Abstract
The anthrax lethal toxin (LT) consists of two subunits, the protective antigen (PA) and the lethal factor (LF), and is essential for anthrax pathogenesis. Several recombinant antibodies directed against PA and intended for medical use have been obtained, but none against LF, despite the recommendations of anthrax experts. Here we describe an anti-LF single-chain variable fragment (scFv) that originated from an immunized macaque (Macaca fascicularis) and was obtained by phage display. Panning of the library of 1.8 × 108 clones allowed the isolation of 2LF, a high-affinity (equilibrium dissociation constant, 1.02 nM) scFv, which is highly neutralizing in the standardized in vitro assay (50% inhibitory concentration, 1.20 ± 0.06 nM) and in an in vivo assay. The scFv neutralizes anthrax LT by inhibiting the formation of the LF-PA complex. The genes encoding 2LF are very similar to those of human immunoglobulin germ line genes, sharing substantial (84.2%) identity with their most similar, germinally encoded counterparts; this feature favors medical applications. These results, and others formerly published, demonstrate that our approach can generate antibody fragments suitable for prophylaxis and therapeutics.
Bacillus anthracis secretes two toxins, the lethal toxin (LT) and the edema toxin, but only the former has been demonstrated to have an essential role in the pathogenesis of anthrax. LT is composed of two subunits, the lethal factor (LF) and the protective antigen (PA), and the edema toxin consists of the edema factor and PA. The PA subunit is the basis of current vaccines against anthrax, which elicit toxin-neutralizing antibodies that protect against the disease (20).
In 2001, anthrax spores sent intentionally through the U.S. postal system infected 11 people and killed 5, despite powerful antibiotherapy and resuscitation. A considerable effort has since been devoted to the development of recombinant antibodies against PA, for use in combination with antibiotics, to confer instant protection and to combat antibiotic-resistant strains (2, 3, 5, 9, 17, 36, 38, 54). PA-specific immunoglobulins have also been purified from immunized U.S. donors (43). However, doubts been raised about the use of anti-PA antibodies alone (3a, 4), since it was feared that PA could be sufficiently modified so as to lose the epitopes recognized but retain biological activity (49); also, such antibodies may prevent simultaneous vaccination. As an alternative to anti-PA, anti-LF antibodies have been considered (4), and antibodies of animal origin against LF have been developed. They have showed activity in assays in vitro evaluating toxin neutralization and protection against infectious challenge (23, 41, 45, 57). A cooperative effect between a monoclonal antibody directed against PA and another directed against LF has also been demonstrated in an animal anthrax model (6). However, no recombinant antibody directed against LF has been reported, as far as we are aware. Furthermore, although many synthetic molecules have been designed to inhibit the enzymatic activity of LF (15, 24, 26, 32, 37, 42, 52, 55), neither their bioavailability nor their tolerance is guaranteed. We report here the first recombinant neutralizing antibody directed against LF, obtained after immunization of a nonhuman primate (NHP) and showing human-like framework regions (FRs), as expected (10, 31). This antibody may be suitable for medical use (7, 16, 40).
MATERIALS AND METHODS
Animal immunization.
After protocol approval by the local ethics committee for animal care, a cynomolgus macaque (Macaca fascicularis) was immunized with LF (List Biological Laboratories, Campbell, CA) and injected (100 μg per injection) subcutaneously first with complete Freud adjuvant (first boost) and then with incomplete Freud adjuvant. The immune response was evaluated by an enzyme-linked immunosorbent assay (ELISA), with the plates (Maxisorp, Nunc, Denmark) coated with LF (10 μg/ml of phosphate-buffered saline [PBS]), and preimmune serum used as a negative control. The reactions were developed with polyclonal anti-macaque immunoglobulin G (IgG) (Fc specific) (Nordimmune, Tilburg, The Netherlands). The titer of the response was measured as the reciprocal of the highest dilution of immune serum giving a signal three times stronger than that of the negative control, at the same dilution.
Construction and screening of the single-chain variable fragment (scFv) γ1/κ phage library.
The first stages of library construction were carried out as described previously (25), except that the amplicons were first inserted into the pGemT vector (Promega, Madison, Wisconsin) to yield one sublibrary of DNA coding for the heavy chain (Fd fragment) and another for the light chain. The cDNA in pGEM was reamplified with two oligucleotide primer sets to introduce restriction sites. A human and a macaque κ oligonucleotide primer set were used as forward oligonucleotide primers, and only a macaque-specific set was used as reverse oligonucleotide primers (Table 1). Each PCR was performed using Red Taq (Sigma, Hamburg, Germany) for 20 cycles (30 s at 94°C, 30 s at 57°C, 30 s at 72°C). The PCR products were separated by agarose gel electrophoresis and purified. The amplified VL (variable region of the light chain) PCR products and VH (variable region of the heavy chain) PCR products were pooled. The library was constructed in two steps: first the VL fragments were inserted into pHAL14, and then the VH fragments were inserted into pHAL14 containing the VL repertoire. The pHAL14 vector is derived from the phagemid vector pHAL1 (18, 22). pHAL14 and the VL fragments were digested with MluI and NotI (New England Biolabs, Frankfurt, Germany), the enzymes were inactivated, pHAL14 was dephosphorylated using calf intestinal phosphatase (MBI Fermentas), and the DNA was purified. VL PCR products (270 ng) were inserted into 1 μg of the dephosporylated pHAL14 preparation in four separate ligation reactions. DNA was precipitated from the reaction mixes with ethanol and sodium acetate, the pellet was washed twice with 70% ethanol, and then four aliquots (25 μl) of XL1-Blue MRF′ (Stratagene, Amsterdam, The Netherlands) were used for electroporation. Plasmids (the VL chain library) were isolated using a Plasmid Midi Kit (QIAGEN, Hilden, Germany). The VL chain library and the VH fragments were digested with NcoI and HindIII (New England Biolabs), and ligation and electroporation were then performed as described for VL. The diversity of the library was estimated to be 1.13 × 108 clones. The library was packaged using a protocol adapted from that for Fab (antigen binding fragment) phage production in reference 22: 2.5 × 1011 Hyperphage particles (19, 48, 53) were used. The packaging of the library was tested by titration and immunoblotting according to reference 22. The library was screened as described elsewhere (1, 46), except that 5, 10, 20, and 40 washes were used for each successive round of panning, with PBS-0.1% Tween 20 as a washing buffer.
TABLE 1.
Primers used for amplification of scFv-coding DNA
| Primer namea | Sequenceb |
|---|---|
| MHMacVH-NcoI_f1 | 5′ gtcctcgca cc atg gcc SAG GTG CAG CTC GAG SAG TCT GGG 3′ |
| MHMacVH-NcoI_f2 | 5′ gtcctcgca cc atg gcc CAG GTG CAG CTR CTC GAG TCK GG 3′ |
| MHMacVH-NcoI_f3 | 5′ gtcctcgca cc atg gcc SAG GTG CAG CTG CTC GAG TCK GG 3′ |
| MHMacVH-NcoI_f4 | 5′ gtcctcgca cc atg gcc CAG GTA CAG CTC GAG CAG TCA GG 3′ |
| MHMacVH-NcoI_f5 | 5′ gtcctcgca cc atg gcc AGG TGC AGC TGC TCG AGT CTG G 3′ |
| MHMacVH-NcoI_f6 | 5′ gtcctcgca cc atg gcc CAG GTG CAG CTA CTR GAG TSG GG 3′ |
| MHMacIgGCH1scFv-HindIII_r | 5′ gtcctcgca aag ctt TGG GCC CTT GGT GGA 3′ |
| MHMacVK-MluI_f1 | 5′ accgcctcc a cgc gta GAH ATC GAG CTC CAN CAG TCT CC 3′ |
| MHMacVK-MluI_f6 | 5′ accgcctcc a cgc gta GAG CTW CAG ATG ACM CAG TCT CC 3′ |
| MHMacKappaCL-NotI_r | 5′ accgcctcc gc ggc cgc GAC AGA TGG TGS AGC CAC 3′ |
Primers' names indicate whether they hybridize to DNA encoding the variable (V) or constant (C) region for the heavy (H) or κ light (K) chain.
Lowercase letters indicate additional sequences to facilitate digestion; boldfaced lowercase letters indicate restriction sites; and capital letters indicate the parts encoding antibody genes.
scFv production, ELISA, and affinity measurements.
Phagemid DNA isolated after the panning process was used to transform the nonsuppressor Escherichia coli strain HB2151 (8) such that it expressed the soluble scFv fragment. Single colonies of randomly chosen transformants were used to inoculate 5 ml of SB (Super Broth) medium supplemented with carbenicillin (50 μg.ml−1) and 1% glucose. Cultures were incubated overnight at 30°C with vigorous shaking (250 rpm). We then inoculated 500 ml of SB medium supplemented with carbenicillin and 0.1% glucose with 500 μl of each culture. The cultures were grown at 30°C until the optical density at 600 nm reached 1.5. Isopropyl-β-d-thiogalactopyranoside (1 mM) was then added to induce gene expression, and the cultures were incubated overnight at 22°C. The cells were harvested by centrifugation at 2,500 × g for 15 min at 4°C. scFv's were extracted with polymyxin B sulfate (47) and purified on a nickel-nitrilotriacetic acid spin column (QIAGEN, Valencia, CA) according to the manufacturer's instructions. An anti-His tag antibody (QIAGEN, Courtaboeuf, France) was used in ELISA to detect scFv's.
Affinities were measured by surface plasmon resonance with a BIAcore X (Biacore, Uppsala, Sweden) instrument. LF was immobilized at a maximum of 240 resonance units on a CM5 chip (Biacore) via amine coupling, according to the manufacturer's instructions. A flow rate of 30 μl/min was maintained during measurements. For each measurement, a minimum of six scFv dilutions (10 to 0.1 μg/ml) in HBS-EP buffer (Biacore) were each tested for 900 s. After each scFv dilution, the chip was regenerated with glycine 1.5 (Biacore), run at 10 μl/min for 30 s. Constants were calculated using a previously described method (21) and were verified by internal consistency tests (51).
In vitro and in vivo neutralizing activity.
The mouse macrophage cell line J774A.1 (ATCC-LGC, Molsheim, France) was plated overnight at 14,000 cells/well in 96-well dishes. LT components—400 ng/ml of PA (List Laboratories) and 40 ng/ml of LF, each diluted in PBS at 1 mg/ml and kept frozen until use—were added simultaneously to scFv or medium alone and incubated for 1 h at 37°C. The incubation product was then added to macrophages and incubated at 37°C for 4 h (34). The CytoTox 96 assay kit (Promega) was used according to the manufacturer's instructions to evaluate cell viability. Each assay was corrected for 100% cell viability (control wells with no toxin and no scFv) and 0% viability (control wells with toxin and no scFv). LF neutralization assays and assays involving the simultaneous use of 2LF and of the PA-neutralizing Fab 35PA83 (25) were performed three times each in triplicate, utilizing the same original J774A.1 cell batch, subcultured <15 times since delivery.
For in vivo assays, Fisher 344 rats (250 to 300 g) (Charles River Laboratories, L'Arbresle, France) were injected with 40 μg of PA (List Laboratories, Campbell, CA) and 8 μg of LF, as described by Ezzell et al. (12), except that the tail vein was used. Four animals were used per group; positive controls received toxin plus PBS, and negative controls received PBS only. Results were interpreted utilizing Fisher's exact test, run on Instat 3.0 software (GraphPad Software, San Diego, CA).
ScFv stability.
ScFv stability was estimated by determining the percentage of scFv still active after 7 days of incubation at 37°C, or after a 2-h incubation at 70°C, as was done in another study (36). Aliquots (50 μg/ml in PBS) were incubated in triplicate and then tested by ELISA, utilizing a freshly thawed aliquot as a control, and signal ratios were calculated.
Gel mobility shift assay.
PA63 (List Laboratories) (6 μg) was incubated with or without LF (2 μg) and with or without 2LF (2.5 μg) in a total volume of 15 μl at 37°C for 20 min. The samples were mixed with 15 μl of native loading buffer and subjected to electrophoresis on a 10 to 20% nondenaturing polyacrylamide gel (Pierce, Brebières, France) using Tris-glycine running buffer. Proteins were detected with Coomassie Plus (Pierce) according to the manufacturer's instructions. The method was adapted from the work of Zhao et al. (57).
Nucleic acid analysis of LF-specific scFv clones.
VL and VH sequences from selected clones were determined by Genome Express (Meylan, France) using primers Mkmyc and MkpelB, respectively (22). The sequences were analyzed online using the International ImMunoGeneTics information system (IMGT) (27) (http://imgt.cines.fr) and were compared with the sequences of the human germ line immunoglobulin genes using IMGT nomenclature (29, 30) and IMGT/V-QUEST (13) and IMGT/JunctionAnalysis (39) software. The new IMGT tools (44) were used for peptide sequence analysis involving classification and comparison of three separate characteristics—hydropathy, volume, and chemical properties—for each amino acid.
Nucleotide sequence accession numbers.
The Macaca fascicularis 2LF-H and 2LF-L sequences (VH and VL domain sequences, respectively) are accessible in GenBank/EMBL under accession numbers AM406799 and AM406800, respectively.
RESULTS
Animal immunization.
One male macaque was immunized with LF. After three LF injections, the titer in the macaque reached 400,000.
Library construction and isolation of scFv's specific for LF.
The last boost was given 3 months after the third LF injection. At this time, no amplicons could be obtained from the bone marrow samples with the primer pairs used. Amplification was possible only after the last boost, and therefore, amplicons were considered as putatively coding for LF-specific antibodies. Ten days after the last boost, the most diverse DNA was obtained: all pairs of primers (nine for the amplification of DNA encoding the Fd fragment and seven for Lκ) allowed DNA amplification. These PCR products were inserted into pGemT to obtain sublibraries of 5 × 106 clones for the DNA encoding the Fd fragment and 5 × 105 clones for the DNA encoding the light (κ) chain. The DNA was transferred to pHAL14 in two steps to produce the final scFv library of 1.13 × 108 clones, containing about 90% full-size inserts. The Hyperphage-packaged library showed strong scFv surface presentation as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blotting, and anti-pIII immunostaining (data not shown). Between the first and the last rounds of panning, there were a 15-fold increase in the number of eluted phages and a 4-fold increase in the phage ELISA signal, indicating enrichment in phages reacting specifically with LF. Fifty clones were isolated, and their DNA was extracted and used to transform E. coli strain HB2151 for expression. Six transformants, those whose periplasmic extracts showed the highest reactivity with LF, were selected, and the corresponding scFv's were purified. In parallel, the scFv-coding DNA fragments of the 50 transformants were sequenced. Only two redundant clones (one in four and one in nine transformants) were found and added to the six selected clones for further testing.
In vitro neutralizing activities and affinity determination.
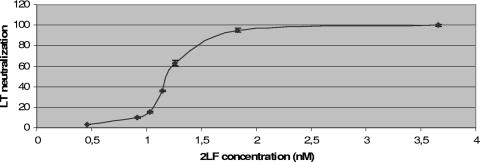
Five of the eight selected clones, including the two redundant clones, showed no neutralizing properties. One clone showed a 50% neutralization value of 4 nM, and the other two had threefold better neutralization properties (data not shown). The affinities of these two clones were measured and found to be 6.98 nM and 1.02 nM (Table 2). The clone with the best affinity was named 2LF; its affinity was confirmed by a competition ELISA (unpublished data), and its 50% neutralization value was measured more precisely as 1.20 nM ± 0.06 nM (mean ± standard deviation) (Fig. 1), representing a molar ratio (2LF/LF) of 2.
TABLE 2.
Affinity constantsa and measurement characteristics of the two selected scFv's
| scFv name | Kon (M−1 s−1) (105) | Koff (s−1) (10−4) | KD (M−1) (10−9) | Maximal resonance units | Chi-square test result |
|---|---|---|---|---|---|
| 2LF | 1.79 | 1.71 | 0.95 | 230 | 0.455 |
| 2.65 | 2.72 | 1.02 | 180 | 0.267 | |
| 2.33 | 2.87 | 1.23 | 150 | 1.31 | |
| 14LF | 6.83 | 4.77 | 6.98 | 190 | 0.533 |
| 8.45 | 3.83 | 4.53 | 240 | 1.15 |
Kon, association constant; Koff, dissociation constant; KD, equilibrium dissociation constant. Values with the most significant chi-square test results are reported.
FIG. 1.
Neutralization capacity of 2LF. LT neutralization was calculated as [(signal in average test wells) − (signal in four no-toxin control wells)]/[(signal in four toxin-only control wells) − (signal in four no-toxin control wells)] and expressed as a function of 2LF concentration (nM). When not visible, the error bars fall within the symbol itself.
ScFv stability.
2LF retained 85% of its activity after a 7-day incubation at 37°C and 6% after a 2-h incubation at 70°C.
Synergy between 2LF and 35PA83.
When concentrations of 2LF (0.9 nM) and 35PA83 (2.34 nM) each corresponding to a 10% neutralization capacity were used together in the neutralization test, the resulting neutralization capacity was 35%. When concentrations of 2LF (1.05 nM) and 35PA83 (2.98 nM) each corresponding to a 20% neutralization capacity were used, toxicity was inhibited by 65%.
In vivo neutralizing activity of 2LF.
To investigate the neutralizing activity in vivo, eight rats were treated with two quantities of 2LF scFv. Administration of 12 μg of 2LF did not delay the time to death for the four rats. Administration of 24 μg of the recombinant antibody fragment resulted in all four rats surviving (considered significant by a two-sided Fisher exact test [P = 0.0286]).
Gel mobility shift assay.
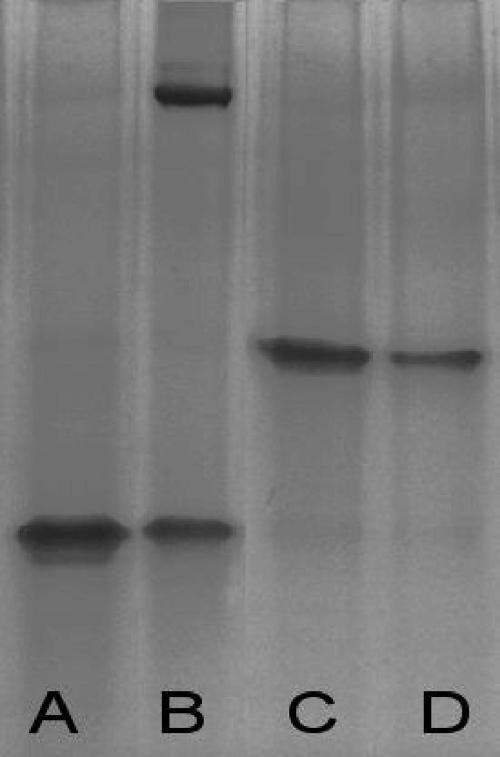
The gel (Fig. 2) shows control lanes (A, B, and C) with bands corresponding to LF, LT (LF plus PA63), and 2LF-LF complexes, respectively. 2LF migrated with the migration front, and PA63 that was not complexed with LF did not enter the gel, presumably because it multimerized, similarly to the formation of heptamers at the surfaces of target cells (data not shown).
FIG. 2.
Gel mobility shift assay. Lane A, LF; lane B, PA63 plus LF, showing the LT (upper band) and LF in excess (lower band); lane C, LF plus 2LF, showing only the complexes; lane D, LF plus PA63 plus 2LF, showing only the LF-2LF complexes and no LT. Control lanes with PA only or scFv only showed no band; PA probably formed aggregates and did not enter the gel, and 2LF migrated with the migration front (data not shown).
When LF and PA63 were coincubated in the presence of 2LF (Fig. 2, lane D), no band corresponding to LT (LF plus PA63) was visible and only a band corresponding to 2LF-LF complexes was detected. This finding indicates that 2LF exerts its neutralization effect by inhibiting the formation of LT (LF plus PA63).
Computational analysis.
The sequences encoding the VH and VL domains of 2LF (called 2LF-H and 2LF-L, respectively) were analyzed with IMGT/V-QUEST software (13, 14, 28) and the IMGT/JunctionAnalysis tool (39) to identify the immunoglobulin germ line V, (D), and J genes from which any particular immunoglobulin chain is derived. The human germ line V, (D), or J alleles found most similar to the 2LF-H nucleotide sequence by IMGT/V-QUEST are IGHV3-30*04, IGHD3-10*01, and IGHJ3*01, and those most similar to 2LF-L are IGKV1-12*01 and IGKJ4*01.
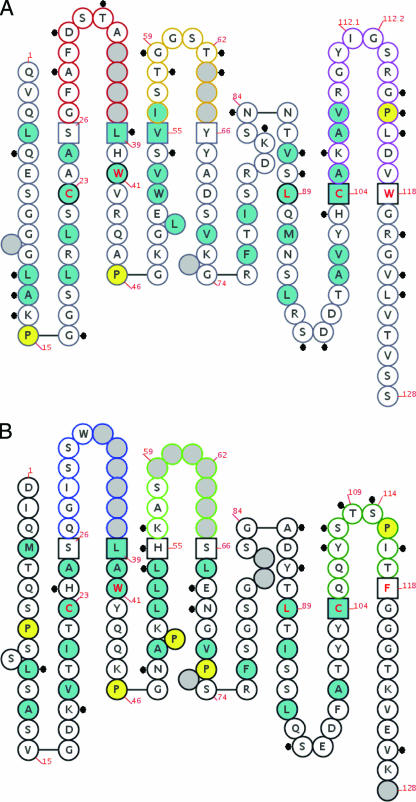
The overall identity between the eight FRs of 2LF and their most similar peptide sequences encoded by human germinal genes is 84.2%. Figure 3 is an IMGT “collier de perle” (pearl necklace) graphical 2-dimensional representation of 2LF; differences between 2LF and amino acids coded by the human germinal genes most similar to 2LF are indicated.
FIG. 3.
IMGT “collier de perle” graphical 2-dimensional representation of 2LF. (A) Fd fragment; (B) light chain. IMGT “collier de perle” representations are displayed according to IMGT unique numbering (29, 30). Dots indicate differences between 2LF and the human genes most similar to 2LF. Hydrophobic amino acids (those with positive hydropathy index values, i.e., I, V, L, F, C, M, and A) and tryptophan (W) are shown in blue circles. All proline (P) residues are shown in yellow circles. The complementarity-determining region (CDR-IMGT) sequences are delimited by amino acids shown in squares (anchor positions), which belong to the neighboring FR (FR-IMGT). Gray circles correspond to missing positions according to IMGT unique numbering. Colors of circle outlines indicate regions: in the VH domain, red for CDR1-IMGT, orange for CDR2-IMGT, and purple for CDR3-IMGT; in the Vκ domain, blue for CDR1-IMGT, green for CDR2-IMGT, and turquoise for CDR3-IMGT.
We used new IMGT tools (44) to analyze the physicochemical properties of 2LF FR residues that differ from the residues encoded by the most similar human germ line genes. The new IMGT tools characterize and compare three separate characteristics for each amino acid. Here, to summarize this analysis, residues were considered to be very similar when no significant difference between two residues was recognized in any of the three characteristics, similar when one difference was found, dissimilar for two differences, and very dissimilar for three differences (Table 3). Only 4 residues of the total of 184 FR residues were very dissimilar, and they mapped in H-FR1 (R versus G at the 16th position of this 2LF FR) (human versus macaque), H-FR4 (V versus T at the 6th position), and L-FR4 (S versus N at the 4th position and T versus A at the 17th position).
TABLE 3.
Localization and evaluation of differences between 2LF framework regions and those encoded by human germ line genes most similar to 2LFa
| Region | No. (%) of residues with the following score:
|
||||
|---|---|---|---|---|---|
| Identical | Very similar | Similar | Dissimilar | Very dissimilar | |
| H-FR1 | 21 | 0 | 2 | 2 | 1 |
| H-FR2 | 15 | 0 | 1 | 1 | 0 |
| H-FR3 | 32 | 0 | 2 | 5 | 0 |
| H-FR4 | 8 | 0 | 1 | 1 | 1 |
| Total H-FR | 76 (81.7) | 0 (0) | 6 (6.4) | 9 (9.8) | 2 (2.1) |
| L-FR1 | 23 | 1 | 1 | 1 | 0 |
| L-FR2 | 14 | 1 | 0 | 2 | 0 |
| L-FR3 | 34 | 0 | 1 | 2 | 2 |
| L-FR4 | 8 | 0 | 1 | 0 | 0 |
| Total L-FR | 79 (86.8) | 2 (2.2) | 3 (3.3) | 5 (5.5) | 2 (2.2) |
| Total FR | 155 (84.2) | 2 (1.1) | 9 (4.9) | 14 (7.7) | 4 (2.2) |
Differences between 2LF framework amino acids and those coded by the most similar human genes are evaluated on a 5-level scale, from identical to very dissimilar (see “Computational analysis” under Results), and located in each framework region.
DISCUSSION
Experts in the development of anti-anthrax antibodies have indicated that the simultaneous use of multitargeted antibodies may be advantageous (4) in view of the efficacy of polyclonal antisera and the fear that one antibody could easily be circumvented (3a, 49). For these reasons, and because a cooperative effect between an anti-LF and an anti-PA antibody has already been described in vivo (6), we have developed an anti-LF scFv which is apparently the first recombinant antibody fragment of this specificity ever obtained. This contrasts with both (i) the large number of anti-PA antibodies that have been developed (17, 36, 38, 50, 56), sometimes by exploiting the availability of lymphocyte donors immunized with anthrax vaccines composed mainly of PA, and (ii) the many synthetic molecules that have been developed to inhibit the enzymatic (proteolytic) activity of LF (15, 24, 26, 32, 37, 42, 52, 55) but whose bioavailability and tolerance are not guaranteed.
With an approach similar to that used by Laffly et al. (25) to obtain anti-PA, a Fab immune library was constructed, starting from a immunized macaque (Macaca fascicularis). This Fab library was successfully panned, according to the phage counts and phage ELISA results, but it appeared to have lost all light chains due to genetic instability, and selected Fd fragments could not be expressed as soluble molecules (unpublished data). By utilizing the pHAL14 vector (18) and starting from the same precloned amplicons used for the Fab library construction, an scFv library was built. Testing of only eight clones isolated after the panning allowed identification of 2LF, an scFv that has a very high affinity (1.02 nM) for LF. Estimations of the stability of 2LF (85% of activity retained after incubation at 37°C and 6% after incubation at 70°C) suggested that it is less stable than previously described scFvs (100% activity retained after incubation at 37°C, and 3.5 to 28% retained after incubation at 70°C [36]); however, this relative instability could be circumvented by 2LF expression as an IgG. The affinity of 2LF for LF is the same as the affinity of LF for heptameric PA (11), its natural binder. The scFv 2LF efficiently inhibited the toxicity of LT in both in vitro (50% neutralizing concentration [CI50], 1.20 ± 0.06 nM) and in vivo assays, neutralizing half the activity of the toxin with a 2LF/LF molar ratio of 2. This ratio is of the same order of magnitude as the antigen binding site/LF ratio of 1.2 observed in vitro by Lim et al. utilizing a murine IgG with an affinity of 2.62 nM (33). The mechanism for LT inhibition by 2LF proved to be competition with PA for LF binding, as assessed by a gel mobility shift assay and an equivalent competitive ELISA (data not shown). This mechanism is shared by other LF-neutralizing antibodies (35). The successful competition is an indirect confirmation of the high affinity of 2LF for LF. The epitope of 2LF is presumably in domain 1 of LF, which binds PA, but precise mapping of this epitope was beyond the scope of this study.
A cooperative effect between an anti-LF and an anti-PA antibody has been described previously (7), so we looked for such cooperation between 2LF and our anti-PA Fab, 35PA83 (26). The assays were performed in vitro, because not enough Fab was available for in vivo tests. In these experiments, 2LF at CI10, in combination with 35PA83 also utilized at CI10, caused 35% neutralization. Similarly, utilization of both antibody fragments at CI20 caused 65% neutralization. The exact interpretation of these data has to take into account the sigmoid-like shape of the curve of neutralization by 2LF; for instance, a concentration corresponding to twice its CI10 causes 100% neutralization (Fig. 1). The neutralization curve for 35PA83 had a similar shape, as shown for other anti-PA antibodies by Wild et al. (56). The combination of 2LF and 35PA83, either at CI10 or at CI20, caused a neutralization effect that was approximately equal to the effect obtained with twice that concentration of 35PA83 and less than the effect obtained with twice the concentration of 2LF. As a consequence, no cooperative effect between 2LF and 35PA83 was evidenced in vitro.
Our strategy of obtaining antibody fragments of NHP origin for therapeutic purposes is covered by patents in Europe (40a) and in the United States (40b); at present, however, in Europe, but not in the United States, no rights are due if the antigen is of nonhuman origin. Both patents state that human and NHP FRs are indistinguishable. This seemed to be the case for a Fab we obtained previously, 35PA83, whose FRs were 92% identical to human germ line-encoded sequences, and this high value was interpreted as predicting good tolerance for medical use of 35PA83 (25). For 2LF, however, the corresponding value is 84.2%, and the presence of NHP-specific sequences, which could potentially be immunogenic in humans, cannot be excluded. To avoid the possible risk of failure in clinical trials, additional screening of further clones to obtain an scFv more closely related to its human counterpart or, alternatively, humanization of 2LF could be considered. The new IMGT tools can be used to evaluate the probability of success for the latter strategy. It is plausible that humanizing all 2LF residues that are very similar or similar to their human counterparts (Table 3) would have little or no effect on the reactivity of the scFv. This process would increase the percentage of identity with human germ line-encoded sequences to 90.2%, thus approaching the value obtained for 35PA83. Such humanization can thus reasonably be considered.
Acknowledgments
We thank Brian Cao (Van Andel Research Institute, Grand Rapids, MI) for advice concerning about the gel mobility shift assay and Eric Quemeneur (CEA, Direction des Sciences du Vivant, Marcoule, France) for fruitful discussions. We thank the “Biologie Appliquée” team (CRSSA) and Saskia Helmsing for excellent technical support.
We gratefully acknowledge the financial support from the Etat Major des Armées (service 125, op3-c/LFR) and the German Ministry of Education and Research (BMBF, SMP “Antibody Factory” in the NGFN2 program).
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Andris-Widhopf, J., C. Rader, P. Steinberger, R. Fuller, and C. F. Barbas III. 2000. Methods for the generation of chicken monoclonal antibody fragments by phage display. J. Immunol. Methods 242:159-181. [DOI] [PubMed] [Google Scholar]
- 2.Athamna, A., M. Athamna, N. Abu-Rashed, B. Medlej, D. J. Bast, and E. Rubinstein. 2004. Selection of Bacillus anthracis isolates resistant to antibiotics. J. Antimicrob. Chemother. 54:424-428. [DOI] [PubMed] [Google Scholar]
- 3.Baillie, L., and T. D. Read. 2001. Bacillus anthracis, a bug with attitude! Curr. Opin. Microbiol. 4:78-81. [DOI] [PubMed] [Google Scholar]
- 3a.Baillie, L., S. Leppla, C. Quinn, P. Swann, F. Top, and S. Welkos. 2003. Expert consultation on monoclonal antibodies for anthrax rPA. http://www3.niaid.nih.gov/biodefense/research/products.htm#5.
- 4.Baillie, L. W. 2006. Past, imminent and future human medical countermeasures for anthrax. J. Appl. Microbiol. 101:594-606. [DOI] [PubMed] [Google Scholar]
- 5.Brook, I., T. B. Elliott, H. I. Pryor II, T. E. Sautter, B. T. Gnade, J. H. Thakar, and G. B. Knudson. 2001. In vitro resistance of Bacillus anthracis Sterne to doxycycline, macrolides and quinolones. Int. J. Antimicrob. Agents 18:559-562. [DOI] [PubMed] [Google Scholar]
- 6.Brossier, F., M. Levy, A. Landier, P. Lafaye, and M. Mock. 2004. Functional analysis of Bacillus anthracis protective antigen by using neutralizing monoclonal antibodies. Infect. Immun. 72:6313-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugelski, P. J., D. J. Herzyk, S. Rehm, A. G. Harmsen, E. V. Gore, D. M. Williams, B. E. Maleeff, A. M. Badger, A. Truneh, S. R. O'Brien, R. A. Macia, P. J. Wier, D. G. Morgan, and T. K. Hart. 2000. Preclinical development of keliximab, a primatized anti-CD4 monoclonal antibody, in human CD4 transgenic mice: characterization of the model and safety studies. Hum. Exp. Toxicol. 19:230-243. [DOI] [PubMed] [Google Scholar]
- 8.Carter, P., H. Bedouelle, and G. Winter. 1985. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 13:4431-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casadevall, A. 2002. Passive antibody administration (immediate immunity) as a specific defense against biological weapons. Emerg. Infect. Dis. 8:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chassagne, S., E. Laffly, E. Drouet, F. Herodin, M. P. Lefranc, and P. Thullier. 2004. A high-affinity macaque antibody Fab with human-like framework regions obtained from a small phage display immune library. Mol. Immunol. 41:539-546. [DOI] [PubMed] [Google Scholar]
- 11.Elliott, J. L., J. Mogridge, and R. J. Collier. 2000. A quantitative study of the interactions of Bacillus anthracis edema factor and lethal factor with activated protective antigen. Biochemistry 39:6706-6713. [DOI] [PubMed] [Google Scholar]
- 12.Ezzell, J. W., B. E. Ivins, and S. H. Leppla. 1984. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect. Immun. 45:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giudicelli, V., D. Chaume, and M. P. Lefranc. 2004. IMGT/V-QUEST, an integrated software program for immunoglobulin and T cell receptor V-J and V-D-J rearrangement analysis. Nucleic Acids Res. 32:W435-W440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giudicelli, V., P. Duroux, C. Ginestoux, G. Folch, J. Jabado-Michaloud, D. Chaume, and M. P. Lefranc. 2006. IMGT/LIGM-DB, the IMGT comprehensive database of immunoglobulin and T cell receptor nucleotide sequences. Nucleic Acids Res. 34:D781-D784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman, M. E., L. Cregar, D. Nguyen, O. Simo, S. O'Malley, and T. Humphreys. 2006. Cationic polyamines inhibit anthrax lethal factor protease. BMC Pharmacol. 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gottlieb, A. B., S. Kang, K. G. Linden, M. Lebwohl, A. Menter, A. A. Abdulghani, M. Goldfarb, N. Chieffo, and M. C. Totoritis. 2004. Evaluation of safety and clinical activity of multiple doses of the anti-CD80 monoclonal antibody, galiximab, in patients with moderate to severe plaque psoriasis. Clin. Immunol. 111:28-37. [DOI] [PubMed] [Google Scholar]
- 17.Harvey, B. R., G. Georgiou, A. Hayhurst, K. J. Jeong, B. L. Iverson, and G. K. Rogers. 2004. Anchored periplasmic expression, a versatile technology for the isolation of high-affinity antibodies from Escherichia coli-expressed libraries. Proc. Natl. Acad. Sci. USA 101:9193-9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hust, M., and S. Dubel. 2005. Phage display vectors for the in vitro generation of human antibody fragments. Methods Mol. Biol. 295:71-96. [DOI] [PubMed] [Google Scholar]
- 19.Hust, M., M. Meysing, T. Schirrmann, M. Selke, J. Meens, G. F. Gerlach, and S. Dubel. 2006. Enrichment of open reading frames presented on bacteriophage M13 using hyperphage. BioTechniques 41:335-342. [DOI] [PubMed] [Google Scholar]
- 20.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson, R., A. Michaelsson, and L. Mattsson. 1991. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. J. Immunol. Methods 145:229-240. [DOI] [PubMed] [Google Scholar]
- 22.Kirsch, M., M. Zaman, D. Meier, S. Dubel, and M. Hust. 2005. Parameters affecting the display of antibodies on phage. J. Immunol. Methods 301:173-185. [DOI] [PubMed] [Google Scholar]
- 23.Kobiler, D., Y. Gozes, H. Rosenberg, D. Marcus, S. Reuveny, and Z. Altboum. 2002. Efficiency of protection of guinea pigs against infection with Bacillus anthracis spores by passive immunization. Infect. Immun. 70:544-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzmic, P., L. Cregar, S. Z. Millis, and M. Goldman. 2006. Mixed-type noncompetitive inhibition of anthrax lethal factor protease by aminoglycosides. FEBS J. 273:3054-3062. [DOI] [PubMed] [Google Scholar]
- 25.Laffly, E., L. Danjou, F. Condemine, D. Vidal, E. Drouet, M. P. Lefranc, C. Bottex, and P. Thullier. 2005. Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob. Agents Chemother. 49:3414-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, L. V., K. E. Bower, F. S. Liang, J. Shi, D. Wu, S. J. Sucheck, P. K. Vogt, and C. H. Wong. 2004. Inhibition of the proteolytic activity of anthrax lethal factor by aminoglycosides. J. Am. Chem. Soc. 126:4774-4775. [DOI] [PubMed] [Google Scholar]
- 27.Lefranc, M. P. 2003. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 31:307-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefranc, M. P. 2005. IMGT, the international ImMunoGeneTics information system: a standardized approach for immunogenetics and immunoinformatics. Immunome Res. 1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lefranc, M. P., E. Duprat, Q. Kaas, M. Tranne, A. Thiriot, and G. Lefranc. 2005. IMGT unique numbering for MHC groove G-DOMAIN and MHC superfamily (MhcSF) G-LIKE-DOMAIN. Dev. Comp. Immunol. 29:917-938. [DOI] [PubMed] [Google Scholar]
- 30.Lefranc, M. P., C. Pommie, Q. Kaas, E. Duprat, N. Bosc, D. Guiraudou, C. Jean, M. Ruiz, I. Da Piedade, M. Rouard, E. Foulquier, V. Thouvenin, and G. Lefranc. 2005. IMGT unique numbering for immunoglobulin and T cell receptor constant domains and Ig superfamily C-like domains. Dev. Comp. Immunol. 29:185-203. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, A. P., K. A. Barber, H. J. Cooper, M. J. Sims, J. Worden, and J. S. Crowe. 1993. Cloning and sequence analysis of kappa and gamma cynomolgus monkey immunoglobulin cDNAs. Dev. Comp. Immunol. 17:549-560. [DOI] [PubMed] [Google Scholar]
- 32.Lewis, J. A., J. Mongan, J. A. McCammon, and S. M. Cohen. 2006. Evaluation and binding-mode prediction of thiopyrone-based inhibitors of anthrax lethal factor. ChemMedChem 1:694-697. [DOI] [PubMed] [Google Scholar]
- 33.Lim, N. K., J. H. Kim, M. S. Oh, S. Lee, S. Y. Kim, K. S. Kim, H. J. Kang, H. J. Hong, and K. S. Inn. 2005. An anthrax lethal factor-neutralizing monoclonal antibody protects rats before and after challenge with anthrax toxin. Infect. Immun. 73:6547-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Little, S. F., S. H. Leppla, and A. M. Friedlander. 1990. Production and characterization of monoclonal antibodies against the lethal factor component of Bacillus anthracis lethal toxin. Infect. Immun. 58:1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Little, S. F., J. M. Novak, J. R. Lowe, S. H. Leppla, Y. Singh, K. R. Klimpel, B. C. Lidgerding, and A. M. Friedlander. 1996. Characterization of lethal factor binding and cell receptor binding domains of protective antigen of Bacillus anthracis using monoclonal antibodies. Microbiology 142:707-715. [DOI] [PubMed] [Google Scholar]
- 36.Maynard, J. A., C. B. Maassen, S. H. Leppla, K. Brasky, J. L. Patterson, B. L. Iverson, and G. Georgiou. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597-601. [DOI] [PubMed] [Google Scholar]
- 37.Menard, A., E. Papini, M. Mock, and C. Montecucco. 1996. The cytotoxic activity of Bacillus anthracis lethal factor is inhibited by leukotriene A4 hydrolase and metallopeptidase inhibitors. Biochem. J. 320:687-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamed, N., M. Clagett, J. Li, S. Jones, S. Pincus, G. D'Alia, L. Nardone, M. Babin, G. Spitalny, and L. Casey. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monod, M. Y., V. Giudicelli, D. Chaume, and M. P. Lefranc. 2004. IMGT/JunctionAnalysis: the first tool for the analysis of the immunoglobulin and T cell receptor complex V-J and V-D-J JUNCTIONs. Bioinformatics 20(Suppl. 1):I379-I385. [DOI] [PubMed] [Google Scholar]
- 40.Newman, R., J. Alberts, D. Anderson, K. Carner, C. Heard, F. Norton, R. Raab, M. Reff, S. Shuey, and N. Hanna. 1992. “Primatization” of recombinant antibodies for immunotherapy of human diseases: a macaque/human chimeric antibody against human CD4. Bio/Technology 10:1455-1460. [DOI] [PubMed] [Google Scholar]
- 40a.Newman, R. A., N. Hanna, and R. W. Raab. 24 May 2006. European patent 1 266 965 B1.
- 40b.Newman, R. A., N. Hanna, and R. W. Raab. 2 December 1997. Recombinant antibodies for human therapy. U.S. patent 5,693,780.
- 41.Paddle, B. M., V. K. Wong, and B. D. Muller. 2006. The cytotoxic effect of anthrax lethal toxin on human lung cells in vitro and the protective action of bovine antibodies to PA and LF. J. Appl. Toxicol. 26:162-168. [DOI] [PubMed] [Google Scholar]
- 42.Panchal, R. G., A. R. Hermone, T. L. Nguyen, T. Y. Wong, R. Schwarzenbacher, J. Schmidt, D. Lane, C. McGrath, B. E. Turk, J. Burnett, M. J. Aman, S. Little, E. A. Sausville, D. W. Zaharevitz, L. C. Cantley, R. C. Liddington, R. Gussio, and S. Bavari. 2004. Identification of small molecule inhibitors of anthrax lethal factor. Nat. Struct. Mol. Biol. 11:67-72. [DOI] [PubMed] [Google Scholar]
- 43.Pittman, P. R., S. F. Leitman, J. G. Oro, S. L. Norris, N. M. Marano, M. V. Ranadive, B. S. Sink, and K. T. McKee, Jr. 2005. Protective antigen and toxin neutralization antibody patterns in anthrax vaccinees undergoing serial plasmapheresis. Clin. Diagn. Lab. Immunol. 12:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pommie, C., S. Levadoux, R. Sabatier, G. Lefranc, and M. P. Lefranc. 2004. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J. Mol. Recognit. 17:17-32. [DOI] [PubMed] [Google Scholar]
- 45.Price, B. M., A. L. Liner, S. Park, S. H. Leppla, A. Mateczun, and D. R. Galloway. 2001. Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid encoding the lethal factor protein. Infect. Immun. 69:4509-4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rader, C., P. Steinberger, and C. F. Barbas III. 2001. Library panning on immobilized antigens, p. 10.12-10.15. In C. F. Barbas III, D. R. Burton, J. K. Scott, and G. J. Silverman (ed.), Phage display. A laboratory manual. CSHL Press, New York, NY.
- 47.Renard, M., L. Belkadi, N. Hugo, P. England, D. Altschuh, and H. Bedouelle. 2002. Knowledge-based design of reagentless fluorescent biosensors from recombinant antibodies. J. Mol. Biol. 318:429-442. [DOI] [PubMed] [Google Scholar]
- 48.Rondot, S., J. Koch, F. Breitling, and S. Dubel. 2001. A helper phage to improve single-chain antibody presentation in phage display. Nat. Biotechnol. 19:75-78. [DOI] [PubMed] [Google Scholar]
- 49.Rosovitz, M. J., P. Schuck, M. Varughese, A. P. Chopra, V. Mehra, Y. Singh, L. M. McGinnis, and S. H. Leppla. 2003. Alanine-scanning mutations in domain 4 of anthrax toxin protective antigen reveal residues important for binding to the cellular receptor and to a neutralizing monoclonal antibody. J. Biol. Chem. 278:30936-30944. [DOI] [PubMed] [Google Scholar]
- 50.Sawada-Hirai, R., I. Jiang, F. Wang, S. M. Sun, R. Nedellec, P. Ruther, A. Alvarez, D. Millis, P. R. Morrow, and A. S. Kang. 2004. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines 2:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schuck, P., and A. P. Minton. 1996. Analysis of mass transport-limited binding kinetics in evanescent wave biosensors. Anal. Biochem. 240:262-272. [DOI] [PubMed] [Google Scholar]
- 52.Shoop, W. L., Y. Xiong, J. Wiltsie, A. Woods, J. Guo, J. V. Pivnichny, T. Felcetto, B. F. Michael, A. Bansal, R. T. Cummings, B. R. Cunningham, A. M. Friedlander, C. M. Douglas, S. B. Patel, D. Wisniewski, G. Scapin, S. P. Salowe, D. M. Zaller, K. T. Chapman, E. M. Scolnick, D. M. Schmatz, K. Bartizal, M. MacCoss, and J. D. Hermes. 2005. Anthrax lethal factor inhibition. Proc. Natl. Acad. Sci. USA 102:7958-7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soltes, G., M. Hust, K. K. Ng, A. Bansal, J. Field, D. I. Stewart, S. Dubel, S. Cha, and E. J. Wiersma. 2007. On the influence of vector design on antibody phage display. J. Biotechnol. 127:626-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stepanov, A. V., L. I. Marinin, A. P. Pomerantsev, and N. A. Staritsin. 1996. Development of novel vaccines against anthrax in man. J. Biotechnol. 44:155-160. [DOI] [PubMed] [Google Scholar]
- 55.Tonello, F., M. Seveso, O. Marin, M. Mock, and C. Montecucco. 2002. Screening inhibitors of anthrax lethal factor. Nature 418:386. [DOI] [PubMed] [Google Scholar]
- 56.Wild, M. A., H. Xin, T. Maruyama, M. J. Nolan, P. M. Calveley, J. D. Malone, M. R. Wallace, and K. S. Bowdish. 2003. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat. Biotechnol. 21:1305-1306. [DOI] [PubMed] [Google Scholar]
- 57.Zhao, P., X. Liang, J. Kalbfleisch, H. M. Koo, and B. Cao. 2003. Neutralizing monoclonal antibody against anthrax lethal factor inhibits intoxication in a mouse model. Hum. Antibodies 12:129-135. [PubMed] [Google Scholar]