Abstract
Forty-eight hepatitis B virus (HBV) E antigen-negative chronic hepatitis B patients received pegylated interferon alfa-2b either alone or with lamivudine for 48 weeks and were followed for an additional 24 weeks. At the end of follow-up, virological response rates (HBV DNA levels of <400 copies/ml) were similar in the monotherapy (24%) and combination therapy (26%) groups.
Several interferon (IFN)-based therapies, including IFN alfa-2b, pegylated IFN (PEG-IFN) alfa-2a, and PEG-IFN alfa-2b, are approved for the treatment of chronic hepatitis B (CHB) (2, 8). These IFN-based therapies are also given in combination with nucleoside/nucleotide analogues (1). Despite the tremendous progress made in the treatment of CHB in recent years, an optimal first-line therapy for CHB remains to be established. Clinical trials with hepatitis B virus (HBV) E antigen (HBeAg)-positive patients show that PEG-IFN alone or in combination with lamivudine provides a greater virological response than lamivudine monotherapy (3, 7). Similarly, in patients with HBeAg-negative CHB, PEG-IFN monotherapy or combination therapy with lamivudine is significantly more effective than lamivudine monotherapy (9). However, it is not clear whether combination therapy with PEG-IFN and lamivudine results in improved therapeutic outcomes compared with PEG-IFN monotherapy (5, 7).
The aim of this study was to assess the use of PEG-IFN alfa-2b in the treatment of patients with HBeAg-negative CHB and to determine whether the addition of lamivudine results in improved therapeutic outcomes.
Patients 18 years of age and older were included in the study if they met the following inclusion criteria: hepatitis B virus surface antigen (HBsAg) positivity for at least 6 months, HBeAg negativity and anti-HBe positivity on two occasions in the past 3 months, serum alanine aminotransferase levels >1.3 times the upper limit of normal on two occasions during the preceding 3 months, HBV DNA positivity (lower limit of detection [LLD], 4 pg/ml), and compensated liver disease with histological evidence of chronic hepatitis. Patients were excluded from participation in the study if they exhibited any other cause of chronic liver disease, received immunosuppressive or antiviral treatment in the previous 6 months, or exhibited hepatocellular carcinoma. This prospective, open-label study was conducted in eight tertiary hospitals between June 2001 and January 2004. The study protocol was approved by the ethics committee of each participating center and by the National Ethical Committee.
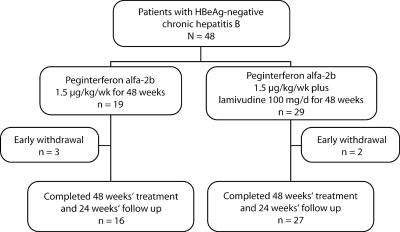
Patients enrolled in the study were randomized (1:1.5 ratio) to treatment with PEG-IFN alfa-2b at 1.5 μg/kg of body weight/week for 48 weeks or with PEG-IFN alfa-2b at 1.5 μg/kg/week plus lamivudine at 100 mg/day for 48 weeks (Fig. 1). All patients were followed up for 24 weeks after the end of treatment. HBsAg, HBeAg, anti-HBe, and anti-HDV were determined by immunoenzymatic assays. Anti-HCV was measured with third-generation UBI HCV EIA 4.0 kits (Organon Teknika, RM Boxtel, The Netherlands). HBV DNA was quantified using a hybridization assay (Hybrid Capture System; Digene Corporation, Gaithersburg, MD) (LLD, 4 pg/ml). HBV DNA was also measured with real-time PCR (LLD, 400 copies/ml) at the end of the follow-up period. The baseline histological evaluation, which was done according to the scoring system of Knodell et al. (6), was based on liver biopsies performed within the 3-month period prior to randomization.
FIG. 1.
Study flow diagram.
Efficacy evaluations (virological and biochemical response) were performed at the end of treatment (week 48) and at the end of the follow-up period (week 72). Study groups were compared using the Fisher exact test and Student t test, where appropriate. Multiple logistical regression analyses were done to determine the independent variables influencing treatment response. A P value of <0.05 was considered statistically significant.
Forty-eight patients were randomized to either combination treatment (n = 29) or monotherapy (n = 19), and each patient received at least one dose of study drug (Fig. 1). Forty-three patients completed the treatment and follow-up periods. The demographic and baseline characteristics of the patients in each group were similar (Table 1). Biochemical and virological response rates at the end of treatment and at the end of the follow-up period were comparable in the monotherapy and combination therapy groups (Table 2). By week 72, two patients (11%) in the monotherapy group and one patient (3%) in the combination therapy group had seroconverted to HBsAg negative.
TABLE 1.
Baseline clinical and laboratory features of the study patients
| Characteristica | Value for groupb
|
|
|---|---|---|
| Monotherapy, PEG-IFN alfa-2b (n = 19) | Combination therapy, PEG-IFN alfa-2b + lamivudine (n = 29) | |
| Gender (n) | ||
| Male | 13 | 20 |
| Female | 6 | 9 |
| Age (yr) | 42.6 ± 10.9 | 43 ± 7.8 |
| ALT (IU/liter) | 130.4 ± 45 | 161.5 ± 127.4 |
| AST (IU/liter) | 80.3 ± 22.9 | 89.2 ± 47.5 |
| Total bilirubin (mg/dl) | 0.8 ± 0.4 | 0.8 ± 0.4 |
| Albumin (g/dl) | 4.3 ± 0.3 | 4.5 ± 0.7 |
| HBV DNA (pg/ml) | 182.3 ± 175.4 | 209.6 ± 207.8 |
| HAI | 7.0 ± 3.2 | 8.3 ± 2.9 |
| Histological stagec (n) | ||
| 1 | 10 | 15 |
| 2 | 3 | 4 |
| 3 | 4 | 8 |
| 4 | 2 | 2 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; HAI, histological activity index.
P > 0.05 for all parameters. Data are presented as means ± standard deviations.
Evaluated by fibrosis score (1, mild; 2, moderate; 3, severe; 4, cirrhosis).
TABLE 2.
Biochemical and virological response rates in treatment groups
| Response and time point |
n (%) for groupd
|
|
|---|---|---|
| Monotherapy, PEG-IFN alfa-2b (n = 19) | Combination therapy, PEG-IFN alfa-2b + lamivudine (n = 29) | |
| Biochemicala | ||
| End of treatment | 10 (53) | 19 (66) |
| End of follow-up | 8 (42) | 14 (48) |
| Virological (HBV DNA level) | ||
| <4 pg/mlb | ||
| End of treatment | 12 (63) | 23 (79) |
| End of follow-up | 7 (37) | 10 (34) |
| <400 copies/ml,c end of follow-up | 5 (26) | 7 (24) |
ALT level normalization.
Determined with the Hybrid Capture System (Digene Corporation).
Determined by real-time PCR.
P > 0.05 for all parameters.
Multivariate analysis showed that the only variable influencing the end of follow-up response was female sex (P < 0.05). The most frequent treatment-related adverse effects in all patients were flu-like symptoms (71%), cytopenia (23%), injection site reactions (10%), pruritus (8%), depression (6%), and thyroiditis (2%). No serious adverse events were reported, and no patient discontinued treatment due to an adverse event.
The results of this study show that PEG-IFN alfa-2b monotherapy and PEG-IFN alfa-2b plus lamivudine provide similar therapeutic outcomes in HBeAg-negative patients with HBV. In both treatment arms, the proportions of patients who had serum alanine aminotransferase normalization and HBV DNA negativity at the end of treatment and at the end of follow-up were similar.
There are two main classes of drugs used in the treatment of CHB: IFN alfa and nucleoside/nucleotide analogues. The limited degree of success achievable with approved drugs, the associated side effects, and the costs of treatment influence both the decision to start treatment and the drug choice. Additionally, treatment for HBeAg-negative CHB differs from treatment for HBeAg-positive CHB in many aspects (13). The advantages and disadvantages associated with these therapies can make selecting a first-line treatment challenging (4). Another controversial issue is whether to use nucleoside/nucleotide analogues alone, IFN alfa alone, or both in combinations. Previous studies showed that the combination of IFN alfa and lamivudine does not increase the rate of treatment success in patients with HBeAg-negative CHB (10-12). In two large-scale, randomized, controlled studies of patients with HBeAg-positive CHB, similar virological response rates occurred whether PEG-IFN alfa-2a or PEG-IFN alfa-2b was administered as monotherapy or in combination with lamivudine (5, 7). In a study conducted by Marcellin et al., PEG-IFN alfa-2a monotherapy was compared with PEG-IFN alfa-2a plus lamivudine combination therapy or lamivudine monotherapy in HBeAg-negative CHB (9). The virological and biochemical response rates observed in the current study are comparable to those obtained by Marcellin et al. even though different PEG-IFNs were used (9).
Future studies assessing recurrence rates in the extended untreated follow-up period are warranted. One of the important handicaps of this study is the limited number of patients included. From the present study, having shown that approximately 50% of the patients exhibited a biochemical response and that approximately 25% of the patients exhibited a virological response at the end of the 6-month follow-up period, the future of PEG-IFN alfa-2b treatment is promising, and the study suggests that PEG-IFN alfa-2b monotherapy may be the best first-line treatment alternative for patients with HBeAg-negative CHB.
Acknowledgments
We acknowledge Maribeth Bogush, Lynn Brown, and Stephanie Klein for editorial assistance in the preparation of the manuscript.
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Asselah, T., M. P. Ripault, C. Castelnau, N. Giuily, N. Boyer, and P. Marcellin. 2005. The current status of antiviral therapy of chronic hepatitis B. J. Clin. Virol. 34(Suppl. 1):S115-S124. [DOI] [PubMed] [Google Scholar]
- 2.Buster, E. H., and H. L. Janssen. 2006. Antiviral treatment for chronic hepatitis B virus infection—immune modulation or viral suppression? Neth. J. Med. 64:175-185. [PubMed] [Google Scholar]
- 3.Chan, H. L. Y., N. W. Y. Leung, A. Y. Hui, V. W. Wong, C. T. Liew, A. M. Chim, F. K. Chan, L. C. Hung, Y. T. Lee, J. S. Tam, C. W. Lam, and J. J. Sung. 2005. A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Ann. Intern. Med. 142:240-250. [DOI] [PubMed] [Google Scholar]
- 4.Chin, R., and S. Locarnini. 2003. Treatment of chronic hepatitis B: current challenges and future directions. Rev. Med. Virol. 13:255-272. [DOI] [PubMed] [Google Scholar]
- 5.Janssen, H. L., M. van Zonneveld, H. Senturk, S. Zeuzem, U. S. Akarca, Y. Cakaloglu, C. Simon, T. M. So, G. Gerken, R. A. de Man, H. G. Niesters, P. Zondervan, B. Hansen, S. W. Schalm, the HBV 99-01 Study Group, and the Rotterdam Foundation for Liver Research. 2005. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 365:123-129. [DOI] [PubMed] [Google Scholar]
- 6.Knodell, R. G., K. G. Ishak, W. C. Black, T. S. Chen, R. Craig, N. Kaplowitz, T. W. Kiernan, and J. Wollman. 1981. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1:431-435. [DOI] [PubMed] [Google Scholar]
- 7.Lau, G. K., T. Piratvisuth, K. X. Luo, P. Marcellin, S. Thongsawat, G. Cooksley, E. Gane, M. W. Fried, W. C. Chow, S. W. Paik, W. Y. Chang, T. Berg, R. Flisiak, P. McCloud, N. Pluck, and the Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. 2005. Peginterferon alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N. Engl. J. Med. 352:2682-2695. [DOI] [PubMed] [Google Scholar]
- 8.Manns, M. P. 2002. Current state of interferon therapy in the treatment of chronic hepatitis B. Semin. Liver. Dis. 22(Suppl. 1):7-13. [DOI] [PubMed] [Google Scholar]
- 9.Marcellin, P., G. K. Lau, F. Bonino, P. Farci, S. Hadziyannis, R. Jin, Z. M. Lu, T. Piratvisuth, G. Germanidis, C. Yurdaydin, M. Diago, S. Gurel, M. Y. Lai, P. Button, N. Pluck, and the Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. 2004. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 351:1206-1217. [DOI] [PubMed] [Google Scholar]
- 10.Santantonio, T., G. A. Niro, E. Sinisi, G. Leandro, M. Insalata, A. Guastadisegni, D. Facciorusso, E. Gravinese, A. Andriulli, and G. Pastore. 2002. Lamivudine/interferon combination therapy in anti-HBe positive chronic hepatitis B patients: a controlled pilot study. J. Hepatol. 36:799-804. [DOI] [PubMed] [Google Scholar]
- 11.Tatulli, I., R. Francavilla, G. L. Rizzo, V. Vinciguerra, E. Ierardi, A. Amoruso, C. Panella, and A. Francavilla. 2001. Lamivudine and alpha-interferon in combination long term for precore mutant chronic hepatitis B. J. Hepatol. 35:805-810. [DOI] [PubMed] [Google Scholar]
- 12.Yurdaydin, C., H. Bozkaya, H. Cetinkaya, T. Sahin, D. Karaoguz, M. Toruner, O. Erkan, A. O. Heper, E. Erden, A. M. Bozdayi, and O. Uzunalimoglu. 2005. Lamivudine vs lamivudine and interferon combination treatment of HBeAg(−) chronic hepatitis B. J. Viral Hepat. 12:262-268. [DOI] [PubMed] [Google Scholar]
- 13.Zarski, J. P., P. Marcellin, V. Leroy, C. Trepo, D. Samuel, N. Ganne-Carrie, K. Barange, V. Canva, M. Doffoel, P. Cales, and the Fédération nationale des Pôles de référence et des Réseaux Hépatites. 2006. Characteristics of patients with chronic hepatitis B in France: predominant frequency of HBe antigen negative cases. J. Hepatol. 45:355-360. [DOI] [PubMed] [Google Scholar]