Abstract
Atypical strains, presumed to be pneumococcus, with ciprofloxacin MICs of ≥4.0 μg/ml and unique sequence variations within the quinolone resistance-determining regions (QRDRs) of the gyrase and topoisomerase genes in comparison with the Streptococcus pneumoniae R6 strain, were examined. These strains were reidentified using phenotypic methods, including detection of optochin susceptibility, bile solubility, and agglutination by serotype-specific antisera, and genotypic methods, including detection of pneumolysin and autolysin genes by PCR, 16S rRNA sequencing, and multilocus sequence typing (MLST). The analysis based on concatenated sequences of the six MLST loci distinguished the “atypical” strains from pneumococci, and these strains clustered closely with S. mitis. However, all these strains and five of nine strains from the viridans streptococcal group possessed one to three gyrA, gyrB, parC, and parE genes whose QRDR sequences clustered with those of S. pneumoniae, providing evidence of horizontal transfer of the QRDRs of the gyrase and topoisomerase genes from pneumococci into viridans streptococci. These genes also conferred fluoroquinolone resistance to viridans streptococci. In addition, the fluoroquinolone resistance determinants of 32 well-characterized Streptococcus mitis and Streptococcus oralis strains from bacteremic patients were also compared. These strains have unique amino acid substitutions in GyrA and ParC that were distinguishable from those in fluoroquinolone-resistant pneumococci and the “atypical” isolates. Both recombinational events and de novo mutations play an important role in the development of fluoroquinolone resistance.
It has been recognized that an overestimation of antimicrobial resistance may result from the misidentification of oral streptococci. Misleading macrolide and penicillin resistance rates in Streptococcus pneumoniae had resulted from the use of one or more phenotypic/genotypic characteristics in the identification of pneumococcus (28, 38). In a study by Neeleman et al. (28), the identities of 141 macrolide-resistant presumptive pneumococcal isolates from 38 laboratories were reexamined. Only 65% (91/141) of the strains were confirmed as S. pneumoniae, while 23% (32/141) were Streptococcus mitis and the remaining streptococcal species. This led to significant overreporting of macrolide-resistant S. pneumoniae. Increasingly, “atypical” pneumococcal isolates that do not have the defining characteristics of S. pneumoniae, such as optochin susceptibility and deoxycholate (bile) solubility, have been identified, while other closely related oral streptococci, such as S. mitis and Streptococcus oralis, are found to harbor the pneumolysin (ply) and/or the autolysin (lytA) genes (13, 14, 39), causing confusion in the presumptive identification of S. pneumoniae in a diagnostic laboratory. Likewise, those strains which are discrepant in phenotype(s) and/or genotype(s) have also been identified from surveillance studies in Asian countries (22).
The development of fluoroquinolone resistance in S. pneumoniae is a global concern, as it is an option for use in the era of drug-resistant S. pneumoniae (24, 25). In most countries, fluoroquinolone resistance remained low, in the range of 0% to 1.8% (1, 33, 35) and the strains were genetically diverse. An exception was seen in Hong Kong, in which a high percentage of levofloxacin (LEV) resistance, ranging from 3.8% to 13.3%, was reported, and the strains were related to the Spanish 23F-1 clone (15, 16). The prevalence of resistance to fluoroquinolone would be misrepresented if S. pneumoniae and other oral streptococci were not correctly identified. A high prevalence of fluoroquinolone resistance in the closely related species S. mitis and S. oralis has been reported, with up to one-third of hospitalized patients having carried fluoroquinolone-resistant viridans streptococci in their oropharynges (12). These viridans streptococci have been demonstrated in vitro as potential donors of the gyrase, topoisomerase, and efflux genes to S. pneumoniae in transformation (3, 12, 19). However, so far, there is little evidence to support the notion that interspecies recombination of fluoroquinolone resistance determinants is a common phenomenon in pneumococci (31, 36), except for a few ciprofloxacin (CIP)-resistant strains that had been described previously (9). On the contrary, recent data are supporting the notion that the reverse is more plausible, with transfer of these genes from pneumococci to other viridans streptococci (36).
Previously, we utilized a rapid screening method using PCR-restriction fragment (RF)-single-strand conformation polymorphism (SSCP) (17) to screen for variants/mutations at the quinolone resistance-determining regions (QRDRs) of the gyrase and topoisomerase genes in CIP-resistant S. pneumoniae (MIC ≥ 4.0 μg/ml). Using PCR-RF-SSCP, we identified a collection of atypical strains, presumed to be pneumococcus, with CIP MICs of ≥4.0 μg/ml and unique patterns suggesting sequence variations within the QRDR in comparison with S. pneumoniae R6 and possible recombinational events with other streptococci. We thus characterized these strains by phenotypic methods, including detection of optochin susceptibility, bile solubility, and agglutination by serotype specific antisera, and genotypic methods, such as detection of the pneumolysin and autolysin genes, 16S rRNA sequencing, and multilocus sequence typing (MLST). In addition, we sought to compare the fluoroquinolone susceptibilities and respective resistance determinants of 32 well-characterized clinical isolates of S. mitis and S. oralis to those of the “atypical” pneumococcal strains.
MATERIALS AND METHODS
Bacterial isolates.
Two groups of nonduplicate clinical isolates were studied: 26 strains, presumptively identified as pneumococci, with MICs of ≥4.0 μg/ml, from respiratory specimens of patients between 2000 and 2003 and 32 isolates of Streptococcus mitis (12) and Streptococcus oralis (20) from blood, cerebrospinal fluid, and body fluid samples of patients isolated between 1994 and 2005. All pneumococcal strains were isolated from patients admitted to the Prince of Wales Hospital, a 1,350-bed teaching hospital in Hong Kong. These strains had been identified based on a standard methodology with hemolysis on blood agar, Gram stain morphology, optochin susceptibility detection, and (where indicated) the ABI32 Strept system (BioMerieux, Marcy d' etoile, France). S. mitis and S. oralis had also been characterized by MLST (7, 18). Optochin susceptibility detection was performed with a 5-μg optochin disk (Oxoid, Basingstoke, Hampshire, England) on horse blood agar plates. The plates were incubated for 18 to 24 h at 35°C in both 5% CO2 and ambient air. A zone of inhibition of ≥14 mm was defined as sensitive. Bile solubility detection was performed according to a standard protocol. S. pneumoniae was suspended in two tubes containing 1 ml 0.85% NaCl saline solution. A few drops of 10% sodium deoxycholate solution were added to one tube while saline was added to the control tube, and the suspensions were incubated at 35°C for 15 min. A positive test was indicated by visible clearing of the suspension. Reference strains used included S. pneumoniae R6 and ATCC 49619, S. mitis ATCC 49456, and S. oralis ATCC 10557.
Antibiotic susceptibility testing, detection of efflux phenotype, and serotypes.
The MICs of CIP and LEV were determined using a broth dilution method according to the Clinical Laboratory Standards Institute (CLSI, formerly NCCLS) (8). S. pneumoniae ATCC 6303 and ATCC 49619 were included as controls. Reduced drug accumulation due to efflux phenotype was examined for isolates with CIP MICs of ≥2 μg/ml using agar dilution in the presence of CIP with or without reserpine (10 μg/ml). The isolates which had fourfold or greater decreases in CIP MIC in the presence of reserpine were considered to have a positive efflux phenotype (6).
DNA isolation and amplification of pneumolysin (ply) and autolysin (lytA) genes by PCR.
DNA was extracted using a Wizard genomic DNA purification kit (Promega, Madison, WI). PCR on the autolysin (lytA) and pneumolysin (ply) genes was performed with a modification of the method of Rudolph et al. (34). DNA amplification was carried out in 10 pmol of each primer, 1× PCR buffer (GE Healthcare), a 200 μM final concentration of each nucleotide (GE Healthcare), 1 unit of Taq DNA Polymerase (GE Healthcare), and approximately 10 ng DNA extract in a 25-μl total volume. The reaction condition used was 96°C for 3 min, followed by 30 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 50 s, and a final extension at 72°C for 5 min. One microliter of the amplified mixture was added for the second-round PCR. The same reaction condition was used, except with 25 cycles. The specificities of the PCRs were examined using DNA extracted from clinical isolates of Streptococcus milleri, S. bovis, S. agalactiae, S. mitis, S. salivarius, S. equinus, S. anginosus, S. constellatus, a group G Streptococcus, and S. pyogenes ATCC 19615.
Analysis of fluoroquinolone resistance determinants by PCR-RF-SSCP.
The mutations and the amino acid substitutions at the QRDRs in the gyrA, gyrB, parC, and parE genes were analyzed by PCR-RF-SSCP as previously described (17). Briefly, for each isolate, PCR amplicons of QRDRs of the gyrA and -B and parC and -E genes were digested with AluI, HinFI, Sau3AI, and MspI, respectively, and analyzed by SSCP. SSCP patterns were correlated with mutations characterized from sequence analyses of PCR amplicons, and these were aligned and compared with the corresponding region in the R6 strain, obtained from the NCBI database.
MLST and sequencing.
An approximately 500-bp internal fragment of each of the six MLST genes (13) was amplified from chromosomal DNA of the isolates by PCR and then sequenced based on the pneumococcal MLST scheme recommended (http://spneumoniae.mlst.net/). Two self-designed primers were used for spi when the recommended primers did not work, and they were spi-up2 (5′-ACG CTT AGA AAG GTA AGT TAT G-3′) and spi-dn2 (5′-GGT TTC TTA AAA TGT TCC GAT AC-3′) (7). A 1,300-bp 16S rRNA gene fragment was amplified by PCR and sequenced. The primer pairs were 16srRNA-8F (5′-AGA GTT TGA TCC TGG (C/T)TC AG-3′) and 16srRNA-R (5′-CGG GAT CCC AGG CCC GGG AAC GTA TTC AC-3′). Sequencing was performed using two additional primers, 16srRNA-247F (5′-GTT GGT GAG GTA ACG GC-3′) and 16srRNA-1088R (5′-CTA GGG CGG TCA TCG GG-3′). All the sequencing was performed with an ABI310 sequencer and an ABI Prism d-rhodamine terminator cycle sequencing kit (Applied Biosystems, Foster City, CA).
MLST and phylogenetic analysis.
The sequences of the six of the seven relevant MLST loci (ddl sequences were omitted from analysis) were concatenated to obtain contigs 2,758 bp in length. A few isolates failed to yield amplification products in each locus. In this case, shorter contigs with gaps marking those gene fragments not available were constructed. Minimum evolution trees were constructed using MEGA 3.1 (23), with bootstrapping assessed by 1,000 replications.
RESULTS
Characteristics of the “atypical pneumococcal” isolates.
The phenotypic and genotypic characteristics of the presumed pneumococcal isolates with CIP MICs of ≥4.0 μg/ml are listed in Table 1. Three groups of isolates were included: those typical of S. pneumoniae, the majority belonging to serotypes 19F and 23F; those which were resistant to optochin, bile insoluble, and negative for pneumolysin and autolysin genes, belonging to the viridans streptococcal group; and those in the “atypical” pneumococcal group. The isolates in the last group were all positive for the ply gene, and all but one were positive for the lytA gene. Two subgroups were identified: six strains resistant to optochin (group “a”) and three susceptible to optochin (group “b”). The respective amino substitutions for these isolates at the QRDR regions of the gyrase and topoisomerase genes are also included in Table 1.
TABLE 1.
Phenotypic and genotypic characteristics of typical and atypical pneumococci and of viridans streptococci, their MICs to fluoroquinolones, and respective amino acid substitutions at the QRDRs of the gyrase and topoisomerase genes
| Group and isolate no. | Characteristicsa
|
Sero-type | Amino acid substitution for indicated position and geneb
|
MIC (μg/ml) for:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
gyrA
|
gyrB
|
parC
|
parE
|
||||||||||||||||||
| Phenotype
|
PCR
|
Ser 81 | Ser 114 | Asp 142 | Ser 494 | Ser 52 | Phe 55 | Ser 58 | Ser 79 | Asp 83 | Asn 91 | Glu 96 | Glu 135 | Lys 137 | Ile 460 | CIP | LEV | ||||
| OPT | Bile | lytA | ply | ||||||||||||||||||
| Typical S. pneumoniae | |||||||||||||||||||||
| L5 | S | P | P | P | NT | Val | 4 | 2 | |||||||||||||
| L6 | S | P | P | P | 23F | Phe | Asn | Val | 8 | 16 | |||||||||||
| L12 | S | P | P | P | 19F | Phe | Phe | Val | 64 | 16 | |||||||||||
| L13 | S | P | P | P | 19F | Tyr | Val | 4 | 2 | ||||||||||||
| L14 | S | P | P | P | NT | Asn | Val | 4 | 2 | ||||||||||||
| L15 | S | P | P | P | 23F | Asn | Asn | Val | 8 | 2 | |||||||||||
| L22 | S | P | P | P | 23F | Asn | Val | 4 | 2 | ||||||||||||
| L24 | S | P | P | P | 23F | Phe | Thr | Asn | Asn | Val | ≥16 | 8 | |||||||||
| Viridans streptococci | |||||||||||||||||||||
| L2 | R | N | N | N | Rough | Gly | Thr | Gly | Asp | - | 4 | 2 | |||||||||
| L7 | R | N | N | N | Rough | Gly | Thr | Gly | Asp | - | 4 | 2 | |||||||||
| L16 | R | N | N | N | Rough | Gly | Thr | Gly | Asp | - | 4 | 1 | |||||||||
| L17 | R | N | N | N | Rough | Gly | Thr | Gly | Asp | - | 4 | 1 | |||||||||
| L18 | R | N | N | N | Rough | Gly | Asn | Thr | Val | 4 | 2 | ||||||||||
| L19 | R | N | N | N | Rough | Gly | Thr | Gly | Asp | Val | 4 | 2 | |||||||||
| L20 | R | N | N | N | Rough | Thr | Gly | Asp | - | 8 | 2 | ||||||||||
| L21 | R | N | N | N | Rough | Thr | Asn | - | 16 | 2 | |||||||||||
| L26 | R | N | N | N | Rough | Phe | Asn | - | 16 | 16 | |||||||||||
| Atypical pneumococci | |||||||||||||||||||||
| a | |||||||||||||||||||||
| L3 | R | N | P | P | Rough | Asn | - | 4 | 1 | ||||||||||||
| L8 | R | N | P | P | Rough | Gly | His | Gly | Asp | Asn | 8 | 2 | |||||||||
| L9 | R | N | P | P | Rough | Gly | Thr | Asn | - | 4 | 2 | ||||||||||
| L10 | R | N | P | P | Rough | Phe | Asn | - | 4 | 2 | |||||||||||
| L4 | R | P | P | P | Rough | - | 4 | 2 | |||||||||||||
| L1 | R | I | P | P | Rough | Phe | Phe | Asp | Asn | - | >32 | 16 | |||||||||
| b | |||||||||||||||||||||
| L25 | S | I | N | P | Rough | Asn | - | 4 | 2 | ||||||||||||
| L27 | S | I | P | P | Rough | Gly | Asp | Asp | - | 4 | 1 | ||||||||||
| L11 | S | I | P | P | Rough | Gly | Asp | Asp | - | 8 | 2 | ||||||||||
Within the atypical pneumococcal group, all isolates possess the lytA and ply genes, but group “a” strains have reduced susceptibilities to optochin, while group “b” strains are optochin susceptible. Isolates may be bile soluble (P), insoluble (N), or incompletely soluble (I). OPT, optochin test; Bile, bile solubility test; lytA, autolysin gene; ply, pneumolysin gene.
Position of amino acid substitution compared to the corresponding amino acid position of S. pneumoniae R6. Bold characters indicate QRDR and amino acid substitutions unique compared to those of R6 and more compatible with those of viridans streptococci. -, not done.
MLST and 16S rRNA sequence analysis.
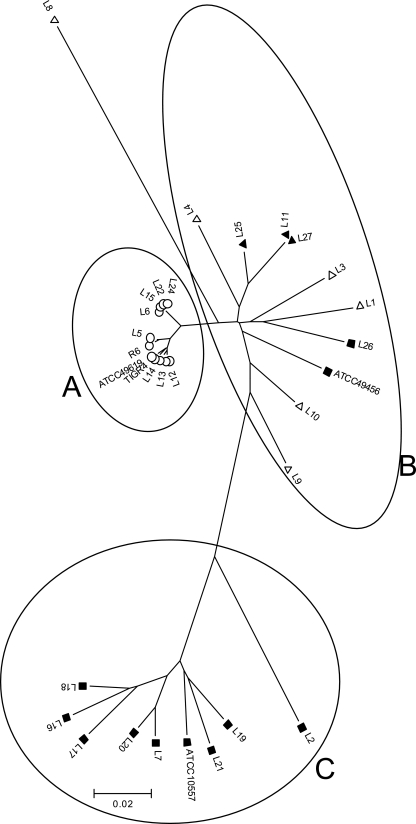
Among the typical S. pneumoniae isolates, only one gave a known allelic profile (L14, spi) from the MLST database (http://spneumoniae.mlst.net/). For the viridans streptococcal and “atypical” isolates, unique sequences were obtained for nearly all loci, except for five known allelic profiles available in the MLST database (http://spneumoniae.mlst.net/). Some of the isolates failed to produce the desired fragment, despite the use of the modified primer set. The analysis based on concatenated sequences of the six housekeeping genes from all the isolates is shown in Fig. 1. Three clusters were identified; isolates in cluster A were all typical S. pneumoniae, and isolates in clusters B and C fell into groups related to S. mitis and S. oralis, respectively. In contrast, there was no clustering with these groups in a phylogenetic tree generated from the 16S rRNA sequences (tree not shown).
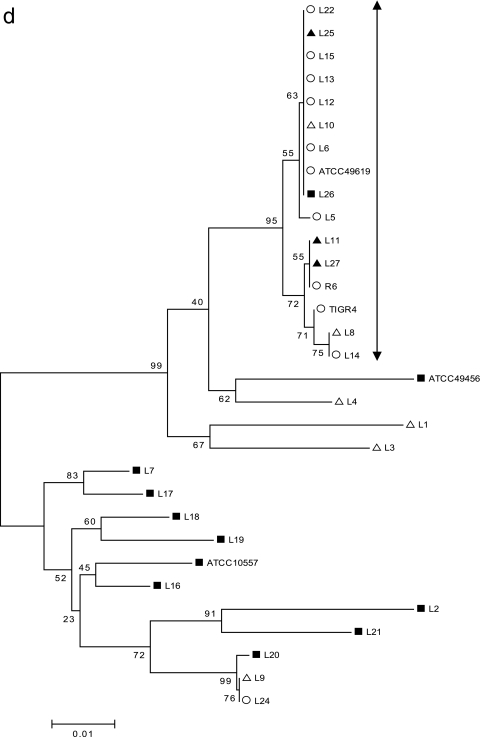
FIG. 1.
Minimum evolution tree showing relationship based on concatenated sequences of six housekeeping genes. The tree was constructed by the neighbor-joining method using MEGA 3.1. Isolates included in group A are those of the typical S. pneumoniae group and ATCC 49619 (Table 1) and are indicated by ○. ▪ represents the viridans streptococcal group, and ▵ or ▴ represents the “atypical pneumococcal” group. Isolates in group B included S. mitis ATCC 49456 and mainly “atypical pneumococcal” strains. Isolates in group C included the viridans streptococcal group and S. oralis ATCC 10557.
Sequence analyses of the QRDRs of gyrase and topoisomerase genes.
Among S. pneumoniae isolates, the major amino acid substitutions were Ser-81-Phe in GyrA; Ser-79-Phe, Asp-83-Tyr or Asn, and Lys-137-Asn in ParC; Ser-494-Thr in GyrB; and Ile-460-Val in ParE. These included the predominant amino acid substitutions described previously associated with fluoroquinolone resistance in pneumococci from Hong Kong (17).
The viridans streptococcal and “atypical” strains showed a number of unique amino acid substitutions within the QRDRs of the GyrA and -B and ParC and -E proteins compared to the corresponding regions of the R6 strain in particular, with amino acid substitutions at Ser-114-Gly in GyrA, Ser-494-Thr in GyrB, and Ser-52-Gly and Asn-91-Asp in Par C.
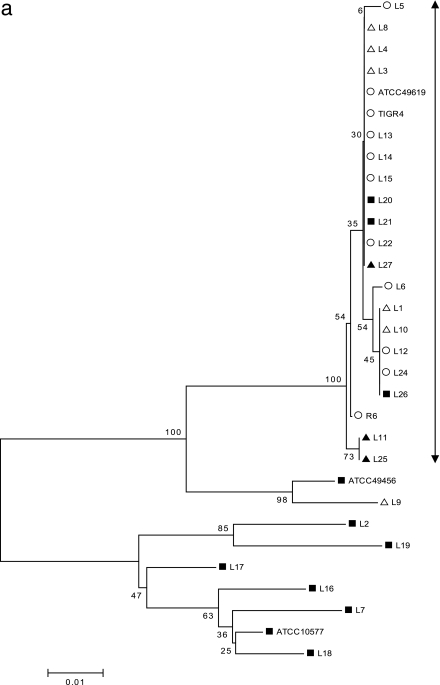
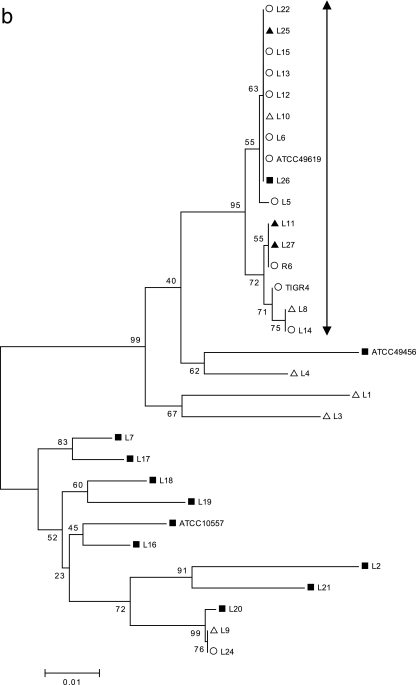
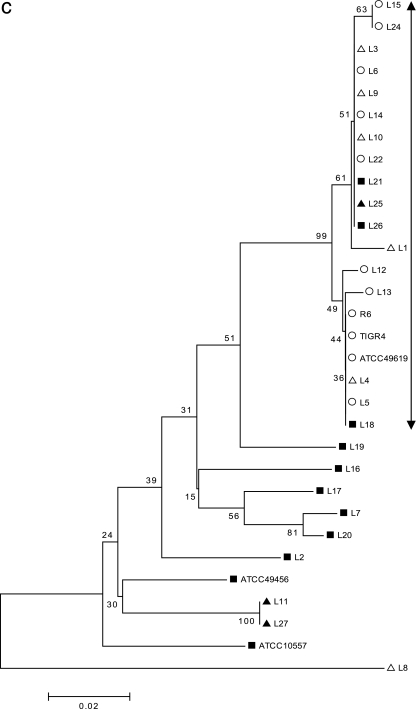
The gene sequences of the QRDR of each of the four genes were aligned (see the supplemental material), and minimum evolution trees were constructed by the neighbor-joining method using MEGA 3.1 (23) (Fig. 2a to d). With 354 bp of the gyrA sequence used for alignment (Fig. 2a), the major group of isolates that descend from a single node included all of the typical S. pneumoniae isolates, R6, and the ATCC 49619 strain. In addition, all the “atypical pneumococcal” isolates except one, L9, clustered within this group, suggesting a common origin in this gyrA region, possibly through horizontal transfer. All the LEV-resistant isolates, including those of atypical strains and viridans streptococci, were present within this cluster. Similarly, with 429 bp of gyrB, 231 bp of parC, and 267 bp of parE used for the analyses (Fig. 2b to d), the major group included all the typical S. pneumoniae isolates, R6, and ATCC 49619, with the exception of one strain (L24). L24 has a unique gyrB amino acid substitution, Ser-494-Thr, which was not present in other S. pneumoniae isolates but was seen in the viridans streptococcal group. This suggests a possible transfer of this gyrB fragment from viridans streptococci into pneumococcus. With parC, six of the nine strains of “atypical pneumococci” also clustered into the major “typical S. pneumoniae” group, suggesting the origin of this gene. Interestingly, with parE, only one (L8) atypical strain was clustered with the “typical S. pneumoniae” group. In all cases, the majority of the viridans streptococci clustered away from the major group, suggesting unique sequences that differ from those of S. pneumoniae.
FIG. 2.
(a) Minimum evolution tree constructed from 354 bp of gyrA gene sequence data using the neighbor-joining method. The scale represents the number of nucleotide substitutions per site. ○ indicates the typical S. pneumoniae group and ATCC 49619; ▪ represents the viridans streptococcal group, S. mitis ATCC 49456, and S. oralis ATCC 10557; and ▵ and ▴ represent “atypical pneumococcal” strains “a” and “b,” respectively (see Table 1 for corresponding isolates). (b) Minimum evolution tree constructed from 429 bp of gyrB gene sequence data using the neighbor-joining method. The scale represents the number of nucleotide substitutions per site. ○ indicates the typical S. pneumoniae group and ATCC 49619; ▪ represents viridans streptococcal group, S. mitis ATCC 49456, and S. oralis ATCC 10557; and ▵ and ▴ represent “atypical pneumococcal” strains “a” and “b,” respectively (see Table 1 for corresponding isolates). (c) Minimum evolution tree constructed from 231 bp of parC gene sequence data using the neighbor-joining method. The scale represents the number of nucleotide substitutions per site. ○ indicates the typical S. pneumoniae group and ATCC 49619; ▪ represents the viridans streptococcal group, S. mitis ATCC 49456, and S. oralis ATCC 10557; and ▵ and ▴ represent “atypical pneumococcal” strains “a” and “b,” respectively (see Table 1 for corresponding isolates). (d) Minimum evolution tree constructed from 267 bp of parE gene sequence data using the neighbor-joining method. The scale represents the number of nucleotide substitutions per site. ○ indicates the typical S. pneumoniae group and ATCC 49619; ▪ represents the viridans streptococcal group, S. mitis ATCC 49456, and S. oralis ATCC 10557. ▵ and ▴ represent “atypical pneumococcal” strains “a” and “b,” respectively (see Table 1 for corresponding isolates).
Fluoroquinolone susceptibility in S. oralis and S. mitis.
The MICs of CIP and LEV were determined for 32 isolates of S. oralis and S. mitis and are listed in Table 2. Fifty-six percent (18/32) of the strains have CIP MICs of ≥4.0 μg/ml, while 9.4% (3/32) have LEV MICs of ≥4.0 μg/ml. The efflux phenotype was detected (using inhibition by reserpine) in all but one strain (HK467, S. mitis) with CIP MICs of ≥2.0 μg/ml and negative in those strains with CIP MICs of ≥32 μg/ml.
TABLE 2.
Fluoroquinolone susceptibilities of S. oralis and S. mitis and their respective amino acid substitutions at the QRDRs of the gyrase and topoisomerase genesa
| Isolate no. | MIC (μg/ml) for:
|
Result for efflux phenotype detection | Amino acid substitution at indicated QRDRb
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | LEV |
gyrA
|
gyrB
|
parC
|
parE
|
||||||||||||
| Ser 81 | Ser 114 | Leu 155 | Ile 385 | Met 483 | Ser 494 | Ser 52 | Ser 79 | Asp 83 | Asn 91 | Glu 135 | Pro 140 | Ala 142 | Arg 447 | ||||
| S. pneumoniae R6 | 0.5 | 0.5 | - | ||||||||||||||
| S. oralis | - | ||||||||||||||||
| ATCC 10557 | 2 | 1 | N | Gly | - | Asp | - | ||||||||||
| HK575 | 1 | 1 | - | Gly | Val | - | Gly | Asp | Ser | - | |||||||
| HK323 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK417 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK496 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK553 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK62 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK122 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK123 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK271 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK300 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK312 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK51 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK299 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK579 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK587 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK386 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK619 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK574 | 8 | 2 | P | Gly | Val | Thr | Thr | Gly | Asp | ||||||||
| HK816 | 32 | 32 | N | Leu | Gly | - | Gly | Tyr | Asp | ||||||||
| HK754 | 128 | ≥32 | N | Leu | Gly | Thr | Gly | Tyr | Asp | Ser | |||||||
| S. mitis | |||||||||||||||||
| ATCC 49456 | 1 | 1 | - | Gly | Gly | Asp | Asp | Ser | |||||||||
| HK260 | <0.5 | <0.25 | - | Gly | - | Gly | Asp | - | |||||||||
| HK141 | 1 | 0.5 | - | Gly | - | Gly | Asp | - | |||||||||
| HK188 | 1 | 0.5 | - | Gly | - | Gly | Asp | Asp | - | ||||||||
| HK651 | 1 | 0.5 | - | Gly | - | Gly | Asp | - | |||||||||
| HK137453 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK680 | 2 | 1 | P | Gly | - | Gly | Asp | ||||||||||
| HK125807 | 2 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK467 | 2 | 1 | N | Gly | Val | - | Gly | Asp | - | ||||||||
| HK666 | 2 | 2 | P | Gly | - | Gly | Asp | Asp | - | ||||||||
| HK135786 | 4 | 1 | P | Gly | - | Gly | Asp | - | |||||||||
| HK29 | 4 | 2 | P | Gly | - | Gly | Asp | - | |||||||||
| HK677 | 128 | ≥32 | N | Leu | Gly | Thr | Gly | Tyr | Asp | - | |||||||
P, positive; N, negative; -, not done.
Position of amino acid substitution compared to the corresponding amino acid position of S. pneumoniae R6.
Mutations at the QRDRs of gyrase and topoisomerase genes in S. oralis and S. mitis.
The QRDR sequences of the parC and gyrA genes were determined for all of the clinical strains, while the QRDR sequences of the parE and gyrB genes were determined for only four strains, for which the CIP MICs were ≥4 μg/ml. The amino acid sequences of QRDRs were analyzed by comparison to susceptible strain R6, and the amino acid substitutions are summarized in Table 2.
The parC and gyrA nucleotide sequences showed high diversity among these strains, with 25.5% and 27.4% variations, respectively (data not shown). Comparisons of amino acid sequences with those of R6 revealed two common substitutions in ParC (Ser-52-Gly and Asn-91-Asp) and one in GyrA (Ser-114-Gly) in all S. oralis and S. mitis isolates. These do not contribute to fluoroquinolone resistance. In the three CIP-resistant strains (MICs ≥ 32 μg/ml; HK816, HK754, and HK677), amino acid substitutions at Ser-79-Tyr and Asp-83-Tyr in ParC and Ser-81-Leu in GyrA were noted. Other substitutions observed (Glu-135-Asp, Pro-140-Ser, and Ala-142-Ser in ParC and Leu-155-Val in GyrA) were present only in strains with CIP MICs of ≤2 μg/ml. For the limited sequence analyses for parE and gyrB, the following amino acid substitutions were noted: Arg-447-Ser in ParE and Ile-385-Val, Met-483-Thr, and Ser-494-Thr in GyrB.
DISCUSSION
The emergence of nontypeable S. pneumoniae and other atypical pneumococci (10, 21, 30) brings a challenge in the identification of pneumococci. As the prevalent capsular types of S. pneumoniae are being eradicated by the use of the seven-valent conjugate vaccine, noncapsulate types become more commonly encountered in a diagnostic laboratory. Both optochin-resistant pneumococci (2, 37) and optochin-susceptible viridans streptococci (5, 26) have been described previously. Based on the analysis of the concatenated sequences of the six MLST loci (13, 14), the “atypical pneumococci” described in this study clustered closely with S. mitis. As with previous reports, DNA-based methods for the detection of the ply and lytA genes did not reliably confirm the identity of S. pneumoniae (13, 20, 32, 39). The 16s rRNA genes of S. mitis, S. oralis, and S. pneumoniae were homologous and do not allow distinction between these species (20, 29) based on sequence analyses. Any possibility for the “atypical” pneumococci to be Streptococcus pseudopneumoniae (2) was also excluded using optochin susceptibility testing in an ambient atmosphere.
However, when the sequences of the QRDRs of the gyrA, gyrB, parC, and parE genes of these “atypical pneumococci” were analyzed, they were found to cluster with sequences of the “typical S. pneumoniae” group, especially for the gyrA and parC genes. All “atypical” strains and five of nine strains from the viridans streptococcal group possessed one to three gyrA, gyrB, parC, and parE genes that were clustered within the S. pneumoniae group. The fact that the atypical strains clustered closely with S. mitis by MLST suggests the occurrence of horizontal transfer from recombination and supports the postulation that horizontal transfer of these genes from pneumococcus into viridans streptococci is probably a more frequent event than vice versa (36).
Substitutions at position 81 of GyrA and positions 79 and 83 of ParC were all important in the development of LEV resistance in S. pneumoniae and viridans streptococci. Interestingly, among the S. mitis and S. oralis isolates isolated in blood/body fluids, position 81 of GyrA was replaced by Leu and position 79 of ParC by Tyr instead of Phe in the pneumococcal or “atypical” isolates. The other amino acid substitutions (Ser-114-Gly at GyrA; Ser-52-Gly, Asn-91-Asp, and Glu-135-Asp at ParC; and Ser-494-Thr at GyrB) have been previously shown not likely to confer CIP resistance (3, 4, 11, 19, 27).
In Hong Kong, the high fluoroquinolone resistance in S. pneumoniae was reported to be due to the dissemination of a variant of the Spain 23F-1 clone (13). In this study, we identified evidence that suggests horizontal transfers of the QRDR of the gyrase and topoisomerase genes from pneumococci into viridans streptococci. These included amino acid substitutions that conferred LEV resistance in viridans streptococcus L26 and the atypical strain L1. The LEV-resistant isolates of S. oralis and S. mitis have a unique amino acid substitution at position 81 by Leu in GyrA, differing from those of fluoroquinolone-resistant pneumococcal and “atypical” isolates. This study reflects the pool of fluoroquinolone resistance determinants that are presently seen among clinical isolates of viridans streptococci.
Previously, it was demonstrated in vitro that recombinational events occurred more frequently from S. mitis or S. oralis to S. pneumoniae (19). Increasingly, members of the pneumococcal clone Spain 23F-1 have been shown to act as frequent donors of fluoroquinolone-nonsusceptible loci (36). Both recombinational events and selective pressure from the use of fluoroquinolones with de novo mutations play an important role in the development of fluoroquinolone resistance.
Supplementary Material
Acknowledgments
This study was supported by an Earmarked Grant (CUHK 4432/03 M) from the Research Grants Council, Hong Kong Special Administrative Region, Hong Kong, China.
We acknowledge the use of the pneumococcal MLST database which is located at Imperial College London and is funded by the Wellcome Trust.
Footnotes
Published ahead of print on 4 June 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Adam, H. J., K. N. Schurek, K. A. Nichol, C. J. Hoban, T. J. Baudry, N. M. Laing, D. J. Hoban, and G. G. Zhanel. 2007. Molecular characterization of increasing fluoroquinolone resistance in Streptococcus pneumoniae in Canada, 1997 to 2005. Antimicrob. Agents Chemother. 51:198-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arbique, J. C., C. Poyart, P. Trieu-Cuot, G. Quesne, G. Carvalho Mda, A. G. Steigerwalt, R. E. Morey, D. Jackson, R. J. Davidson, and R. R. Facklam. 2004. Accuracy of phenotypic and genotypic testing for identification of Streptococcus pneumoniae and description of Streptococcus pseudopneumoniae sp. nov. J. Clin. Microbiol. 42:4686-4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balsalobre, L., M. J. Ferrandiz, J. Linares, F. Tubau, and A. G. de la Campa. 2003. Viridans group streptococci are donors in horizontal transfer of topoisomerase IV genes to Streptococcus pneumoniae. Antimicrob. Agents Chemother. 47:2072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bast, D. J., D. E. Low, C. L. Duncan, L. Kilburn, L. A. Mandell, R. J. Davidson, and J. C. de Azavedo. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borek, A. P., D. C. Dressel, J. Hussong, and L. R. Peterson. 1997. Evolving clinical problems with Streptococcus pneumoniae: increasing resistance to antimicrobial agents, and failure of traditional optochin identification in Chicago, Illinois, between 1993 and 1996. Diagn. Microbiol. Infect. Dis. 29:209-214. [DOI] [PubMed] [Google Scholar]
- 6.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chi, F., O. Nolte, C. Bergmann, M. Ip, and R. Hakenbeck. 2007. Crossing the barrier: Evolution and spread of a major class of mosaic pbp2x in Streptococcus pneumoniae, S. mitis and S. oralis. Int. J. Med. Microbiol. doi: 10.1016/j-ijmm.2007.02.009. [DOI] [PubMed]
- 8.Clinical Laboratory Standards Institute. 2004. Performance standards for antimicrobial susceptibility testing, M100-S14. Clinical Laboratory Standards Institute, Wayne, PA.
- 9.de la Campa, A. G., L. Balsalobre, C. Ardanuy, A. Fenoll, E. Perez-Trallero, J. Linares, and Spanish Pneumococcal Infection Study Network G03/103. 2004. Fluoroquinolone resistance in penicillin-resistant Streptococcus pneumoniae clones, Spain. Emerg. Infect. Dis. 10:1751-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denys, G. A., and R. B. Carey. 1992. Identification of Streptococcus pneumoniae with a DNA probe. J. Clin. Microbiol. 30:2725-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez, I., M. Georgiou, F. Alcaide, D. Balas, J. Linares, and A. G. de la Campa. 1998. Fluoroquinolone resistance mutations in the parC, parE, and gyrA genes of clinical isolates of viridans group streptococci. Antimicrob. Agents Chemother. 42:2792-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerin, F., E. Varon, A. B. Hoï, L. Gutmann, and I. Podglajen. 2000. Fluoroquinolone resistance associated with target mutations and active efflux in oropharyngeal colonizing isolates of viridans group streptococci. Antimicrob. Agents Chemother. 44:2197-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanage, W. P., T. Kaijalainen, E. Herva, A. Saukkoriipi, R. Syrjanen, and B. J. Spratt. 2005. Using multilocus sequence data to define the pneumococcus. J. Bacteriol. 187:6223-6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanage, W. P., B. J. Spratt, K. M. E. Turner, and C. Fraser. 2006. Modelling bacterial speciation. Philos. Trans. R. Soc. Lond. B 261:2039-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho, P. L., R. W. Yung, D. N. Tsang, T. L. Que, M. Ho, W. H. Seto, T. K. Ng, W. C. Yam, and W. W. Ng. 2001. Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J. Antimicrob. Chemother. 48:659-665. [DOI] [PubMed] [Google Scholar]
- 16.Ho, P. L., T. L. Que, S. S. Chiu, R. W. H. Yung, T. K. Ng, D. N. C. Tsang, W. H. Seto, and Y. L. Lau. 2004. Fluoroquinolone and other antimicrobial resistance in invasive pneumococci, Hong Kong, 1995-2001. Emerg. Infect. Dis. 7:1250-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ip, M., S. S. Chau, F. Chi, A. Qi, and R. W. Lai. 2006. Rapid screening of fluoroquinolone resistance determinants in Streptococcus pneumoniae by PCR-restriction fragment length polymorphism and single-strand conformational polymorphism. J. Clin. Microbiol. 44:970-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ip, M., F. Chi, S. S. L. Chau, M. Hui, J. Tang, and P. K. S. Chan. 2006. Use of the housekeeping genes, gdh (zwf) and gki, in multilocus sequence typing (MLST) to differentiate S. pneumoniae from S. mitis and S. oralis. Diagn. Microbiol. Infect. Dis. 56:321-324. [DOI] [PubMed] [Google Scholar]
- 19.Janoir, C., I. Podglajen, M. D. Kitzis, C. Poyart, and L. Gutmann. 1999. In vitro exchange of fluoroquinolone resistance determinants between Streptococcus pneumoniae and viridans streptococci and genomic organization of the parE-parC region in S. mitis. J. Infect. Dis. 180:555-558. [DOI] [PubMed] [Google Scholar]
- 20.Kawamura, Y., X. Hou, F. Sultana, H. Miura, and T. Ezaki. 1995. Determination of 16S rRNA sequences of Streptococcus mitis and Streptococcus gordonii and phylogenetic relationships among members of the genus Streptococcus. Int. J. Syst. Bacteriol. 45:406-408. [DOI] [PubMed] [Google Scholar]
- 21.Kellogg, J. A., D. A. Bankert, C. J. Elder, J. L. Gibbs, and M. C. Smith. 2001. Identification of Streptococcus pneumoniae revisited. J. Clin. Microbiol. 39:3373-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko, K. S., W. S. Oh, K. R. Peck, J. H. Lee, N. Y. Lee, and J. H. Song. 2005. Phenotypic and genotypic discrepancy of Streptococcus pneumoniae strains isolated from Asian countries. FEMS Immunol. Med. Microbiol. 45:63-70. [DOI] [PubMed] [Google Scholar]
- 23.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 24.Mandell, L. A., J. G. Bartlett, S. F. Dowell, T. M. File, Jr., D. M. Musher, and C. Whitney. 2003. Update of practice guidelines for the management of community-acquired pneumonia in immunocompetent adults. Clin. Infect. Dis. 37:1405-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandell, L. A., T. J. Marrie, R. F. Grossman, A. W. Chow, R. H. Hyland, Canadian Infectious Disease Society, and Canadian Thoracic Society. 2000. Summary of Canadian guidelines for the initial management of community-acquired pneumonia: an evidence-based update by the Canadian Infectious Disease Society and the Canadian Thoracic Society. Can. Respir. J. 7:371-382. [DOI] [PubMed] [Google Scholar]
- 26.Martin-Galiano, A. J., L. Balsalobre, A. Fenoll, and A. G. de la Campa. 2003. Genetic characterization of optochin-susceptible viridans group streptococci. Antimicrob. Agents Chemother. 47:3187-3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muñoz, R., and A. G. De La Campa. 1996. ParC subunit of DNA topoisomerase IV of Streptococcus pneumoniae is a primary target of fluoroquinolones and cooperates with DNA gyrase A subunit in forming resistance phenotype. Antimicrob Agents Chemother. 40:2252-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neeleman, C., C. H. W. Klaassen, D. M. Klomberg, H. A. de Valk, and J. W. Mouton. 2004. Pneumolysin is a key factor in misidentification of macrolide-resistant Streptococcus pneumoniae and is a putative virulence factor of S. mitis and other streptococci. J. Clin. Microbiol. 42:4355-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picard, F. J., D. Ke, D. K. Boudreau, M. Boissinot, A. Huletsky, D. Richard, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2004. Use of tuf sequences for genus-specific PCR detection and phylogenetic analysis of 28 streptococcal species. J. Clin. Microbiol. 42:3686-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pikis, A., J. M. Campos, W. J. Rodriguez, and J. M. Keith. 2001. Optochin resistance in Streptococcus pneumoniae: mechanism, significance, and clinical implications. J. Infect. Dis. 184:582-590. [DOI] [PubMed] [Google Scholar]
- 31.Pletz, M. W. R., L. McGee, B. Beall, C. G. Whitney, and K. P. Klugman. 2005. Interspecies recombination in type II topoisomerase genes is not a major cause of fluoroquinolone resistance in invasive Streptococcus pneumoniae isolates in the United States. Antimicrob Agents Chemother. 49:779-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poyart, C., G. Quesne, S. Coulon, P. Berche, and P. Trieu-Cuot. 1998. Identification of streptococci to species level by sequencing the gene encoding the manganese-dependent superoxide dismutase. J. Clin. Microbiol. 36:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter, S. S., K. P. Heilmann, S. E. Beekmann, N. J. Miller, C. L. Rice, and G. V. Doern. 2005. The molecular epidemiology of Streptococcus pneumoniae with quinolone resistance mutations. Clin. Infect. Dis. 40:225-235. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph, K. M., A. J. Parkinson, C. M. Black, and L. W. Mayer. 1993. Evaluation of polymerase chain reaction for diagnosis of pneumococcal pneumonia. J. Clin. Microbiol. 31:2661-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song, J. H., S. I. Jung, S. K. Kwan, Y. K. Na, J. S. Son, H. H. Chang, H. K. Ki, W. S. Oh, J. Y. Suh, K. R. Peck, N. Y. Lee, Y. H. Yang, Q. Lu, A. Chongthaleong, C. H. Chiu, M. K. Lalitha, J. Perera, T. T. Yee, G. Kumarasinghe, F. Jamal, A. Kamarulzaman, N. Parasakthi, P. H. Van, C. Carlos, T. So, T. K. Ng, and A. Shibl. 2004. High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob. Agents Chemother. 48:2101-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanhope, M. J., S. L. Walsh, J. A. Becker, M. J. Italia, K. A. Ingraham, M. N. Gwynn, T. Mathie, J. A. Poupard, L. A. Miller, J. R. Brown, and H. Amrine-Madsen. 2005. Molecular evolution perspectives on intraspecific lateral DNA transfer of topoisomerase and gyrase loci in Streptococcus pneumoniae, with implications for fluoroquinolone resistance development and spread. Antimicrob. Agents Chemother. 49:4315-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verhelst, R., T. Kaijalainen, T. De Baere, G. Verschraegen, G. Claeys, L. Van Simaey, C. De Ganck, and M. Vaneechoutte. 2003. Comparison of five genotypic techniques for identification of optochin-resistant pneumococcus-like isolates. J. Clin. Microbiol. 41:3521-3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wester, C. W., D. Ariga, C. Nathan, T. W. Rice, J. Pulvirenti, R. Patel, F. Kocka, J. Ortiz, and R. A. Weinstein. 2002. Possible overestimation of penicillin resistant Streptococcus pneumoniae colonization rates due to misidentification of oropharyngeal streptococci. Diagn. Microbiol. Infect. Dis. 42:263-268. [DOI] [PubMed] [Google Scholar]
- 39.Whatmore, A. M., A. Efstratiou, A. P. Pickerill, K. Broughton, G. Woodard, D. Sturgeon, R. George, and C. G. Dowson. 2000. Genetic relationships between clinical isolates of Streptococcus pneumoniae, Streptococcus oralis, and Streptococcus mitis: characterization of “atypical” pneumococci and organisms allied to S. mitis harboring S. pneumoniae virulence factor-encoding genes. Infect. Immun. 68:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.