Abstract
β-d-2′-Deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130) is an effective inhibitor of hepatitis C virus (HCV) replication in vitro. The purpose of this study was to evaluate the single-dose pharmacokinetics of PSΙ-6130 in rhesus monkeys following intravenous (i.v.) and oral administration. Noncompartmental analysis of the serum data obtained following oral and i.v. administration was performed. Pharmacokinetic studies with rhesus monkeys indicated slow and incomplete absorption with a mean absorption time (MAT) of 4.6 h and an oral bioavailability of 24.0% ± 14.3% (mean ± standard deviation), with comparable mean apparent half-lives following i.v. (4.54 ± 3.98 h) and oral (5.64 ± 1.13 h) administrations. The average percentages of the total dose recovered unchanged and in deaminated form in the urine were 32.9% ± 12.6% and 18.9% ± 6.6% (i.v.) and 6.0% ± 3.9% and 3.9% ± 1.0% (oral), respectively. The total bioavailability, taking into account the parent drug and its deaminated metabolite 2′-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6206), was 64% ± 26%. PSI-6130 was present in the cerebrospinal fluid after oral and i.v. dosing. However, no deamination of radiolabeled PSI-6130 was detected after 8 h of incubation in monkey and human whole blood. An N4-modified prodrug of PSI-6130 (PSI-6419) was orally administered to monkeys, but it failed to improve the oral bioavailability of PSI-6130. Further studies are warranted to improve the oral bioavailability and reduce the deamination of PSI-6130 in order to explore the potential of this drug for the treatment of HCV-infected individuals.
Hepatitis C virus (HCV) is an important viral pathogen which has a vast impact on human health and well-being, since about 170 million people are infected globally (4, 11). Treatment options are inadequate because of the limited number of available therapeutic modalities that produce a sustained virological response (15, 17). Therefore, new agents are needed for more efficacious therapies. β-d-2′-Deoxy-2′-fluoro-2′-C-methylcytidine (PSI-6130) is a specific HCV RNA-dependent RNA polymerase (RdRp) inhibitor with a 90% effective antiviral concentration (EC90) of 5.4 ± 2.6 μM in Huh-7 replicon cells, with no cytotoxicity or cytostasis at this concentration (8). The median inhibitory concentration of PSI-6130 in HepG2 cells was greater than 100 μM in a 4-day assay. The triphosphate of PSI-6130 was found to be equally active against wild-type HCV NS5B and in a replicon containing the S282T mutation (18). This mutation has been selected by a variety of base-modified 2′-methyl ribonucleosides (4, 16).
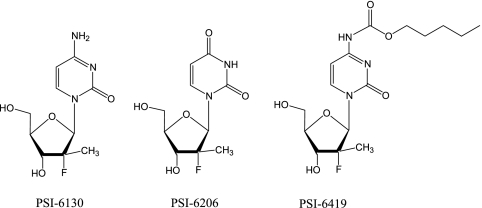
The objective of this study was to assess the single-dose pharmacokinetics (PK) of PSI-6130 in rhesus monkeys given 33.3 mg/kg by the intravenous (i.v.) and oral routes. This dose was selected for comparison with previous studies of the PK of antiviral nucleosides in rhesus monkeys (6, 7, 20, 21). A prodrug of PSI-6130, β-d-(2S)-2′-deoxy-2′-fluoro-2′-methyl-N4-pentyloxycarbonylcytidine (PSI-6419), was also synthesized and administered orally in an attempt to improve the oral bioavailability and reduce the deamination of PSI-6130 (Fig. 1) (10). Studies of the stability of radiolabeled PSI-6130 in whole human and monkey blood were also conducted.
FIG. 1.
Structures of β-d-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6130), β-d-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6206), and d-(2S)-2′-deoxy-2′-fluoro-2′-methyl-N4-pentyloxycarbonylcytidine (PSI-6419; PSI-6130 prodrug).
MATERIALS AND METHODS
Chemicals.
The synthesis of PSI-6130 (molecular weight = 259.2), its deaminated metabolite 2′-deoxy-2′-fluoro-2′-C-methyluridine (PSI-6206; molecular weight = 260.2) (Fig. 1), and the internal standard, lamivudine (3TC), have been described elsewhere (8). The chemical purity of each compound was greater than 98% by high-performance liquid chromatography (HPLC) and spectral analysis.
5-3H-PSI-6130 (10.0 Ci/mmol) and [6-3H]deoxycytidine (specific activity = 26.1 Ci/mmol) were synthesized by Moravek Biochemicals, Inc. (Brea, CA). Tetrabutylammonium phosphate was purchased from Alltech Associates, Inc. (Deerfield, IL). Scintillation liquid, Eco Lite, was purchased from Valeant Pharmaceuticals International (Costa Mesa, CA). Fresh monkey blood containing EDTA as an anticoagulant was kindly provided by the Yerkes National Primate Research Center, Emory University, Atlanta, GA, and human blood containing EDTA was obtained by venipuncture from healthy volunteers at the VA Medical Center, Atlanta, GA. Acetonitrile (HPLC grade) and all other chemicals (analytical grade) were purchased from Fisher Scientific (Fair Lawn, NJ).
Synthesis of β-d-(2S)-2′-deoxy-2′-fluoro-2′-methyl-N4-pentyloxycarbonylcytidine (PSI-6419; a potential prodrug of PSI-6130).
To a solution of β-d-(2S)-2′-deoxy-2′-fluoro-2′-methylcytidine (750 mg, 2.89 mmol) in anhydrous pyridine (15 ml) was added trimethylsilylchloride (1.88 ml, 14.47 mmol), and the solution was stirred at room temperature for 2 h. Amyl chloroformate (2.20 ml, 14.47 mmol) was added, and the solution was stirred at room temperature overnight. The solution was cooled to 0°C, and water (3 ml) was added. After stirring at 0°C for 20 min, NH4OH (28%, 3 ml) was added and the mixture was stirred at room temperature for another 20 min. The solvent was removed by evaporation at reduced pressure, and the residue was purified by flash chromatography on silica gel eluting with CH2Cl2-methanol (95:5) to give 845 mg (78%) of the title compound as a white solid (molecular weight = 373.38). 1H nuclear magnetic resonance analysis of PSI-6419 (dimethyl sulfoxide-d6): δ 10.78 (s, 1H, NH), 8.41 (d, J = 7.7 Hz, 1H, H-6), 7.06 (d, J = 7.7 Hz, 1H, H-5), 6.12 (d, J = 18.5 Hz, 1H, H-1′), 5.68 (d, J = 6.5 Hz, 1H, OH), 5.35 (bs, 1H, OH), 4.10 (t, J = 6.0 Hz, 2H, OCH2), 3.88, 3.67 (2m, 4H, H-3′, H-4′, H-5′), 1.60 (m, 2H, CH2), 1.31 (m, 4H, 2 CH2), 1.21 (d, J = 22.3 Hz, 3H, CH3-2′), 0.88 (t, J = 6.7 Hz, 3H, CH3).
Stability of radiolabeled PSI-6130 in monkey and human whole blood.
3H-labeled PSI-6130 (10 μM, 1,000 dpm/pmol) was incubated in either monkey or human blood at 37°C for selected periods of time (0, 0.25, 0.50, 1, 2, 4, 8, 10, 24, and 48 h). An aliquot of 200 μl was taken at each time period and centrifuged at 14,000 rpm (Eppendorf 5415C centrifuge) for 5 min. The supernatant was collected, 500 μl of acetonitrile was added, and then the mixture was vortexed. The sample was recentrifuged at 14,000 rpm for 5 min, and the supernatant was air dried. Residues were resuspended in 100 μl of water, and aliquots were injected into the HPLC column.
HPLC analysis of radiolabeled PSI-6130.
The compounds were separated with an ion-pairing mobile phase by reverse-phase HPLC with a C18 column (Columbus, 4.6 by 250 mm, 5-μm particle diameter; Phenomenex, Torrance, CA) with a Varian Pro Star 210 HPLC apparatus with a manual injector (Varian, Walnut Creek, CA). The mobile phase consisted of (A) 25 mM ammonium acetate with 5 mM tetrabutylammonium phosphate, pH 7.0, and (B) methanol. Elution was performed with a multistage linear gradient of solvent B of 0 to 30% at a constant flow of 1.0 ml/min. The radioactivity of the eluting tritiated compounds was measured with a 500TR Radiometric Flo-One radiochromatography analyzer (Perkin-Elmer, Life and Analytical Sciences, Wellesley, MA).
PK studies with rhesus monkeys.
Three female rhesus monkeys (Macaca mulatta) weighing 6.0 to 6.7 kg were used for the PK studies. The animals were maintained at the Yerkes National Primate Research Center at Emory University, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care, in accordance with guidelines established by the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (19) of the National Institutes of Health. To determine the single-dose PK after administration of the oral dose, the monkeys were administered PSI-6130 at 33.3 mg/kg by gastric intubation in a total volume of 10 ml of water, followed by a further 3 ml of water. The single-dose PK following drug administration by the i.v. route were determined after a washout period of at least 4 weeks. The monkeys were given a single bolus dose of PSI-6130 at 33.3 mg/kg i.v. in 10 ml of pyrogen-free, sterile, normal saline via the femoral vein. After a further washout period of 5 to 6 weeks, a single dose of the PSI-6130 prodrug PSI-6419 of 33.3 mg/kg was administered to the same monkeys by gastric intubation in a total volume of 10 ml of water, followed by a further 3 ml of water. Animals were maintained on their backs on a heating pad and covered with a blanket under anesthesia for 4 h after dosing. Anesthesia was performed with a mixture of ketamine HCl (60 mg) and tiletamine HCl plus zolazepam HCl (Telazol; 20 mg) administered intramuscularly. Animals were monitored for alertness, and additional anesthetic doses (30 to 60 mg of ketamine HCl) were administered as needed. Blood samples were collected through the femoral vein at 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after dosing. Cerebrospinal fluid (CSF) samples were collected from treated monkeys at 1 h after drug administration by a cisternal or lumbar tap with a 22-gauge needle. The monkeys were catheterized for urine collection. Urine samples were collected at 0 to 0.25, 0.25 to 0.5, 0.5 to 1, 1 to 1.5, 1.5 to 2, 2 to 3, 3 to 4, 4 to 6, and 6 to 8 h after drug administration. Serum, CSF, and urine samples were kept on ice and then frozen at −70°C until used for analyses.
HPLC assays of PSI-6130 in monkey serum, urine, and CSF samples.
PSI-6130 concentrations in monkey fluid samples were assayed with a Hitachi HPLC system (Tokyo, Japan) equipped with a model L-7100 pump, a model L-7400 UV detector, and a model L-7200 autosampler with a C18 reverse-phase column (Columbus, 4.6 by 250 mm, 5-μm particle diameter; Phenomenex, Torrance, CA). The mobile phase was isocratic for the first 20 min and consisted of 1% phosphate buffer (10 mM K2HPO4, pH 7.5), 2% acetonitrile, and 97% water. A gradient elution was then used from 20 to 34 min in which the buffer ratio was kept at 1% and the acetonitrile ratio was changed from 2 to 7% at 23.5 min and reduced to 3% at 34 min. The flow rate was maintained at 0.7 ml/min. The retention times of PSI-6130, PSI-6206, and 3TC were 20 min, 31 min, and 34.3 min, respectively. The compounds were detected at a wavelength of 270 nm.
Preparation of standards and method validation.
Standard solutions of PSI-6130, PSI-6206, and PSI-6419 were prepared in deionized water. Calibration curves for PSI-6130, PSI-6206, and PSI-6419 were prepared by diluting standard solutions in monkey serum derived from the same monkeys prior to drug administration. The concentrations measured ranged from 0.1 to 100 μg/ml. (Micrograms per milliliter were converted to micromolar concentrations by multiplying by 3.86.) Dilutions of standard solutions for calibration curves were prepared in urine and deionized water with concentrations that ranged from 0.1 to 100 μg/ml and from 0.1 to 25 μg/ml for analysis of urine and CSF samples, respectively. The HPLC method used was validated according to the Food and Drug Administration's Guidelines for Industry Bioanalytic Method Validation protocol (22). The intraday accuracy and relative standard deviations of the assay methodologies for serum were evaluated by assaying six samples per concentration on the same day. For interday accuracy and precision, samples were analyzed on three separate days. The intraday and interday relative standard deviations were less than 10%, and the intraday and interday accuracies were greater than 90%. The standard curves were linear over concentrations ranging from 0.2 to 100 μg/ml (r2, >0.99). The limit of detection of the assay was 0.1 μg/ml (0.38 μΜ for PSI-6130 and PSI-6206 and 0.27 μΜ for PSI-6419).
Extraction procedure.
For serum samples, 10 μl of 3TC (200 μg/ml) was added as an internal standard to a 50- to 100-μl serum sample in a microcentrifuge tube. Acetonitrile (450 μl) was then added, followed by thorough vortexing and centrifugation at 9,000 × g for 5 min. The supernatant was evaporated to dryness, and the samples were reconstituted with water (100 μl) and recentrifuged at 11,000 × g for 3 min. A 25- to 50-μl supernatant sample was injected into the HPLC column. The percent recovery of PSI-6130 was calculated by comparing the mean peak areas for six extracted serum samples with those of the three standard samples with the same amount of nucleoside. Low (1 μg/ml), medium (10 μg/ml), and high (100 μg/ml) concentrations were investigated. The percent extraction recovery was calculated with the formula 100 × Aextracted/Astandard, where Aextracted is the peak area of drug extracted from the biological fluid and Astandard is the peak area of the same amount of the drug without extraction. The extraction recovery of PSI-6130 was greater than 92%.
Ten microliters of a 3TC solution (200 μg/ml) was added to 100-μl CSF samples as an internal standard, and 75 μl was injected into the HPLC column. The urine samples collected after oral and i.v. drug administrations were diluted 20- and 50-fold, respectively. A 10-μl volume of 3TC (200 μg/ml) was added to 100-μl diluted urine samples, and then 25 to 50 μl was injected into the HPLC column.
PK analysis.
Noncompartmental PK analysis was performed on the serum concentration-versus-time data of PSI-6130 derived from each monkey and on the pooled data obtained following i.v. and oral dosing (33.3 mg/kg), with a nonlinear regression curve fitting program (WinNonlin, version 5.0, 2005; Pharsight Corp., Cary, NC). The resulting PK parameters are summarized in Table 1. The terminal elimination constant (λz) was calculated with a weighting factor of 1/observed (12, 13). The areas under the serum concentration-versus-time curve (AUC) and the first-moment curve (AUMC) from time zero to the last sample time determined were calculated by a trapezoidal method, with extrapolation to infinity by making use of λz The fraction of the oral dose absorbed (F) = AUCoral × 100/AUCi.v., where AUCoral and AUCi.v. are the extrapolated AUCs after oral and i.v. dosing, respectively. The mean absorption time (MAT) = MRToral − MRTi.v., where MRToral and MRTi.v. represent the mean residence times extrapolated to infinity after oral and i.v. dosing, respectively (12, 13). The relative percent exposure to PSI-6206 in serum relative to PSI-6130 and the deaminated metabolite after i.v. and oral dosing (%Exp6206-oral and %Exp6206-i.v., respectively) were calculated with the equations %Exp6206-oral = AUCoral-6206/(AUCoral-6130 + AUCoral-6206) × 100 and %Exp6206-i.v. = AUCi.v.-6206/(AUCi.v.-6130 + AUCi.v.-6206) × 100. Since a large fraction of the oral dose was converted into PSI-6206, the total oral bioavailability relative to that determined after i.v. administration was calculated as Ftotal = (AUCoral-6130 + AUCoral-6206) × 100/(AUCi.v.-6130 + AUCi.v.-6206).
TABLE 1.
Noncompartmental PK parameters of PSI-6130 after i.v. and oral administration of 33.3 mg/kg to three rhesus monkeys
| Parametera | Unit | Value following i.v. administration
|
Value following oral administration
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-1b | M-2 | M-3 | Mean | SD | Pooled | M-1 | M-2 | M-3 | Mean | SD | Pooled | ||
| r2 | 1.00 | 0.97 | 0.86 | 0.94 | 0.07 | 0.98 | 0.92 | 0.43 | 0.44 | 0.60 | 0.28 | 0.56 | |
| λz | h−1 | 0.08 | 0.40 | 0.25 | 0.24 | 0.16 | 0.16 | 0.14 | 0.10 | 0.14 | 0.13 | 0.02 | 0.14 |
| t1/2β | h | 9.09 | 1.74 | 2.78 | 4.54 | 3.98 | 4.25 | 5.08 | 6.94 | 4.90 | 5.64 | 1.13 | 5.09 |
| Tmax | h | 0.25 | 0.25 | 0.25 | 0.25 | 0.00 | 0.25 | 2.00 | 3.00 | 1.00 | 2.00 | 1.00 | 2.00 |
| Cmax | μM | 146 | 142 | 174 | 154.2 | 17.4 | 154 | 10.2 | 11.9 | 6.81 | 9.64 | 2.59 | 8.45 |
| AUC | μM h | 147 | 253 | 303 | 235.2 | 79.7 | 235 | 36.4 | 69.2 | 26.8 | 44.1 | 22.2 | 52.7 |
| Vβ/(Vβ/F) | liters kg−1 | 11.4 | 1.3 | 1.7 | 4.8 | 5.7 | 3.5 | 23.5 | 13.1 | 29.2 | 21.9 | 8.1 | 17.6 |
| CL/(CL/F) | liters h−1 kg−1 | 0.87 | 0.51 | 0.42 | 0.60 | 0.24 | 0.55 | 3.21 | 1.31 | 4.13 | 2.88 | 1.44 | 2.39 |
| MRT | h | 2.59 | 2.29 | 2.62 | 2.50 | 0.18 | 2.40 | 5.09 | 10.25 | 5.99 | 7.11 | 2.76 | 7.21 |
| Vss | liters kg−1 | 2.26 | 1.16 | 1.11 | 1.51 | 0.65 | 1.31 | ||||||
| AUMC | μM h2 | 383 | 581 | 795 | 586 | 205 | 564 | 203 | 1004 | 186 | 464 | 467 | 386 |
| %Exp6206 | % | 33.7 | 20.1 | 10.0 | 19.7 | 11.9 | 19.4 | 81.1 | 50.9 | 79.2 | 69.9 | 17.0 | 69.2 |
| CSF6130 | μM | 6.49 | 0.98 | 3.58 | 3.67 | 2.78 | 0.38 | 0.45 | 0.0 | 0.28 | 0.25 | ||
| CSF6206 | μM | 2.45 | 0.99 | 1.00 | 1.38 | 0.66 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | ||
| MAT | h | 2.49 | 7.96 | 3.38 | 4.61 | 2.93 | 4.81 | ||||||
| F | % | 27.1 | 38.6 | 10.2 | 24.0 | 14.3 | 22.8 | ||||||
| Ftotal | % | 95.2 | 62.8 | 44.3 | 63.9 | 25.7 | 59.6 | ||||||
Cmax and Tmax, observed peak concentration in serum and time of maximum concentration after dose administration, respectively; λz, first-order elimination rate constant from serum; t1/2β, apparent half-life; AUC, area under the serum concentration-versus-time curve extrapolated to infinity; CL, systemic clearance after i.v. administration; Vβ, distribution volume after i.v. administration; CL/F, systemic clearance/F after oral administration; Vβ/F, distribution volume/F after oral administration; Vss, steady-state distribution volume; AUMC, area under first-moment curve extrapolated to infinity; r2, linear correlation coefficient of observed versus predicted concentrations in serum for the terminal phase of elimination used to calculate λz; %Exp6206 = AUC6206/(AUC6130 + AUC6206) × 100; F, PSI-6130 oral bioavailability; Ftotal, total bioavailability (PSI-6130 plus PSI-6206); MAT, mean absorption time.
M-1, monkey 1; M-2, monkey 2; M-3, monkey 3.
RESULTS
Studies of radiolabeled PSI-6130 stability in human and monkey blood.
The stability of PSI-6130 in monkey and human whole blood was measured with radiolabeled PSI-6130 over a period of 0 to 48 h at 37°C (Table 2). PSI-6130 remained unchanged up to 8 h. The percentages of PSI-6130 deamination to the corresponding uracil nucleoside (PSI-6206) at 10 h in human and monkey blood were 0.88% ± 0.36% and 2.8% ± 1.1%, respectively, and at 48 h, the percentages were 16.3% ± 1.7% and 20.0% ± 1.2%, respectively.
TABLE 2.
Stability of 10 μM 3H-PSI-6130 at 1,000 dpm/pmol in whole human and monkey blood at 37°C
| Time (h) | % Remaininga in:
|
|||
|---|---|---|---|---|
| Whole human blood
|
Whole monkey blood
|
|||
| PSI-6130 | PSI-6206 | PSI-6130 | PSI-6206 | |
| 0 | 100 | BLDb | 100 | BLD |
| 0.25 | 100 | BLD | 100 | BLD |
| 0.50 | 100 | BLD | 100 | BLD |
| 1 | 100 | BLD | 100 | BLD |
| 2 | 100 | BLD | 100 | BLD |
| 4 | 100 | BLD | 100 | BLD |
| 8 | 100 | BLD | 100 | BLD |
| 10 | 99.1 ± 0.4 | 0.88 ± 0.4 | 97.2 ± 1.1 | 2.8 ± 1.1 |
| 24 | 95.1 ± 0.9 | 4.9 ± 0.9 | 93.1 ± 0.8 | 6.9 ± 0.8 |
| 48 | 83.7 ± 1.7 | 16.3 ± 1.7 | 80.0 ± 1.2 | 20.0 ± 1.2 |
Each time point was determined in replicate.
BLD, below the limit of detection.
PK studies with rhesus monkeys.
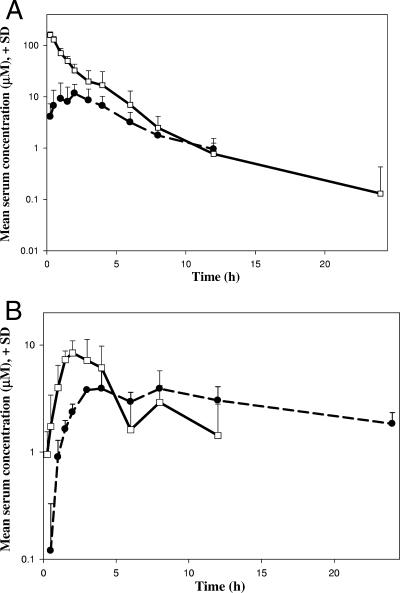
Single-dose PK studies were performed with three rhesus monkeys given 33.3 mg/kg by the oral and i.v. routes of administration. A bimodal absorption profile was observed following oral administration. The first peak concentration in serum (Cmax) varied from 6.8 to 11.9 μΜ and was achieved from 1 to 3 h (Tmax) in the three animals, followed by a drop at ∼6 h. The concentrations of PSI-6130 in serum increased again slightly, producing a second local maximum at around 8 h in all three monkeys. Figure 2A and B depict the mean concentrations of PSI-6130 in serum following i.v. and oral administrations, respectively. The noncompartmental PK analysis was performed on the i.v. and oral serum data from each monkey and on the pooled data. The PK parameters are summarized in Table 1. The lower regression coefficients (r2) observed for the terminal slopes of the elimination phase may result from the bimodal serum maxima observed for PSI-6130 after oral dosing (Fig. 2B).
FIG. 2.
Mean (+ standard deviation [SD]) concentrations in serum of PSI-6130 (squares) and the deaminated metabolite PSI-6206 (circles) after administration of PSI-6130 at 33.3 mg/kg by the i.v. (A) and oral (B) routes.
The average oral bioavailability of PSI-6130 was 24.0% ± 14.3%, the MAT was 4.6 ± 2.9 h, and the terminal half-life (t1/2β) for the oral dose was 5.64 h. The total bioavailability (PSI-6130 plus PSI-6206) ranged from 44 to 95%, and the relative percent exposures to PSI-6206 following oral and i.v. dosing were 70 and 20%, respectively.
The serum concentrations declined in a biexponential manner following i.v. administration. The apparent t1/2β following the i.v. route was 4.54 h. The mean volume of distribution predicted by extrapolation was 4.78 liters kg−1, and the steady-state volume of distribution was 1.51 liters kg−1. The average systemic clearance values following administration of oral and i.v. doses (CL/F and CL) were 2.88 and 0.60 liters h−1 kg−1, respectively. The percentages of the dose recovered unchanged in urine within 8 h after administration of oral and i.v. doses to three monkeys were 10, 3, and 5% and 18, 38, and 42%, respectively, while the percentages recovered in deaminated form were 6, 3, and 4% and 27, 17, and 16%, for the oral and i.v. doses, respectively. The average concentrations of PSI-6130 detected in the CSF 1 h after administration were 0.3 ± 0.2 and 3.8 ± 3.4 μM for the oral and i.v. doses, respectively. In contrast, the average concentration of PSI-6206 in CSF samples after i.v. administration was 1.5 ± 0.8 μM. However, the concentration of PSI-6206 in CSF samples was below the limit of detection (≤0.38 μM) following oral administration.
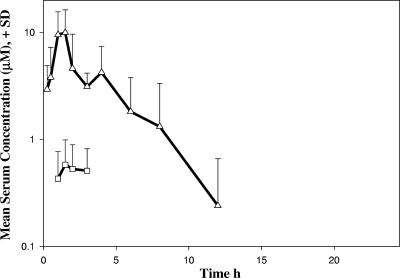
The prodrug PSI-6419 was administered orally to the same three monkeys at a dose of 33.3 mg/kg. The serum concentration profiles of PSI-6130 and PSI-6419 versus time following oral PSI-6419 administration are shown in Fig. 3. On the basis of the relatively low maximal concentration of PSI-6130 in serum (0.8 μM) achieved in three monkeys, PSI-6419 failed to convert efficiently into PSI-6130 in serum.
FIG. 3.
Mean concentrations in serum of PSI-6130 (squares) and its prodrug PSI-6419 (triangles) versus time following oral administration of PSI-6419 (dose, 33.3 mg/kg). SD, standard deviation.
DISCUSSION
The RdRp (NS5B) is essential for the replication of HCV RNA and is an attractive target for designing HCV chemotherapeutic agents. Like 2′-methylcytidine and 2′-deoxy-2′-fluoro-2′-C-methylcytidine, PSI-6130 in its 5′-triphosphate form is an HCV RdRp inhibitor. PSI-6130 demonstrates an excellent inhibitory effect on HCV in Huh-7 replicon cells in vitro, with an EC90 of 5.4 μΜ, and it is also effective against mutant virus containing the S282T mutation in the pol region (8, 18). Therefore, the purpose of this study was to determine whether PSI-6130 is a suitable candidate for testing in humans. The stability of radiolabeled PSI-6130 was evaluated in monkey and human whole blood, and the single-dose PK of PSI-6130 was measured in rhesus monkeys, with a 33.3-mg/kg dose administered via the oral and i.v. routes. The single-dose oral PK of a prodrug of PSI-6130 (PSI-6419) in the monkeys was also studied.
PSI-6130 was deaminated to PSI-6206, which was detected in serum and urine after administration by both routes. However, PSI-6130 was found to be stable for at least 8 h when incubated with monkey and human whole blood at 37°C (Table 2). Furthermore, PSI-6130 demonstrated less deamination in human blood than in monkey blood (16% versus 20%) after 48 h of incubation. The PSI-6206 metabolite demonstrated no antiviral activity in Huh-7 replicon cells and is nontoxic up to a concentration of 100 μΜ in HepG2 cells (8). Racemic β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(±)-FTC, Racivir, Rcv] was also deaminated by cytidine deaminase after administration to rhesus monkeys (14, 20). However, the (−)-enantiomer of FTC (emtricitabine) was not deaminated by the enzyme (20).
PSI-6130 exhibited a complex bimodal PK profile after single-dose oral administration to rhesus monkeys. A noncompartmental PK analysis was performed on the serum data from each monkey and the pooled data obtained from each route of administration. PSI-6130 was slowly and incompletely absorbed (MAT = 4.61 h), and a Cmax of 9.6 ± 2.6 μΜ was achieved between 1 and 3 h (Tmax). The concentration of PSI-6130 increased to a second local maximum at 8 h, which might arise from a discontinuous absorption profile in different regions of the intestine or through enterohepatic cycling. A similar absorption pattern has been reported for the nucleoside agent clevudine (β-l-2′-fluoro-5-methyl-arabinofuranosyluracil) in rats (23). The average relative percent exposure of PSI-6206 following administration by the oral route (70%) was higher than for the i.v. route (20%), suggesting that deamination may take place predominantly during first-pass metabolism in the liver or in the gastrointestinal lining. A higher AUC ratio (AUCFTU/AUCFTC) of the deaminated metabolite (2,3′-dideoxy-5-fluoro-3′-thiauridine [FTU]) was also reported for RCV after oral versus i.v. (2:1) administration (20). The total oral bioavailability, taking into account the AUCs of both PSI-6130 and PSI-6206, was 44 to 95%, indicating incomplete absorption. This was also suggested by the larger extrapolated volume of distribution for the oral route (Vβ/F = 21.9 liters/kg) compared to the i.v. route of administration (Vβ = 4.8 liters/kg). The average apparent t1/2β of PSI-6130 in rhesus monkeys following i.v. (4.54 h) and oral (5.64 h) administrations were comparable and higher than those commonly observed for nucleosides in previous studies with rhesus monkeys, including zidovudine, 2′,3′-dideoxycytidine, stavudine, and 2′,3′-dideoxyinosine (t1/2β values, <1.82 h) (1, 6, 7, 20, 21). The excretion of a small fraction of the dose in urine unchanged (6.0% ± 3.9%) or as PSI-6206 (3.9% ± 1.0%) within 8 h of dosing may be a result of incomplete urinary excretion, given the slow absorption (overall Tmax = 1 to 3 h and secondary local Tmax at 8 h) and the long t1/2β. PSI-6130 was detected in the CSF 1 h after administration by both routes. Although neurological complications in HCV-infected patients occur predominantly in the peripheral nervous system and involvement of the central nervous system is rarely reported, penetration of the CSF may still be important because of other coinfections (2, 3, 5, 9).
Taken together, these results suggest that the oral bioavailability of PSI-6130 warrants improvement. Therefore, a prodrug of PSI-6130, PSI-6419, was synthesized and administered orally to the same monkeys. However, this prodrug did not produce high levels of PSI-6130 in serum (Fig. 3). Therefore, other prodrug approaches should be considered as this drug moves to the clinic (10). In pursuit of this objective, Pharmasset, Inc., in collaboration with F. Hoffmann-La Roche, Ltd., recently reported the initiation of phase 1 studies with R7128, another prodrug of PSI-6130 (www.pharmasset.com).
It should be noted that despite the relatively low oral bioavailability and deamination of PSI-6130, it penetrated the CSF and reached concentrations in the serum of monkeys following administration by both routes that were greater than the in vitro EC90 against HCV in Huh-7 replicon cells. It is known that deamination of a cytidine analogue is less efficient in humans than in monkeys (14). Therefore, further studies of PSI-6130 with other species are warranted to further assess its potential for the treatment of HCV-infected humans.
Acknowledgments
This work was supported in part by NIH grants 5R37-AI-041980, 4R37-AI-025899, 5P30-AI-50409 (CFAR), and RR000165 (to Harold M. McClure) and the Department of Veterans Affairs.
R. F. Schinazi is the principal founder and a former director of Pharmasset, Inc. He is also a major shareholder of Pharmasset, Inc., and Idenix Pharmaceuticals, Inc.
Footnotes
Published ahead of print on 11 June 2007.
Dedicated to our friend and colleague, Harold M. McClure (1937 to 2004).
REFERENCES
- 1.Boudinot, F. D., R. F. Schinazi, J. M. Gallo, H. M. McClure, K. J. Doshi, P. C. Kambhampathi, and C. K. Chu. 1990. 3′-Azido-2′,3′-dideoxyuridine (AzddU): comparative pharmacokinetics with 3′-azido-3′-deoxythymidine (AZT) in monkeys. AIDS Res. Hum. Retrovir. 6:219-228. [DOI] [PubMed] [Google Scholar]
- 2.Cacoub, P., D. Saadoun, N. Limal, J. M. Leger, and T. Maisonobe. 2005. Hepatitis C virus infection and mixed cryoglobulinaemia vasculitis: a review of neurological complications. AIDS 3:S128-S134. [DOI] [PubMed] [Google Scholar]
- 3.Cappellari, A., L. Origgi, M. F. Spina, K. G. Yiannopoulou, G. Meola, M. Vanoli, A. Ciammola, F. Gregorini, R. Scorza, and N. Bresolin. 2006. Central nervous system involvement in HCV-related mixed cryoglobulinemia. Electromyogr. Clin. Neurophysiol. 46:149-158. [PubMed] [Google Scholar]
- 4.Carroll, S. S., J. E. Tomassini, M. Bosserman, K. Getty, M. W. Stahlhut, A. B. Eldrup, B. Bhat, D. Hall, A. L. Simcoe, R. LaFemina, C. A. Rutkowski, B. Wolanski, Z. Yang, G. Migliaccio, R. De Francesco, L. C. Kuo, M. MacCoss, and D. B. Olsen. 2003. Inhibition of hepatitis C virus RNA replication by 2′-modified nucleoside analogs. J. Biol. Chem. 278:11979-11984. [DOI] [PubMed] [Google Scholar]
- 5.Casato, M., D. Saadoun, A. Marchetti, N. Limal, C. Picq, P. Pantano, D. Galanaud, R. Cianci, P. Duhaut, J. C. Piette, M. Fiorilli, and P. Cacoub. 2005. Central nervous system involvement in hepatitis C virus cryoglobulinemia vasculitis: a multicenter case-control study using magnetic resonance imaging and neuropsychological tests. J. Rheumatol. 32:484-488. [PubMed] [Google Scholar]
- 6.Chen, H., F. D. Boudinot, C. K. Chu, H. M. McClure, and R. F. Schinazi. 1999. Pharmacokinetics of (−)-β-d-dioxolane guanine and prodrug (−)-β-d-2,6-diaminopurine dioxolane in rats and monkeys. AIDS Res. Hum. Retrovir. 15:1625-1630. [DOI] [PubMed] [Google Scholar]
- 7.Chen, H., S. B. Pai, S. J. Hurwitz, C. K. Chu, Y. Glazkova, H. M. McClure, M. Feitelson, and R. F. Schinazi. 2003. Antiviral activity and pharmacokinetics of 1-(2,3-dideoxy-2-fluoro-β-l-glyceropent-2-enofuranosyl)cytosine. Antimicrob. Agents Chemother. 47:1922-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, J. L., L. Hollecker, J. C. Mason, L. J. Stuyver, P. M. Tharnish, S. Lostia, T. R. McBrayer, R. F. Schinazi, K. A. Watanabe, M. J. Otto, P. A. Furman, W. J. Stec, S. E. Patterson, and K. W. Pankiewicz. 2005. Design, synthesis, and antiviral activity of 2′-deoxy-2′-fluoro-2′-C-methylcytidine, a potent inhibitor of hepatitis C virus replication. J. Med. Chem. 48:5504-5508. [DOI] [PubMed] [Google Scholar]
- 9.Clifford, D. B., Y. Yang, and S. Evans. 2005. Neurologic consequences of hepatitis C and human immunodeficiency virus coinfection. J. Neurovirol. 11:67-71. [DOI] [PubMed] [Google Scholar]
- 10.De Clercq, E., and H. J. Field. 2006. Antiviral prodrugs—the development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 147:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eldrup, A. B., M. Prhavc, J. Brooks, B. Bhat, T. P. Prakash, Q. Song, S. Bera, N. Bhat, P. Dande, P. D. Cook, C. F. Bennett, S. S. Carroll, R. G. Ball, M. Bosserman, C. Burlein, L. F. Colwell, J. F. Fay, O. A. Flores, K. Getty, R. L. LaFemina, J. Leone, M. MacCoss, D. R. McMasters, J. E. Tomassini, D. Von Langen, B. Wolanski, and D. B. Olsen. 2004. Structure-activity relationship of heterobase-modified 2′-C-methyl ribonucleosides as inhibitors of hepatitis C virus RNA replication. J. Med. Chem. 47:5284-5297. [DOI] [PubMed] [Google Scholar]
- 12.Gabrielsson, J., and D. Weiner (ed.). 2000. Pharmacokinetic and pharmacodynamic data analysis: concepts and applications, 3rd ed. Apotekarsocieteten, Stockholm, Sweden.
- 13.Gibaldi, M., and D. Perrier. 1982. Pharmacokinetics, 2nd ed., Marcel Dekker, Inc., New York, NY.
- 14.Hurwitz, S. J., M. J. Otto, and R. F. Schinazi. 2005. Comparative pharmacokinetics of Racivir, (±)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine, in rats, rabbits, dogs, monkeys and HIV-infected humans. Antivir. Chem. Chemother. 16:117-127. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay, K. L., C. Trepo, T. Heintges, M. L. Shiffman, S. C. Gordon, J. C. Hoefs, E. R. Schiff, Z. D. Goodman, M. Laughlin, R. Yao, J. K. Albrecht, and the Hepatitis Interventional Therapy Group. 2001. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 34:395-403. [DOI] [PubMed] [Google Scholar]
- 16.Ludmerer S. W., D. J. Graham, E. Boots, E. M. Murray, A. Simcoe, E. J. Markel, J. A. Grobler, O. A. Flores, D. B. Olsen, D. J. Hazuda, and R. L. LaFemina. 2005. Replication fitness and NS5B drug sensitivity of diverse hepatitis C virus isolates characterized by using a transient replication assay. Antimicrob. Agents Chemother. 49:2059-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McHutchison, J. G., S. C. Gordon, E. R. Schiff, M. L. Shiffman, W. M. Lee, V. K. Rustgi, Z. D. Goodman, M. H. Ling, S. Cort, and J. K. Albrecht. 1998. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N. Engl. J. Med. 339:1485-1492. [DOI] [PubMed] [Google Scholar]
- 18.Murakami, E., H. Bao, M. Ramesh, T. R. McBrayer, T. Whitaker, H. M. Micolochick Steuer, R. F. Schinazi, L. J. Stuyver, A. Obikhod, M. J. Otto, and P. A. Furman. 2007. Mechanism of activation of β-d-2′-deoxy-2′-fluoro-2′-C-methylcytidine and inhibition of hepatitis C virus NS5B RNA polymerase. Antimicrob. Agents Chemother. 51:503-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 20.Schinazi, R. F., F. D. Boudinot, S. Ibrahim, C. Manning, H. M. McClure, and D. C. Liotta. 1992. Pharmacokinetics and metabolism of racemic 2′,3′-dideoxy-5-fluoro-3′-thiacytidine in rhesus monkeys. Antimicrob. Agents Chemother. 36:2432-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schinazi, R. F., F. D. Boudinot, K. J. Doshi, and H. M. McClure. 1990. Pharmacokinetics of 3′-fluoro-3′-deoxythymidine and 3′-deoxy-2′,3′-didehydrothymidine in rhesus monkeys. Antimicrob. Agents Chemother. 34:1214-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah, V. P., K. K. Midha, J. W. A. Findlay, H. M. Hill, J. D. Hulse, I. J. McGilveray, G. McKay, K. J. Miller, R. N. Patnaik, M. L. Powell, A. Tonelli, C. T. Viswanathan, and A. Yacobi. 2000. Bioanalytical method validation—a revisit with a decade of progress. Pharm. Res. 17:1551-1557. [DOI] [PubMed] [Google Scholar]
- 23.Wright, J. D., T. Ma, C. K. Chu, and F. D. Boudinot. 1996. Discontinuous oral absorption pharmacokinetic model and bioavailability of 1-(2-fluoro-5-methyl-β-l-arabinofuranosyl)uracil (l-FMAU) in rats. Biopharm. Drug Dispos. 17:197-207. [DOI] [PubMed] [Google Scholar]