were therefore interested in examining the effects of subacute high-dose treatment with DS-96. No differences in polyamine levels (putrescine, spermidine, spermine, N1-acetylspermidine) between control mice and those that received 40 mg/kg per day DS-96 for 5 days were noted (data not shown).
The experimental studies described in this paper establish that DS-96 binds LPS and attenuates its toxicity with a potency very similar to that of PMB. The inhibition of early cellular activation events, namely, p38 MAPK phosphorylation and NF-κB translocation, in conjunction with the inhibition of the more distal response events of cytokine and NO production and the lack of activity against non-LPS stimuli clearly show that the mechanism of action is via sequestration of LPS. DS-96 was found to exhibit potent anti-endotoxin activities not only in whole human blood ex vivo but also in a murine model of endotoxic shock.
Particularly instructive are the results of the time course experiment with mice (Fig. 7C), which show that if LPS-sequestering compounds, such as DS-96, are ever to find utility in the clinic, they will have to be used as prophylactic agents rather than in treatment of established sepsis, for once the inflammatory cascades are set in motion, LPS sequestrants would be of no value. This may indeed be feasible and indeed desirable, not only since many of the therapeutic strategies that target downstream processes, such as blockade of TNF-α or IL-1β, have failed (29, 46) but also because the predisposing factors for septic shock are very well recognized (28). It may be possible to institute LPS sequestrant therapy as an adjunct to conventional antimicrobial chemotherapy—with the important proviso that the therapeutic index is sufficiently high. We therefore have examined the effects of large multiples of therapeutic doses of DS-96 in the murine model and have not found any detectable toxicity. Escalating dose regimens and histopathology studies are currently in progress, as are pharmacokinetic experiments using liquid chromatography-mass spectrometry to determine the plasma half-life of the compound.
In summary, we have described here a detailed characterization of DS-96, a novel monoalkylated homospermine derivative, exhibiting true LPS-sequestering and -neutralizing properties in a panel of in vitro assays and with a murine model of endotoxic shock. The potency of DS-96 rivals that of PMB and, in some key assays, including a murine model of endotoxic shock, is superior to that of PMB.
Acknowledgments
This work was supported by NIH grant 1R01 AI50107.
Footnotes
Published ahead of print on 4 June 2007.
REFERENCES
- 1.Beamer, L. J., S. F. Carroll, and D. Eisenberg. 1999. The three-dimensional structure of human bactericidal/permeability-increasing protein: implications for understanding protein-lipopolysaccharide interactions. Biochem. Pharmacol. (Oxford) 57:225-229. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharjya, S., S. A. David, V. I. Mathan, and P. Balaram. 1997. Polymyxin B nonapeptide: conformations in water and in the lipopolysaccharide-bound state determined by two-dimensional NMR and molecular dynamics. Biopolymers 41:251-265. [Google Scholar]
- 3.Bone, R. C. 1996. The sepsis syndrome. Definition and general approach to management. Clin. Chest Med. 17:175-181. [DOI] [PubMed] [Google Scholar]
- 4.Burns, M. R., S. A. Jenkins, S. J. Wood, K. Miller, and S. A. David. 2006. Structure-activity relationships in lipopolysaccharide neutralizers: design, synthesis, and biological evaluation of a 540-membered amphipathic bisamide library. J. Comb. Chem. 8:32-43. [DOI] [PubMed] [Google Scholar]
- 5.Burns, M. R., S. J. Wood, K. A. Miller, T. Nguyen, J. R. Cromer, and S. A. David. 2005. Lysine-spermine conjugates: hydrophobic polyamine amides as potent lipopolysaccharide sequestrants. Bioorg. Med. Chem. 13:2523-2536. [DOI] [PubMed] [Google Scholar]
- 6.Cavaillon, J. M., and M. Adib-Conquy. 2007. Determining the degree of immunodysregulation in sepsis. Contrib. Nephrol. 156:101-111. [DOI] [PubMed] [Google Scholar]
- 7.Cook, E. B., J. L. Stahl, L. Lowe, R. Chen, E. Morgan, J. Wilson, R. Varro, A. Chan, F. M. Graziano, and N. P. Barney. 2001. Simultaneous measurement of six cytokines in a single sample of human tears using microparticle-based flow cytometry: allergics vs. non-allergics. J. Immunol. Methods 254:109-116. [DOI] [PubMed] [Google Scholar]
- 8.David, S. A. 2001. Towards a rational development of anti-endotoxin agents: novel approaches to sequestration of bacterial endotoxins with small molecules. J. Mol. Recognit. 14:370-387. [DOI] [PubMed] [Google Scholar]
- 9.David, S. A., P. Balaram, and V. I. Mathan. 1995. Characterization of the interaction of lipid A and lipopolysaccharide with human serum albumin: implications for an endotoxin-carrier function for albumin. J. Endotoxin Res. 2:99-106. [Google Scholar]
- 10.David, S. A., V. I. Mathan, and P. Balaram. 1995. Interactions of linear dicationic molecules with lipid A: structural requisites for optimal binding affinity. J. Endotoxin Res. 2:325-336. [Google Scholar]
- 11.Dinarello, C. A. 1996. Cytokines as mediators in the pathogenesis of septic shock. Curr. Top. Microbiol. Immunol. 216:133-165. [DOI] [PubMed] [Google Scholar]
- 12.Durando, M. M., R. J. MacKay, S. Linda, and L. A. Skelley. 1994. Effects of polymyxin B and Salmonella typhimurium antiserum on horses given endotoxin intravenously. Am. J. Vet. Res. 55:921-927. [PubMed] [Google Scholar]
- 13.Fenton, M. J., and D. T. Golenbock. 1998. LPS-binding proteins and receptors. J. Leukoc. Biol. 64:25-32. [DOI] [PubMed] [Google Scholar]
- 14.Ferguson, A. D., E. Hofmann, J. Coulton, K. Diedrichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 15.Funato, Y., H. Baumhover, D. Grantham-Wright, J. Wilson, E. Ernst, and H. Sepulveda. 2002. Simultaneous measurement of six human cytokines using the Cytometric Bead Array System, a multiparameter immunoassay system for flow cytometry. Cytometry Res. 12:93-103. [Google Scholar]
- 16.Gough, M., R. E. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite and [15-N]nitrate in biological fluids. Anal. Biochem. 126:131. [DOI] [PubMed] [Google Scholar]
- 18.Greiss, P. 1879. Bemerkungen zu der abhandlung der H.H. Weselsky und Benedikt “Ueber einige azoverbindungen.” Chem. Ber. 12:426-427. [Google Scholar]
- 19.Guo, J. X., S. J. Wood, S. A. David, and G. H. Lushington. 2006. Molecular modeling analysis of the interaction of novel bis-cationic ligands with the lipid A moiety of lipopolysaccharide. Bioorg. Med. Chem. Lett. 16:714-717. [DOI] [PubMed] [Google Scholar]
- 20.Han, J., J. D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 21.Helander, I. M., I. Kilpelainen, and M. Vaara. 1994. Increased substitution of phosphate groups in lipopolysaccharides and lipid A of the polymyxin-resistant pmrA mutants of Salmonella typhimurium: a 31P-NMR study. Mol. Microbiol. (Oxford) 11:481-487. [DOI] [PubMed] [Google Scholar]
- 22.Holst, O., A. J. Ulmer, H. Brade, and E. T. Rietschel. 1994. On the chemistry and biology of bacterial endotoxic lipopolysaccharides, p. 281-308. In N. Masihi (ed.), Immunotherapy of infections. Marcel Dekker, Inc., New York, NY.
- 23.Hurley, J. C. 1995. Antibiotic-induced release of endotoxin. A therapeutic paradox. Drug Saf. 12:183-195. [DOI] [PubMed] [Google Scholar]
- 24.Khownium, K., S. J. Wood, K. A. Miller, R. Balakrishna, T. B. Nguyen, M. R. Kimbrell, G. I. Georg, and S. A. David. 2006. Novel endotoxin-sequestering compounds with terephthalaldehyde-bis-guanylhydrazone scaffolds. Bioorg. Med. Chem. Lett. 16:1305-1308. [DOI] [PubMed] [Google Scholar]
- 25.Martin, G. S., D. M. Mannino, S. Eaton, and M. Moss. 2003. The epidemiology of sepsis in the United States from 1979 through 2000. New Engl. J. Med. 348:1546-1554. [DOI] [PubMed] [Google Scholar]
- 26.Miller, K. A., E. V. K. Suresh Kumar, S. J. Wood, J. R. Cromer, A. Datta, and S. A. David. 2005. Lipopolysaccharide sequestrants: structural correlates of activity and toxicity in novel acylhomospermines. J. Med. Chem. 48:2589-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nummila, K., I. Kilpelainen, U. Zahringer, M. Vaara, and I. M. Helander. 1995. Lipopolysaccharides of polymyxin B-resistant mutants of Escherichia coli are extensively substituted by 2-aminoethyl pyrophosphate and contain aminoarabinose in lipid A. Mol. Microbiol. (Oxford) 16:271-278. [DOI] [PubMed] [Google Scholar]
- 28.Opal, S. M., and R. L. J. Yu. 1998. Antiendotoxin strategies for the prevention and treatment of septic shock. New approaches and future directions. Drugs 55:497-508. [DOI] [PubMed] [Google Scholar]
- 29.Quezado, Z. M. N., S. M. Banks, and C. Natanson. 1995. New strategies for combatting sepsis: the magic bullets missed the mark...but the search continues. Trends Biotechnol. 13:56-63. [DOI] [PubMed] [Google Scholar]
- 30.Raetz, C. R., T. A. Garrett, C. M. Reynolds, W. A. Shaw, J. D. Moore, D. C. Smith, Jr., A. A. Ribeiro, R. C. Murphy, R. J. Ulevitch, C. Fearns, D. Reichart, C. K. Glass, C. Benner, S. Subramaniam, R. Harkewicz, R. C. Bowers-Gentry, M. W. Buczynski, J. A. Cooper, R. A. Deems, and E. A. Dennis. 2006. Kdo2-lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J. Lipid Res. 47:1097-1111. [DOI] [PubMed] [Google Scholar]
- 31.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zähringer, U. Seydel, F. Di Padova, et al. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 32.Rietschel, E. T., T. Kirikae, U. F. Schade, A. J. Ulmer, O. Holst, H. Brade, G. Schmidt, U. Mamat, H. D. Grimmecke, S. Kusumoto, and U. Zähringer. 1993. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology 187:169-190. [DOI] [PubMed] [Google Scholar]
- 33.Rossignol, D. P., and M. Lynn. 2002. Antagonism of in vivo and ex vivo response to endotoxin by E5564, a synthetic lipid A analogue. J. Endotoxin Res. 8:483-488. [DOI] [PubMed] [Google Scholar]
- 34.Seiler, N. 2004. Catabolism of polyamines. Amino Acids 26:217-233. [DOI] [PubMed] [Google Scholar]
- 35.Seiler, N., L. Badolo, B. Duranton, F. Vincent, Y. Schneider, F. Gosse, and F. Raul. 2000. Effect of the polyamine oxidase inactivator MDL 72527 on N(1)-(n-octanesulfonyl)spermine toxicity. Int. J. Biochem. Cell Biol. 32:1055-1068. [DOI] [PubMed] [Google Scholar]
- 36.Seiler, N., B. Duranton, F. Vincent, F. Gosse, J. Renault, and F. Raul. 2000. Inhibition of polyamine oxidase enhances the cytotoxicity of polyamine oxidase substrates. A model study with N(1)-(n-octanesulfonyl)spermine and human colon cancer cells. Int. J. Biochem. Cell Biol. 32:703-716. [DOI] [PubMed] [Google Scholar]
- 37.Storm, D. R., and K. Rosenthal. 1977. Polymyxin and related peptide antibiotics. Annu. Rev. Biochem. 46:723-763. [DOI] [PubMed] [Google Scholar]
- 38.Udenfriend, S., S. Stein, P. Bohlen, W. Dairman, W. Leimgruber, and M. Weigle. 1972. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science 178:871-872. [DOI] [PubMed] [Google Scholar]
- 39.Ulevitch, R. J. 2000. Molecular mechanisms of innate immunity. Immunol. Res. 21:49-54. [DOI] [PubMed] [Google Scholar]
- 40.Vesentini, S., M. Soncini, A. Zaupa, V. Silvestri, G. B. Fiore, and A. Redaelli. 2006. Multi-scale analysis of the toraymyxin adsorption cartridge. Part I: molecular interaction of polymyxin B with endotoxins. Int. J. Artif. Organs 29:239-250. [DOI] [PubMed] [Google Scholar]
- 41.Wallace, H. M., and A. V. Fraser. 2004. Inhibitors of polyamine metabolism: review article. Amino Acids 26:353-365. [DOI] [PubMed] [Google Scholar]
- 42.Weisz, A., S. Oguchi, L. Cicatiello, and H. Esumi. 1994. Dual mechanism for the control of inducible-type NO synthase gene expression in macrophages during activation by interferon-gamma and bacterial lipopolysaccharide. Transcriptional and post-transcriptional regulation. J. Biol. Chem. 269:8324-8333. [PubMed] [Google Scholar]
- 43.Wood, S. J., K. A. Miller, and S. A. David. 2004. Anti-endotoxin agents. 1. Development of a fluorescent probe displacement method for the rapid identification of lipopolysaccharide-binding agents. Comb. Chem. High Throughput Screen. 7:239-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wood, S. J., K. A. Miller, and S. A. David. 2004. Anti-endotoxin agents. 2. Pilot high-throughput screening for novel lipopolysaccharide-recognizing motifs in small molecules. Comb. Chem. High Throughput Screen. 7:733-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood, S. J., K. A. Miller, G. H. Lushington, M. R. Burns, and S. A. David. 2006. Anti-endotoxin agents. 3. Rapid identification of high-affinity lipopolysaccharide-binding compounds in a substituted polyamine library. Comb. Chem. High Throughput Screen. 9:27-36. [DOI] [PubMed] [Google Scholar]
- 46.Zeni, F., B. Freeman, and C. Natanson. 1997. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit. Care Med. 25:1097-1100. [DOI] [PubMed] [Google Scholar]