Abstract
Several recent outbreaks of Clostridium difficile-associated disease (CDAD) have been attributed to the emergence of an epidemic strain with increased resistance to fluoroquinolone antibiotics. Some clinical studies have suggested that fluoroquinolones with enhanced antianaerobic activity (i.e., gatifloxacin and moxifloxacin) may have a greater propensity to induce CDAD than ciprofloxacin and levofloxacin do. We examined the effects of subcutaneous fluoroquinolone treatment on in vitro growth of and toxin production by epidemic and nonepidemic C. difficile isolates in cecal contents of mice and evaluated the potential for these agents to inhibit fluoroquinolone-susceptible isolates during treatment. When C. difficile isolates were inoculated into cecal contents collected 2 days after the final antibiotic dose, gatifloxacin and moxifloxacin promoted significantly more growth and toxin production than ciprofloxacin and levofloxacin did. During treatment, gatifloxacin and moxifloxacin inhibited growth of fluoroquinolone-susceptible but not fluoroquinolone-resistant isolates. Ciprofloxacin and levofloxacin promoted growth of C. difficile when administered at higher doses (i.e., 20 times the human dose in mg/kg of body weight), and levofloxacin inhibited growth of fluoroquinolone-susceptible, but not fluoroquinolone-resistant, C. difficile isolates when administered in combination with ceftriaxone. Thus, fluoroquinolones with enhanced antianaerobic activity (i.e., gatifloxacin and moxifloxacin) promoted C. difficile growth to a greater extent than did ciprofloxacin and levofloxacin in this model. However, our findings suggest that fluoroquinolones may exert selective pressure favoring the emergence of epidemic fluoroquinolone-resistant C. difficile strains by inhibiting fluoroquinolone-susceptible but not fluoroquinolone-resistant isolates during treatment and that agents such as levofloxacin or ciprofloxacin can exert such selective pressure when administered in combination with antibiotics that disrupt the anaerobic microflora.
Several cities in North America and Europe are currently experiencing significant outbreaks of Clostridium difficile-associated disease (CDAD) (6, 8, 9, 11). These outbreaks have been attributed to the emergence of an epidemic strain of C. difficile that exhibits increased resistance to fluoroquinolone antibiotics, including the C8-methoxyfluoroquinolones gatifloxacin and moxifloxacin (6, 8). Although fluoroquinolones were previously considered a relatively uncommon cause of CDAD, they have been implicated as an important risk factor for disease due to the epidemic strain (6, 8, 9, 11). Because gatifloxacin and moxifloxacin have enhanced antianaerobic activity, it has been proposed that these agents might promote CDAD to a greater degree than ciprofloxacin and levofloxacin (2, 3, 15). However, ciprofloxacin and levofloxacin have also been associated with CDAD in clinical studies (6, 7, 9, 11, 17). Using a mouse model, we previously demonstrated that antibiotics that disrupt the anaerobic microflora (e.g., clindamycin and ceftriaxone) promoted in vitro growth of (≥2-log increase in density after inoculation of 4 log10 CFU/ml) and toxin production by nonepidemic C. difficile isolates in cecal contents, whereas antibiotics that cause minimal disruption of the anaerobic microflora, including levofloxacin, did not (≥1-log decrease in density) (12). Piperacillin-tazobactam, an agent with inhibitory activity against many C. difficile isolates, suppressed the organism during treatment, but overgrowth and toxin production were promoted if exposure occurred after treatment during the period of recovery of the indigenous microflora (12).
In this study, we used the same mouse model to examine the effects of different fluoroquinolone antibiotics on growth of and toxin production by epidemic and nonepidemic strains of C. difficile in cecal contents. We hypothesized that gatifloxacin and moxifloxacin would promote C. difficile growth and toxin production to a greater degree than ciprofloxacin and levofloxacin would. In addition, we hypothesized that fluoroquinolones may exert selective pressure favoring the emergence of epidemic fluoroquinolone-resistant C. difficile strains by inhibiting fluoroquinolone-susceptible but not fluoroquinolone-resistant isolates during treatment and that agents such as levofloxacin or ciprofloxacin can exert such selective pressure when administered in combination with antibiotics that disrupt the anaerobic microflora.
MATERIALS AND METHODS
C. difficile strains.
Six strains of C. difficile were studied. Strain 1 was ATCC 9689. The remaining five strains were clinical isolates from Cleveland: strain 2 was typed as J29 or J30 by restriction endonuclease analysis (performed in Dale Gerding's laboratory), strains 3 and 4 were epidemic strains typed as BI9 and BI6-8-17 by restriction endonuclease analysis typing, strain 5 was an epidemic strain typed as NAP1 (North American pulsed-field gel electrophoresis type 1), and strain 6 was a nonepidemic strain. All of the strains produced toxins A and B, and the epidemic strains had positive PCRs for the binary toxin gene cdtB (data not shown).
Susceptibility testing.
Broth dilution MICs of the test antibiotics for the C. difficile isolates were determined using standard methods for susceptibility testing of anaerobic bacteria (10). Clinical and Laboratory Standards Institute interpretative categories for resistance were used. For the fluoroquinolones, trovafloxacin breakpoints were used because this is the only fluoroquinolone for which resistance breakpoints were provided for anaerobes.
Bioassay for antibiotic concentrations.
The concentrations of fluoroquinolone antibiotics in stool were determined by an agar diffusion assay with Escherichia coli as the indicator strain (13).
Toxin assay.
To determine toxin production, a commercially available kit for detection of C. difficile toxin (Diagnostic Hybrids, Inc., Athens, OH) was utilized as recommended by the manufacturer. The cecal content supernatants were serially diluted 10-fold in specimen diluent. Following dilution, samples were added to microtiter plates containing human fibroblast cells and observed by bright-field microscopy, at 24 and 48 h, for evidence of C. difficile toxin cytopathic effect.
Mouse model of colonization resistance to C. difficile.
The mouse model that was used was adapted from the model of colonization resistance to C. difficile infection developed by Borriello et al. (1). These investigators demonstrated that antibiotics that promoted in vitro growth and toxin production by C. difficile in cecal emulsions of hamsters also caused C. difficile disease in hamsters, whereas antibiotics that did not promote in vitro growth and toxin production did not cause disease (1). We have previously found that this model yields similar results in mice and hamsters treated with clindamycin or aztreonam (1, 12). The experimental protocol was approved by the Animal Care Committee of the Cleveland Veterans Affairs Medical Center.
In the first set of experiments, C. difficile strains 1 to 4 were used to compare the effects of the different antibiotics on growth and toxin production by nonepidemic and epidemic isolates. Female CF-1 mice weighing 25 to 30 g (Harlan Sprague-Dawley, Indianapolis, IN) were housed in individual cages with plastic filter tops to prevent cross-contamination among animals. Four fluoroquinolone antibiotics were studied, including ciprofloxacin (Bayer, West Haven, CT), levofloxacin (Ortho-McNeil, Titusville, NJ), moxifloxacin (Bayer), and gatifloxacin (Bristol-Myers Squibb, Princeton, NJ). Ceftriaxone (Roche, Nutley, NJ) and clindamycin (Pharmacia & Upjohn, Kalamazoo, MI) were included as positive controls. Mice received daily subcutaneous injections (0.2-ml total volume) of saline, ciprofloxacin (0.4 mg/day), levofloxacin (0.375 mg/day), gatifloxacin (0.2 mg/day), moxifloxacin (0.2 mg/day), ceftriaxone (2.0 mg/day), and clindamycin (1.4 mg/day) for 4 days. The doses of antibiotics were equal to the usual human doses administered over a 24-hour period (milligrams of antibiotic per gram of body weight). Stool samples were collected on day 4 of antibiotic treatment for determination of concentrations of fluoroquinolone antibiotics. Two days after the final antibiotic dose, mice were killed by CO2 asphyxiation. Because ciprofloxacin and levofloxacin did not promote significant overgrowth of C. difficile, the effects of higher daily doses of 12 and 20 times the initial dosage were also assessed.
After the mice were killed, the cecum was removed and opened longitudinally. Cecal contents were collected and transferred to an anaerobic chamber (Coy Laboratories, Grass Lake, MI) within 5 min. The cecal contents were diluted threefold (vol/vol) in sterile prereduced phosphate-buffered saline (PBS). A final concentration of 104 CFU/ml of each strain was added to separate aliquots of the cecal contents of individual mice. The C. difficile strains were prepared for inoculation by serially diluting 24-hour broth cultures in sterile prereduced PBS. After incubation for 24 h, the samples were diluted in sterile PBS and plated on prereduced cefoxitin-cycloserine-fructose agar (Becton Dickinson, Cockeysville, MD) containing 1% taurocholic acid sodium salt (Sigma, St. Louis, MO) to quantify C. difficile (12). Experiments were performed twice with six total mice per group.
Examination of fluoroquinolone selective pressure.
A second set of experiments was performed to evaluate the potential for the fluoroquinolones to exert selective pressure by inhibiting fluoroquinolone-susceptible but not fluoroquinolone-resistant C. difficile isolates during treatment. Mice (six per group) received daily antibiotic treatment for 4 days as described above but were killed 2 h after administration of the final antibiotic dose. Antibiotic treatment groups included levofloxacin, gatifloxacin, moxifloxacin, ceftriaxone, and ceftriaxone in combination with levofloxacin. C. difficile strains 5 (fluoroquinolone resistant) and 1 and 6 (fluoroquinolone susceptible) were studied. Growth in cecal contents was examined as described above. Finally, to further assess the potential for antibiotics to inhibit C. difficile during treatment, we examined the ability of clindamycin to inhibit clindamycin-susceptible isolates (strains 5 and 6) during treatment but to promote the same strains during the period of recovery of the indigenous microflora. Mice received 4 days of treatment with subcutaneous clindamycin, and cecal contents were collected either 2 h or 2 days after completion of treatment to assess growth of C. difficile.
Statistical analysis.
One-way analysis of variance was performed to compare C. difficile densities and toxin production among the treatment groups. P values were adjusted for multiple comparisons using the Scheffe correction. Computations were performed with the use of Stata software (version 5.0; Stata, College Station, TX). P < 0.05 was considered significant.
RESULTS
Mouse model of colonization resistance to C. difficile.
Preliminary experiments demonstrated that results were similar when cecal contents were collected 1, 2, or 3 days after the final antibiotic dose (data not shown). For these experiments, contents were collected 2 days after the final antibiotic dose. The broth dilution MICs for the six test strains are shown in Table 1.
TABLE 1.
MICs for the six Clostridium difficile test strains
| Antibiotic | MIC (μg/ml) for strain:a
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Ciprofloxacin | 16 | 128 | 128 | 128 | >256 | 32 |
| Levofloxacin | 8 | 256 | 256 | >256 | >256 | 8 |
| Gatifloxacin | <2 | 32 | 64 | 32 | 256 | 4 |
| Moxifloxacin | <2 | 32 | 64 | 64 | 256 | 2 |
| Ceftriaxone | 32 | 64 | 32 | 32 | 16 | 32 |
| Clindamycin | >256 | >256 | >256 | >256 | 2 | 2 |
MICs were determined by broth dilution. Strains 3, 4, and 5 were epidemic strains. The resistance breakpoints (μg/ml) based on Clinical and Laboratory Standards Institute interpretive categories for resistance were 8 for each of the fluoroquinolones, 64 for ceftriaxone, and 8 for clindamycin.
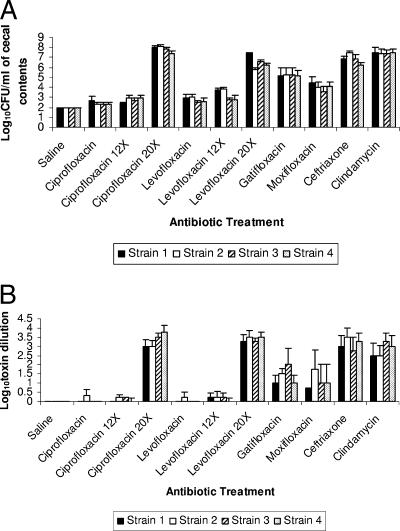
None of the cecal contents had detectable concentrations of C. difficile prior to inoculation of the test strains (level of detection, ∼2 log10 CFU/ml). Figure 1A demonstrates the effect of antibiotic treatment on growth of the C. difficile strains 1 to 4 in cecal contents. None of the cecal contents of the saline-treated mice had detectable levels of C. difficile. About 50% of the cecal contents of mice that received prior treatment with the lower dose of ciprofloxacin and levofloxacin had low levels of C. difficile, but the densities of growth did not differ significantly from those for saline controls (P > 0.96). However, both ciprofloxacin and levofloxacin promoted overgrowth when administered at 20 times the usual human dose (P < 0.001), and levofloxacin promoted overgrowth of the nonepidemic strains (1 and 2) when administered at 12 times the usual human dose (P < 0.05), whereas ciprofloxacin did not (P = 0.91). When data for all four strains were included, the cecal contents of gatifloxacin- and moxifloxacin-treated mice had increased density of C. difficile in comparison to saline controls (P ≤ 0.02), whereas contents of the mice receiving the equivalent lower doses of ciprofloxacin and levofloxacin did not (P > 0.96).
FIG. 1.
Effect of antibiotic treatment on growth of (A) and toxin production by (B) Clostridium difficile in the cecal contents of mice. Strains 3 and 4 were epidemic BI strains. Mice received daily subcutaneous antibiotic treatment for 5 days. Two days after the final antibiotic dose, cecal contents were collected and inoculated with 104 CFU/ml of the C. difficile test strains. Samples were incubated anaerobically for 48 h, and then serial dilutions were plated onto selective medium for quantification of C. difficile and assayed for toxin production. Mean toxin titers are expressed as the reciprocal of the highest serial 10-fold dilution that gave positive results. For ciprofloxacin and levofloxacin, 12× and 20× refer to 12 and 20 times the initial dosage, respectively. Error bars represent standard errors.
When data from individual strains were assessed separately, gatifloxacin treatment was associated with increased density of each of the four strains (P < 0.001), whereas moxifloxacin was associated with significantly increased density of strains 1, 2, and 4 (P < 0.02). The cecal contents of mice that received prior treatment with ceftriaxone and clindamycin supported growth of each of the C. difficile isolates (P < 0.001 for each group in comparison to saline controls). There was no difference in the densities of the nonepidemic (1 and 2) and epidemic (3 and 4) strains (P ≥ 0.51). The concentrations (mean ± standard deviation) of ciprofloxacin, levofloxacin, gatifloxacin, and moxifloxacin in stool were 226.5 ± 23.1, 58.1 ± 68.5, 76.6 ± 79.4, and 44.0 ± 47.7 μg/g, respectively.
Figure 1B shows the toxin levels of the C. difficile strains. Significantly increased levels of toxin were produced in cecal contents of mice that received prior treatment with ceftriaxone, clindamycin, gatifloxacin, high-dose ciprofloxacin (20 times the initial dose), and high-dose levofloxacin in comparison to the saline, ciprofloxacin, and levofloxacin groups (P < 0.04). There was a nonsignificant trend toward greater toxin levels in the moxifloxacin-treated group than in the saline-treated group (P = 0.27). The levels of toxin associated with the epidemic strains (3 and 4) did not differ from those of the nonepidemic strains (P = 0.85).
Examination of fluoroquinolone selective pressure.
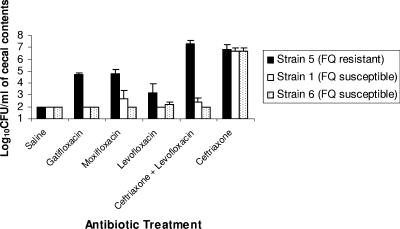
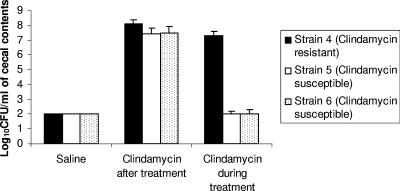
Figure 2 shows the effects of gatifloxacin, moxifloxacin, and levofloxacin with or without concurrent ceftriaxone on the density of fluoroquinolone-susceptible (strains 1 and 6) and fluoroquinolone-resistant (strain 5) C. difficile isolates during treatment. All of the antibiotic treatment groups promoted increased density of the fluoroquinolone-resistant C. difficile isolate in comparison to the saline controls (P < 0.05 for all comparisons); however, the density was significantly lower in the levofloxacin group than in the other treatment groups (P < 0.03). The fluoroquinolone-susceptible isolates grew to high concentrations in cecal contents of ceftriaxone-treated mice but were inhibited in each of the fluoroquinolone-treated groups, including the combination of ceftriaxone and levofloxacin. Similarly, Fig. 3 demonstrates that two clindamycin-susceptible C. difficile isolates were inhibited in cecal contents collected during clindamycin treatment but that their growth was promoted when the contents were collected 2 days after completion of treatment.
FIG. 2.
Effect of antibiotic treatment on growth of Clostridium difficile in the cecal contents of mice during treatment. Mice received daily subcutaneous antibiotic treatment for 5 days. Two hours after the final antibiotic dose, cecal contents were collected and inoculated with 104 CFU/ml of the C. difficile test strains. Samples were incubated anaerobically for 48 h, and then serial dilutions were plated onto selective medium for quantification of C. difficile. Error bars represent standard errors. FQ, fluoroquinolone.
FIG. 3.
Effect of clindamycin treatment on growth of clindamycin-resistant (strain 4) and clindamycin-susceptible (strains 5 and 6) Clostridium difficile strains in the cecal contents of mice during or after treatment. Mice received daily subcutaneous clindamycin treatment for 5 days. Two hours (during treatment) or 2 days (after treatment) after the final antibiotic dose, cecal contents were collected and inoculated with 104 CFU/ml of the C. difficile test strains. Samples were incubated anaerobically for 48 h, and then serial dilutions were plated onto selective medium for quantification of C. difficile. Error bars represent standard errors.
DISCUSSION
Our findings provide support for the hypothesis that gatifloxacin, and to a lesser extent moxifloxacin, may promote greater growth of and toxin production by epidemic and nonepidemic C. difficile strains than ciprofloxacin and levofloxacin. These data are consistent with two recent observational studies in which rates of CDAD increased after a formulary switch from levofloxacin to gatifloxacin or moxifloxacin and again decreased after a change back to levofloxacin (2, 15). However, it is notable that cecal contents of gatifloxacin- and moxifloxacin-treated mice did not support growth of fluoroquinolone-susceptible C. difficile isolates during treatment, presumably because the level of the antibiotics was sufficient to inhibit growth. Such inhibitory activity may in part explain the infrequent association of fluoroquinolones with CDAD prior to the emergence of fluoroquinolone-resistant strains, including the epidemic strain. Our findings suggest that fluoroquinolones may exert selective pressure on C. difficile strains circulating in hospitals, favoring proliferation of fluoroquinolone-resistant epidemic strains (i.e., inhibition of fluoroquinolone-susceptible nonepidemic strains and promotion of fluoroquinolone-resistant epidemic strains).
Monotherapy with ciprofloxacin and levofloxacin at dosages equal to the usual human doses on a milligram/kilogram basis did not promote significant growth of C. difficile in cecal contents. However, these agents did appear to have an adverse impact on resistance to growth of C. difficile at these dosages (i.e., low levels of C. difficile were detectable in cecal contents of 50 to 60% of mice treated with these agents, whereas none were detected in cecal contents of saline-treated mice). Ciprofloxacin and levofloxacin did promote C. difficile growth when administered at higher dosages (Fig. 2). In addition, levofloxacin inhibited growth of fluoroquinolone-susceptible, but not fluoroquinolone-resistant, C. difficile isolates when administered in combination with ceftriaxone. These data are consistent with reports indicating that monotherapy with agents such as ciprofloxacin and levofloxacin may be less likely to be associated with CDAD than use of these agents in combination with other antibiotics (4, 16). However, our findings also suggest that ciprofloxacin and levofloxacin may exert selective pressure on C. difficile, particularly when administered in combination or in sequence with other antibiotics that disrupt the indigenous microflora. In clinical practice, fluoroquinolone antibiotics are commonly administered in combination with other agents; in a review of antimicrobial usage at our institution, we found that more than half of levofloxacin use was in combination or in sequence with other agents (unpublished data).
In addition to the fluoroquinolones, clindamycin also exhibited the potential to suppress clindamycin-susceptible C. difficile isolates during treatment (Fig. 3). These data are consistent with recent studies demonstrating that clindamycin use may be a specific risk factor for disease associated with clindamycin-resistant C. difficile strains (5). Restriction of clindamycin was an effective control measure for outbreaks associated with clindamycin-resistant strains (5). In contrast, Loo et al. (6) found that clindamycin was not a risk factor for CDAD in the setting of an outbreak due to epidemic C. difficile strains that were clindamycin susceptible.
Our study has some limitations. First, although the mouse model used demonstrated good correlation with CDAD in hamsters (1), it has not been confirmed that this model correlates well with disease in humans. Second, the pharmacokinetics of antibiotics differ in mice and humans; however, the levels of the study drugs in the stool of mice receiving equal dosages on a mg/kg basis were similar to stool concentrations measured in human volunteers receiving oral or parenteral fluoroquinolones (14). Third, we did not examine the effect of treatment with oral fluoroquinolones. However, we previously demonstrated that orogastric and subcutaneous administration of levofloxacin yielded similar results in this model (12). Finally, higher doses of ciprofloxacin and levofloxacin did result in promotion of overgrowth and toxin production by C. difficile strains. If prolonged duration of therapy or altered elimination results in higher levels of drug in the colon of humans, our findings may underestimate the propensity of these agents to induce CDAD in patients. Further work is therefore needed to confirm the relevance of our findings to clinical situations.
Acknowledgments
This work was supported by an Advanced Research Career Development Award from the Department of Veterans Affairs to C.J.D.
Footnotes
Published ahead of print on 11 June 2007.
REFERENCES
- 1.Borriello, S. P., F. E. Barclay, and A. R. Welch. 1988. Evaluation of the predictive capability of an in-vitro model of colonization resistance to Clostridium difficile infection. Microb. Ecol. Health Dis. 1:61-64. [Google Scholar]
- 2.Gaynes, R., D. Rimland, E. Killum, K. Lowery, T. M. Johnson, G. Killgore, and F. C. Tenover. 2004. Outbreak of Clostridium difficile infection in a long-term care facility: association with gatifloxacin use. Clin. Infect. Dis. 38:640-645. [DOI] [PubMed] [Google Scholar]
- 3.Gerding, D. N. 2004. Clindamycin, cephalosporins, fluoroquinolones, and Clostridium difficile-associated diarrhea: this is an antimicrobial resistance problem. Clin. Infect. Dis. 38:646-648. [DOI] [PubMed] [Google Scholar]
- 4.Golledge, C. L., C. F. Carson, G. L. O'Neill, R. A. Bowman, and T. V. Riley. 1992. Ciprofloxacin and Clostridium difficile-associated diarrhea. J. Antimicrob. Chemother. 30:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Johnson, S., M. H. Samore, K. A. Farrow, G. E. Killgore, F. C. Tenover, D. Lyras, J. I. Rood, P. DeGirolami, A. L. Baltch, M. E. Rafferty, S. M. Pear, and D. N. Gerding. 1999. Epidemics of diarrhea caused by a clindamycin-resistant strain of Clostridium difficile in four hospitals. N. Engl. J. Med. 341:1645-1651. [DOI] [PubMed] [Google Scholar]
- 6.Loo, V. G., L. Poirier, M. A. Miller, et al. 2005. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N. Engl. J. Med. 353:2442-2449. [DOI] [PubMed] [Google Scholar]
- 7.McCusker, M. E., A. D. Harris, E. Perencevich, and M. C. Roghmann. 2003. Fluoroquinolone use and Clostridium difficile-associated diarrhea. Emerg. Infect. Dis. 9:730-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2500-2505. [DOI] [PubMed] [Google Scholar]
- 9.Muto, C. A., M. Pokrywka, K. Shutt, et al. 2005. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect. Control Hosp. Epidemiol. 26:273-280. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria, 6th ed. Approved standard M11-A6. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 11.Pepin, J., N. Saheb, M. Coulombe, et al. 2005. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin. Infect. Dis. 41:1254-1260. [DOI] [PubMed] [Google Scholar]
- 12.Pultz, N. J., and C. J. Donskey. 2005. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 49:3529-3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolfe, R. D., and S. M. Finegold. 1983. Intestinal β-lactamase activity in ampicillin-induced, Clostridium difficile-associated ileocecitis. J. Infect. Dis. 147:227-235. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan, A., C. Edlund, and C. E. Nord. 2001. Effect of antimicrobial agents on the ecological balance of human microflora. Lancet Infect. Dis. 1:101-114. [DOI] [PubMed] [Google Scholar]
- 15.von Baum, H., A. Sigge, M. Bommer, W. V. Kern, R. Marre, H. Dohner, P. Kern, and S. Reuter. 2006. Moxifloxacin prophylaxis in neutropenic patients. J. Antimicrob. Chemother. 58:891-894. [DOI] [PubMed] [Google Scholar]
- 16.Wistrom, J., S. R. Norrby, E. B. Myhre, S. Eriksson, G. Granstrom, L. Lagergren, G. Englund, C. E. Nord, and B. Svenungsson. 2001. Frequency of antibiotic-associated diarrhea in 2462 antibiotic-treated hospitalized patients: a prospective study. J. Antimicrob. Chemother. 47:43-50. [DOI] [PubMed] [Google Scholar]
- 17.Yip, C., M. Loeb, S. Salama, L. Moss, and J. Olde. 2001. Quinolone use as a risk factor for nosocomial Clostridium difficile-associated diarrhea. Infect. Control Hosp. Epidemiol. 22:572-575. [DOI] [PubMed] [Google Scholar]