Abstract
Capreomycin is used for the treatment of multidrug-resistant tuberculosis (MDR-TB), but it is limited therapeutically by its severe side effects. The objectives of the present studies were (i) to design low-density porous capreomycin sulfate particles for efficient pulmonary delivery to improve local and systemic drug bioavailability and capacity to reduce the bacillary load in the lungs in a manner similar to that achieved with intramuscular injections; (ii) to determine pharmacokinetic parameters after pulmonary administration of these capreomycin particles; and (iii) to evaluate the efficacy of these particles in treating animals in a small-aerosol-inoculum guinea pig model of TB. Capreomycin particles were manufactured by spray drying and characterized in terms of size and drug content. Pharmacokinetic parameters were determined by noncompartmental methods with healthy guinea pigs after administration of capreomycin particles by insufflation. The efficacy of the particles was evaluated by histopathological analysis and in terms of wet organ weight and bacterial burden in TB-infected animals. Lungs of animals receiving a 14.5-mg/kg dose of capreomycin particles showed significantly lower wet weights and smaller bacterial burdens than those of animals receiving any other treatment. These results were supported by histopathological analysis. The feasibility of inhaling capreomycin in a novel powder form, with the ultimate objective of the treatment of MDR-TB, is demonstrated by pharmacokinetic and pharmacodynamic studies with guinea pigs. If applied to humans with MDR-TB, such a therapeutic approach might simplify drug delivery by eliminating injections and might reduce adverse effects through lowering the dose.
Worldwide, there is an increase in multiple drug-resistant tuberculosis (MDR-TB), including reports of outbreaks of extensive MDR-TB in several countries (4, 36). Patients suffering from MDR-TB have infections which are resistant to at least two key drugs in the first-line regimen, namely, rifampin and isoniazid, in part as a consequence of poor compliance to the current first-line regimen (approximately 23% of active TB patients presently complete first-line treatment) (5). To treat MDR-TB, other, second-line antibiotics are needed and therapy lasts up to 2 years, typically requiring parenteral injection (2).
Among the drugs injected is the polypeptide antibiotic capreomycin, the only second-line drug specifically indicated for MDR-TB. Capreomycin is a complex of four microbiologically active components that has been shown to be effective in combination with other appropriate anti-TB drugs in the treatment of pulmonary Mycobacterium tuberculosis infections when the primary agents (in particular isoniazid and rifampin) have become ineffective as a result of bacterial resistance or toxicity. The drug is painful to receive by injection and is associated with serious side effects, such as anorexia, thirst, anemia, and notably, nephrotoxicity and damage to the auditory and vestibular divisions of cranial nerve VIII (8, 14, 26). The usual dose for humans is 20 mg/kg of body weight/day (total dose, 1 g) for 60 to 120 days injected intramuscularly (i.m.), posing significant challenges in delivering a full course to severely emaciated and chronically ill patients (11).
Targeted drug delivery may maintain local therapeutic concentrations and minimize systemic exposure, thus decreasing side effects. The efficacy of aerosolized rifampin-polylactic-co-glycolic acid particles (3 to 5 μm) for the treatment of pulmonary TB has been reported previously (27, 32, 33). However, large porous particles (LPPs) are known to aerosolize more efficiently because they do not readily form aggregates and they disperse more easily in response to airflow shear forces than do smaller, nonporous particles. Also, LPPs exhibiting relatively low water solubility may release drugs into the lung environment over extended periods of time, since their large size prevents phagocytosis by macrophages (10).
The main objectives of this study were (i) to determine key parameters of capreomycin disposition after administration of powders for inhalation in a guinea pig model of TB and (ii) to demonstrate the feasibility of pulmonary administration of capreomycin particles and its efficacy in treating TB-infected guinea pigs, utilizing a novel dry powder formulation that permits room-temperature stability and high-efficiency delivery. An anticipated advantage of simplified pulmonary delivery is the elimination of injections, thereby reducing the burden on clinically qualified personnel for administration of the drug. Formulation of capreomycin in LPP powders that possess optimized aerosol physical properties would allow the drug to be delivered to the lungs, resulting in enhanced local tissue concentrations and excellent systemic absorption owing to the natural absorptive capacity of pulmonary epithelia. Thus, efficacious doses of capreomycin by inhalation may be much lower than those administered by injection.
MATERIALS AND METHODS
Materials.
Leucine was obtained from Sigma-Aldrich (St. Louis, MO), and acetonitrile, USP-grade ethanol, and methanol were purchased from Pharmco Products Inc. (Brookfield, CT). Water was obtained from a Millipore Corp. Milli-Q water purification system (Billerica, MA). Capreomycin sulfate was contributed by Eli Lilly and Co.
Manufacture and characterization of capreomycin particles.
Particles for inhalation were manufactured from a capreomycin solution containing 80% capreomycin sulfate and 20% l-leucine in 50% ethanol, using a Niro Mobile Minor spray dryer (Columbia, MD) at a feed flow rate of 80 ml/min, with an inlet temperature of 189 to 192°C, as described elsewhere (10, 34).
The resulting powders were characterized in terms of morphology, volume diameter, and aerodynamic diameter. Morphology of the particles was visualized with a LEO 982 field emission scanning electron microscope (Carl Zeiss, Inc., Thornwood, NY). Volume diameter was determined by light diffraction using a HELOS system with a RODOS dry dispersing unit (Sympatec Inc., Lawrenceville, NJ), and aerodynamic diameter was determined using an eight-stage Andersen Cascade Impactor (Copley Scientific Limited, Nottingham, United Kingdom). The capreomycin sulfate content in the powders was determined by high-performance liquid chromatography (HPLC) (1100 series system; Agilent Technologies, Palo Alto, CA), using a mobile phase consisting of 22:78 methanol-phosphate buffer with 0.3% (wt/wt) heptafluorobutyric acid at 1.0 ml/min and 25°C in a C18 reverse-phase column (Agilent ZORBAX Eclipse XDB-C18 column).
Pharmacokinetic (PK) studies.
Male guinea pigs weighing 836 ± 162.3 g were employed in these studies. Animals were housed with a 12-h light/12-h dark cycle in a constant-temperature environment of 22°C. A standard diet and water were supplied ad libitum. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of North Carolina. Twenty-four hours before the study, each animal underwent cannulation of the right external jugular vein for continuous blood sampling (12).
Animals were randomly divided into five groups receiving capreomycin as follows: intravenously (i.v.; n = 6) and i.m. (n = 8) in solution at a dose of 20 mg/kg and as dry powder insufflated by the pulmonary route at low (n = 6), medium (n = 6), and high (n = 8) nominal doses of 1.4, 7.2, and 14.5 mg/kg. These doses were based on the dose reduction estimated from direct delivery of antibiotics to the lungs by insufflation (circumventing oropharyngeal deposition) and on the practical limitations of easily delivering large masses of dry powders to the guinea pigs. Blood samples (0.35 ml) were collected from each animal into heparinized tubes at 0, 0.08, 0.25, 0.5, 1.0, 1.5, 2, 3, 4, 5, 6, 8, and 12 h. After collection of the last blood sample, animals were euthanized by exsanguination and bronchoalveolar lavage (BAL) was conducted (5 ml sterile saline).
Plasma and BAL samples were analyzed for drug content by a modified HPLC assay (29). The limit of detection was 0.5 μg/ml, the intraday variation was 8.64%, and the interday variation was 7.15%. The PK parameters area under the curve (AUC), apparent total body clearance (CL), mean residence time (MRT), half-life (t1/2), volume of distribution (Vss or V_F), and elimination rate constant (K) were obtained by noncompartmental methods (WinNonlin; Pharsight Corporation, Mountain View, CA). Maximum capreomycin concentrations (Cmax) and time to obtain the maximum capreomycin concentration (Tmax) were determined from the nonfitted plasma-versus-time profile for each individual animal. Mean absorption time (MAT) was estimated by subtracting the average MRT for the i.v. group from the MRT of each animal in all other treatment groups.
Pharmacodynamic studies.
Animals were infected via the respiratory route with nebulized suspensions (2 × 105 CFU/ml) of Mycobacterium tuberculosis (strain H37Rv), employing an aerosol exposure chamber (University of Wisconsin) (35). Animals remained untreated until 4 weeks following infection, when the bacterial burden is known to plateau (22). Animals were then assigned to different groups of six guinea pigs each to receive capreomycin daily for 4 weeks by i.m. injection or by inhalation using a custom-designed dry powder dosing chamber.
Prior to pharmacodynamic studies, the aerodynamic particle size of capreomycin particles was characterized by inertial impaction as a measure of delivery efficiency in the dry powder dosing chamber. Based on a 10% estimated delivery efficiency (15, 25, 31), the mass of capreomycin particles loaded into the dry powder dosing chamber was increased 10-fold for daily treatments. The following six treatment groups were evaluated: i.m. capreomycin solution (20 mg/kg); inhaled capreomycin LPPs at nominal doses of 1.4, 7.2, and 14.5 mg/kg; inhaled placebo (leucine:dipalmitoyl-phosphocholine) LPPs; and untreated controls. Body weights of each animal and changes in behavior or signs of adverse effects were recorded throughout the study.
Eight weeks after infection and 4 weeks after treatment initiation, animals were sacrificed and necropsy was performed. The lungs, spleen, and liver were inspected to determine the number of superficial lesions. The left lung lobe and a portion of the spleen were placed in 10% neutral buffered formalin for histopathological evaluation. For bacteriology, the right lung lobe and the remaining spleen tissue were homogenized. Aliquots of diluted homogenates (lung and spleen) were inoculated onto M7H10 agar plates (Hardy Diagnostics, Santa Maria, CA), and plates were incubated at 37°C for 3 weeks. Visible bacterial CFU (expressed as a logarithm to base 10) were counted.
Formalin-fixed lung and spleen tissues from representative animals in each group (at least two per group) were submitted for histopathological analysis as a confirmatory measure of bacteriology. Tissues were embedded in paraffin wax, sectioned with a microtome (Leica Microsystems, Bannockburn, IL) at 5 μm, and mounted on glass slides. Microscopic examinations were conducted on hematoxylin-eosin-stained slides for each tissue by a pathologist who was blinded with respect to the treatment received by any of the animals. The following parameters were scored: percentage of tissue affected by granulomas, granuloma size, percentage of granulomas affected by caseous necrosis, amount of caseous necrosis, and lymphocyte involvement.
Statistical analysis.
Data for PK and pharmacodynamic studies were subjected to analysis of variance and the least-squares significant difference multiple comparison method. A probability level of 5% (P < 0.05) was considered statistically significant.
RESULTS
Particle manufacture and characterization.
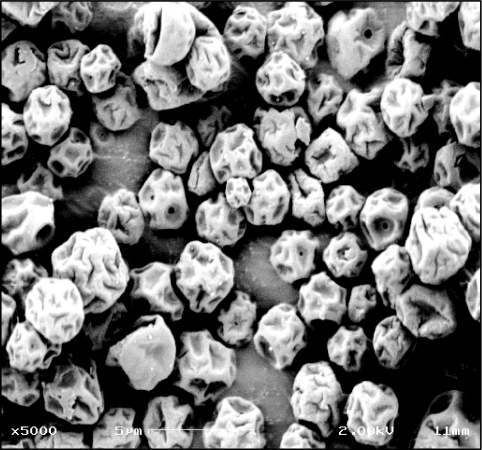
Evaluation by microscopy showed that capreomycin particles were thin-walled structures (Fig. 1). The drug content in particles was 59.5% ± 1.2% (73.8% ± 1.5% capreomycin sulfate). Sizing methods revealed particles with a volume diameter of 4.19 ± 0.05 μm and desirable aerodynamic properties for powders that deposit in the respiratory tract, with a mass median aerodynamic diameter of 4.99 ± 0.02 μm and fine particle fractions (FPFTD < 5.8 μm and FPFTD < 3.3 μm) of 47.7% ± 0.7% and = 8.8% ± 0.5%, respectively.
FIG. 1.
Scanning electron micrograph of spray-dried capreomycin dry powder. Bar = 5 μm.
PK studies.
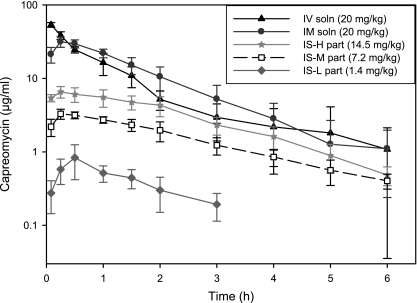
Figure 2 shows the average capreomycin plasma concentration-time curves. For animals treated with capreomycin i.v., the terminal phase of the plasma concentration-time curves was biphasic, whereas for all other treatments it was linear and parallel among the different doses. Statistically comparable capreomycin concentrations were observed after 1 h for the i.v. and i.m. groups (Fig. 2) and after 2 h for the i.v. group and for animals receiving 14.5 mg/kg capreomycin particles by insufflation (Fig. 2, IS-H). Capreomycin was not detected in the BAL samples from animals in any of the treatment groups, reflecting the efficient absorption of the drug by the airway epithelium.
FIG. 2.
Average plasma concentration-time curves (log scale) after administration of capreomycin at different doses and with different treatments, namely, capreomycin solution given i.v. and i.m. and capreomycin particles given by insufflation at high (IS-H part), medium (IS-M part), and low (IS-L part) doses (data are averages ± standard deviations; n = 6 to 8). Individual animals showed detectable capreomycin plasma concentrations at 8 h, but the group average was under the limit of detection and thus was not included.
PK parameters obtained by noncompartmental methods are presented in Table 1. The AUC0-t correlated with the capreomycin dose administered, with the i.v. and i.m. groups having the largest AUC and animals receiving 1 mg of particles by insufflation having the smallest one. Capreomycin CL was comparable among animals in all insufflated groups, and that of animals receiving 14.5 mg/kg of particles was also statistically comparable to capreomycin CL after i.v. administration. Capreomycin was eliminated faster (K) when administered i.v., i.m., or by insufflation of 1.4 mg/kg of particles than after insufflation of 7.2 mg/kg of particles. The t1/2 and MRT of capreomycin were longest after insufflation of 7.2 or 14.5 mg/kg of particles than those after any other treatment, which can be correlated with the longer absorption times (MAT) exhibited by these groups. The absolute bioavailability of capreomycin after insufflation of 7.2 or 14.5 mg/kg of particles was ∼59%.
TABLE 1.
PK parameters obtained by noncompartmental analysis after administration of different capreomycin formulations by different routesa
| Treatment route (formulation) | AUC0-t (μg·h/ml) | CL_F (ml/h·kg) | K (h−1) | t1/2 (h) | MRT (h) | Cmax (μg/ml) | Tmax (h) | MAT (h) | F0-t |
|---|---|---|---|---|---|---|---|---|---|
| i.v. (20 mg/kg; solution) | 49.29 ± 10.712 | 0.42 ± 0.102,3 | 0.92 ± 0.101 | 0.75 ± 0.084 | 0.88 ± 0.093 | 53.56 ± 3.931 | 0.08 ± 0.003 | 1.00 ± 0.001 | |
| i.m. (20 mg/kg; solution) | 58.20 ± 10.981 | 0.35 ± 0.063 | 0.68 ± 0.181,2,3 | 1.09 ± 0.303,4 | 1.44 ± 0.302 | 32.33 ± 3.752 | 0.36 ± 0.281 | 0.44 ± 0.202 | 1.20 ± 0.231 |
| Insufflation | |||||||||
| 14.5 mg/kg; powder | 16.95 ± 3.943 | 0.54 ± 0.111,2 | 0.48 ± 0.132,3 | 1.53 ± 0.361,2 | 1.80 ± 0.121 | 6.70 ± 1.263 | 0.31 ± 0.121,2 | 0.80 ± 0.121 | 0.54 ± 0.112 |
| 7.2 mg/kg; powder | 8.20 ± 1.454 | 0.52 ± 0.102 | 0.45 ± 0.113 | 1.68 ± 0.371 | 1.72 ± 0.181 | 3.34 ± 0.494 | 0.38 ± 0.141 | 0.72 ± 0.181 | 0.59 ± 0.122 |
| 1.4 mg/kg; powder | 1.15 ± 0.365 | 0.67 ± 0.251 | 0.71 ± 0.421,2 | 1.22 ± 0.562,3 | 1.01 ± 0.313 | 0.92 ± 0.385 | 0.5 ± 0.311 | —b | 0.41 ± 0.142 |
Data are averages ± standard deviations (n = 6 to 8). Numeric superscripts show the relative ranks of values (starting from the highest values). When the means are not significantly different, the same superscript is used.
MAT could not be estimated for this low dose.
Pharmacodynamic studies.
The efficacy of capreomycin formulations delivered by aerosol and injection in the treatment of TB was evaluated in the guinea pig model. The efficacy of treatment was measured in terms of reduction of the bacterial burden in the lungs and spleen and by wet tissue weights and histopathology as supportive parameters. The TB-infected guinea pigs tolerated the administration of multiple doses of capreomycin solution (i.m.) and capreomycin particles (pulmonary), with no signs of adverse effects.
Wet lung and spleen weights for treated animals were employed as an indication of the degree of inflammation of each organ (6, 7, 9, 13, 18, 24). Figure 3 shows the organ weights corrected by body weight at the time of necropsy. The wet lung weight of animals receiving 14.5 mg/kg of aerosolized capreomycin particles was significantly smaller than that of untreated controls and animals receiving 1.4 mg/kg of aerosolized capreomycin particles. In addition, the wet lung weight of animals receiving 20 mg/kg of capreomycin solution by i.m. injection was significantly smaller than that of untreated controls. No significant differences were found in the wet spleen weights of animals receiving the different treatments.
FIG. 3.
Lung and spleen wet weights at necropsy for TB-infected guinea pigs receiving different treatments daily for 4 weeks (data are averages plus or minus standard deviations; n = 6). *, significantly smaller than untreated control values.
Figure 4 shows the number of viable bacteria per ml (CFU/ml) of organ homogenate for lungs and spleens of animals receiving the different treatments daily for 4 weeks. Animals receiving an estimated 14.5-mg/kg delivered dose of aerosolized capreomycin particles showed a significantly smaller bacterial burden in the lungs (3.52 ± 0.20 CFU/ml; P < 0.05) than did untreated controls (4.58 ± 0.20 CFU/ml), placebo-treated animals (4.21 ± 0.36 CFU/ml), and animals receiving estimated 1.4- and 7.2-mg/kg delivered doses of aerosolized capreomycin particles (4.02 ± 0.32 and 4.01 ± 0.29 CFU/ml, respectively). Animals receiving 20 mg/kg capreomycin solution by i.m. injection also showed a significantly smaller bacterial burden in the lungs (3.84 ± 0.34 CFU/ml) than did untreated controls. These positive results were supported by histopathological analysis, which revealed that a small percentage of the lung lobe was affected by small granulomas in animals treated with 20 mg/kg i.m. and with 14.5 mg/kg inhaled capreomycin and that only a few of these granulomas exhibited caseous necrosis and mild to moderate lymphocyte involvement (Fig. 5).
FIG. 4.
Number of viable bacteria per ml of tissue homogenate (CFU/ml) at necropsy in lung and spleen tissues of TB-infected guinea pigs receiving the following different treatments daily for 4 weeks: no treatment (untreated controls), i.m. injection of capreomycin (20 mg/kg), placebo (equivalent to 1.8 mg/kg), and aerosolized capreomycin particles at estimated deposited doses of 0.18, 0.90, and 1.8 mg/kg (corresponding to 1.4-, 7.2-, and 14.5-mg/kg nominal doses, respectively) (data are averages plus or minus standard deviations; n = 6). *, significantly smaller than untreated control values; **, significantly smaller than placebo-treated and untreated control values; #, significantly smaller than the value for any other treatment.
FIG. 5.
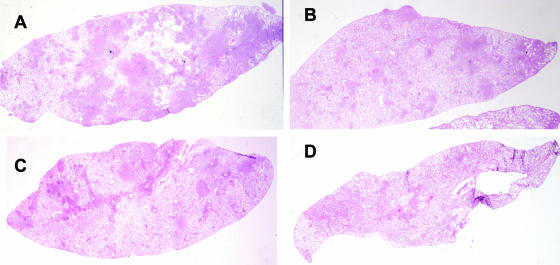
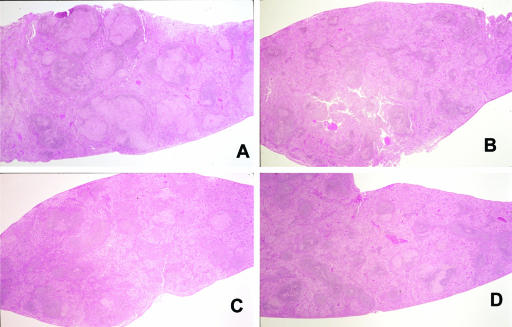
Lung histopathology of TB-infected guinea pigs. (A) Untreated control; (B) guinea pig receiving 20 mg/kg capreomycin solution i.m.; (C) guinea pig receiving 1.4 mg/kg capreomycin particles; (D) guinea pig receiving 14.5 mg/kg capreomycin particles. Guinea pigs in groups C and D received capreomycin particles in the dry powder dispersion chamber by passive inhalation.
The bacterial burdens in the spleens of animals receiving capreomycin i.m. were significantly smaller (at least 1.9-log reduction) (Fig. 4) than those for any other treatment (P < 0.05), and there were no significant differences among other treatment groups. Histopathological analysis also indicated a positive effect in the spleens of these animals and those receiving the high dose of capreomycin particles compared to other treatment groups, based on smaller granulomas and a smaller percentage of white pulp affected by these granulomas (Fig. 6).
FIG. 6.
Spleen histopathology of TB-infected guinea pigs. (A) Untreated control; (B) guinea pig receiving 20 mg/kg capreomycin solution i.m.; (C) guinea pig receiving 1.4 mg/kg capreomycin particles; (D) guinea pig receiving 14.5 mg/kg capreomycin particles. Guinea pigs in groups C and D received capreomycin particles in the dry powder dispersion chamber by passive inhalation.
DISCUSSION
The primary objective of this study was to demonstrate the feasibility and viability of simple pulmonary administration of capreomycin, utilizing a novel and pharmaceutically scaleable dry powder formulation prepared from materials that allow for varying the chemical composition, incorporating one or many drugs into the same particle, therefore enabling therapies that require combinations of pharmaceutical agents and excipients (e.g., amino acids, sugars, lipids, and polymers), extended shelf-life stability, and a controlled particle dissolution rate in the lungs (1, 17). Low-density powders such as LPPs can be aerosolized, permitting the efficient delivery of large drug masses from relatively simple inhalers (30).
The low-inoculum aerosol infection guinea pig model of TB closely mimics important aspects of the human disease (22). PK parameters after administration of capreomycin solutions or suspensions have been determined predominantly for mice and humans (3, 8, 14, 16, 19-21, 28). There are no reports of these studies for guinea pigs, a species known to have a different drug disposition from that of other rodents. Furthermore, it is expected that drug disposition will be different in healthy animals from that in infected animals. The present studies investigated the disposition of capreomycin in healthy animals to define initial PK parameters, which will require subsequent evaluation and redefinition in infected animals.
PK studies performed with guinea pigs showed that capreomycin plasma concentrations were higher 2 h after i.v. or i.m. administration than those for animals receiving the highest dose of insufflated particles. This initial difference in plasma concentration was expected given the differences in the nominal doses administered (20 mg/kg versus 14.5 mg/kg); however, plasma concentrations were comparable among these groups in the period 2 to 6 h after administration. Moreover, noncompartmental analysis demonstrated that capreomycin was cleared (CL) at a lower rate when delivered by the pulmonary route than when given i.v. or i.m., resulting in a significantly longer t1/2. Thus, it was proposed that given the comparably high drug concentrations in the lungs achieved by inhalation relative to those reported for other inhaled TB drugs (34) and the relatively good bioavailability achieved by inhalation (Table 1), efficacy might be achieved in an infection model at an inhaled dose below the dose required to achieve efficacy by conventional parenteral routes of delivery.
To test this hypothesis, capreomycin was delivered by aerosol and injection to guinea pigs in a TB infection model. The lungs of animals receiving the highest nominal dose of capreomycin particles by passive inhalation in the dry powder dosing chamber showed significantly smaller degrees of inflammation, bacterial burdens (CFU/ml), and percentages of lung tissue affected by granulomas and caseous necrosis (by histopathology) than those for any other treatment. In contrast, only animals treated by i.m. injection showed a significantly reduced bacterial burden in the spleen; however, histopathological analysis of spleens revealed a positive effect of treatment with the high dose of capreomycin particles and the i.m. solution. Given these results, we postulated that despite the initially higher systemic concentrations achieved after i.m. administration, the local capreomycin concentrations in the lung were higher in animals receiving inhaled powders, resulting in the reduction of the bacterial burden of the lungs. Although local concentrations were determined by BAL at the end of the PK study, determination of capreomycin levels in BAL samples at earlier time points would be desirable to confirm this assumption. The requirement of terminating the life of an animal for each time point to acquire BAL samples necessitates a separate extensive study, which was considered beyond the scope of the present work.
The lack of statistical significance in differences in the degree of inflammation and bacterial burden in the lungs and spleens of animals receiving the medium and low nominal doses as well as in the spleens of animals receiving the high dose of capreomycin particles can be explained by the actual deposited dose of particles in the lungs of these animals. Capreomycin plasma concentration-versus-time profiles as well as AUC and bioavailability were determined after delivering the high, medium, and low nominal doses to healthy animals in the dry powder dispersion chamber. Nominal doses of 14.5 mg/kg (high), 7.2 mg/kg (medium), and 1.4 mg/kg (low) were delivered to animals, and it is estimated that they received deposited doses of 1.8, 0.9, and 0.18 mg/kg, respectively. Given these relatively low deposited doses, the positive responses of reductions in the bacterial burdens and wet weights of the lungs of animals receiving the high dose of capreomycin particles by passive inhalation are noteworthy. The differences in bacterial burdens and wet weights of the spleens of animals receiving medium and low doses of particles were statistically insignificant with respect to untreated controls, which might be anticipated from the low systemic doses achieved by inhalation. In contrast, statistically significant differences were observed following i.m. administration, where high systemic concentrations of drug were achieved. While little has been published of relevance to this subject, a mouse study of inhaled nebulized capreomycin demonstrated a significantly higher deposited lung dose than that achieved in the present study (23), resulting in a reduction in bacterial burden in the spleen. Therefore, there is reason to believe that deposition of sufficient drug (higher than that achieved in the present study) in the lungs of guinea pigs would potentially result in therapeutic systemic concentrations.
The results of the present study demonstrate the practical feasibility of safe and efficacious inhaled capreomycin therapy as a means of treating TB. The pharmaceutically sound principles on which the particles are manufactured are suitable for immediate clinical trial supply production. Such trials would demonstrate the utility of pulmonary delivery in the following phase I and II studies: (i) a phase Ia, single-dose escalation study with healthy volunteers, using the inhaled capreomycin formulation; and (ii) phase Ib/IIa studies with newly diagnosed MDR-TB patients to observe the fall in serial sputum colony counts over a period of 16 weeks and to determine the relative rate of sputum conversion, duration of infectivity, symptomatology, and radiography for patients receiving capreomycin injections versus inhaled capreomycin.
Acknowledgments
This work was supported by the National Institutes of Health under grant 5 U01 61336-02.
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Ansel, H., L. Allen, and N. Popovich. 1999. Pharmaceutical dosage forms and drug delivery systems. Lippincott Williams & Wilkins, Philadelphia, PA.
- 2.Bastian, I., and R. Colebunders. 1999. Treatment and prevention of multidrug-resistant tuberculosis. Drugs 58:633-661. [DOI] [PubMed] [Google Scholar]
- 3.Black, H. R., R. S. Griffith, and A. M. Peabody. 1966. Absorption, excretion, and metabolism of capreomycin in normal and diseased states. Ann. N. Y. Acad. Sci. 135:974-982. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000-2004. Morb. Mortal. Wkly. Rep. 55:301-305. [PubMed] [Google Scholar]
- 5.Chaulk, C. P. 1995. Eleven years of community-based directly observed therapy for tuberculosis. JAMA 275:945-951. [PubMed] [Google Scholar]
- 6.Demidov, S. V., V. G. Myasnikov, E. F. Chernushenko, and L. Osipova. 1991. Effect of thymus preparations and antituberculosis drugs on immunologic reactivity and the course of the tuberculosis process in experimental animals. Probl. Tuberk. 12:52-54. [PubMed] [Google Scholar]
- 7.Devi, T. I., R. P. Gupta, R. K. Dhawan, V. V. S. Murti, B. Ramamurthy, T. Ramakrishnan, and T. Venkitasubramanian. 1988. Chemotherapy of experimental tuberculosis with N-(2-naphthyl)glycine hydrazide dihydrochloride. I. Acute toxicity and effect on wet weight, dry weight, glycogen and total lipids of organs. Indian J. Clin. Biochem. 3:37-44. [Google Scholar]
- 8.Donomae, I. 1966. Capreomycin in the treatment of pulmonary tuberculosis. Ann. N. Y. Acad. Sci. 135:1011-1038. [DOI] [PubMed] [Google Scholar]
- 9.Drandarska, I., V. Kussovski, S. Nikolaeva, and N. Markova. 2005. Combined immunomodulating effects of BCG and Lentinan after intranasal application in guinea pigs. Int. Immunopharmacol. 5:795-803. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, D. 1997. Large porous particles for pulmonary drug delivery. Science 276:1868-1871. [DOI] [PubMed] [Google Scholar]
- 11.Francis J. Curry Tuberculosis Reference Center and California Department of Health Services. 2004. Drug-resistant tuberculosis: a survival guide for clinicians. Francis J. Curry National Tuberculosis Center, San Francisco, CA.
- 12.García-Contreras, L., and A. J. Hickey. 2002. Pharmacokinetics of aerosolized rifampicin in the guinea pig, p. 699-701. In R. N. Dalby, P. R. Byron, J. Peart, and S. Farr (ed.), Respiratory drug delivery VIII. Davis Horwood International Publishing, Ltd., Raleigh, NC.
- 13.García-Contreras, L., V. V. Sethuraman, M. Kazantseva, V. Godfrey, and A. Hickey. 2006. Evaluation of dosing regimen of respirable rifampicin biodegradable microspheres in the treatment of tuberculosis in the guinea pig. J. Antimicrob. Chemother. 58:980-986. [DOI] [PubMed] [Google Scholar]
- 14.Garfield, J., J. Jones, N. Cohen, J. Daly, and J. McClement. 1966. The auditory, vestibular and renal effects of capreomycin in humans. Ann. N. Y. Acad. Sci. 135:1039-1046. [DOI] [PubMed] [Google Scholar]
- 15.Gonda, I. 1992. Targeting by deposition, p. 61-82. In A. J. Hickey (ed.), Pharmaceutical inhalation aerosol technology, vol. 54. Marcel Dekker, New York, NY. [Google Scholar]
- 16.Gyselen, A., L. Verbist, J. Prignot, and J. Cosemans. 1965. Capreomycin in the re-treatment of patients with pulmonary tuberculosis. Tubercle 46:243-249. [DOI] [PubMed] [Google Scholar]
- 17.Hinds, W. 1998. Aerosol technology: properties, behavior and measurement of airborne particles. Wiley Publishers, New York, NY.
- 18.Jiang, S., D. Zhu, Y. Jiang, X. Luo, and Q. Chen. 2005. Therapeutic effect of eukaryotic expression plasmid psIL-12 on murine infection with Mycobacterium tuberculosis. Zhonghua Weishengwuxue He Mianyixue Zazhi 24:373-376. [Google Scholar]
- 19.Le Conte, P., F. Le Gallou, G. Potel, L. Struillou, D. Baron, and H. B. Drugeon. 1994. Pharmacokinetics, toxicity, and efficacy of liposomal capreomycin in disseminated Mycobacterium avium beige mouse model. Antimicrob. Agents Chemother. 38:2695-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee, S. H., J. C. J. M. Shin, E. Y. Lee, D. H. Kim, J.-W. Suh, and J. Chang. 2003. The impurities of capreomycin make a difference in the safety and pharmacokinetic profiles. Int. J. Antimicrob. Agents 22:81-83. [DOI] [PubMed] [Google Scholar]
- 21.Lehmann, C. R., L. E. Garrett, R. E. Winn, P. D. Springberg, S. Vicks, D. K. Porter, W. P. Pierson, J. D. Wolny, G. L. Brier, and H. R. Black. 1988. Capreomycin kinetics in renal impairment and clearance by hemodialysis. Am. Rev. Respir. Dis. 138:1312-1313. [DOI] [PubMed] [Google Scholar]
- 22.McMurray, D. 1994. Guinea pig model of tuberculosis, p. 135-148. In B. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, DC.
- 23.NIH. 3 December 2003, posting date. Aerosol delivery of MTB and chemotherapeutics: pitfalls and plans. NIH, Bethesda, MD. http://www3.niaid.nih.gov/Biodefense/Research/AEROSOL/lauravia.pdf.
- 24.Niu, X., C. Liu, Y. Li, M. Li, and H. Wang. 1998. Therapeutic action of Luolining capsules on tuberculotic guinea pigs. Zhongguo Yaoke Daxue Xuebao 29:142-144. [Google Scholar]
- 25.Niven, R. W. 1996. Atomization and nebulizers, p. 273-312. In A. J. Hickey (ed.), Inhalation aerosols. Physical and biological basis for therapy, vol. 94. Marcel Dekker, New York, NY. [Google Scholar]
- 26.Obrien, R. J. 1993. The treatment of tuberculosis, p. 207-240. In L. B. Reichman and E. S. Hershfield (ed.), Tuberculosis. Marcel Dekker, New York, NY.
- 27.O'Hara, P., and A. J. Hickey. 2000. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: manufacture and characterization. Pharm. Res. 17:955-961. [DOI] [PubMed] [Google Scholar]
- 28.Organick, A. B., and E. M. Wilson. 1968. Multiple drugs in retreatment of chronic pulmonary tuberculosis. Results with capreomycin and ethambutol. Dis. Chest 53:560-570. [DOI] [PubMed] [Google Scholar]
- 29.Rossi, C., G. Fardella, I. Chiappini, L. Perioli, C. Vescovi, M. Ricci, S. Giovagnoli, and S. Scuota. 2004. UV spectroscopy and reverse-phase HPLC as novel methods to determine capreomycin of liposomal formulations. J. Pharm. Biomed. Anal. 36:249-255. [DOI] [PubMed] [Google Scholar]
- 30.Scheuch, F., T. Meyer, K. Sommerer, H. Lichte, and A. Pohner. 1999. Measuring in vivo deposition of large porous particles J. Aerosol Med. 12:138. [Google Scholar]
- 31.Smith, S. J., and J. A. Bernstein. 1996. Therapeutic uses of lung aerosols, p. 233-269. In A. J. Hickey (ed.), Inhalation aerosols. Physical and biological basis for therapy. Marcel Dekker, Inc., New York, NY.
- 32.Suarez, S., P. O'Hara, M. Kazantseva, C. Newcomer, R. Hopfer, D. McMurray, and A. Hickey. 2001. Airways delivery of rifampicin microparticles for the treatment of tuberculosis. J. Antimicrob. Chemother. 48:431-434. [DOI] [PubMed] [Google Scholar]
- 33.Suarez, S., P. O'Hara, M. Kazantseva, C. Newcomer, R. Hopfer, D. McMurray, and A. Hickey. 2001. Respirable PLGA microspheres containing rifampicin for the treatment of tuberculosis: screening in an infectious disease model. Pharm. Res. 19:1315-1319. [DOI] [PubMed] [Google Scholar]
- 34.Tsapis, N., D. Bennett, K. O'Driscoll, K. Shea, M. M. Lipp, K. Fu, R. W. Clarke, D. Deaver, D. Yamins, J. Wright, C. A. Peloquin, D. A. Weitz, and D. A. Edwards. 2003. Direct lung delivery of para-aminosalicylic acid by aerosol particles. Tuberculosis (Edinburgh) 83:379-385. [DOI] [PubMed] [Google Scholar]
- 35.Wiegeshaus, E. H., D. N. McMurray, A. A. Grover, G. E. Harding, and D. W. Smith. 1970. Host-parasite relationships in experimental airborne tuberculosis. III. Relevance of microbial enumeration to acquired resistance in guinea pigs. Am. Rev. Respir. Dis. 170:422-429. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. 2004. World Health Organization/International Union Against Tuberculosis and Lung Disease global project on anti-tuberculosis drug resistance surveillance. Antituberculosis drug resistance in the world report no. 3. World Health Organization, Geneva, Switzerland.