Abstract
The antiinflammatory cytokine transforming growth factor β (TGF-β) plays an important role in Chagas disease, a parasitic infection caused by the protozoan Trypanosoma cruzi. In the present study, we show that SB-431542, an inhibitor of the TGF-β type I receptor (ALK5), inhibits T. cruzi-induced activation of the TGF-β pathway in epithelial cells and in cardiomyocytes. Further, we demonstrate that addition of SB-431542 greatly reduces cardiomyocyte invasion by T. cruzi. Finally, SB-431542 treatment significantly reduces the number of parasites per infected cell and trypomastigote differentiation and release. Taken together, these data further confirm the major role of the TGF-β signaling pathway in both T. cruzi infection and T. cruzi cell cycle completion. Our present data demonstrate that small inhibitors of the TGF-β signaling pathway might be potential pharmacological tools for the treatment of Chagas disease.
Chagas disease is a widely distributed, debilitating disease process affecting 16 to 18 million people in Latin America that is caused by the intracellular protozoan Trypanosoma cruzi (18). In this disease, transforming growth factor β (TGF-β) has been shown to play a crucial role in parasite infection and multiplication and in cardiac fibrosis (2, 12, 21). It has been demonstrated that antiinflammatory cytokines such as TGF-β can promote parasite survival (8, 20). It has also been shown that cell infection by T. cruzi is markedly enhanced by TGF-β and requires fully functional TGF-β receptors (9, 17). In support of these observations, we have shown that extracellular addition of anti-TGF-β antibodies inhibits T. cruzi infection of cardiomyocytes (23). It has also been shown that T. cruzi infection induces the expression of TGF-β in different models (19, 20), which facilitates parasite survival in mice (20). Moreover, we have also shown that the parasite directly activates latent TGF-β at the surfaces of infected cells (23). Once in the cytoplasm, the amastigote forms of T. cruzi internalize host cell TGF-β, thereby regulating their own intracellular life cycle (22). Taken together, these data clearly indicate an important role for TGF-β in T. cruzi infection and Chagas disease development.
TGF-β belongs to a family of structurally related multifunctional polypeptides participating in the regulation of development, tissue remodeling, differentiation, angiogenesis, inflammation, immune regulation, and fibrosis (14). TGF-β signaling is initiated by ligand binding to a transmembrane receptor with intracellular serine/threonine kinase activity, known as TGF-β receptor-II (TβRΙΙ) (15). Upon ligand binding, TβRII phosphorylates and stimulates the serine/threonine kinase activity of TβRΙ, also known as activin receptor-like kinase 5 (ALK5). Upon activation, ALK5 phosphorylates the cytoplasmic signaling proteins Smad-2 and -3, which then associate with Smad-4, translocate into the nucleus as a multiprotein complex, and stimulate the transcription of TGF-β-responsive genes.
In the present study, we tested the effects of an ALK5 inhibitor, 4-(5-benzo[1,3]dioxol-5-yl-4-pyridin-2-yl-1H-imidazol-2-yl)-benzamide (SB-431542), on the infection of cardiomyocytes by T. cruzi. We demonstrate that the inhibition of ALK5 activity by SB-431542 decreases T. cruzi invasion of cardiomyocytes, inhibits intracellular parasite differentiation, induces parasite apoptosis, and decreases trypomastigote release. Small inhibitors of the TGF-β signaling pathway may therefore represent new pharmacological tools in the treatment of Chagas disease.
MATERIALS AND METHODS
Parasites.
Trypomastigotes of the Y and Dm28c strains of T. cruzi were obtained from the blood of infected mice at the peak of parasitemia (16) and from the supernatants of infected cultured Vero cells on day 4 postinfection as previously described (5), respectively, and were maintained in serum-free medium with 2% bovine serum albumin. All procedures were carried out in accordance with the guidelines established by the Fiocruz Committee of Ethics for the Use of Animals, resolution 242/99.
Cells and cultures.
Cardiomyocytes from mouse embryos were obtained from primary cultures as previously described (16) and maintained in Eagle's medium (Sigma, Saint-Quentin Fallavier, France) supplemented with 7% fetal calf serum (FCS) (Sigma), 100 μg/ml gentamicin (Sigma), 1 mM l-glutamine (Sigma), and 2.5 mM CaCl2. The Mv1Lu mink lung epithelial cell line, stably transfected with a construct in which luciferase expression is driven by the plasminogen activator inhibitor promoter (C32) (1), was maintained in the same medium with 5% FCS, 1,000 U/ml penicillin, and 50 μg/ml streptomycin.
Infection assay.
Cardiomyocytes were seeded in 24-well plates (1 × 105 cells/well) for 24 h at 37°C under an atmosphere of 5% CO2. Cultures were incubated with fresh medium containing 10 μM SB-431542 (Tocris Bioscience, Bristol, United Kingdom) or vehicle for 1 h prior to the addition of 2 ng/ml TGF-β1 (R&D Systems, Abingdon, United Kingdom) or trypomastigotes of the Dm28c clone or Y strain in a parasite-to-host cell proportion of 10:1. At the time indicated, cells were washed with phosphate-buffered saline (PBS), fixed in Bouin's solution, and stained with Giemsa stain. The percentage of cardiomyocytes containing parasites and the number of parasites per infected cell were determined by counting 400 cells/slide on two distinct coverslips at 4, 24, 48, 72, and 96 h postinfection. Analysis was performed with a Zeiss microscope at a magnification of ×400. Data are means ± standard deviations from three independent experiments.
Measurement of TGF-β activation.
C32 cells were plated in 24-well tissue culture plates (1 × 105 cells/per well) in 5% FCS. After 3 h, the medium was changed (0.1% FCS) and cells were incubated with TGF-β1 (2 ng/ml) or live trypomastigotes (1 ×106 to 4 ×106 parasites), with or without SB-431542 (10 μM), as indicated in Fig. 1. After 20 h, cells were harvested and assayed for luciferase activity (Promega, Charbonnières, France). Luciferase activity was measured with a luminometer (Berthold, Monolight 2010). Data are means ± standard deviations from three independent experiments.
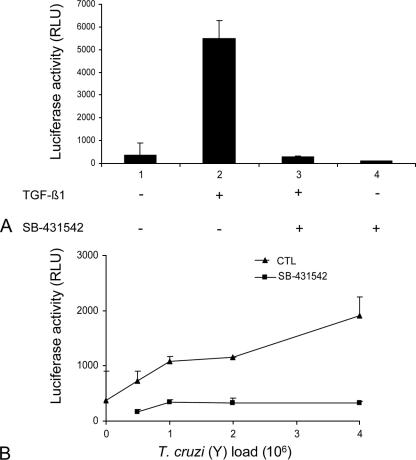
FIG. 1.
SB-431542 treatment inhibits T. cruzi-induced TGF-β signaling in C32 cells. C32 cells were incubated with 2 ng/ml TGF-β1 (A) or different doses of T. cruzi trypomastigotes of the Y strain (1 × 106 to 4 × 106 parasites) (B) in the presence or absence of SB-431542 (10 μM). After 20 h, cells were harvested and assayed for luciferase activity. The luminescence produced by the reaction was recorded as relative light units (RLU).
Immunoblot analysis.
Cells were either left untreated or treated with SB-431542 (10 μM) for 1 h; then TGF-β1 (2 ng/ml) or T. cruzi Dm28c was added for 1 h. Cardiomyocytes were then washed twice with PBS and lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1 mM EGTA, 100 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml pepstatin, 10 μg/ml leupeptin, and 1 μg aprotinin, pH 8.0). Proteins in the lysates (20 μg/lane) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12%) and analyzed by immunoblotting with polyclonal rabbit anti-phospho-Smad2 (Cell Signaling Technology, Beverly, MA). The same membrane was stripped and reprobed with a monoclonal antibody against α-tubulin given to us by D. Job (INSERM U366, CEA-G, Grenoble, France) to confirm equal protein loading.
Apoptosis detection.
Apoptotic events were analyzed using the terminal deoxynucleotidyltransferase-mediated fluorescein dUTP nick end labeling (TUNEL) technique (Boehringer Mannheim, Mannheim, Germany). Cardiomyocyte cultures grown on coverslips were fixed for 20 min at 4°C with 4% paraformaldehyde solution. Samples were washed twice with PBS, treated for 2 min at 4°C with 0.1% Triton X-100 in 0.1% sodium citrate, washed again, and incubated with the TUNEL reaction mixture for 60 min at 37°C. For negative controls, cells were incubated in the absence of the terminal transferase; for positive controls, the cultures were pretreated for 40 min at room temperature with 5 μg/ml DNase I prior to the TUNEL procedure. After staining, samples were further incubated with 10 μg/ml 4′,6′-diamidino-2-phenylindole for DNA staining in order to enable visualization of parasites and cell nuclei and to allow direct quantification of the parasite infection levels. Samples were mounted with 2.5% 1,4-diazabicyclo-(2,2,2)-octane (DABCO) and examined immediately using a Zeiss photomicroscope equipped with epifluorescence. Images were captured using the Quips Smart Capture (Vysis) software.
Statistics.
For comparison of the mean values for control and treated cells, a nonparametric Student t test was used.
RESULTS
SB-431542 treatment inhibits the T. cruzi-induced TGF-β signaling pathway in C32 cells.
We and others have previously demonstrated that T. cruzi infection activates the TGF-β signaling pathway and that this pathway is required for T. cruzi infection (17, 23). We therefore wanted to study the effect of the ALK5 inhibitor SB-431542 on T. cruzi-induced TGF-β signaling. To test this, we used a mink lung epithelial cell clone (C32) that stably expresses the firefly luciferase reporter gene under the control of repeated TGF-β response elements from the PAI-1 gene promoter (1). As a control, we could show that the addition of 10 μM SB-431542 completely inhibited luciferase activity induced by TGF-β1 (Fig. 1A). Incubation of C32 cells with increasing amounts of live T. cruzi trypomastigotes (Y strain) increased luciferase activity as a function of parasite load, indicating that parasites were able to stimulate the production of active TGF-β by C32 cells (Fig. 1B). Addition of SB-431542 (10 μM) completely inhibited the T. cruzi-induced TGF-β reporter gene activity at all the parasitic loads tested (Fig. 1B).
SB-431542 treatment inhibits T. cruzi-induced TGF-β signaling in cardiomyocytes.
Having demonstrated that the TGF-β inhibitor SB-431542 blocks T. cruzi-induced TGF-β activation in epithelial cells, we then tested its effects in cardiomyocytes, a major target of T. cruzi infection. Again, as a control, we showed that the addition of SB-431542 (10 μM) to cardiomyocyte cultures completely inhibited TGF-β1-induced Smad2 phosphorylation (Fig. 2, lanes 2 and 3). In order to test the effects of T. cruzi infection on Smad2 phosphorylation, we used the T. cruzi Dm28c clone, which invades target cells more rapidly than the Y strain (7). Figure 2 shows that T. cruzi infection induced Smad2 phosphorylation within 1 h. Addition of SB-431542 (10 μM) completely inhibited T. cruzi-induced Smad2 phosphorylation (Fig. 2, lanes 4 and 5).
FIG. 2.
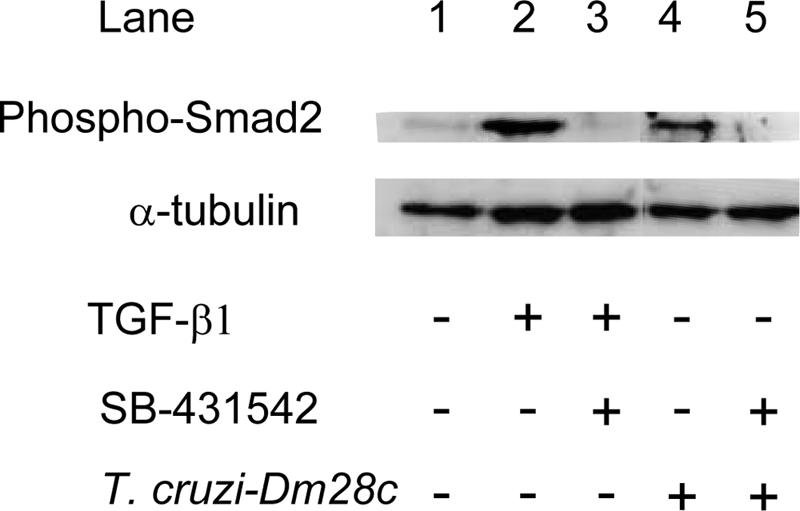
SB-431542 treatment inhibits T. cruzi-induced phosphorylation of Smad2 in cardiomyocytes. Cardiomyocytes were treated with 2 ng/ml TGF-β1 or infected with T. cruzi (Dm28c) for 1 h in the presence or absence of 10 μM SB-431542. Cell lysates (20 μg) were resolved on a 12% sodium dodecyl sulfate-polyacrylamide gel and immunoblotted with anti-phosphoSmad2 and anti-α-tubulin antibodies.
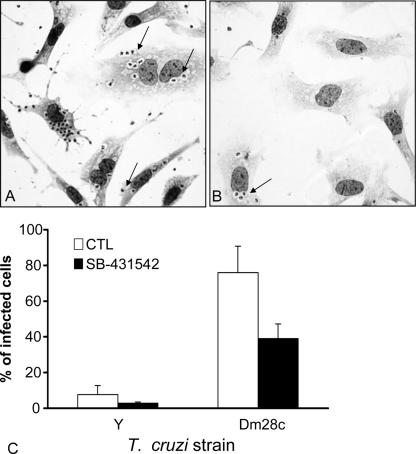
SB-431542 treatment decreases T. cruzi invasion of cardiomyocytes.
Given that active extracellular TGF-β and functional TGF-β receptors are required for T. cruzi entry into cardiomyocytes (17, 23), we wondered whether SB-431542 could change the rates of invasion of these cells by T. cruzi. To investigate this, cardiomyocytes were either left untreated or treated for 1 h with 10 μM SB-431542 and then infected with live parasites (T. cruzi Dm28c or Y strain) for 4 h. As shown in Fig. 3A and B, the addition of 10 μM SB-431542 reduced the percentage of cells infected with Dm28c. Quantification of the percentage of infected cells is shown in Fig. 3C. SB-431542 strongly inhibited invasion by T. cruzi Dm28c (39% of SB-431542-treated cells were infected versus 75% of untreated cells) and also by the Y strain (2.8% of SB-431542-treated cells were infected versus 7.7% of untreated cells) (Fig. 3C). To test the specificity of this effect, we used wortmannin, an inhibitor of a phosphatidylinositol 3-kinase, and H89, an inhibitor of protein kinase A, and found much weaker inhibition (data not shown). To address the possibility of a direct toxic effect of SB-431542 on live parasites, we treated live trypomastigotes with 10 μM SB-431542 for 24 h at 37°C and found no alteration (data not shown) in parasite morphology, motility, or viability (assessed by trypan blue dye exclusion).
FIG. 3.
SB-431542 treatment decreases T. cruzi invasion of cardiomyocytes. Cardiomyocytes were incubated with trypomastigotes of the Dm28c (A, B, and C) or Y (C) strain in a parasite-to-host cell proportion of 10:1 with or without SB-431542 (10 μM). Cells were fixed 4 h postinfection and stained with Giemsa stain. (A and B) Cells were incubated without an inhibitor (A) or with SB-431542 (B). Arrows indicate infected cells. Magnification, ×400. (C) Results are expressed as the percentage of cardiomyocytes containing parasites as measured by counting 400 cells per slide on two distinct coverslips. *, P < 0.05.
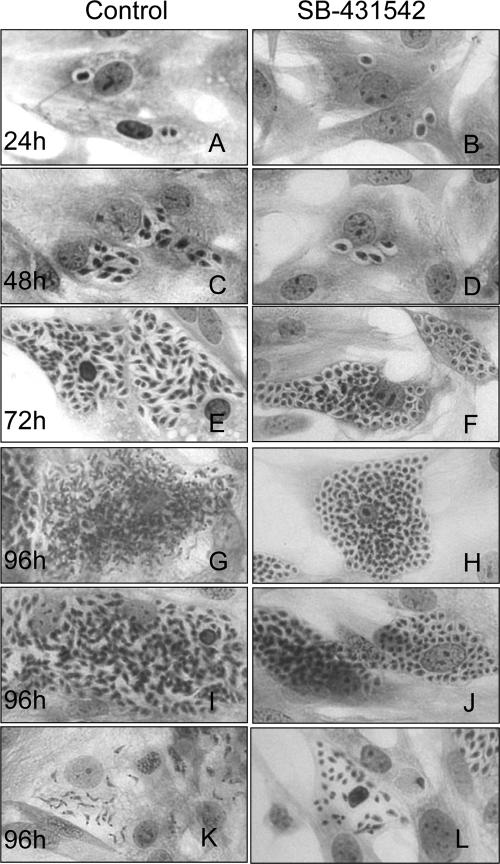
SB-431542 treatment inhibits the intracellular T. cruzi cell cycle.
Since we have recently shown that TGF-β is implicated in T. cruzi infection but also controls its intracellular life cycle (22), we examined whether the TGF-β inhibitor SB-431542 could affect the intracellular parasite cycle. For this purpose, cells were treated throughout the experiment with or without SB-431542. Our results showed that the addition of SB-431542 (10 μM) reduced the number of intracellular amastigotes (Fig. 4). Quantification of the number of intracellular parasites during the cycle showed that there was already a significant difference in the mean number of intracellular parasites at the beginning of the infection (24 h) (Table 1). This difference increased throughout the infection, reaching the highest differences at 96 h (163 versus 58 parasites in control versus SB-431542-treated cells, respectively). As shown in Table 1, treatment with SB-431542 also strongly inhibited trypomastigote release (106 versus 19 parasites in control versus SB-431542-treated cells, respectively). The numbers of nontrypomastigote parasites (both amastigotes and epimastigote-like parasites) released were similar in SB-431542-treated cells and control cells. When we looked more precisely at the forms of intracellular parasites present in untreated cells at 96 h postinfection, we observed cells with differentiated trypomastigote forms (Fig. 4G), cells with parasites in differentiation forms (Fig. 4I), and disrupted cells with trypomastigotes (Fig. 4K). In contrast, in cells treated with SB-431542, we observed mainly undifferentiated parasites (Fig. 4H, J, and L), even on disrupted cells (Fig. 4L).
FIG. 4.
SB-431542 treatment inhibits the T. cruzi intracellular cell cycle. Cardiomyocytes were incubated with trypomastigotes of the Y strain in a parasite-to-host cell proportion of 10:1 with or without SB-431542 (10 μM). Cells were fixed 24, 48, 72, and 96 h postinfection, as indicated, and stained with Giemsa stain. Magnification, ×400.
TABLE 1.
SB-431542 treatment inhibits the intracellular T. cruzi cell cyclea
| Treatment | No. of parasites/infected cell after the following time postinfection:
|
No. of parasites released (104) at 96 h
|
% of TUNEL-positive parasitesc at the following time postinfection:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | Trypomastigote | Nontrypomastigoteb | 72 h | 96 h | |
| CTL | 2.0 ± 0.4 | 9.0 ± 1.1 | 66 ± 43 | 163 ± 50 | 106 ± 8.5 | 50 ± 47 | 16.3 ± 7.1 | 15.9 ± 19.04 |
| SB-431542 | 1.4 ± 0.1** | 4.4 ± 1.4** | 38 ± 20** | 58 ± 30** | 19 ± 27* | 53 ± 40 | 47.9 ± 20.3 | 26.5 ± 12.5 |
Cardiomyocytes were incubated with trypomastigotes of the Y strain in a parasite-to-host cell proportion of 10:1 with or without SB 431542 (10 μM). Cells were fixed and stained with Giemsa stain 24, 48, 72, and 96 h postinfection. Results were determined by counting 400 cells on two distinct slides. **, P < 0.01; *, P < 0.05.
Includes amastigote and epimastigote-like forms of the parasite.
Apoptotic parasites, quantified by using the TUNEL method.
As first described by de Souza et al. (7), intracellular parasites can undergo apoptosis. We therefore studied if the decrease in the number of parasites could be due to an effect of SB-431542 (10 μM) on T. cruzi apoptosis. As shown in Table 1, we found that SB-431542 treatment increased T. cruzi apoptotic rates. This effect was more pronounced at 72 h than at 96 h.
DISCUSSION
Due to the known roles of TGF-β in Chagas disease, antagonizing the biological effects of TGF-β represents an attractive experimental strategy to combat this disease. This study shows that by using the low- molecular-weight ALK5 inhibitor SB-431542 to block the activation of TβRI (ALK5), we are able to significantly inhibit T. cruzi invasion of cardiomyocytes, to arrest the intracellular life cycle of the parasite, and to strongly inhibit trypomastigote release.
The present demonstration that modulation of intracellular TGF-β signal transduction by SB-431542 inhibits T. cruzi infection in cardiac myocytes confirms that the ALK5/Smad2 signaling pathway is required for parasite colonization of the heart and that it probably plays a direct role in Chagas cardiomyopathy. Furthermore, we show in the present work that SB-431542 not only inhibits T. cruzi invasion but also strongly reduces trypomastigote release. This result is important, because it demonstrates that inhibiting TGF-β signaling not only blocks T. cruzi invasion but can also act at a later stage during the release of mature parasites that will infect other cells and amplify the infection. The fact that SB-431542 inhibits the intracellular parasite cell cycle is in accordance with our previous work where we demonstrated that T. cruzi is able to internalize TGF-β and that this host TGF-β could have a direct role in the biology of the parasite (22). We found that SB-431542 treatment decreases the number of parasites within each cell. This decrease can be attributed, at least partially, to the increase in the rate of parasite apoptosis induced by SB-431542. However, we cannot completely exclude the possibility that SB-431542 could also have an effect on parasite proliferation. It has been demonstrated that T. cruzi infection induces the production of nitric oxide (NO), which could contribute to parasite killing by murine macrophages. TGF-β, which is produced during the infection, is a potent suppressor of NO production (8). In the absence of an active TGF-β pathway, there could be exacerbated NO production and the consumption of arginine by inducible NO synthase activation. Increased NO generation could indirectly impair parasite survival and growth and directly kill parasites by inducing their apoptosis. The other interesting point that we also show here is that in the presence of SB-431542, we mainly observed infected cells with undifferentiated parasites. We will need to further elucidate whether the TGF-β signaling pathway is implicated in the differentiation of amastigotes into trypomastigotes or whether the inhibition of differentiation is due to a decrease in the proliferation of amastigotes, which then do not reach a density that allows them to differentiate and to induce host cell disruption.
SB-431542 is a synthetic small molecule that has been identified as a specific inhibitor of TGF-β signals mediated by ALK4, ALK5, or ALK7 in cultured mammalian cells (4, 11, 13). This inhibitor binds to the ATP binding site of the type I receptor kinases and blocks phosphorylation of the downstream effectors Smad2 and Smad3. SB-431542 has recently been described as able to prevent Smad2 phosphorylation in vivo in Xenopus and Danio embryos (10) but has not yet been investigated in other in vivo animal models. Two orally active TGF-β inhibitors have recently been described as blockers of TGF-β1-induced lung fibrosis (3) and dimethylnitrosamine-induced liver fibrosis (6). This suggests that inhibition of TGF-β signaling could potentially be employed in the reversal of the fibrosis observed in Chagas disease.
In summary, we demonstrated that SB-431542, a kinase inhibitor of TβRI (ALK5), strongly inhibits T. cruzi infectivity in cardiomyocytes and, most importantly, is able to arrest parasite multiplication and release. Considering the crucial role of TGF-β in T. cruzi infection and differentiation, as well as in the fibrosis that accompanies Chagas disease, as well as the current lack of effective therapeutic approaches during the chronic phase, it would be relevant to test orally active inhibitors of TGF-β signaling that present good pharmacokinetic and pharmacodynamic properties for the control of T. cruzi infection in preclinical animal models.
Acknowledgments
We thank J. LaMarre (University of Guelph, Guelph, Ontario, Canada) for review of the manuscript and N. Laping (Glaxo Smith Kline) and Mirian Pereira (Oswaldo Cruz Institute) for helpful suggestions.
This work was supported by the INSERM-FIOCRUZ collaborative research program, by grants from PAPES/FIOCRUZ, IOC, CNPq, and FAPERJ to the Brazilian laboratories, and by recurrent funding from INSERM and CEA to the French laboratory.
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Abe, M., J. G. Harpel, C. N. Metz, I. Nunes, D. J. Loskutoff, and D. B. Rifkin. 1994. An assay for transforming growth factor-beta using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal. Biochem. 216:276-284. [DOI] [PubMed] [Google Scholar]
- 2.Araujo-Jorge, T. C., M. C. Waghabi, A. M. Hasslocher-Moreno, S. S. Xavier, L. Higuchi Mde, M. Keramidas, S. Bailly, and J. J. Feige. 2002. Implication of transforming growth factor-β1 in Chagas disease myocardiopathy. J. Infect. Dis. 186:1823-1828. [DOI] [PubMed] [Google Scholar]
- 3.Bonniaud, P., P. J. Margetts, M. Kolb, J. A. Schroeder, A. M. Kapoun, D. Damm, A. Murphy, S. Chakravarty, S. Dugar, L. Higgins, A. A. Protter, and J. Gauldie. 2005. Progressive transforming growth factor β1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am. J. Respir. Crit. Care Med. 171:889-898. [DOI] [PubMed] [Google Scholar]
- 4.Callahan, J. F., J. L. Burgess, J. A. Fornwald, L. M. Gaster, J. D. Harling, F. P. Harrington, J. Heer, C. Kwon, R. Lehr, A. Mathur, B. A. Olson, J. Weinstock, and N. J. Laping. 2002. Identification of novel inhibitors of the transforming growth factor β1 (TGF-β1) type 1 receptor (ALK5). J. Med. Chem. 45:999-1001. [DOI] [PubMed] [Google Scholar]
- 5.Cavallesco, R., and M. E. Pereira. 1988. Antibody to Trypanosoma cruzi neuraminidase enhances infection in vitro and identifies a subpopulation of trypomastigotes. J. Immunol. 140:617-625. [PubMed] [Google Scholar]
- 6.de Gouville, A. C., V. Boullay, G. Krysa, J. Pilot, J. M. Brusq, F. Loriolle, J. M. Gauthier, S. A. Papworth, A. Laroze, F. Gellibert, and S. Huet. 2005. Inhibition of TGF-β signaling by an ALK5 inhibitor protects rats from dimethylnitrosamine-induced liver fibrosis. Br. J. Pharmacol. 145:166-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza, E. M., T. C. Araujo-Jorge, C. Bailly, A. Lansiaux, M. M. Batista, G. M. Oliveira, and M. N. Soeiro. 2003. Host and parasite apoptosis following Trypanosoma cruzi infection in in vitro and in vivo models. Cell Tissue Res. 314:223-235. [DOI] [PubMed] [Google Scholar]
- 8.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 9.Hall, B. S., and M. A. Pereira. 2000. Dual role for transforming growth factor β-dependent signaling in Trypanosoma cruzi infection of mammalian cells. Infect. Immun. 68:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho, D. M., J. Chan, P. Bayliss, and M. Whitman. 2006. Inhibitor-resistant type I receptors reveal specific requirements for TGF-β signaling in vivo. Dev. Biol. 295:730-742. [DOI] [PubMed] [Google Scholar]
- 11.Inman, G. J., F. J. Nicolas, J. F. Callahan, J. D. Harling, L. M. Gaster, A. D. Reith, N. J. Laping, and C. S. Hill. 2002. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62:65-74. [DOI] [PubMed] [Google Scholar]
- 12.Khan, R., and R. Sheppard. 2006. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118:10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laping, N. J., E. Grygielko, A. Mathur, S. Butter, J. Bomberger, C. Tweed, W. Martin, J. Fornwald, R. Lehr, J. Harling, L. Gaster, J. F. Callahan, and B. A. Olson. 2002. Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol. Pharmacol. 62:58-64. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence, D. A. 2001. Latent-TGF-β: an overview. Mol. Cell. Biochem. 219:163-170. [DOI] [PubMed] [Google Scholar]
- 15.Massague, J., and R. R. Gomis. 2006. The logic of TGFβ signaling. FEBS Lett. 580:2811-2820. [DOI] [PubMed] [Google Scholar]
- 16.Meirelles, M. N., T. C. de Araujo-Jorge, C. F. Miranda, W. de Souza, and H. S. Barbosa. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 41:198-206. [PubMed] [Google Scholar]
- 17.Ming, M., M. E. Ewen, and M. E. Pereira. 1995. Trypanosome invasion of mammalian cells requires activation of the TGF β signaling pathway. Cell 82:287-296. [DOI] [PubMed] [Google Scholar]
- 18.Moncayo, A. 2003. Chagas disease: current epidemiological trends after the interruption of vectorial and transfusional transmission in the Southern Cone countries. Mem. Inst. Oswaldo Cruz 98:577-591. [DOI] [PubMed] [Google Scholar]
- 19.Samudio, M., S. Montenegro-James, E. Kasamatsu, M. Cabral, A. Schinini, A. Rojas De Arias, and M. A. James. 1999. Local and systemic cytokine expression during experimental chronic Trypanosoma cruzi infection in a Cebus monkey model. Parasite Immunol. 21:451-460. [DOI] [PubMed] [Google Scholar]
- 20.Silva, J. S., D. R. Twardzik, and S. G. Reed. 1991. Regulation of Trypanosoma cruzi infections in vitro and in vivo by transforming growth factor beta (TGF-β). J. Exp. Med. 174:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waghabi, M. C., C. M. Coutinho, M. N. Soeiro, M. C. Pereira, J. J. Feige, M. Keramidas, A. Cosson, P. Minoprio, F. Van Leuven, and T. C. Araujo-Jorge. 2002. Increased Trypanosoma cruzi invasion and heart fibrosis associated with high transforming growth factor β levels in mice deficient in α2-macroglobulin. Infect. Immun. 70:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waghabi, M. C., M. Keramidas, S. Bailly, W. Degrave, L. Mendonca-Lima, N. Soeiro Mde, N. Meirelles Mde, S. Paciornik, T. C. Araujo-Jorge, and J. J. Feige. 2005. Uptake of host cell transforming growth factor-beta by Trypanosoma cruzi amastigotes in cardiomyocytes: potential role in parasite cycle completion. Am. J. Pathol. 167:993-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waghabi, M. C., M. Keramidas, J. J. Feige, T. C. Araujo-Jorge, and S. Bailly. 2005. Activation of transforming growth factor beta by Trypanosoma cruzi. Cell. Microbiol. 7:511-517. [DOI] [PubMed] [Google Scholar]