Abstract
Apricitabine is a novel deoxycytidine analogue reverse transcriptase inhibitor that is under development for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. Apricitabine is phosphorylated to its active triphosphate by deoxycytidine kinase, which is also responsible for the intracellular phosphorylation of lamivudine (3TC) and emtricitabine (FTC); hence, in vitro studies were performed to investigate possible interactions between apricitabine and these agents. Human peripheral blood mononuclear cells (PBMC) were incubated for 24 h with various concentrations of 3H-labeled or unlabeled apricitabine, 3TC, or FTC. Intracellular concentrations of parent compounds and their phosphorylated derivatives were measured by high-performance liquid chromatography. In other experiments, viral reverse transcriptase activity was measured in PBMC infected with HIV-1 bearing M184V in the presence of various concentrations of apricitabine and 3TC. [3H]apricitabine and [3H]3TC were metabolized intracellularly to form mono-, di-, and triphosphates. 3TC and FTC (1 to 10 μM) produced concentration-dependent decreases in apricitabine phosphorylation; in contrast, apricitabine at concentrations of up to 30 μM had no effect on the phosphorylation of 3TC or FTC. The combination of apricitabine and 3TC reduced the antiviral activity of apricitabine against HIV-1: apricitabine concentrations producing 50% inhibition of viral reverse transcriptase were increased two- to fivefold in the presence of 3TC. These findings suggest that nucleoside reverse transcriptase inhibitors with similar modes of action may show biochemical interactions that affect their antiviral efficacy. It is therefore essential that potential interactions between combinations of new and existing agents be thoroughly investigated before such combinations are introduced into clinical practice.
Agents that target the virus-encoded reverse transcriptase (RT) make up most of the current regimens used for the therapy of human immunodeficiency virus (HIV) infection. The enzyme catalyzes reverse transcription of viral RNA to proviral DNA, which is an essential step in the replication of HIV. Three non-nucleoside RT inhibitors and eight nucleoside or nucleotide RT inhibitors (NRTIs) are currently approved for therapy of HIV infection. However, the emergence of viral resistance as a result of mutations in the RT gene represents a major challenge to therapy. Thus, new agents with activity against drug-resistant strains of HIV type 1 (HIV-1) presenting different patterns of resistance remain a requirement for the effective treatment of HIV infection (2).
One challenge to such developments is the complex nature of pharmacokinetic and pharmacodynamic interactions between antiretroviral agents of the same and different classes. Apricitabine [ATC; (−)2′-deoxy-3′-oxa-4′-thiocytidine (formerly known as AVX754, SPD754, and BCH10618], is a novel heterosubstituted deoxycytidine analogue (Fig. 1) and is a potent inhibitor of HIV replication in vitro, with concentrations producing 50% inhibition (EC50) against HIV-1 ranging from 1.0 to 10.0 μM in T-cell lines and from 0.1 to 3.0 μM in peripheral blood mononuclear cellc (PBMC). In addition to potent antiviral activity, ATC shows a low potential for mitochondrial and cytotoxicity in vitro (11).
FIG. 1.
Structures of ATC, 3TC, and FTC.
Despite the structural similarity of ATC to lamivudine (3TC) and its fluorinated derivative emtricitabine (FTC) (Fig. 1), ATC retains antiretroviral activity against clinical isolates and laboratory strains of HIV-1 with mutations conferring resistance to 3TC, showing only an approximately twofold reduction in susceptibility (1). Similarly, ATC retains a high level of activity against viruses resistant to zidovudine (AZT), with up to five thymidine-associated mutations conferring a median 1.8-fold reduction in susceptibility (1).
ATC is metabolized intracellularly into its monophosphate (MP), diphosphate (DP), and the active triphosphate (TP) derivatives, as well as two additional phosphorylated metabolites (UM1 and UM2), whose structures are yet to be identified (6). The formation of these phosphorylated metabolites is dependent upon the initial intracellular conversion of ATC to ATC-MP, a step catalyzed by deoxycytidine kinase (dCK) (6). The same enzyme is responsible for the initial intracellular phosphorylation of 3TC and FTC, as well as the naturally occurring deoxynucleosides deoxycytidine, deoxyadenosine, and deoxyguanosine. As a result, there exists the potential for competition between ATC and other deoxycytidine analogues for phosphorylation.
ATC has shown additive to slightly synergistic antiviral activity against wild-type HIV-1 laboratory strains in PBMC or MT-4 cells when combined with 3TC, stavudine (d4T), dideoxyinosine (ddI), tenofovir, abacavir, AZT, saquinavir, or neviparine (11). Such combination studies are usually sufficient to identify the potential for antagonistic effects on the antiviral activity of pairs of antiviral agents that would compromise their clinical antiretroviral activity if they were to be coadministered. However, the precision of these studies is not high: in most studies a 20 to 25% reduction in antiviral activity of the combination relative to the combined activity of both components tested separately is regarded as the lower limit of detection of an antagonistic effect of a pair of drugs. The lack of precision of such assays therefore suggests that more detailed studies are warranted in cases when drugs use the same cellular processes for activation or distribution to their site of action. In the case of ATC, the key enzyme responsible for its intracellular metabolism to ATC triphosphate is the same one that is used by both 3TC and FTC. Because of this mechanistic potential for a drug-drug interaction, it was decided that more detailed studies of the effects of 3TC and ATC on each other's intracellular metabolism and the impact this may have upon the results of combination studies were appropriate.
MATERIALS AND METHODS
ATC and its MP and TP derivatives were synthesized at Shire Biochem. (Laval, Canada), and [3H]ATC (15Ci/mmole) was generated by International Isotope Clearing House, Inc. (Leawood, KS). [5-3H]3TC (17.1 Ci/mmol), [5-3H]FTC (5 Ci/mmol), unlabeled 3TC, unlabeled FTC, and their phosphorylated derivatives were obtained from Moravek BioChemical (Brea, CA). Stock solutions of ATC, 3TC, and FTC were prepared at 50 mM in dimethyl sulfoxide and diluted further in culture medium.
Isolation of human PBMC.
Heparinized blood (100 to 300 ml) from a single healthy male donor was diluted 1:1 with phosphate-buffered saline (PBS), and 24-ml aliquots were layered onto 18 ml of Lymphocyte-Mammal (Cederlane, Hornby, Canada) and centrifuged at 800 × g for 30 min at room temperature. The mononuclear cell layer was collected, washed once in PBS, and pelleted by centrifugation. The cells were then resuspended in 5 ml of PBS, and the residual erythrocytes were lysed by the addition of 3 volumes of cold water for 30 s. Erythrocyte lysis was stopped by the addition of 1 volume of 0.6 M sodium chloride made up to 50 ml with PBS, and the mononuclear cells were pelleted by centrifugation.
Culture of PBMC.
The PBMC were suspended in RPMI 1640 supplemented with l-glutamine and 10% heat-inactivated fetal bovine serum (Gibco, Burlington, Canada) before transfer into 75-cm2 tissue culture flasks. For studies involving the use of activated PBMC, the cells were then stimulated with 5 μg of phytohemagglutinin M (PHA-M) (Roche, Laval, Canada)/ml and 10 U of interleukin-2 (Roche)/ml before incubation at 37°C in a humidified 5% CO2 atmosphere for 72 h prior to use. The cell viability was determined by visual inspection.
Intracellular metabolism in PBMC.
Activated or resting PBMC were plated in 25-cm2 culture flasks with 4 ml of medium at densities of 0.75 × 106 and 1.0 × 106 cells/ml, respectively. Initially, the PBMC were incubated for 24 h with 2.6 μM [3H]ATC alone or in the presence of 2.6 μM unlabeled 3TC. Similarly, PBMC were incubated for 24 h with 2.6 μM [3H]3TC alone or with 2.6 μM unlabeled ATC.
In the second experiment activated PBMC were incubated for 24 h with 3, 10, or 30 μM [3H]ATC in the presence of 1, 3, or 10 μM unlabeled 3TC or FTC. The range of concentrations of each compound was chosen to reflect the range of plasma concentrations anticipated after oral administration in humans.
Activated PBMC were also incubated for 24 h with 1, 3, or 10 μM [3H]3TC in the presence of 3, 10, or 30 μM ATC. This procedure was repeated with 1, 3, or 10 μM [3H]FTC in the presence of 3, 10, or 30 μM unlabeled ATC.
At the end of each incubation period cells were harvested by centrifugation at 800 × g for 10 min. at 4°C, followed by one wash with 10 ml of cold PBS. The cell pellets were resuspended in 1 ml of cold PBS and centrifuged at 4°C for 1 min at 14,000 rpm in a microcentrifuge. The pellets were resuspended in 150 μl of cold PBS. The nucleotides or nucleosides were extracted by adding cold methanol to the cells to a final concentration of 60%. The mixtures were then vortex mixed and held at −20°C overnight.
The cell extracts were centrifuged at 14,000 rpm in a microcentrifuge for 5 min at 4°C, and the methanol supernatants were transferred into fresh Eppendorf tubes, and the methanol was evaporated under a gentle flow of nitrogen. The dry residues were stored at −85°C until the high-pressure liquid chromatography (HPLC) analysis was performed.
HPLC analysis.
The stored radioactive residues were dissolved in 130 μl of sterile deionized water, and 100 μl was injected per sample for HPLC analysis, using a C18 reverse-phase column (YMC-005-A, 5 μm, 100 Å, 4.6 mm [inner diameter] by 250 mm; Waters Corp., Milford, MA). The mobile phase consisted of a constant aqueous solution of sodium dihydrogen phosphate (7.5 mM) at pH 6.8 at 1 ml/min. for 50 min. The absorbance of the unlabeled standards was detected at 276 nm with a Waters Associates photodiode array UV detector (LC module 1), and the radioactivity measurements were performed with an online radioactivity detector (Canberra Packard Canada, Ltd., Montreal, Canada) with a liquid scintillation cell using Insta-gel as the scintillation liquid. Chemically synthesized ATC-MP, ATC-TP, and the mono-, di-, and triphosphates of 3TC and FTC were used as analytical standards at 15 to 30 nmol each. Peak areas were quantified by using Millennium software (Waters Corp.). The lower limit of quantification of each metabolite was 0.01 pmol/106 cells, with an accuracy within 7%.
Enzymatic digestion of metabolites.
In order to identify which peaks correspond to nucleotides or other phosphorylated derivatives of ATC or 3TC, dry extracts prepared from 3 × 106 PHA-activated PBMC that had been incubated for 24 h with labeled nucleoside were suspended in 130 μl of PBS containing 4.7 U of alkaline phosphatase. After incubation at 37°C for 3 h, the digested samples were extracted with methanol as described above.
Combination studies with HIV-1 RT carrying the M184V mutation.
For each assay, 96-well culture plates were used to create a checkerboard of drug concentrations, whereby ATC was titrated vertically and 3TC was titrated horizontally. ATC was used in doubling dilutions from 20 to 0.625 μM. 3TC was titrated down from 60 μM in doubling dilutions to 1.9 μM. In a second study, a 2.5-fold dilution range was used for each compound from 50 μM down for ATC and 200 μM down for 3TC. All combinations were repeated in triplicate for each experiment. Control cultures were performed in which the antiviral activity of each compound was determined in the absence of the other.
PBMC stimulated for 3 days with PHA were infected with HIV-1 M184V (provided by M. Wainberg, McGill University AIDS Centre, Montreal, Canada) at a multiplicity of infection of 0.02. After 3 h at 37°C the cells were washed and divided into aliquots to give 2 × 105 cells per well into wells already holding medium containing the previously prepared concentrations of drugs.
At day 4 postinfection half of the medium was removed and replaced with fresh medium containing the respective drug combinations. At 7 days postinfection HIV-1 replication was determined by quantifying the viral RT activity in harvested supernatant fluid. These data were analyzed by plotting dose-response curves for ATC in the presence of increasing combinations of 3TC.
HIV RT assays.
Poly(rA)(dT) at 0.2 μg/ml was used in a 50-μl reaction volume containing 25 μM Tris (pH 7.9), 150 μM KCl, 5 mM MgCl2, 0.5 mM EGTA, 5 mM dithiothreitol, 0.3 mM glutathione, 2.5% polyethylene glycol, 0.5% Triton X-100, 0.6 μM [3H]dTTP (Amersham), and 10 μl of the virus culture supernatant. After overnight incubation at 37°C, the DNA in each sample was precipitated with 50 μl of 16% trichloroacetic acid (TCA) solution per well. The plates were shaken for 2 min and then kept on ice for 30 min before 100 μl of 5% TCA was added per well. Aliquots (200 μl) were transferred onto Millipore multiscreen 96-well HVPP filter plates which were prewetted with 100 μl of 5% TCA.
After aspirating the samples, the plates were washed with 200 μl of 5% TCA per well, and then 150 μl of 5% TCA was added to each well for 2 min. The filter plates were removed from the vacuum apparatus and dried before 50 μl of liquid scintillation cocktail (Supermix; Fisher Scientific, Montreal, Canada) was added to each well. The radioactivity was measured on a Wallac Trilux counter (Perkin-Elmer, Woodbridge, Canada).
Data analysis.
For the whole study nonlinear regression analysis was performed by using Prism (GraphPad Software, San Diego, CA).
RESULTS
Effects of 3TC and FTC on the intracellular metabolism of ATC and vice versa.
Incubation of PBMC in 2.6 μM [3H]ATC or [3H]3TC resulted in their intracellular metabolism, yielding their MP, DP and TP derivatives. The intracellular concentrations observed are detailed in Table 1.
TABLE 1.
Intracellular concentrations of ATC, 3TC, and their phosphorylated metabolites in PBMC after 24 h of incubation
| PBMC and agent | Intracellular concn (μM)
|
|||
|---|---|---|---|---|
| Parenta | MP | DP | TP | |
| Resting PBMC | ||||
| ATC | 0.39 | 0.11 | 0.58 | 0.33 |
| 3TC | 0.1 | 0.12 | 1.01 | 0.73 |
| Activated PBMC | ||||
| ATC | 1.19 | 0.23 | 0.69 | 0.54 |
| 3TC | 0.18 | 0.52 | 1.66 | 0.66 |
Since the volume of a eukaryotic cell is approximately 1 pmol, an intracellular concentration of 1 pmol/106 cells corresponds approximately to an intracellular concentration of 1 μM (8).
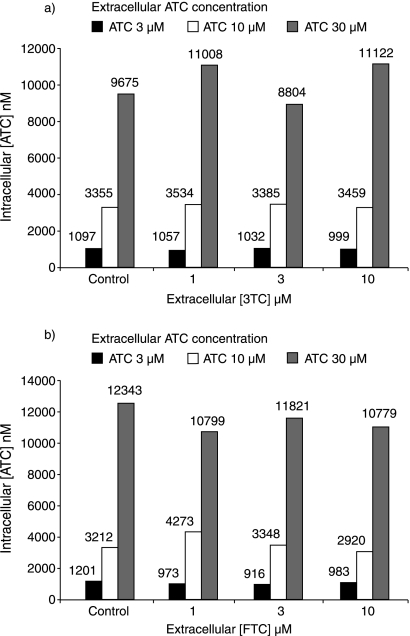
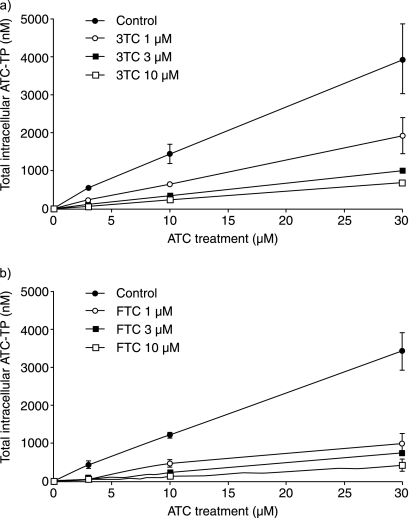
In the second experiment, in which PBMC were incubated in the presence of two compounds, the intracellular concentration of ATC did not change substantially with increasing concentrations of 3TC or FTC but increased proportionately with increasing extracellular ATC levels (Fig. 2). However, as the extracellular concentrations of 3TC and FTC were increased, the intracellular concentrations of all of the ATC phosphorylated metabolites decreased in a concentration-dependent manner. The extent of inhibition ranged from 2-fold at the highest ATC and lowest 3TC concentrations up to 10-fold at the lowest ATC concentration with the highest concentration of 3TC. Likewise, the presence of extracellular FTC reduced the levels of the intracellular phosphorylated metabolites of ATC by between 3- and 11-fold (Fig. 3).
FIG. 2.
Intracellular concentrations of ATC at various extracellular concentrations of ATC and 3TC (a) and FTC (b).
FIG. 3.
Intracellular concentrations of ATC-TP at various extracellular concentrations of ATC and 3TC (a) and FTC (b) in activated human PBMC.
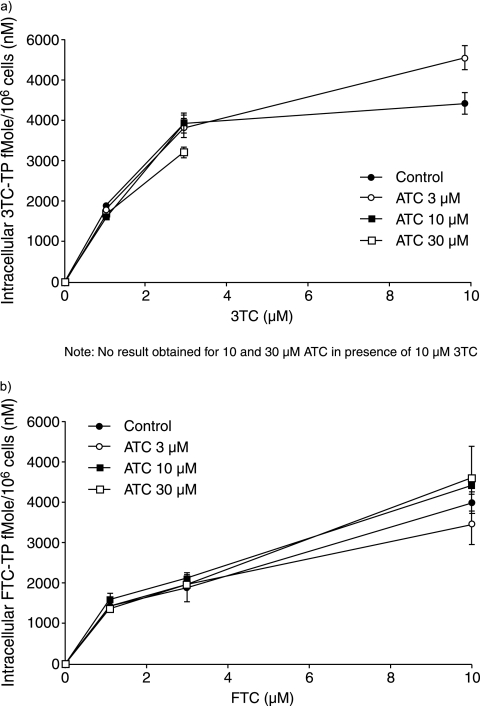
In contrast to the effect of 3TC and FTC on ATC phosphorylation, no changes in the intracellular concentrations of 3TC or FTC phosphorylated metabolites were observed in the presence of ATC at concentrations of up to 30 μM (Fig. 4).
FIG. 4.
Intracellular concentrations of 3TC-TP (a) and FTC-TP (b) at various extracellular concentrations of ATC and 3TC (a) or FTC (b) in activated human PBMC.
Antiviral activity of 3TC and ATC in combination against recombinant HIV-1 carrying the M184V RT mutation.
The observed intracellular interaction between 3TC and ATC was in direct contradiction to previous observations indicating apparent additive antiviral activity when used in combination (11). In an attempt to provide a virological confirmation of this interaction, we conducted a study of the antiviral activity of ATC in the presence of 3TC against HIV carrying the M184V reverse transcriptase mutation. This mutation confers high-level resistance to 3TC, but <2-fold resistance to ATC (1). The use of this virus would therefore allow the pharmacological effects of 3TC on ATC metabolism to be segregated from its own antiviral activity.
In the first experiment, the EC50 value for ATC was 1.9 μM against HIV-1 carrying the M184V mutation. For 3TC, however, only ca. 45% inhibition of replication was evident at 60 μM, and the EC50 value was estimated to be 68 μM. Combination of 3TC with ATC across the range of concentrations studied resulted in a reduction in the observed activity of ATC against the mutant M184V HIV-1, which was unrelated to the 3TC concentration. The resultant EC50 values, determined based on the assumption that 3TC did not contribute to the observed antiviral activity, were raised two- to fivefold (Table 2). EC90 values for ATC could only be calculated in two instances and were approximately twofold higher than the observed value of ATC alone.
TABLE 2.
Observed antiviral activity of ATC against HIV-1 carrying M184V, when in combination with 3TC
| EC type | EC (μM)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Expt A, at 3TC concn (μM)a of:
|
Expt B, at 3TC concn (μM)b of:
|
|||||||||||||
| 0 | 1.88 | 3.75 | 7.5 | 15 | 30 | 60 | 0 | 2 | 5.1 | 12.8 | 32 | 80 | 200 | |
| ATC EC50 | 1.9 | 4.4 | 5.1 | 4.4 | 9.7 | 4.7 | 5.0 | 1.4 | 3.0 | 5.3 | 6.3 | 6.7 | 5.0 | 3.9 |
| ATC EC90 | 11.9 | 15.6 | >20 | 18.7 | >20 | >20 | >20 | 17.9 | 35.2 | 30.4 | 49.6 | >50 | >50 | >50 |
3TC estimated EC50 of 68 μM.
3TC estimated EC50 of 320 μM.
In the second experiment, despite the use of higher concentrations of 3TC, once again the maximum inhibition of viral replication observed was ca. 45% (at 200 μM), and the EC50 value for 3TC in the present study was estimated to be 320 μM. A similar reduction in the inhibitory effect of ATC on mutant M184V HIV-1 replication was observed when ATC was combined with the higher concentrations of 3TC, a finding consistent with the results from the first experiment in this part of the study (Table 2).
DISCUSSION
Current treatment of HIV infection typically involves the use of at least three antiretroviral agents in combination. NRTIs form the backbone of highly active antiviral therapy regimens recommended for the treatment of HIV disease (22). The dependence of NRTIs upon common intracellular phosphorylation pathways for pharmacological activation (21) renders them susceptible to interactions with other nucleoside analogues using the same phosphorylation pathway.
The intracellular interactions between nucleoside analogues are complex and depend on the particular enzymes involved in each nucleoside's metabolism. In general, nucleosides that are closely structurally related often share a common pathway and compete for phosphorylation, which may limit their potential to be used in combination. For example, when AZT and d4T are incubated in combination, AZT is preferentially phosphorylated and the formation of d4TTP is reduced (13-16). This antagonism of d4T phosphorylation by AZT may contribute to the observed poor clinical efficacy of AZT/d4T combination therapy (12).
Similarly, since dCK is responsible for the monophosphorylation of many deoxycytidine analogues, including ATC, 3TC, and FTC, an interaction may be expected between any two of these when used in combination. Indeed, although data showing an interaction between 3TC and FTC have not thus far been published, the similar resistance profiles, mechanisms of action, and phosphorylation pathways for the two drugs negates any rationale to combine these agents, and their coadministration is contraindicated (Emtriva [FTC] prescribing information; Gilead Sciences, Inc., Foster City, CA). These studies of the interaction between ATC and 3TC and between ATC and FTC are therefore the first studies to investigate both the in vitro metabolic and pharmacodynamic interactions of combinations of two cytidine analogues at a range of concentrations consistent with those expected in patients treated with them in clinical practice (10, 17, 19, 23).
The observation that all phosphorylated intracellular metabolites of ATC were reduced by a similar extent is consistent with the hypothesis that this interaction is mediated by the effect of 3TC or one or more of its intracellular metabolites on dCK, which catalyzes the intracellular conversion of ATC to ATC-MP. The data also showed that the presence of either 3TC or FTC inhibited intracellular metabolism of ATC in both resting and activated PBMC to a similar extent. This was not unexpected since high levels of 3TC's phosphorylated metabolites are found in both resting and activated PBMC (Table 1) (9). Since the extracellular concentration of either 3TC or FTC was increased, the intracellular concentrations of all of the ATC phosphorylated metabolites decreased in a concentration-dependent manner, a finding consistent with the known dependencies of the intracellular concentrations of 3TC and its intracellular metabolites upon extracellular 3TC concentration (18).
The intracellular concentration of ATC itself did not change substantially with increasing concentrations of either 3TC or FTC but increased proportionally with the extracellular concentration of ATC. This suggests that entry of ATC into cells is not affected by either 3TC or FTC and is again consistent with the proposition that it is the monophosphorylation of ATC by dCK that is inhibited by the presence of 3TC or FTC or one or more of their intracellular metabolites.
The reduction in the intracellular concentration of ATC-TP in the presence of 3TC and FTC occurs at extracellular concentrations of these agents that are observed in human plasma after administration of the approved dose. Substantial increases in either the plasma concentrations of ATC would be required in the presence of these concentrations of 3TC or FTC to overcome the inhibitory effects of 3TC or FTC and achieve the same levels of ATC-TP that are achieved when PBMC are exposed only to ATC. Conversely, however, ATC does not inhibit the intracellular metabolism of either 3TC or FTC.
The interaction between 3TC and ATC that was observed in the intracellular metabolism studies was in direct contradiction to earlier studies, in which it had been shown that 3TC and ATC have additive antiviral activity against wild-type HIV-1 when used in combination. The failure of conventional antiviral combination studies to detect the one-way interaction that was observed between ATC and 3TC or FTC represents a previously unrecognized limitation of this approach to investigate the potential for antiviral antagonism between pairs of antiviral agents that may compete for intracellular activation. In addition to the unidirectional nature of the interaction, the observed differences between the in vitro and the clinical antiretroviral activities of the two drugs contributed to the poor sensitivity of the standard combination assay to detect the interaction. In in vitro assays of anti-HIV activity, 3TC is approximately 30- to 40-fold more potent than ATC (11). The results of individual short-term monotherapy studies suggest that their clinical antiretroviral activities are only ∼4-fold different (3, 5). The two drugs have similar pharmacokinetic properties (17); hence, various concentrations of drug and triphosphate over time in vivo, as opposed to constant levels throughout in vitro experiments are unlikely to account for the difference between their relative antiviral activities in vitro and in humans. This difference in relative antiviral activity creates the potential for misleading results from in vitro combination studies. Thus, if 3TC and ATC are tested at a concentration ratio equivalent to the ratio of their in vitro antiviral activities, there may be insufficient 3TC present to exert any significant antagonistic effect upon ATC activity. On the other hand, if they are tested in vitro at a concentration ratio equivalent to their clinical antiretroviral activities, the ratio of the concentration of 3TC relative to its EC50 value would be 10 times greater than that of ATC. As a result, 3TC would be expected to contribute >90% of the observed antiviral activity of the combination, preventing observation of a unidirectional antagonism of the antiviral activity of ATC by 3TC.
In this case it was possible to establish that the observed effects on intracellular metabolism of ATC do result in a reduction in the antiviral activity of ATC. This was achieved by performing combination studies with HIV carrying the M184V mutation, which allowed the pharmacological effects of 3TC or FTC on the intracellular metabolism of ATC to be segregated from their own antiretroviral activities. The activity of ATC against M184V HIV-1 was reduced by two- to fivefold when ATC was combined with 3TC. The reduction in ATC antiviral activity by 3TC may in fact be even higher, since it was assumed that 3TC contributed no antiviral activity against the M184V variant in the combination, whereas it has been shown that 3TC retains a low level of antiretroviral activity against viruses carrying the M184V mutation (20). Indeed, in the present study 45% inhibition of M184V HIV-1 replication by 3TC alone was still observed at the top concentration of 3TC used. This suggests that part of the antiviral activity of the 3TC/ATC combination observed at the highest 3TC concentration was due to the residual antiviral activity of 3TC. Hence, the reduction in ATC antiviral activity in the presence of 3TC may be higher than indicated here.
Recently, a phase II study has shown that the antiretroviral activity of the developmental deoxycytidine analogue dexelvucitabine was significantly reduced in the presence of either 3TC or FTC (4). In vitro combination studies with dexelvucitabine and 3TC showed only a marginal effect of 3TC upon intracellular dexelvucitabine triphosphate concentrations and combination studies suggested additivity (7). The discrepancy between these in vitro observations and clinical outcomes underscores the limitations of simple in vitro combination studies and the importance of understanding pharmacokinetic/pharmacodynamic relationships in vivo using relevant viral replication kinetics, tissues, cells, and concentrations of drugs. These results suggest that caution is appropriate when combining nucleoside analogues that are closely structurally related are considered. Indeed, when considerations of patterns of cross-resistance and toxicity profiles are also taken into account, there would appear to be much to recommend combinations of different nucleobase analogues (thymidine, deoxycytidine, deoxyadenosine, or deoxyguanosine) rather than combining NRTIs belonging to the same nucleobase type (i.e., two deoxycytidine analogues, two thymidine analogues, etc.).
These data demonstrate that NRTIs with similar modes of action may interact at a biochemical level, thus impacting on their antiviral potential. It is, therefore, essential that potential pharmacokinetic and pharmacodynamic interactions of new NRTIs with existing agents are investigated preclinically in order to ensure that patients do not receive combination regimens with suboptimal potency.
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Bethell, R. C., Y. S. Lie, and N. T. Parkin. 2005. In vitro activity of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor (NRTI), against 215 HIV-1 isolates resistant to other NRTIs. Antivir. Chem. Chemother. 16:295-302. [DOI] [PubMed] [Google Scholar]
- 2.Blaise, P., P. Clevenbergh, D. Vaira, M. Moutschen, and P. Dellamonica. 2002. HIV resistance to antiretroviral drugs: mechanisms, genotypic and phenotypic resistance testing in clinical practice. Acta Clin. Belg. 57:191-201. [DOI] [PubMed] [Google Scholar]
- 3.Cahn, P., I. Cassetti, R. Wood, P. Phanuphak, L. Shiveley, R. C. Bethell, and J. Sawyer. 2006. Efficacy and tolerability of 10-day monotherapy with apricitabine in antiretroviral-naive, HIV-infected patients. AIDS 20:1261-1268. [DOI] [PubMed] [Google Scholar]
- 4.Cohen, C., C. Katlama, R. Murphy, J. Gathe, C. Brinson, G. Richmond, P. M. Girard, J. Fessel, A. Liappis, E. Puglia, B. Rodwick, J. Nadler, W. O'Brien, K. Arasteh, M. Otto, S. Viitanen-Erickson, and R. Levy. 2005. Antiretroviral activity and tolerability of Reverset (D-d4FC), a new fluoro-cytidine nucleoside analog, when used in combination therapy in treatment-experienced patients: results of phase IIb study RVT-203. Abstr. Third IAS, abstr. WeOaLB0103.
- 5.Delehanty, J., C. Wakeford, L. Hulett, J. Quinn, B. Mccreedy, M. Almond, D. Miralles, and F. Rousseau. 1999. A phase I/II randomized, controlled study of FTC versus 3TC in HIV-infected patients. Abstr. Sixth CROI, abstr. 16.
- 6.De Muys, J. M., H. Gourdeau, N. Ba-Nguyen, D. L. Taylor, P. S. Ahmed, T. Mansour, C. Locas, N. Richard, M. A. Wainberg, and R. F. Rando. 1999. Anti-human immunodeficiency virus type 1 activity, intracellular metabolism, and pharmacokinetic evaluation of 2′-deoxy-3′-oxa-4′-thiocytidine. Antimicrob. Agents Chemother. 43:1835-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson-Viitanen, S., J.-T. Wu, G. Shi, S. Unger, R. W. King, B. Fish, R. Klabe, R. Geleziunas, K. Gallagher, M. J. Otto, and R. F. Schinazi. 2003. Cellular pharmacology of D-D4FC, a nucleoside analog active against drug-resistant HIV. Antivir. Chem. Chemother. 14:39-47. [DOI] [PubMed] [Google Scholar]
- 8.Furman, P. A., J. Fyfe, M. St. Clair, K. Weinhold, J. Rideout, G. Freeman, S. Lehrman, D. Bolognesi, S. Broder, and H. Mitsuya. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, W. Y., R. Agbaria, J. S. Driscoll, and H. Mitsuya. 1994. Divergent Anti-HIV activity and anabolic phosphorylation of 2′-3′-dideoxynucleoside analogs in resting and activated human cells. J. Biol. Chem. 269:12633-12638. [PubMed] [Google Scholar]
- 10.Gish, R. G., N. W. Leung, T. L. Wright, H. Trinh, W. Lang, H. A. Kessler, L. Fang, L. H. Wang, J. Delehanty, A. Rigney, E. Mondou, A. Snow, and F. Rousseau. 2002. Dose range study of pharmacokinetics, safety, and preliminary antiviral activity of emtricitabine in adults with hepatitis B virus infection. Antimicrob. Agents Chemother. 46:1734-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu, Z., B. Allard, J. M. deMuys, J. Lippens, R. F. Rando, N. Ba-Nguyen, C. Ren, P. McKenna, D. L. Taylor, and R. C. Bethell. 2006. The in vitro antiretroviral activity and in vitro toxicity profile of SPD754, a new deoxycytidine nucleoside reverse transcriptase inhibitor for the treatment of HIV infection. Antimicrob. Agents Chemother. 50:625-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havlir, D. V., C. Tierney, G. H. Friedland, R. B. Pollard, L. Smeaton, J. P. Sommadossi, L. Fox, H. Kessler, K. H. Fife, and D. D. Richman. 2000. In vivo antagonism with zidovudine plus stavudine combination therapy. J. Infect. Dis. 182:321-325. [DOI] [PubMed] [Google Scholar]
- 13.Hoggard, P. G., S. D. Sales, S. Kewn, D. Sunderland, S. H. Khoo, C. A. Hart, and D. J. Back. 2000. Correlation between intracellular pharmacological activation of nucleoside analogues and HIV suppression in vitro. Antivir. Chem. Chemother. 11:353-358. [DOI] [PubMed] [Google Scholar]
- 14.Hoggard, P. G., S. Kewn, M. G. Barry, S. H. Khoo, and D. J. Back. 1997. Effects of drugs on 2′,3′-dideoxy-2′,3′-didehydrothymidine phosphorylation in vitro. Antimicrob. Agents Chemother. 41:123-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoggard, P., S. Khoo, M. Barry, and D. Back. 1996. Intracellular metabolism of zidovudine and stavudine in combination. J. Infect. Dis. 174:671-672. [DOI] [PubMed] [Google Scholar]
- 16.Ho, H. T., and M. J. Hitchcock. 1989. Cellular pharmacology of 2′,3′-dideoxy-2′,3′-didehydrothymidine, a nucleoside analog active against HIV. Antimicrob. Agents Chemother. 33:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holdich, T., L. A. Shiveley, and J. Sawyer. 2007. Effect of lamivudine on the plasma and intracellular pharmacokinetics of apricitabine, a novel nucleoside reverse transcriptase inhibitor, in healthy volunteers. Antimicrob. Agents Chemother. 51:2943-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kewn, S., G. J. Veal, P. G. Hoggard, M. G. Barry, and D. J. Back. 1997. Lamivudine (3TC) phosphorylation and drug interactions in vitro. Biochem. Pharmacol. 54:589-595. [DOI] [PubMed] [Google Scholar]
- 19.Molina, J. M., G. Peytavin, S. Perusat, C. Lascoux-Combes, D. Sereni, W. Rozenbaum, and G. Chene. 2004. Pharmacokinetics of emtricitabine, didanosine, and efavirenz administered once-daily for the treatment of HIV-infected adults (pharmacokinetic substudy of the ANRS 091 trial). HIV Med. 5:99-104. [DOI] [PubMed] [Google Scholar]
- 20.Quan, Y., B. G. Brenner, M. Oliveira, and M. A. Wainberg. 2003. Lamivudine can exert a modest antiviral effect against HIV type 1 containing the M184V mutation. Antimicrob. Agents Chemother. 47:747-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray, A. S. 2005. Intracellular interactions between nucleos(t)ide inhibitors of HIV reverse transcriptase. AIDS Rev. 7:113-125. [PubMed] [Google Scholar]
- 22.U.S. Department of Health and Human Services. 2006. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC. [Online.] http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 23.Yuen, G. J., Y. Lou, N. F. Bumgarner, J. P. Bishop, G. A. Smith, V. R. Otto, and D. D. Hoelscher. 2004. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 mg once daily and 150mg twice daily. Antimicrob. Agents Chemother. 48:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]