Abstract
A 269-kilobase conjugative plasmid, pK29, from a Klebsiella pneumoniae strain was sequenced. The plasmid harbors multiple antimicrobial resistance genes, including those encoding CMY-8 AmpC-type and CTX-M-3 extended-spectrum β-lactamases in the common backbone of IncHI2 plasmids. Mechanisms for dissemination of the resistance genes are highlighted in comparative genomic analyses.
Plasmid-mediated β-lactamases play a key role in the increasing multidrug resistance in the Enterobacteriaceae worldwide, among which CTX-M-type extended-spectrum β-lactamases (ESBLs) and AmpC-type β-lactamases are two major contributors in recent years (3, 14, 17, 19). In general, ESBLs confer resistance to oxyimino-cephalosporins but not cephamycins and are inhibited by β-lactamase inhibitors, while AmpC-type β-lactamases provide resistance to cephamycins and oxyimino-cephalosporins and are refractory to β-lactamase inhibitors. Although reports of multiple β-lactamases in a single pathogen are increasing for the Enterobacteriaceae, especially Klebsiella pneumoniae (1, 9, 15, 22, 26), the complete sequence information for a plasmid that encodes both an ESBL and an AmpC-type β-lactamase has not been reported.
In Taiwan, CTX-M- and SHV-type ESBLs and CMY- and DHA-type AmpC-type enzymes are the most common β-lactamases that can confer resistance to extended-spectrum cephalosporins in clinical K. pneumoniae isolates (24, 26). Here we report the sequencing, annotation, and comparative genomic analysis of an IncHI2 plasmid isolated from a nosocomial K. pneumoniae strain. The plasmid carries both CTX-M- and CMY-type β-lactamase genes.
Three K. pneumoniae isolates from three patients were collected from the National Cheng Kung University Hospital in Taiwan during a 1-month period in 2001. These three isolates shared identical antimicrobial susceptibility and plasmid profiles (data not shown). Conjugal transfer was performed by using one of the K. pneumoniae isolates, NK29, as the donor and Escherichia coli J53 Azir as the recipient, following a previously described protocol (11). Transconjugants were obtained at an efficiency of 10−4 to 10−5 transconjugants/donor at 25°C. DNA sequencing of the plasmid was determined as part of the process of sequencing the entire genome of K. pneumoniae NK29, using a shotgun approach. Sequence assembly, annotation, and analysis followed previously described protocols (4).
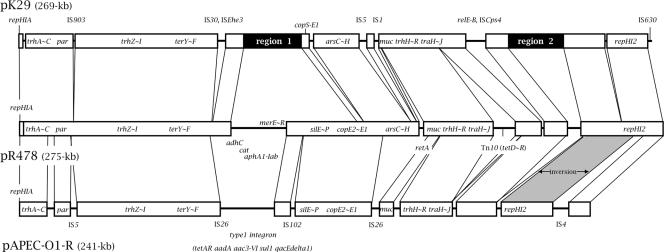
The plasmid pK29 was determined to be a 269,674-bp circular plasmid with a G+C content of 46%. A total of 310 open reading frames (ORFs) were identified. Since pK29 carries the backbone of the IncHI2 plasmids, we compared its nucleotide sequence with those of pR478 and pAPEC-O1-R, the IncHI2 plasmids with complete nucleotide sequences available to date. Plasmid pR478 (275 kb) was from Serratia marcescens clinical isolates, and plasmid pAPEC-O1-R (241 kb) was from avian pathogenic E. coli strains (6, 12).
The common regions of the three plasmids contain essential genes for plasmid replication, maintenance, and transmission. These include the repHIA and repHI2 regions for replication initiation, the trh and tra genes for conjugation, and the par region for plasmid segregation (Fig. 1).
FIG. 1.
Map of pK29 and comparison of pK29 with the other two IncHI2 plasmids, pR478 and pAPEC-O1-R. The lines between the three plasmids indicate the homologous sequence blocks which are >99% identical (white squares). Inversion between the homologous regions is shaded. The important features are indicated, as follows: repHIA and repHI2, replication origins; trh and tra, conjugal transfer genes; parAB, plasmid partitioning genes; muc, mucAB mutagenesis genes. The ter, ars, cop, sil, and mer genes encode products conferring tellurite, arsenic, copper, silver, and mercury resistance, respectively. Other genes and their products are as follows: adhC, alcohol dehydrogenase; cat, putative chloramphenicol acetyltransferase; aphA1-Iab, aminoglycoside phosphotransferase; tet, tetracycline resistance proteins; aadA, putative streptomycin adenyltransferase; aac3-VI, gentamicin resistance protein; sul-1, dihydropteroate synthetase; and qacEdelta1, quaternary ammonium transporter protein. The two regions unique to pK29 (regions 1 and 2) are shown in black.
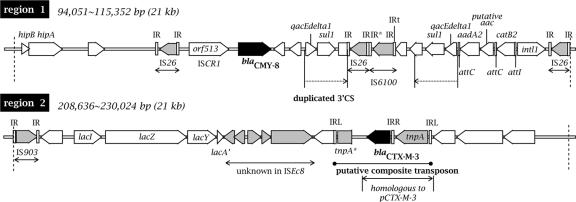
Two antimicrobial resistance gene-containing regions (Fig. 1, regions 1 and 2) are unique to pK29. Region 1 (bp 94,051 to 115,352) encodes HipBA multidrug tolerance protein homologs (13) and carries a type I integron containing the antimicrobial resistance gene cassettes aadA2, aac (putative), and catB2 (Fig. 2). In the integron region, the inverted repeat IRt was identified at the right end of IS6100 but the IRi was not found. The integron is flanked by two complete IS26 elements. In addition, a partial duplicate of the 3′-conserved sequence of the integron, including the qacEdelta1 and sul-1 genes, was identified on the left of the integron, followed by an AmpC β-lactamase gene, blaCMY-8. No intl1 or gene cassettes were found on the left of the duplicate 3′-conserved sequence.
FIG. 2.
Schematic diagram of regions 1 and 2, which are unique to pK29. The boundaries between the regions and the known sequence from pR478 are indicated by dashed lines. The ORFs are shown as arrows, with the arrowheads indicating the direction of transcription. The β-lactamase genes are shown in black. The ORFs related to IS are shown in gray. Unmarked white arrows are hypothetical genes. IR, inverted repeats; IR*, incomplete inverted repeats. The genes and their products are as follows: sul-1, dihydropteroate synthetase; qacEdelta1, quaternary ammonium transporter protein; aadA2, streptomycin adenyltransferase; putative aac, putative aminoglycoside 6′-N-acetyltransferase (80% identical to AAC6-II of Acinetobacter baumannii [GenBank accession no. ABL95928]); catB2, chloramphenicol acetyltransferase; and intl1, integrase. In region 2, the ISEcp1 element to the left of blaCTX-M-3 is truncated (tnpA*). The region homologous to plasmid pCTX-M-3 is indicated.
The AmpC β-lactamase blaCMY-8 was first identified in a 25-kb plasmid of a K. pneumoniae epidemic strain from southern Taiwan (25). The originally determined 1,973-bp spanning sequence of blaCMY-8 (GenBank accession no. AF167990), which contains part of the upstream orf513 and the downstream hypothetical ORF, can be aligned perfectly to that of pK29. The association of blaCMY-8 with orf513 (recently renamed insertion sequence common regions [ISCR1]) (23) indicates that the blaCMY-8 gene is mobilized with these type I integron-associated elements. In pK29, an additional IS26 element was identified at the left end of orf513 (Fig. 2). Thus, the blaCMY-8 region and the nearby type I integron region represent two small composite transposon-like elements, and the entire 14-kb region bounded by the two external IS26 elements could also be regarded as a large composite transposon (Fig. 2). We did not, however, find direct repeats or other supportive evidence for the transpositional acquisition of any of these putative composite transposons. The insertion sequences likely contributed to the formation of the region as a result of multiple transposition and recombination events.
Region 2 (bp 208,636 to 230,024) contains a lacIZY gene cluster followed by several IS-related conserved hypothetical genes and a 3.8-kb putative composite transposon. This contains a blaCTX-M-3 ESBL gene flanked by two convergently positioned ISEcp1 elements at both ends, with that on the left being truncated (Fig. 2).
The CTX-M-3 ESBL was first found among clinical Enterobacteriaceae isolates from Warsaw (7) and, soon after, from Taiwan (27). The finding of identical CTX-M enzymes in widely separated parts of the world has been suggested to be the result of independent evolution (17). However, the blaCTX-M-3 genes of both pK29 and pCTX-M-3, the plasmid from Poland (GenBank accession no. AF550415), were found to reside in a 3-kb homologous region with >99% identity, including a 1,656-bp ISEcp1 element upstream of the β-lactamase gene. Recent studies on the frequent association of blaCTX-M and ISEcp1-like insertion sequences have demonstrated that the IS may be responsible for the mobilization and expression of the β-lactamase gene (20, 21). Thus, the presence of blaCTX-M-3 in different geographical regions is likely to be the consequence of horizontal transmission assisted by the nearby ISEcp1 element.
The plasmid pK29 carries both hipBA and relEB homologs. Known as type II toxin-antitoxin modules, the products of these genes are important in the segregation maintenance of plasmids (8, 10). Recently, some of them have been suggested to play a role in the formation of persister cells that exhibit multidrug tolerance (13). pK29 also carries a mucAB operon for mutagenesis (16). By facilitating the formation of persister cells and enhancing the mutation rate, these genes may provide additional advantages to bacterial survival in the presence of lethal factors such as antibiotics.
The transconjugant E. coli J53/pK29 revealed resistance or decreased susceptibilities to cefotaxime, cefoxitin, ceftriaxone, amoxicillin-clavulanic acid, aztreonam, ceftazidime, cefepime, chloramphenicol, and sulfamethoxazole (Table 1). The resistance phenotype is consistent with the resistance genes identified on the plasmid. Both NK29 and the J53/pK29 transconjugant tested negative for ESBL by the CLSI ESBL confirmatory disk diffusion test (5), likely due to the presence of the AmpC β-lactamases. Thus, the coexistence of ESBL and AmpC β-lactamases not only limits treatment options but also complicates routine phenotypic detection of ESBLs, a problem of increasing concern for clinical microbiology laboratories (2, 17, 18).
TABLE 1.
MICs of different antimicrobials and ESBL test results for the clinical strain K. pneumoniae NK29, its plasmid pK29 transconjugant E. coli J53/pK29, and the reference strain E. coli J53
| Antimicrobial agent | MIC (μg/ml)a
|
Zone of inhibition (mm) by disk diffusion test
|
||||
|---|---|---|---|---|---|---|
| K. pneumoniae NK29 | E. coli J53/pK29 transconjugant | E. coli J53 | K. pneumoniae NK29 | E. coli J53/pK29 transconjugant | E. coli J53 | |
| Aminoglycosides | ||||||
| Gentamicin | 3 | 1.5 | 0.25 | |||
| Kanamycin | 32 | 32 | 8 | |||
| β-Lactams | ||||||
| Amoxicillin-clavulanic acid | 48 | 48 | 8 | |||
| Aztreonam | 4 | 2 | 0.064 | |||
| Cefepime | 2 | 1 | 0.094 | |||
| Cefotaxime | 32 | 24 | 0.064 | |||
| Cefoxitin | >256 | >256 | 4 | |||
| Ceftazidime | 16 | 1.5 | 0.19 | |||
| Ceftriaxone | 24 | 16 | 0.19 | |||
| Imipenem | 0.25 | 0.25 | 0.25 | |||
| Other drugs | ||||||
| Chloramphenicol | 96 | 96 | 4 | |||
| Ciprofloxacin | 0.047 | 0.012 | 0.012 | |||
| Sulfamethoxazole | >512 | >512 | ≤64 | |||
| Tetracycline | 8 | 2 | 2 | |||
| Trimethoprim-sulfamethoxazole | 0.50 | 0.064 | 0.047 | |||
| Drugs for ESBL testingb | ||||||
| CAZ/CAZ + CLA | 16/>4.0 | 1.5/1.0 | <0.5/0.25 | |||
| CTX/CTX + CLA | >16/>1.0 | >16/>1.0 | <0.25/0.064 | |||
| CAZ vs CAZ + CLA | 17 vs 17 | 27 vs 27 | 33 vs 33 | |||
| CTX vs CTX + CLA | 13 vs 15 | 16 vs 17 | 31 vs 31 | |||
Based on Etest results, except for kanamycin and sulfamethoxazole MICs, which are from broth microdilution tests. For sulfamethoxazole, the lowest concentration tested was 64 μg/ml, and thus the actual MIC for J53 could be lower.
CAZ, ceftazidime; CTX, cefotaxime; CLA, clavulanic acid. The ceftazidime results were all negative, and the cefotaxime results were indeterminate for NK29 and the J53/pK29 transconjugant based on MIC results. The isolates all tested negative for ESBLs by the CLSI disk diffusion confirmatory test (5).
This is the first report of a completely sequenced plasmid that carries both CTX-M-type ESBL and CMY-type AmpC β-lactamase genes. The finding of the coexistence of these genes in such a transmissible plasmid that can propagate in different hosts provides further insight into the mechanisms of transmission of these β-lactamases in Taiwan, where plasmid-mediated ESBLs and AmpC β-lactamases are prevalent.
Nucleotide sequence accession number.
The annotated sequence of pK29 has been submitted to the GenBank nucleotide sequence database under accession number EF382672.
Acknowledgments
We thank George A. Jacoby, Lahey Clinic, Massachusetts, for providing the E. coli J53 Azir strain.
This project was funded by a grant from the National Science Council (NSC-95-3112-B-400-011) and by an intramural grant from the National Health Research Institutes (CL-095-PP-01).
Footnotes
Published ahead of print on 25 May 2007.
REFERENCES
- 1.Alvarez, M., J. H. Tran, N. Chow, and G. A. Jacoby. 2004. Epidemiology of conjugative plasmid-mediated AmpC β-lactamases in the United States. Antimicrob. Agents Chemother. 48:533-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Black, J. A., E. S. Moland, and K. T. Thomson. 2005. AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β-lactamases. J. Clin. Microbiol. 43:3110-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, Y. T., H. Y. Hsu, L. H. Li, T. L. Liao, K. M. Wu, Y. R. Shiau, J. J. Yan, I. J. Su, S. F. Tsai, and T. L. Lauderdale. 2006. Complete nucleotide sequence of pK245, a 98-kilobase plasmid conferring quinolone resistance and extended-spectrum-beta-lactamase activity in a clinical Klebsiella pneumoniae isolate. Antimicrob. Agents Chemother. 50:3861-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 9th ed. Document M2-A9. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Gilmour, M. W., N. R. Thomson, M. Sanders, J. Parkhill, and D. E. Taylor. 2004. The complete nucleotide sequence of the resistance plasmid R478: defining the backbone components of incompatibility group H conjugative plasmids through comparative genomics. Plasmid 52:182-202. [DOI] [PubMed] [Google Scholar]
- 7.Gniadkowski, M., I. Schneider, A. Palucha, R. Jungwirth, B. Mikiewicz, and A. Bauernfeind. 1998. Cefotaxime-resistant Enterobacteriaceae isolates from a hospital in Warsaw, Poland: identification of a new CTX-M-3 cefotaxime-hydrolyzing β-lactamase that is closely related to the CTX-M-1/MEN-1 enzyme. Antimicrob. Agents Chemother. 42:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gotfredsen, M., and K. Gerdes. 1998. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol. 29:1065-1076. [DOI] [PubMed] [Google Scholar]
- 9.Hanson, N. D., K. S. Thomson, E. S. Moland, C. C. Sanders, G. Berthold, and R. G. Penn. 1999. Molecular characterization of a multiply resistant Klebsiella pneumoniae encoding ESBLs and a plasmid-mediated AmpC. J. Antimicrob. Chemother. 44:377-380. [DOI] [PubMed] [Google Scholar]
- 10.Hayes, F. 2003. Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301:1496-1499. [DOI] [PubMed] [Google Scholar]
- 11.Jacoby, G. A., and P. Han. 1996. Detection of extended-spectrum β-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli. J. Clin. Microbiol. 34:908-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, T. J., Y. M. Wannemeuhler, J. A. Scaccianoce, S. J. Johnson, and K. L. Nolan. 2006. Complete DNA sequence, comparative genomics, and prevalence of an IncHI2 plasmid occurring among extraintestinal pathogenic Escherichia coli isolates. Antimicrob. Agents Chemother. 50:3929-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keren, I., D. Shah, A. Spoering, N. Kaldalu, and K. Lewis. 2004. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli. J. Bacteriol. 186:8172-8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livermore, D. M., and N. Woodford. 2006. The β-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 15.Moland, E. S., S. G. Hong, K. S. Thomson, D. H. Larone, and N. D. Hanson. 2007. Klebsiella pneumoniae isolate producing at least eight different β-lactamases, including AmpC and KPC β-lactamases. Antimicrob. Agents Chemother. 51:800-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mortelmans, K. 2006. Isolation of plasmid pKM101 in the Stocker laboratory. Mutat. Res. 612:151-164. [DOI] [PubMed] [Google Scholar]
- 17.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum beta-lactamase: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., and J. Segreti. 2006. Overview of the epidemiological profile and laboratory detection of extended-spectrum beta-lactamases. Clin. Infect. Dis. 42:S153-S163. [DOI] [PubMed] [Google Scholar]
- 19.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel, L., J. W. Decousser, and P. Nordmann. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a blaCTX-M β-lactamase gene. Antimicrob. Agents Chemother. 47:2938-2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirel, L., M. F. Lartigue, J. W. Decousser, and P. Nordmann. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 49:447-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song, W., K. M. Lee, H. S. Kim, J. S. Kim, J. Kim, S. H. Jeong, and K. H. Roh. 2006. Clonal spread of both oxyimino-cephalosporin- and cefoxitin-resistant Klebsiella pneumoniae isolates co-producing SHV-2a and DHA-1 β-lactamase at a burns intensive care unit. Int. J. Antimicrob. Agents. 28:520-524. [DOI] [PubMed] [Google Scholar]
- 23.Toleman, M. A., P. M. Bennett, and T. R. Walsh. 2006. ISCR elements: novel gene-capturing systems of the 21st century? Microbiol. Mol. Biol. Rev. 70:296-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan, J. J., P. R. Hsueh, J. J. Lu, F. Y. Chang, J. M. Shyr, J. H. Wan, Y. C. Liu, Y. C. Chuang, Y. C. Yang, S. M. Tsao, H. H. Wu, L. S. Wang, T. P. Lin, H. M. Wu, H. M. Chen, and J. J. Wu. 2006. Extended-spectrum β-lactamases and plasmid-mediated AmpC enzymes among clinical isolates of Escherichia coli and Klebsiella pneumoniae from seven medical centers in Taiwan. Antimicrob. Agents Chemother. 50:1861-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan, J. J., S. M. Wu, S. H. Tsai, J. J. Wu, and I. J. Su. 2000. Prevalence of SHV-12 among clinical isolates of Klebsiella pneumoniae producing extended-spectrum β-lactamases and identification of a novel AmpC enzyme (CMY-8) in southern Taiwan. Antimicrob. Agents Chemother. 44:1438-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan, J. J., W. C. Ko, H. M. Wu, S. H. Tsai, C. L. Chuang, and J. J. Wu. 2004. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J. Clin. Microbiol. 42:5337-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan, J. J., W. C. Ko, S. H. Tsai, H. M. Wu, Y. T. Jin, and J. J. Wu. 2000. Dissemination of CTX-M-3 and CMY-2 β-lactamases among clinical isolates of Escherichia coli in southern Taiwan. J. Clin. Microbiol. 38:4320-4325. [DOI] [PMC free article] [PubMed] [Google Scholar]