Abstract
Six tetraoxanes had 50% inhibitory concentrations in the range of 10 to 100 ng/ml against Plasmodium falciparum, whereas the corresponding hexaoxonanes had minimal antimalarial activity. The lack of iron-mediated reactivity of the hexaoxonanes may explain their low activity compared to the tetraoxanes, the latter of which are able to undergo iron(II)-mediated activation.
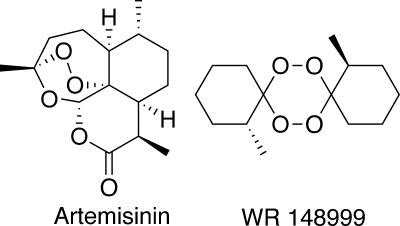
The antimalarial artemisinin (qinghaosu) contains a pharmacophoric peroxide bond within its 1,2,4-trioxane heterocycle. The complex structure of artemisinin is an incentive to identify more synthetically accessible antimalarial peroxides (10, 13, 15, 18). One of the most structurally simple classes of antimalarial synthetic peroxides are 1,2,4,5-tetraoxanes (2, 5, 6, 12, 16), as exemplified by WR 148999 (Fig. 1). Tetraoxanes such as WR 148999 (20) differ considerably in structure from artemisinin, are readily prepared in one step from substituted cyclohexanones, and possess good antimalarial activity, although they are significantly less active than the semisynthetic artemisinins.
FIG. 1.
Structures of artemisinin and WR 148999.
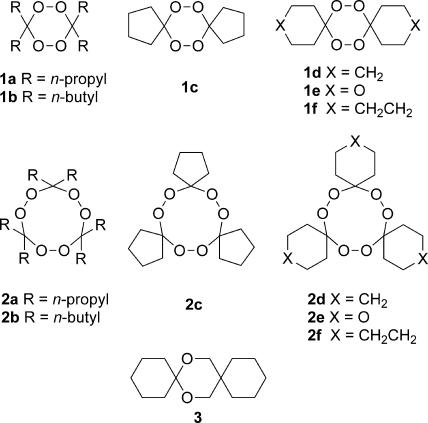
In this study, we disclose the comparative antimalarial activities of six pairs of 1,2,4,5-tetraoxanes (peroxide dimers) 1a-1f and 1,2,4,5,7,8-hexaoxonanes (peroxide trimers) 2a-2f (Fig. 2) to further elucidate the structure-activity-relationship of 1,2,4,5-tetraoxanes and, to our knowledge, record for the first time the antimalarial activity of 1,2,4,5,7,8-hexaoxonanes. The latter group of synthetic peroxides has been investigated primarily as synthetic precursors to macrocyclic lactones of perfumery interest (17). As previously described (7, 8), tetraoxanes 1a-1f and hexaoxonanes 2a-2f were prepared either by peroxidation of the corresponding ketone derivatives in H2SO4/CH3CN or by ozonolysis of the corresponding ketone O-methyl oximes. 1,3-Dioxolane 3, the nonperoxidic isostere of tetraoxane 1d, was prepared by ketalization of cyclohexanone with 1,1-dimethanolcyclohexane (TsOH, refluxing toluene) in 35% yield after Kugelrohr distillation (melting point 53 to 56°C) and characterized by 1H and 13C NMR and elemental analysis.
FIG. 2.
Structures of 1,2,4,5-tetraoxanes 1a to 1f, 1,2,4,5,7,8-hexaoxonanes 2a to 2f, and nonperoxidic isostere 3.
As previously described (6), in vitro and in vivo antimalarial activities were measured using the chloroquine-resistant K1 and chloroquine-sensitive NF54 strains of Plasmodium falciparum- and P. berghei-infected mice, respectively. Groups of three P. berghei-infected MORO mice were treated 1 day postinfection with 100-mg/kg oral and subcutaneous doses of compounds dissolved or suspended in a solubilizing 3% ethanol and 7% Tween 80 vehicle. Antimalarial activity was measured by determining the percent reduction in parasitemia on day 3 postinfection compared to an untreated control group.
The data in Table 1 show a clear demarcation in the antimalarial activities of tetraoxanes 1 and hexaoxonanes 2 against P. falciparum. Tetraoxanes 1 had 50% inhibitory concentrations (IC50s) in the range of 10 to 100 ng/ml, whereas all hexaoxonanes had minimal antimalarial activity; only three of these (2a, 2d, and 2f) had IC50s of <10,000 ng/ml. The previously reported IC50s against the P. falciparum W2 and D6 clones for 1d (20 and 26 ng/ml) and 1e (68 and 100 ng/ml) (11) and against the P. falciparum FCB1 strain for 1a (48 ng/ml) and 1f (21 ng/ml) (21) are quite similar to those that we measured against the P. falciparum K1 and NF54 isolates. Of tetraoxanes 1, only 1f was as potent as WR 148999. However, each of tetraoxanes 1a to 1f was much less active than WR 148999 against P. berghei. Apparently, the two methyl groups flanking the central 1,2,4,5-tetraoxane heterocycle in WR 148999, which provide substantial steric hindrance to the peroxide bonds, are critical for good in vivo activity. Ketal 3, the nonperoxidic isostere of tetraoxane 1d, was devoid of antimalarial activity demonstrating that at least one of the two peroxide bonds of 1d (and other tetraoxanes) is required for antimalarial activity.
TABLE 1.
Activity of 1,2,4,5-tetraoxanes 1, 1,2,4,5,7,8-hexaoxonanes 2 and nonperoxidic isostere 3 against P. falciparum in vitro and P. berghei in vivo
| Compounda | IC50 (ng/ml)b K1/NF54 | Activity (%)c (p.o./s.c.) |
|---|---|---|
| 1a | 40/23 | 21/0 |
| 1b | 71/88 | 47/5 |
| 1c | 51/23 | 35/84 |
| 1d | 30/44 | 0/26 |
| 1e | 78/44 | 22/0 |
| 1f | 14/11 | 60/56 |
| 2a | 7400/5500 | ND |
| 2b | >10,000/>10,000 | ND |
| 2c | >10,000/>10,000 | ND |
| 2d | 770/2700 | ND |
| 2e | >10,000/>10,000 | ND |
| 2f | 2000/3200 | ND |
| 3 | >10,000/>10,000 | ND |
| WR 148999* | 7.2/10 | 99.7/>99.99 |
| Artemisinin* | 2.8/3.4 | 98/>99.99 |
*, Data from Dong et al. (6).
Mean from n = 2 to 3 determinations. Individual measurements differed by <50%.
Mean from n = 3 determinations. Individual measurements differed by <10%. ND, not determined. Data are presented as values for peroral (p.o.)/subcutaneous (s.c.) administrations.
A long-standing hypothesis (9, 14, 15) to account for the antimalarial specificity of artemisinin is that the peroxide bond undergoes reductive activation by heme released by parasite hemoglobin digestion. This irreversible redox reaction produces carbon-centered free radicals or carbocations that alkylate heme or parasite proteins (9). Since the antimalarial activity of tetraoxanes 1 is peroxide bond dependent and hexaoxonanes 2 are without significant antimalarial activity, we wondered whether this observation could be explained in part by the relative susceptibilities of 1 and 2 to reaction with iron(II). To this end, we investigated the reaction of the tetraoxane/hexaoxonane pair 1d/2d with inorganic ferrous iron using previously established standardized conditions (3). In this experiment, pseudo-first-order reaction rate constants for 1d and 2d (0.03 mM) with FeSO4 (3 mM) in 50% acetonitrile-water at 37°C (n = 3) under an argon atmosphere were determined. The experiment was corrected for nonspecific degradation in iron-free controls. Tetraoxane 1d underwent complete degradation in the presence of FeSO4 with a pseudo-first order rate constant (k) of 0.38 ± 0.04 h−1. In contrast, hexaoxonane 2d did not undergo any significant degradation in the presence of FeSO4 over the 15 h time course studied (k < 0.007 h−1 − the upper limit of the 95% confidence interval). The lack of iron-mediated reactivity of 2d may explain the low antimalarial activity of the hexaoxonanes 2 compared to the tetraoxanes 1, the latter of which are able to undergo iron(II)-mediated activation. For comparison, the pseudo-first-order rate constant (k) for artemisinin is 0.054 ± 0.006 h−1 (3).
The difference in iron-mediated reactivity between 1d and 2d can be explained by considering their peroxide bond steric accessibility as shown by molecular modeling using Spartan'06 (Wavefunction, Inc.) (Fig. 3). The peroxide bonds in 1d appear to be fairly accessible, whereas the peroxide bonds in 2d are shielded by the three cyclohexane rings so that an iron(II) complex would be sterically prohibited from undergoing inner-sphere electron transfer with the peroxide bond antibonding σ* (LUMO) orbitals. Similar arguments have been put forth to explain the low antimalarial activity of sterically congested 1,2,4-trioxolanes (4), 1,2,4-trioxanes (19), and 1,2,4-trioxepanes (1).
FIG. 3.
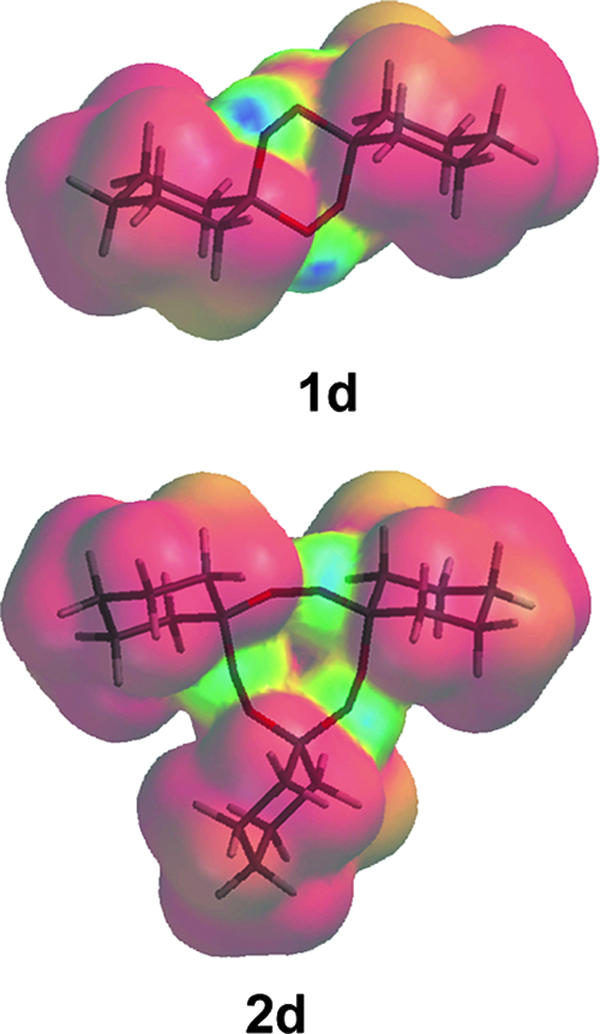
Modeled structures of 1,2,4,5-tetraoxane 1d and 1,2,4,5,7,8-hexaoxonane 2d depicted with LUMOs (blue) mapped on isodensity surfaces.
Acknowledgments
This investigation received financial support from the UNDP/WORLD BANK/WHO Special Programme for Research and Training in Tropical Diseases (TDR ID no. 960275).
We thank Christian Scheurer, Christopher Snyder, and Josefina Santo-Tomas for technical assistance with the antimalarial assays.
Footnotes
Published ahead of print on 7 May 2007.
REFERENCES
- 1.Amewu, R., A. V. Stachulski, N. G. Berry, S. A. Ward, J. Davies, G. Labat, J.-F. Rossignol, and P. M. O'Neill. 2006. Synthesis of 1,2,4-trioxepanes via application of thiol-olefin co-oxygenation methodology. Bioorg. Med. Chem. Lett. 16:6124-6130. [DOI] [PubMed] [Google Scholar]
- 2.Amewu, R., A. V. Stachulski, S. A. Ward, N. G. Berry, P. G. Bray, J. Davies, G. Labat, L. Vivas, and P. M. O'Neill. 2006. Design and synthesis of orally active dispiro 1,2,4,5-tetraoxanes; synthetic antimalarials with superior activity to artemisinin. Org. Biomol. Chem. 4:4431-4436. [DOI] [PubMed] [Google Scholar]
- 3.Creek, D. J., F. C. K. Chiu, R. J. Prankerd, S. A. Charman, and W. N. Charman. 2005. Kinetics of iron-mediated artemisinin degradation: effect of solvent composition and iron salt. J. Pharm. Sci. 94:1820-1829. [DOI] [PubMed] [Google Scholar]
- 4.Creek, D. J., F. C. K. Chiu, R. J. Prankerd, K. J. McCullough, Y. Dong, J. L. Vennerstrom, W. N. Charman, and S. A. Charman. 4 June 2007, posting date. Iron-mediated degradation kinetics of substituted dispiro-1,2,4-trioxolane antimalarials. J. Pharm. Sci. doi: 10.1002/jps.20958. [DOI] [PubMed]
- 5.Dong, Y. 2002. Synthesis and antimalarial activity of 1,2,4,5-tetraoxanes. Mini Rev. Med. Chem. 2:113-123. [DOI] [PubMed] [Google Scholar]
- 6.Dong, Y., H. Matile, J. Chollet, R. Kaminsky, J. K. Wood, and J. L. Vennerstrom. 1999. Synthesis and antimalarial activity of eleven dispiro-1,2,4,5-tetraoxane analogs of WR 148999 7,8,15,16-tetraoxadispiro[5.2.5.2] hexadecanes substituted at the 1 and 10 positions with unsaturated and polar functional groups. J. Med. Chem. 42:1477-1480. [DOI] [PubMed] [Google Scholar]
- 7.Dong, Y., and J. L. Vennerstrom. 1998. Dispiro-1,2,4,5-tetraoxanes via ozonolysis of cycloalkanone O-methyl oximes: a comparison with the peroxidation of cycloalkanones in acetonitrile sulfuric acid media. J. Org. Chem. 63:8582-8585. [Google Scholar]
- 8.Dong, Y., and J. L. Vennerstrom. 2001. Differentiation between 1,2,4,5-tetraoxanes and 1,2,4,5,7,8-hexaoxonanes using 1H and 13C NMR analyses. J. Heterocyclic Chem. 38:463-466. [Google Scholar]
- 9.Jefford, C. W. 2001. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr. Med. Chem. 8:1803-1826. [DOI] [PubMed] [Google Scholar]
- 10.Jefford, C. W. 2004. Synthetic peroxides as antimalarials. Curr. Opin. Investig. Drugs 5:866-872. [PubMed] [Google Scholar]
- 11.Jefford, C. W., J.-C. Rossier, and W. K. Milhous. 2000. The structure and antimalarial activity of some 1,2,4-trioxanes, 1,2,4,5-tetroxanes, and bicyclic endoperoxides: implications for the mode of action. Heterocycles 52:1345-1352. [Google Scholar]
- 12.Kim, H.-S., Y. Shibata, Y. Wataya, K. Tsuchiya, A. Masuyama, and M. Nojima. 1999. Synthesis and antimalarial activity of cyclic peroxides, 1,2,4,5,7-pentoxocanes and 1,2,4,5-tetroxanes. J. Med. Chem. 42:2604-2609. [DOI] [PubMed] [Google Scholar]
- 13.McCullough, K. J., and M. Nojima. 2001. Recent advances in the chemistry of cyclic peroxides. Curr. Org. Chem. 5:601-636. [Google Scholar]
- 14.Meshnick, S. R. 2002. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 32:1655-1660. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill, P. M., and G. H. Posner. 2004. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47:2945-2964. [DOI] [PubMed] [Google Scholar]
- 16.Solaja, B. A., N. Terzic, G. Pocsfalvi, L. Gerena, B. Tinant, D. Opsenica, and W. K. Milhous. 2002. Mixed steroidal 1,2,4,5-tetraoxanes: antimalarial and antimycobacterial activity. J. Med. Chem. 45:3331-3336. [DOI] [PubMed] [Google Scholar]
- 17.Story, P. R., and P. Busch. 1972. Modern methods for the synthesis of macrocyclic compounds. Adv. Org. Chem. 8:67-95. [Google Scholar]
- 18.Tang, Y., Y. Dong, and J. L. Vennerstrom. 2004. Synthetic peroxides as antimalarials. Med. Res. Rev. 24:425-448. [DOI] [PubMed] [Google Scholar]
- 19.Tang, Y., Y. Dong, X. Wang, K. Sriraghavan, J. K. Wood, and J. L. Vennerstrom. 2005. Dispiro-1,2,4-trioxane analogs of a prototype dispiro-1,2,4-trioxolane: mechanistic comparators for artemisinin in the context of reaction pathways with iron(II). J. Org. Chem. 70:5103-5110. [DOI] [PubMed] [Google Scholar]
- 20.Vennerstrom, J. L., H.-N. Fu, W. Y. Ellis, A. L. Ager, L. Gerena, and W. K. Milhous. 1992. Dispiro-1,2,4,5-tetraoxanes: a new class of antimalarial peroxides. J. Med. Chem. 35:3023-3027. [DOI] [PubMed] [Google Scholar]
- 21.Zmitek, K., S. Stavber, M. Zupan, D. Bonnet-Delpon, S. Charneau, P. Grellier, and J. Iskra. 2006. Synthesis and antimalarial activities of novel 3,3,6,6-tetraalkyl-1,2,4,5-tetraoxanes. Bioorg. Med. Chem. 14:7790-7795. [DOI] [PubMed] [Google Scholar]