Abstract
Human African trypanosomiasis is a devastating disease with only a few treatment options, including pentamidine. Diamidine compounds such as pentamidine, DB75, and DB820 are potent antitrypanosomal compounds. Previous investigations have shown that diamidines accumulate to high concentrations in trypanosomes. However, the mechanism of action of this class of compounds remains unknown. A long-hypothesized mechanism of action has been binding to DNA and interference with DNA-associated enzymes. The fluorescent diamidines, DB75 and DB820, have been shown to localize not only in the DNA-containing nucleus and kinetoplast of trypanosomes but also to the acidocalcisomes. Here we investigate two series of analogs of DB75 and DB820 with various levels of in vitro antitrypanosomal activity to determine whether any correlation exists between trypanosome accumulation, distribution, and in vitro activity. Despite wide ranges of in vitro antitrypanosomal activity, all of the compounds investigated accumulated to millimolar concentrations in trypanosomes over a period of 8 h. Interestingly, some of the less potent compounds accumulated to concentrations much higher than those of more potent compounds. All of the compounds were localized to the DNA-containing nucleus and/or kinetoplast, and many were also found in the acidocalcisomes. Accumulation in the nucleus and kinetoplast should be important to the mechanism of action of these compounds. The acidocalcisomes may also play a role in the mechanism of action of these compounds. This investigation suggests that the extent of accumulation alone is not responsible for killing trypanosomes and that organelle-specific accumulation may not predict in vitro activity.
Diamidine compounds, such as pentamidine, propamidine, and diminazene, have been used for many years as chemotherapeutic agents for infections caused by a variety of microbes, including parasites and fungi. Pentamidine has been used for almost 60 years as a treatment for human African trypanosomiasis and is also used to treat leishmaniasis and the opportunistic infection Pneumocystis pneumonia (32). Diminazene has been used widely for treatment of animal trypanosomiasis (9) and has also been used in humans (25). Recently, pafuramidine, or DB289, a methamidoxime prodrug of the diamidine DB75 (furamidine), has been developed as an oral treatment for early-stage sleeping sickness caused by Trypanosoma brucei gambiense. DB289 is currently in phase III clinical trials in sub-Saharan Africa (6). In addition to DB75 and DB289, a library of diamidines and prodrugs has been synthesized, with various activities against many parasites (1-3, 14-18, 32).
Although diamidines have been used therapeutically for over half a century, their mechanism of action is not well understood. Many mechanisms of action have been proposed (32), but one mechanism of action of diamidines that has often been hypothesized is binding to DNA in the nucleus or kinetoplast, leading to interference of DNA-associated enzymes, such as topoisomerase II (27, 34). Pentamidine has also been shown to linearize kinetoplast DNA isolated from trypanosomes (27), which could play an important role in the compound's mechanism of action. Furthermore, ultrastructural studies of whole trypanosomes have demonstrated that diamidines such as pentamidine have an impact on the kinetoplasts of trypanosomes, perhaps leading to the disintegration of kinetoplast DNA (33). However, neither quantitative nor qualitative investigations have been performed to determine in which compartments pentamidine localizes in trypanosomes. This is not surprising, as pentamidine is not a fluorescent compound and therefore its intracellular distribution cannot be tracked using microscopy.
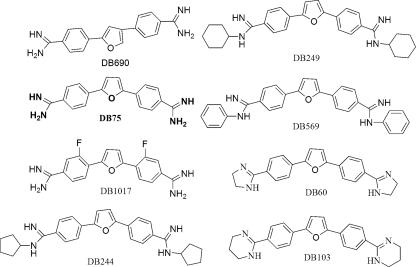
Several innately fluorescent compounds related to pentamidine have been investigated qualitatively in cancer cells (19, 20) to determine effects of structural modifications on nuclear distribution. Compounds tested included DB75, the substituted diamidines DB569, DB244, and DB249, and the imidazoline DB60 (Fig. 1). Lansiaux et al. found that of the three substituted diamidines, only the N-phenyl-substituted compound DB569 had an altered distribution in cells. While the other compounds were localized to the nuclei of cancer cells, DB569 was found only in the mitochondria (20).
FIG. 1.
Structures of diphenyl furan diamidines, presented in order of IC50 values against T. b. brucei S427 trypanosomes. DB75, which is shown in bold, represents the prototypical diamidine in this series. All other compounds are modifications of DB75.
More recently, the accumulation and distribution in trypanosomes of two fluorescent analogs of pentamidine, namely, DB75 and its aza analog, DB820, were described (24). Both compounds accumulated within trypanosomes to millimolar concentrations both in vitro and in an in vivo mouse model, with concentrations in trypanosomes approximately 1000-fold or greater than the environmental concentrations. These compounds not only were distributed in the nucleus and kinetoplast, the DNA-containing organelles of trypanosomes, but were also found to be localized to acidocalcisomes. Although DNA binding has been hypothesized to be the mechanism of action for diamidines, accumulation in the acidocalcisomes or other organelles may also play an important role in killing these parasites.
This paper describes the in vitro activity, DNA binding ability, accumulation, and cellular distribution of 13 structurally related compounds. Because some are intensely fluorescent and others are not as fluorescent, we combined qualitative microscopy studies to determine the intracellular localization of the compounds with quantitative high-pressure liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC/MS) to determine the concentrations of compounds within trypanosomes. The compounds investigated, which have the same core structure, do have several structural modifications that could influence activity and DNA binding as well as accumulation and distribution within trypanosomes. Here we investigate two series of compounds, diphenyl furans (including DB75) and aza analogs of diphenyl furans (including DB820), to determine whether any correlation exists between trypanosome accumulation and intracellular distribution and in vitro activity.
MATERIALS AND METHODS
Antitrypanosomal diamidines.
Diamidine compounds were synthesized as previously described (3, 7, 16, 20). Stock solutions of the compounds were prepared at a concentration of 1 mM in sterile distilled water.
In vitro antitrypanosomal activity.
To determine 50% inhibitory concentrations (IC50 values), T. b. brucei S427 and T. b. rhodesiense STIB900 trypanosomes (2 × 104 per ml) were cultured at 37°C and 5% CO2 with serial dilutions of the diamidines for 72 h, using a previously described method (26). The T. b. brucei strain S427 is routinely used at the University of North Carolina (UNC) for in vitro activity, compound accumulation, and intracellular distribution studies. The T. b. rhodesiense strain STIB900 is routinely used at the Swiss Tropical Institute for screening diamidine compounds for both in vitro and in vivo activity (15). Experiments were performed in triplicate with each trypanosome strain. IC50 values were determined using the Hill equation and are reported as averages for at least three determinations.
DNA binding by thermal melting.
Thermal melting experiments were conducted with a Cary 300 Bio UV-visible spectrophotometer (Walnut Creek, CA) as previously described (35). DNA samples for melting studies were added to 1 ml of MES buffer (0.01 M morpholineethanesulfonic acid [MES], 0.1 M NaCl, 0.001 M EDTA, pH 6.2) in 1-cm-path-length reduced-volume quartz cells. Experiments were generally conducted at a concentration of 5 × 10−5 M in base pairs for poly(dA)·poly(dT) or 1 × 10−6 M in duplex for the self-complementary oligomer d(CGCGAATTCGCG)2 (The Midland Certified Reagent Company, Inc., Midland, TX), which has a single AT binding site for the diamidines. The concentration of DNA was determined by measuring the absorbance at 260 nm. For the diamidine-DNA complexes, a ratio of compound to DNA oligomer duplex of 1:1 or a ratio of compound to DNA base pairs with poly(dA)·poly(dT) of 0.6 was used. A thermistor fixed into a reference cuvette was used to monitor the temperature, and the heating rate was 0.5°C/min. The data were collected as absorbance (A) versus temperature (T) by a computer which also controlled the instrument. The melting temperature (Tm) values for DNA alone or for the complex of DNA and compound were determined by first-derivative analysis (plot of ΔA/ΔT versus T), where the maximum marks the Tm at the inflection point in the sigmoidal melting curve (A versus T; ΔTm = Tm for complex − Tm for DNA).
Fluorescence spectra of diamidines in aqueous solution.
Fluorescence scans to determine excitation and emission maxima were performed using a Perkin-Elmer luminescence spectrophotometer (LS 50B) using FL Winlab, version 4.00.02, software (Perkin-Elmer, Wellesley, MA). Compounds were prepared as 500 nM solutions in distilled water. One milliliter of each solution was transferred to a disposable cuvette (Fisher Scientific), and the fluorescence spectrum was determined. Fluorescence intensity measurements were then determined using the λex and λem maxima.
For some compounds, the fluorescence properties were determined in the presence of DNA. Briefly, fluorescence emission spectra were obtained with a Cary Eclipse spectrofluorometer with software provided to control the instrument and collect the fluorescence data. Typically, the fluorescence intensity for a compound at a concentration of 1 μM was measured at 25°C in MES buffer. Compounds were excited at their maximum excitation wavelength, determined as described above, and fluorescence emission spectra were collected. A solution of each compound was titrated with aliquots of a stock solution of Clostridium perfringens genomic DNA (Sigma) containing 72% AT base pairs, and the samples were rescanned for emission spectra.
In vitro accumulation of diamidines in S427 trypanosomes.
To determine the in vitro accumulation of diamidines within trypanosomes over time, approximately 106 trypanosomes were incubated with a 7.5 μM concentration of each diamidine for periods ranging from 1 to 8 h. At each time point, trypanosomes were washed twice in fresh drug-free medium, lysed, and extracted in 4 volumes of 8:1 methanol-0.1 N HCl (vol/vol). Half of each sample was evaporated in a Zymark (Hopkinton, MA) Turbovap-LV instrument under nitrogen gas (7 lb/in2) and reconstituted in HPLC solvent A (described in detail in “HPLC analysis of diamidines”). The remainder of the sample was retained for future analysis if needed. Diamidine concentrations in trypanosomes were determined as described previously (24).
In vitro distribution of diamidines in S427 trypanosomes.
S427 trypanosomes were cultured in 24-well plates (Corning Costar, Fisher Scientific) at a starting concentration of 105 trypanosomes/ml. Diamidines were added to each well to a final concentration of 500 nM. Trypanosomes were incubated with the diamidines for 8 h at 37°C, and then trypanosomes were examined under a light microscope for motility and viability. Trypanosomes were washed twice in 1 ml fresh medium which contained no diamidine and resuspended in 20 μl of freshly isolated mouse blood. A small drop of blood was placed on a microscope slide, and thin films were prepared. Thin films were used to reduce the photobleaching that occurs when viewing live trypanosomes under a UV-fluorescence cube. No differences in intracellular distribution of fluorescence were seen when live trypanosomes were viewed under fluorescent light.
Fluorescence microscopy of diamidines in trypanosomes.
Thin films were mounted with a drop of glycerol before observation. A Nikon Microphot FXA microscope (Garden City, NJ) with a 60-DM 1.4-numerical-aperture objective lens, a mercury lamp, and an Optronics DEI 750 charge-coupled device camera (Goleta, CA) was used for fluorescence microscopy. The microscope was equipped with a Nikon UV2A cube that limits excitation wavelengths to 330 to 380 nm and emission wavelengths to ≥420 nm. The UV2A cube allows for the visualization of the blue fluorescence seen in the nucleus and kinetoplast and the yellow/orange fluorescence seen in the acidocalcisomes due to interactions of diamidines with polyphosphates in these organelles (31).
Distribution of DB75 in acidocalcisomes.
Trypanosoma brucei brucei S427 trypanosomes were cultured in CBMEM as previously described (24). For various time points from 5 min to 1 h, 106 trypanosomes were pretreated with a stock solution of 5 μM monensin (Sigma) prepared in ethanol or 20 mM ammonium chloride. After pretreatment, trypanosomes were washed and then DB75 was added to a final concentration of 7.5 μM. DB75 was incubated with trypanosomes for 2 h, and then trypanosomes were washed twice and resuspended in freshly isolated mouse blood (20 μl). Thin films were prepared from a drop of blood and viewed with a Nikon Microphot FXA microscope equipped with a 60-DM 1.4-numerical-aperture objective lens, a mercury lamp, and a QImaging Micropublisher 3.3 charge-coupled device digital camera (QImaging Corporation, Surrey, British Columbia, Canada) for fluorescence microscopy. QCapture, version 3.0, imaging software was used to capture images (QImaging Corporation). The microscope was equipped with a Nikon UV2A cube that limits excitation wavelengths to 330 to 380 nm and emission wavelengths to ≥420 nm. Acridine orange (Sigma) was also used as a marker for acidocalcisome accumulation. Acridine orange has been shown in the past to accumulate in acidocalcisomes and to be displaced by alkalinizing agents (12).
HPLC analysis of diamidines.
HPLC analytical methods for DB1017, DB244, DB249, DB103, DB60, DB935, and DB829 were based on analytical methods previously described for DB75 and DB820 (24, 30). HPLC solvent A consisted of 15 mM ammonium formate, 30 mM formic acid in HPLC-grade water, while solvent B contained the same buffer system prepared in 4:1 acetonitrile-HPLC-grade water. An elution gradient was used to resolve each diamidine, with starting conditions of 95% solvent A and 5% solvent B, which increased linearly over 10 to 15 min, followed by a 3- to 5-min reequilibration to starting conditions. An Agilent Zorbax Bonus RP (New Castle, DE) column (2.1 mm by 50 mm; 3.5 μm) was used for compound resolution. Compounds were detected using their maximum excitation and emission wavelengths (see Table 3).
TABLE 3.
Fluorescence properties of aqueous solutions (500 nM) of diphenyl furan and aza analogs and changes in fluorescence of select analogs bound to DNA
| Compound | λex max | λem max | Fluorescence intensity at max | % Change in fluorescence when bound to DNAa |
|---|---|---|---|---|
| DB690 | 323 | 452 | 51 | ↑270 |
| DB75 | 356 | 458 | 464 | ↓17 |
| DB1017 | 354 | 455 | 840 | ND |
| DB244 | 356 | 450 | 625 | ND |
| DB249 | 356 | 453 | 920 | ND |
| DB60 | 373 | 458 | 630 | ↓23 |
| DB103 | 352 | 437 | 860 | ND |
| DB867 | 370 | 481 | 51 | ↑360 |
| DB820 | 359 | 455 | 318 | ↓50 |
| DB935 | 358 | 461 | 330 | ND |
| DB829 | 359 | 433 | 701 | ↓86 |
| DB1057 | 352 | 470 | 16 | ND |
| DB879 | 337 | 435 | 17 | ND |
Determined at a concentration of 1 μM. ND, not determined.
MS analysis of diamidines.
DB690, DB867, DB1057, and DB879 concentrations were determined by LC/MS. To verify results, analyses of DB103 and DB60 were conducted via HPLC with fluorescence detection as well as via LC/MS. Depending on the molecular weight of the test diamidine, either DB75 or DB103 was used as an internal standard. Automated sample analysis was performed using Analyst software (version 1.4.1; Applied Biosystems, Foster City, CA). The Analyst-controlled HPLC-MS/MS system consisted of two Shimadzu Scientific (Columbia, MD) solvent delivery pumps, a thermostated (6°C) LEAP HTC autosampler (Carrboro, NC), and an Applied Biosystems API4000 triple-quadrupole mass spectrometer. Reversed-phase gradient chromatography was used to elute the diamidines from an Aquasil (C18; 5 μm; 50 mm by 2.1 mm) analytical column (Torrance, CA) at a flow rate of 0.75 ml/min, following injection of 4-μl sample. Starting conditions for each injection were 95%-5% water-methanol, with 0.1% formic acid (vol/vol) in each. The relative amounts of water and methanol were held constant for 0.5 min while the column eluted to waste. After 0.5 min, the eluent was directed to the mass spectrometer, and the relative amount of methanol was increased linearly to 90% at 3 min postinjection. This amount of methanol was held for 0.5 min to wash the column. The column was reequilibrated under the starting conditions for the final 0.5 min. Total run time was 4 min. The mass spectrometer was connected to the HPLC system by a Turbo ion spray interface. Nitrogen from a Peak Scientific (Bedford, MA) nitrogen generator was used as the curtain, nebulizer, and collision gas. User-controlled voltages, gas pressures, and source temperature were optimized for the detection of the parent and product ions of the diamidines. All diamidines were analyzed in positive-ion mode, using multiple reaction monitoring.
RESULTS
In vitro antitrypanosomal activity of diamidines.
Two series of diamidines were investigated, namely, the diphenyl furan series, which includes DB75, and the aza analogs, which include DB820 (Fig. 1 and 2). Compounds in the diphenyl furan series had the greatest structural diversity, with modifications to the core structure, N substitutions on the diamidines, and cyclic diamidines. Compounds in the aza series had less structural diversity, with modifications to the core structure only. All compounds were active in vitro, although activities against T. b. brucei S427 and T. b. rhodesiense STIB900 varied greatly (Tables 1 and 2).
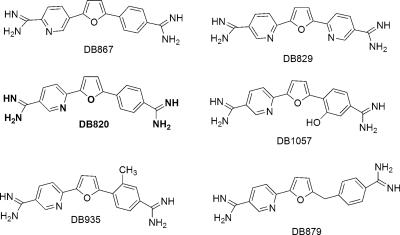
FIG. 2.
Structures of the aza analog diamidines, presented in order of IC50 values against T. b. brucei S427 trypanosomes. DB820, which is shown in bold, represents the prototypical diamidine in this series. All other compounds are modifications of DB820.
TABLE 1.
In vitro activity and DNA binding (ΔTm) of select diphenyl furans
| Compound | pKa | clogDa | IC50 value (nM)
|
ΔTm
|
||
|---|---|---|---|---|---|---|
| S427 | STIB 900 | Poly(dA)·poly(dT) | (dCGCGAATTCGCG)2 | |||
| DB690 | 10.8, 11.6 | −2.8 | 2.1 | 1.7 | 17.1 | 10.4 |
| DB75 | 10.4, 11.8 | >−2.0 | 7 | 3.2 | 26.2 | 8.9 |
| DB1017 | 10.5, 11.3 | −1.2 | 14.8 | 8.0 | 15.2 | 8.2 |
| DB244 | 10.3, 11.1 | 0.2 | 100 | 60 | 27 | 16 |
| DB249 | 10.3, 11.1 | 1.0 | 121 | 68 | 27 | 16.8 |
| DB60 | 8.8, 9.5 | −0.4 | 251 | 245 | 19.3 | 11.0 |
| DB103 | 9.5, 10.2 | −1.3 | 260 | 33 | 27 | 15.1 |
clogD values (at pH 7.4) and pKa values were calculated using ChemAxon MarvinSketch. DB75 and DB1017 pKa and logD values were measured by J. Saulter (by a UV spectrophotometric method).
TABLE 2.
In vitro activity and DNA binding (ΔTm) of select aza analogs
| Compound | pKa | clogDa | IC50 value (nM)
|
ΔTm
|
||
|---|---|---|---|---|---|---|
| S427 | STIB 900 | Poly(dA)·poly(dT) | (dCGCGAATTCGCG)2 | |||
| DB867 | 8.5, 11.1 | −2.8 | 3.3 | 2.1 | 23.3 | 10.4 |
| DB820 | 10.5, 11.8 | −3.8 | 3.4 | 6.5 | 20.0 | 11.2 |
| DB935 | 9.9, 11.3 | −4.5 | 10 | 9.7 | 16.7 | 10.6 |
| DB829 | 9.6, 10.3 | −3.3 | 13 | 19 | 16.0 | 8.4 |
| DB1057 | 7.4, 11.6 | −2.7 | 32 | 32 | 16.4 | 10.4 |
| DB879 | 9.9, 11.6 | −3.5 | 314 | 147 | 3.1 | 0.4 |
clogD values (at pH 7.4) and pKa values were calculated using ChemAxon MarvinSketch.
The majority of the diamidines used in this study have two positive charges at physiological pH (pH 7.0) and lower pH values. pKa values for DB75, for example, have been determined to be around pH 11 (Table 1). Calculated logD and calculated pKa values, obtained from ChemAxon MarvinSketch, are shown in Tables 1 and 2 for both series of compounds. ChemAxon's program calculated values consistent with measured values. This program was a better predictor than was ACD LogD Suite v 4.5 (Advanced Chemistry Development, Inc., Toronto, Canada). As seen in Tables 1 and 2, pKa values for all the diamidines are approximately 10 or greater, indicating that the compounds will be doubly charged at physiological pH. Most compounds, with the exception of DB244 and DB249, are hydrophilic at pH 7.4. DB244 and DB249 are both slightly lipophilic at this pH, likely due to the substitution of bulky cyclic groups off the diamidines, which could mask the positive charges.
(i) Diphenyl furans.
Diamidines in the diphenyl furan series had IC50 values ranging between 2 and 260 nM, a >100-fold difference in activity, against the S427 strain (Table 1). The three compounds with unmodified diamidines (DB690, DB75, and DB1017) were the most potent. Changes in bond positioning (DB690) or the addition of functional groups to the core nucleus (DB1017) had only a modest (two- to threefold) effect on activity compared to that of DB75. Compounds which had substituted amidines (DB244 and DB249) had intermediate potency against S427 trypanosomes, with IC50 values of 100 to 150 nM. The cyclic diamidines, the imidazoline DB60, and the tetrahydropyrimidyl DB103 were the least potent compounds in this series against S427 trypanosomes. Against the STIB900 strain, the trend in activity for diphenyl furans was similar to that for the S427 strain, with six of the seven compounds having the same rank order against both strains (Table 1). The only exception was DB103, which had an IC50 value of over 100 nM for the S427 strain but was much more active against the STIB900 strain (IC50 value of 33 nM).
(ii) Aza analogs.
All aza analogs had unmodified amidine moieties. Five of the six compounds were potent, with activities against T. b. brucei S427 varying only 10-fold (Table 2). DB867 was the most potent compound, with an IC50 value of 3.3 nM. The positioning of the nitrogen in the aromatic rings had only minor effects (DB867, DB820, and DB829). As with the diphenyl furans, there was little impact on activity when functional groups were added to the aromatic rings (DB935 and DB1057). Between DB867 and DB1057, there was only a 10-fold difference in IC50 values. DB879, which has a methylene group inserted between the furan and phenyl rings, was much less potent than the other compounds in this series. IC50 values for the compounds in this series against the STIB900 strain were similar for all compounds and were in the same rank order. DB879 was about twofold more potent against STIB900 trypanosomes than against S427 trypanosomes but overall was still not a very potent compound.
Thermal melting analysis of DNA binding.
Increases in Tm values for compound-DNA oligomer and compound-polymer complexes are shown in Tables 1 and 2. The majority of the compounds in these two series were potent DNA binders and bound strongly to both poly(dA)·poly(dT), which has multiple AT binding sites, and the d(CGCGAATTCGCG) oligomer duplex, which has a single AT binding site for diamidines (Tables 1 and 2). Studies with a wide array of diamidines have shown that evaluation of increases in the melting temperature of DNA upon addition of the compounds provides a rapid method for ranking diamidines according to their binding affinities (23).
All of the diphenyl furans were potent binders, with ΔTm values of >15°C for poly(dA)·poly(dT) and >8°C for the d(CGCGAATTCGCG) oligomer duplex. The largest increases in the melting temperature were seen with DB244, DB249, DB103, and DB75 (Table 1), but there was no correlation between activity of the diphenyl furans and DNA binding affinity (r2 = 0.09). With the aza analogs (Table 2), there appeared to be a correlation between the activity and the increase in melting temperature with the poly(dA)·poly(dT) oligomer and the d(CGCGAATTCGCG) oligomer duplex (r2 = 0.9; slope, −0.05 and 0.03, respectively), but this correlation was driven by the low ΔTm values determined with DB879. When DB879 is removed from the series, no correlation exists (r2 = 0.4 and 0.03 for the two oligomers, respectively). DB879, the least potent compound investigated, had little effect on the melting temperature of the DNA complex (Table 2).
Intracellular accumulation of diamidines in T. b. brucei S427.
DB75 and DB820 were previously shown to accumulate to approximately 12 mM and 3 mM, respectively, after a 24-h in vitro incubation with 7.5 μM of either compound (24). Since the compounds chosen for investigation have a wide range of in vitro activities, we chose the concentration of 7.5 μM to incubate with trypanosomes for comparison to previous work. After investigating the accumulation of several of the diamidines, the pattern of intracellular concentration was similar to that reported previously (24). However, the toxicity of some of the compounds became an issue with the accumulation experiments (increasing cell death when incubated for longer than 8 h), so investigations were limited to 8 h.
Due to poor fluorescence intensity, some compounds were analyzed and quantified by LC/MS instead of HPLC, which was used for the more fluorescent compounds. DB75, DB1017, DB244, DB249, DB103, DB60, DB935, DB820, and DB829 were analyzed using HPLC, while DB690, DB867, DB1057, and DB879 were analyzed by LC/MS.
(i) Diphenyl furans.
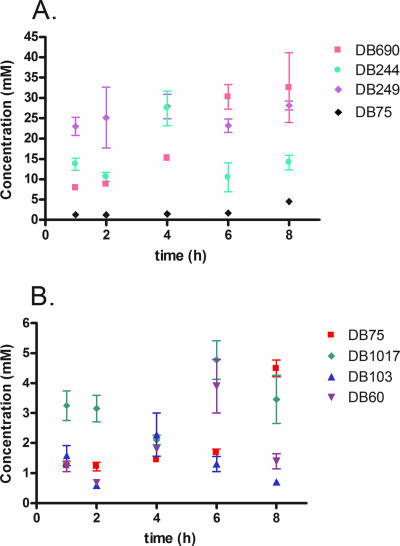
All of the diphenyl furans accumulated to high concentrations, with peak concentrations ranging from 1 mM to 30 mM (Fig. 3), after 8 h of incubation at 7.5 μM. Three of the diphenyl furans (DB690, DB244, and DB249) investigated accumulated to higher peak concentrations in trypanosomes than those seen previously for DB75 (4.5 ± 0.3 mM at 8 h) (Fig. 3A). DB690, the most potent diphenyl furan investigated, as well as DB244 and DB249, with intermediate potencies, accumulated to peak concentrations of approximately 30 mM (Fig. 3A). The other diphenyl furans accumulated in trypanosomes to peak concentrations similar to that of DB75 (Fig. 3B). Thus, there appears to be no trend linking the potency of the diamidines and accumulation of the diphenyl furans in trypanosomes over time.
FIG. 3.
In vitro accumulation of diphenyl furans (7.5 μM) over 8 h. (A) Accumulation of diphenyl furans to concentrations much higher than that of DB75. (B) Accumulation at lower millimolar levels over 8 h. Accumulation, which was determined by HPLC or LC/MS analysis, is presented as the mean concentration ± standard error. Data for DB569 are not shown, as it killed trypanosomes rapidly, and accumulation was unable to be determined.
(ii) Aza analogs.
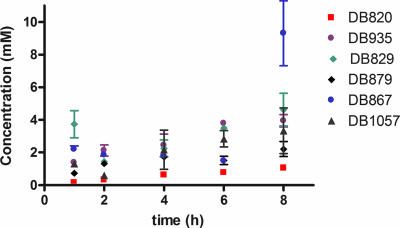
Compounds in the aza analog series accumulated linearly over 8 h in trypanosomes, for the most part (Fig. 4), except for DB867, which accumulated in a nonlinear manner. DB820 had a peak concentration of 1.0 ± 0.2 mM at 8 h. DB867, the most potent compound in this series (Table 2), which has the nitrogen in the pyrimidine ring moved meta- to the furan ring, accumulated to a concentration of 9.3 ± 2.0 mM after 8 h, which is significantly greater than the accumulation of DB820. DB935, which has a methyl substituent on the phenyl ring, accumulated to concentrations in trypanosomes which were fourfold higher than that of DB820, despite being threefold less potent. All compounds in the aza analog series accumulated to higher concentrations than DB820, including DB879, the least potent diamidine investigated, as seen in Fig. 4.
FIG. 4.
In vitro accumulation of aza analogues (7.5 μM) over 8 h. Data are presented as mean concentrations ± standard errors. Accumulation was determined by HPLC or LC/MS analysis.
Fluorescence spectra of diamidines in aqueous solution.
The fluorescence spectra of diamidines were determined for developing analysis methods and for the fluorescence microscopy studies. The fluorescence of the diamidines varied in terms of intensity and excitation (λex) and emission (λem) wavelengths (Table 3).
(i) Diphenyl furans.
In the diphenyl furan series, four compounds had similar fluorescence spectra in water. DB75, DB1017, DB244, and DB249 had excitation maxima around 355 nm, with maximum emission around 455 nm. Of these compounds, DB249 had the highest emission intensity, and DB75 had the lowest emission intensity. Other structural modifications to DB75 tended to shift either the excitation wavelength, by about 20 nm (DB690 or DB60), or the emission maximum, by 20 nm (DB103), in either direction. In addition to shifting fluorescence spectra, some of the modifications also greatly decreased the fluorescence intensity, as seen with DB690 (Table 3).
The fluorescence properties of select diphenyl furan diamidines were also investigated in the presence of AT-rich DNA. The fluorescence of DB690, which is only weakly fluorescent in solution, increased almost 300% in the presence of DNA, while the fluorescence of DB75 decreased 17% in the presence of DNA (Table 3). The fluorescence intensity of DB60 also decreased 23% when bound to DNA.
(ii) Aza analogs.
In the aza series, only DB820 and DB935 had similar excitation and emission spectra, with a maximum excitation wavelength of about 360 nm and emission around 460 nm. The spectra of other compounds were shifted in either the excitation or emission wavelength or both. Placing the nitrogen in the pyrimidine ring ortho- to the amidine (DB867), as opposed to meta- to the amidine (DB820), shifted the excitation maximum 10 nm, and the emission maximum was also shifted 20 nm. This modification also decreased the fluorescence intensity approximately 60-fold. However, having two nitrogens—one in each pyrimidine ring (DB867)—increased the fluorescence intensity twofold over that of DB820 while shifting the maximum emission wavelength down 20 nm. The addition of either an alcohol group to the phenyl ring or a methylene linker between the phenyl and furan ring decreased the fluorescence intensity dramatically (Table 3).
The fluorescence of select diamidines was investigated in the presence of AT-rich DNA. As with DB690 in the diphenyl furan series, the fluorescence of the weakly fluorescent DB867 analog was greatly enhanced (almost 400%) with increasing concentrations of DNA (Table 3). The very fluorescent DB820 and DB829 molecules exhibited decreased fluorescence when bound to DNA (50% and 86%, respectively).
Intracellular distribution of diamidines in T. b. brucei S427.
The majority of the compounds in both series were distributed in the following three organelles in the trypanosomes: the kinetoplast, the nucleus, and the acidocalcisomes (see below). A concentration of 500 nM was chosen for microscopy studies for several reasons, including prevention of fluorescence signal saturation of the compounds and the toxicity of some compounds at higher concentrations. For some compounds that were less fluorescent, distribution at 5 μM was also investigated, but no difference in the fluorescence intensity within trypanosomes was identified.
(i) DB75 localization to the acidocalcisomes.
After a 2-hour incubation in vitro with S427 trypanosomes, DB75 accumulated in the nucleus, kinetoplast, and acidocalcisomes (Fig. 5, first column). However, when ammonium chloride or monensin was used as a pretreatment before DB75 was added, accumulation in the acidocalcisomes was not seen (Fig. 5, columns 2 and 3). A 60-min pretreatment of ammonium chloride was needed to prevent DB75 accumulation in acidocalcisomes, although partial inhibition of acidocalcisome localization was seen after 30 min. The sodium ionophore monensin, on the other hand, needed to be incubated with trypanosomes for only 15 min before DB75 was added to abolish acidocalcisome accumulation. Partial inhibition of accumulation was seen after 5 min. Additionally, monensin prevented acridine orange fluorescence in the acidocalcisomes of trypanosomes after only 15 min (data not shown). We were unable to use fixation and colocalization techniques due to issues with redistribution of DB75 or loss of fluorescence (30).
FIG. 5.
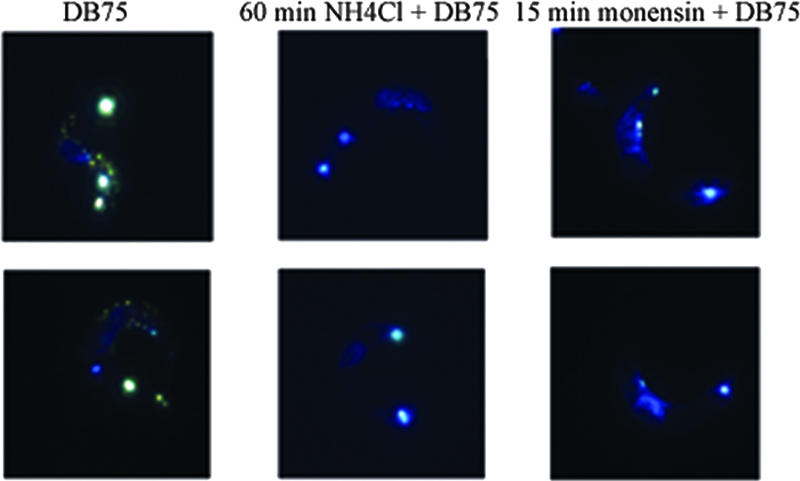
Distribution of DB75 (7.5 μM) in trypanosomes alone (first column) and after either a 60-min pretreatment of ammonium chloride (NH4Cl) or a 15-min pretreatment of monensin.
(ii) Diphenyl furans.
DB75 accumulated in the nucleus, kinetoplast, and acidocalcisomes after 8 h of in vitro incubation (Fig. 6). There were four compounds in the diphenyl furan series that had similar fluorescence spectra and relatively similar fluorescence intensities, namely, DB75, DB1017, DB244, and DB249. Compounds with similar fluorescence properties can be compared directly, but it must be considered that each compound may behave differently when accumulated in cells (i.e., fluorescence could be shifted, enhanced, or quenched upon sequestration in organelles, and this may be different for each compound). Like DB75, DB1017 appeared to be localized to the kinetoplast, nucleus, and acidocalcisomes (based on comparison of localization between DB75 and DB1017). The color shift in the acidocalcisomes with this compound was more orange, in contrast to the yellow seen in DB75-stained acidocalcisomes. DB244 and DB249 were both found only in the nucleus and kinetoplast (Fig. 6).
FIG. 6.
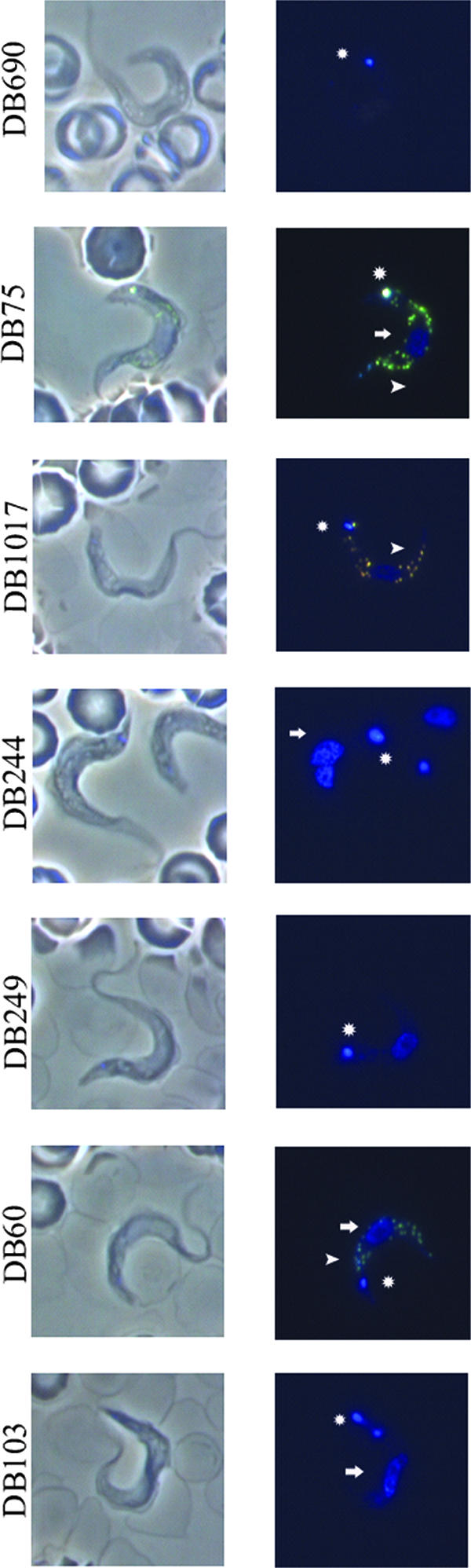
Fluorescence microscopy of the diphenyl furans (listed in order of IC50 values) at 8 h following incubation with 500 nM of each compound. Images at the top depict phase-contrast images of trypanosomes, while those at the bottom are fluorescence images of the same trypanosomes. Arrows, nucleus; circles, kinetoplast; arrowheads, acidocalcisomes.
For the rest of the diphenyl furans, the excitation and/or emission wavelengths were shifted slightly, and no direct comparisons can be made as to their relative fluorescence intensities. DB690 appeared to accumulate only in the kinetoplast, as shown in Fig. 6. However, due to the weak innate fluorescence of DB690 (Table 3), it is possible that there was some low-level fluorescence in other organelles in the trypanosomes which we were unable to observe. Additionally, the peak excitation of DB690 is below the limit of detection of the UV2A cube, and we likely did not see the compound excited at its maximal excitation wavelength. The imidazoline DB60 was localized to the kinetoplast, nucleus, and acidocalcisomes. DB103 accumulated only in the nucleus and the kinetoplast (Fig. 6).
(iii) Aza analogs.
DB820, as shown previously, was localized to the nucleus, kinetoplast, and (faintly) the acidocalcisomes (Fig. 7). DB935, the only compound with similar excitation and emission wavelengths to those of DB820, was localized to the nucleus and kinetoplast. The most potent analog, DB867, appeared in the nucleus, kinetoplast, and acidocalcisomes (Fig. 7). Despite being weakly fluorescent in solution, the compound exhibited intense fluorescence in trypanosomes, which may be related to an increase in fluorescence when bound to DNA (Table 3). DB1057 was found in the nucleus and kinetoplast, while DB829 was found in the nucleus, kinetoplast, and acidocalcisomes. DB879, the least potent of the DB820 analogs, was located very faintly in the kinetoplast. The compound is weakly fluorescent, which may affect the appearance of fluorescence seen in the trypanosome, although DB879 concentrated in trypanosomes to levels comparable to those of the other aza analogs.
FIG. 7.
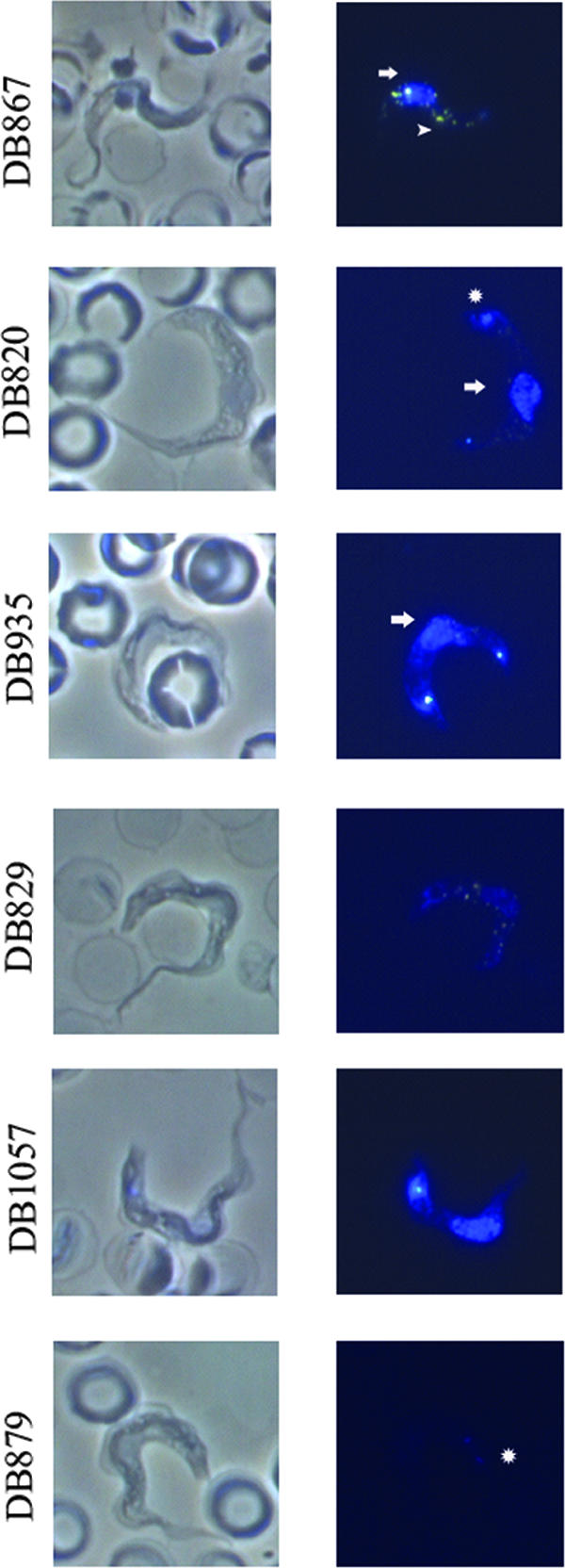
Fluorescence microscopy of the aza analogs (listed in order of IC50 values) at 8 h following incubation with 500 nM of each compound. Images at the top depict phase-contrast images of trypanosomes, while those at the bottom are fluorescence images of the same trypanosomes. Arrows, nucleus; circles, kinetoplast; arrowheads, acidocalcisomes.
DISCUSSION
Diphenyl furan and aza diphenyl furan diamidines represent two potent classes of antitrypanosomal compounds. The compounds shown here had a >150-fold range of in vitro activities, and some had different organelle distributions within the cells. However, despite the wide range of activities and distributions and, for the most part, similar DNA binding properties, the compounds accumulated to similar concentrations in the cells, with only a few exceptions.
Eight compounds in the diphenyl furan series were investigated. The substituted and cyclic diamidines were the least potent compounds in this series, and the fact that modification to the diamidine affected their potency reinforces the importance of the moiety in the potential mechanism of action of these compounds. Other modifications of DB75 had little effect on the IC50 value, changing potency only approximately twofold.
In the aza analog series, the only modification to have a major effect on potency was the methylene linker in DB879, which also decreased DNA binding, likely due to the change in curvature of the molecule. This illustrates the importance of a threshold DNA binding activity necessary for antitrypanosomal activity of these compounds.
DNA binding for the two series of compounds, as determined by measuring the ΔTm in the presence of compound, was similar for most of the compounds. With the exception of DB879, all of the compounds tested increased the melting temperature of a poly(dA)·poly(dT) complex >15°C. With DB879, the methylene linker appeared to inhibit DNA binding. Surprisingly, differing effects were seen when the fluorescence of select diamidines was measured when the compounds were bound to DNA. The least fluorescent analogs, DB690 and DB867, exhibited increased fluorescence when bound to AT-rich DNA, while more fluorescent analogs had decreased fluorescence when bound to DNA. The DNA used for this experiment was chosen because of its high AT content. However, conclusions about changes in fluorescence in the trypanosome organelles cannot be made until they are measured with isolated kinetoplast and nuclear DNA.
We previously proposed (34) that interaction with DNA alone was insufficient to generate biological activity. In one possible mechanism for biological activity of the dications, however, DNA binding would be an initial requirement but specific secondary effects, which occur subsequent to binding, would be required for activity. Such effects could be topological changes induced in DNA as a result of binding that cause errors in DNA structure, with resulting instability and eventual destruction. It is also possible that binding could result in direct inhibition of DNA-targeted enzymes or control proteins, which could also lead to cell death. These secondary effects could vary significantly among compounds with similar binding affinities for DNA and could account for the different activities of compounds with similar uptake rates and DNA affinities. The set of compounds in this paper that have similar DNA binding and uptake would make an excellent test set for testing the possible influence of secondary effects, after DNA binding, on biological activity.
Changes to the structure of the compounds also resulted in changes in the accumulation of the compounds in trypanosomes. Surprisingly, some of the more potent compounds, including DB75 and DB820, accumulated to lesser extents than did some less active compounds, such as DB244 and DB249. Additionally, in the aza analog series, all of the compounds investigated accumulated to higher concentrations in trypanosomes than that of the very potent compound DB820 (Fig. 4).
There also does not appear to be any correlation between which organelles the compounds are distributed to and overall accumulation in trypanosomes. For example, DB820, one of the most potent compounds examined, had the lowest accumulation in trypanosomes. Of the three compounds that accumulated to the highest concentrations in trypanosomes, one appeared to be localized to the kinetoplast only (DB690), while the other two were localized to the nucleus and kinetoplast (DB244 and DB249). DB690 was the most potent of the compounds tested, while DB244 and DB249 had intermediate IC50 values, yet all three accumulated to similar concentrations in trypanosomes. However, as stated previously, DB690 is a weakly fluorescent compound with a peak excitation wavelength below the limit of detection of the UV2A cube used for fluorescence microscopy. It is thus possible that we did not see the true distribution of DB690 in trypanosomes, even accounting for the enhancement of DB690 fluorescence when it was bound to AT-rich DNA (Table 3). This indicates that neither concentration nor distribution in trypanosomes can be predicted from in vitro activity. Additionally, the site of localization in trypanosomes cannot be predicted from the accumulation in the cells, or vice versa.
It is likely from these series of diamidines that DNA binding in either the nucleus or kinetoplast is important, especially considering that, for the most part, all of these compounds are excellent DNA binders. Further studies are needed to determine relative amounts of diamidines in each organelle as well as relative affinities for DNA-associated proteins found in either or both organelles. Only then can organelle-specific mechanisms of action be determined. Additionally, changes in fluorescence intensity and/or shifts in excitation or emission wavelengths may occur when compounds bind DNA, as shown in Table 3. Although the DNA used for that investigation was AT-rich, to be certain of relative fluorescence changes in the nucleus or kinetoplast, DNAs from both organelles must be isolated and binding and fluorescence changes measured. With the dramatic changes seen here, these measurements should be determined routinely in the future in order to extrapolate from DNA binding to distribution studies.
Tools used for investigating the transport and uptake of compounds in trypanosomes include rapid transport experiments with various inhibitors that prevent uptake via particular transporters. Transport of diamidines has been attributed to uptake via the P2 transporter (5, 8, 22, 29), and in the case of pentamidine, HAPT1 and LAPT1 are also involved in uptake and accumulation (4). Lanteri et al. were able to inhibit uptake of DB75 for 30 s with inhibitors of the P2 transporter, such as adenosine, adenine, pentamidine, and diminazene (21, 22), indicating that it is likely a substrate of the P2 transporter. However, transport studies with all of the compounds investigated here have not been implemented, and further investigation is needed to determine the impact of transporters on long-term accumulation. The high levels of DB690, DB244, and DB249 in trypanosomes may indicate that others transporters or transport processes are involved in the uptake of these compounds.
The effects of endogenous substrates of the transporters, such as adenosine, can be assayed in vitro in antitrypanosomal activity experiments to determine if the presence of other substrates affects the potency (i.e., increases the IC50 value) of the test compounds (13). When an IC50 value was determined for DB75 in the presence of 1 mM adenosine (a substrate of the P1 and P2 transporters), we found that the IC50 value increased 10-fold over that of controls without adenosine (data not shown). However, there was no effect seen in the presence of inosine, a substrate for the P1 transporter. This phenomenon has also been shown with the adenosine antimetabolite tubercidin in trypanosomes, where the addition of 1 mM adenosine or inosine decreased the antitrypanosomal IC50 value of tubercidin four- to fivefold (13). The decrease in potency of DB75 in the presence of excess adenosine is likely due to less DB75 getting into trypanosomes, but this should be evaluated further over time to determine if there is a significant reduction in the amount of DB75 accumulating in the trypanosomes.
Additionally, the impact of increasing the lipophilicity of compounds such as DB244 and DB249 on the transport properties should be investigated further. It is possible that other transporters or uptake processes are involved in the accumulation of the more lipophilic compounds. Is it possible that these compounds are accumulated by both passive diffusion and active transport? Both DB244 and DB249, according to calculations performed using ChemAxon MarvinSketch, are lipophilic at physiological pH (pH 7.4) and could potentially diffuse across trypanosome membranes.
We also attempted to investigate the accumulation and distribution of another compound in the diphenyl furan series, DB569. DB569 is an N-phenyl-substituted diamidine. In cancer cells, DB569 was found to be localized to the mitochondria instead of the nucleus (20). However, this compound was rapidly toxic to trypanosomes, inducing cell death in 1 h. At lower concentrations, the substituted diamidine appeared to be widely distributed throughout trypanosomes (data not shown). Increased lipophilicity could be one reason that DB569 rapidly kills trypanosomes, due to high levels accumulating in trypanosomes. The pKa of DB569 was determined to occur at pH 6.9 (average) (data not shown), indicating that at the pH of the medium (pH 7.4), over one-half of the drug was un-ionized, in contrast to <1% un-ionized drug with DB75, which has a pKa of around 11. The logD of DB569 at pH 7.4 was calculated to be 5.1 using ChemAxon MarvinSketch, indicating that the substituted diamidine is highly lipophilic at this pH and would likely diffuse across cell membranes more rapidly than unsubstituted diamidines. DB569 is an interesting diamidine, as it has very different physicochemical properties from those of other diamidines we have examined. Additionally, due to its rapid killing of trypanosomes, it may have different mechanisms of action. It is clear that more than one approach is needed to fully characterize the processes of transport and accumulation of diamidines in trypanosomes. One interesting avenue would be to investigate the long-term accumulation of diamidines in trypanosomes that lack the P2 transporter.
All of the compounds investigated in both series of diamidines were found in the nucleus, the kinetoplast, or both. A few of the compounds, with various potencies, also accumulated in the acidocalcisomes. Due to previous evidence to support DNA binding as a mechanism of action, primarily studied with pentamidine (27, 28), our original hypothesis when we began working with DB75 and DB820 was that their mechanism of action would be related to DNA binding as well. It is still not understood what impact the acidocalcisomes could have on the mechanism of action. It may be that even for this closely related group of compounds, there could potentially be two or three mechanisms of action, involving DNA in the nucleus and kinetoplast as well as some mechanism in the acidocalcisomes. As dicationic molecules, the diamidines may accumulate in the low-pH acidocalcisomes and interfere with homeostasis mechanisms in trypanosomes.
Many questions still remain about the mechanisms of action of these compounds. The lack of correlation between accumulation and activity of the compounds is intriguing. Why do some of the less active compounds accumulate to levels much higher than those of the more active compounds? There is also likely a time dependency to the amount of drug that is necessary to kill trypanosomes.
Investigations with DB569, DB75, and DB820 indicated that only about 30 min to 1 h of drug treatment is necessary to cause trypanosome death. With DB75 and DB820, death occurred 24 to 48 h after washing away the drug (data not shown), but with DB569, death occurred within 1 h, which may indicate a different mechanism of action from that of DB75 and DB820. DB569 has also been shown to cause apoptosis in Trypanosoma cruzi, to a greater extent than DB75 (10). However, no indication of the time which this process needs to take place was indicated. In vivo, DB569 resulted in increased survival of mice infected with T. cruzi and in decreased cardiac parasitism but was unable to clear parasitemia (11). Since we were unable to investigate the accumulation of DB569 in our trypanosomes, we cannot compare it completely to other substituted diamidines, such as DB244 and DB249. However, the differences in killing seen between DB569 and the other compounds in this series are very intriguing and require further investigation, especially since DB569 would appear to not be very potent in a typical in vitro screen for antitrypanosomal activity and in light of its different physicochemical properties.
In summary, the accumulation and distribution of a diphenyl furan or an aza analog in trypanosomes are not indicative of its antitrypanosomal activity. In actuality, there may be a minimum in vitro accumulation of a diamidine in trypanosomes necessary for killing. From this study, it seems that a minimum accumulation concentration of 1 mM in trypanosomes is necessary for this type of compound. DB820, the compound that accumulated the least in trypanosomes, was also one of the most potent compounds. Whether this minimum accumulation level would hold true for other series of diamidines remains to be investigated. The overall accumulation level depends on a variety of factors, including which transporters play a role in accumulation and the relative affinities for the transporters. The factors that affect transport, accumulation, and ultimately the mechanism of action will be investigated further using this series of diamidines as well as other, more structurally diverse diamidines.
Acknowledgments
We thank the Bill and Melinda Gates Foundation for financial support.
Footnotes
Published ahead of print on 21 May 2007.
REFERENCES
- 1.Boykin, D. 2002. Antimicrobial activity of the DNA minor groove binders furamidine and analogs. J. Braz. Chem. Soc. 13:763-771. [Google Scholar]
- 2.Boykin, D. W., A. Kumar, J. Spychala, M. Zhou, R. J. Lombardy, W. D. Wilson, C. C. Dykstra, S. K. Jones, J. E. Hall, R. R. Tidwell, et al. 1995. Dicationic diarylfurans as anti-Pneumocystis carinii agents. J. Med. Chem. 38:912-916. [DOI] [PubMed] [Google Scholar]
- 3.Boykin, D. W., A. Kumar, G. Xiao, W. D. Wilson, B. C. Bender, D. R. McCurdy, J. E. Hall, and R. R. Tidwell. 1998. 2,5-Bis[4-(N-alkylamidino)phenyl]furans as anti-Pneumocystis carinii agents. J. Med. Chem. 41:124-129. [DOI] [PubMed] [Google Scholar]
- 4.Bray, P. G., M. P. Barrett, S. A. Ward, and H. P. de Koning. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 19:232-239. [DOI] [PubMed] [Google Scholar]
- 5.Carter, N. S., B. J. Berger, and A. H. Fairlamb. 1995. Uptake of diamidine drugs by the P2 nucleoside transporter in melarsen-sensitive and -resistant Trypanosoma brucei brucei. J. Biol. Chem. 270:28153-28157. [DOI] [PubMed] [Google Scholar]
- 6.Croft, S. L., M. P. Barrett, and J. A. Urbina. 2005. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 21:508-512. [DOI] [PubMed] [Google Scholar]
- 7.Das, B. P., and D. W. Boykin. 1977. Synthesis and antiprotozoal activity of 2,5-bis(4-guanylphenyl)furans. J. Med. Chem. 20:531-536. [DOI] [PubMed] [Google Scholar]
- 8.de Koning, H. P. 2001. Transporters in African trypanosomes: role in drug action and resistance. Int. J. Parasitol. 31:512-522. [DOI] [PubMed] [Google Scholar]
- 9.de Koning, H. P., L. F. Anderson, M. Stewart, R. J. Burchmore, L. J. Wallace, and M. P. Barrett. 2004. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 48:1515-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Souza, E. M., R. Menna-Barreto, T. C. Araujo-Jorge, A. Kumar, Q. Hu, D. W. Boykin, and M. N. Soeiro. 2006. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology 133:75-79. [DOI] [PubMed] [Google Scholar]
- 11.de Souza, E. M., G. M. Oliveira, D. W. Boykin, A. Kumar, Q. Hu, and N. Soeiro Mde. 2006. Trypanocidal activity of the phenyl-substituted analogue of furamidine DB569 against Trypanosoma cruzi infection in vivo. J. Antimicrob. Chemother. 58:610-614. [DOI] [PubMed] [Google Scholar]
- 12.Docampo, R., and S. N. Moreno. 1999. Acidocalcisome: a novel Ca2+ storage compartment in trypanosomatids and apicomplexan parasites. Parasitol. Today 15:443-448. [DOI] [PubMed] [Google Scholar]
- 13.Geiser, F., A. Luscher, H. P. de Koning, T. Seebeck, and P. Maser. 2005. Molecular pharmacology of adenosine transport in Trypanosoma brucei: P1/P2 revisited. Mol. Pharmacol. 68:589-595. [DOI] [PubMed] [Google Scholar]
- 14.Ismail, M. A., R. K. Arafa, R. Brun, T. Wenzler, Y. Miao, W. D. Wilson, C. Generaux, A. Bridges, J. E. Hall, and D. W. Boykin. 2006. Synthesis, DNA affinity, and antiprotozoal activity of linear dications: terphenyl diamidines and analogues. J. Med. Chem. 49:5324-5332. [DOI] [PubMed] [Google Scholar]
- 15.Ismail, M. A., A. Batista-Parra, Y. Miao, W. D. Wilson, T. Wenzler, R. Brun, and D. W. Boykin. 2005. Dicationic near-linear biphenyl benzimidazole derivatives as DNA-targeted antiprotozoal agents. Bioorg. Med. Chem. 13:6718-6726. [DOI] [PubMed] [Google Scholar]
- 16.Ismail, M. A., R. Brun, J. D. Easterbrook, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2003. Synthesis and antiprotozoal activity of aza-analogues of furamidine. J. Med. Chem. 46:4761-4769. [DOI] [PubMed] [Google Scholar]
- 17.Ismail, M. A., R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2004. Dicationic biphenyl benzimidazole derivatives as antiprotozoal agents. Bioorg. Med. Chem. 12:5405-5413. [DOI] [PubMed] [Google Scholar]
- 18.Ismail, M. A., R. Brun, T. Wenzler, F. A. Tanious, W. D. Wilson, and D. W. Boykin. 2004. Novel dicationic imidazo[1,2-a]pyridines and 5,6,7,8-tetrahydro-imidazo[1,2-a]pyridines as antiprotozoal agents. J. Med. Chem. 47:3658-3664. [DOI] [PubMed] [Google Scholar]
- 19.Lansiaux, A., L. Dassonneville, M. Facompre, A. Kumar, C. E. Stephens, M. Bajic, F. Tanious, W. D. Wilson, D. W. Boykin, and C. Bailly. 2002. Distribution of furamidine analogues in tumor cells: influence of the number of positive charges. J. Med. Chem. 45:1994-2002. [DOI] [PubMed] [Google Scholar]
- 20.Lansiaux, A., F. Tanious, Z. Mishal, L. Dassonneville, A. Kumar, C. E. Stephens, Q. Hu, W. D. Wilson, D. W. Boykin, and C. Bailly. 2002. Distribution of furamidine analogues in tumor cells: targeting of the nucleus or mitochondria depending on the amidine substitution. Cancer Res. 62:7219-7229. [PubMed] [Google Scholar]
- 21.Lanteri, C. 2005. Mechanisms of uptake and action of DB75 [2,5-bis(4-amidinophenyl)furan] in African trypanosomes. Ph.D. dissertation. University of North Carolina, Chapel Hill.
- 22.Lanteri, C. A., M. L. Stewart, J. M. Brock, V. P. Alibu, S. R. Meshnick, R. R. Tidwell, and M. P. Barrett. 2006. Roles for the Trypanosoma brucei P2 transporter in DB75 uptake and resistance. Mol. Pharmacol. 70:1585-1592. [DOI] [PubMed] [Google Scholar]
- 23.Mallena, S., M. P. Lee, C. Bailly, S. Neidle, A. Kumar, D. W. Boykin, and W. D. Wilson. 2004. Thiophene-based diamidine forms a “super” at binding minor groove agent. J. Am. Chem. Soc. 126:13659-13669. [DOI] [PubMed] [Google Scholar]
- 24.Mathis, A. M., J. L. Holman, L. M. Sturk, M. A. Ismail, D. W. Boykin, R. R. Tidwell, and J. E. Hall. 2006. Accumulation and intracellular distribution of antitrypanosomal diamidine compounds DB75 and DB820 in African trypanosomes. Antimicrob. Agents Chemother. 50:2185-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peregrine, A. S., and M. Mamman. 1993. Pharmacology of diminazene: a review. Acta Trop. 54:185-203. [DOI] [PubMed] [Google Scholar]
- 26.Raz, B., M. Iten, Y. Grether-Buhler, R. Kaminsky, and R. Brun. 1997. The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop. 68:139-147. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro, T. A., and P. T. Englund. 1990. Selective cleavage of kinetoplast DNA minicircles promoted by antitrypanosomal drugs. Proc. Natl. Acad. Sci. USA 87:950-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shapiro, T. A., V. A. Klein, and P. T. Englund. 1989. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J. Biol. Chem. 264:4173-4178. [PubMed] [Google Scholar]
- 29.Stewart, M. L., S. Krishna, R. J. Burchmore, R. Brun, H. P. de Koning, D. W. Boykin, R. R. Tidwell, J. E. Hall, and M. P. Barrett. 2005. Detection of arsenical drug resistance in Trypanosoma brucei with a simple fluorescence test. Lancet 366:486-487. [DOI] [PubMed] [Google Scholar]
- 30.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75) and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 91:131-143. [DOI] [PubMed] [Google Scholar]
- 31.Tijssen, J. P., H. W. Beekes, and J. Van Steveninck. 1982. Localization of polyphosphates in Saccharomyces fragilis, as revealed by 4′,6-diamidino-2-phenylindole fluorescence. Biochim. Biophys. Acta 721:394-398. [DOI] [PubMed] [Google Scholar]
- 32.Werbovetz, K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147-157. [PubMed] [Google Scholar]
- 33.Williamson, J. 1979. Effects of trypanocides on the fine structure of target organisms. Pharmacol. Ther. 7:445-512. [DOI] [PubMed] [Google Scholar]
- 34.Wilson, W. D., B. Nguyen, F. Tanious, A. Mathis, J. E. Hall, C. Stephens, and D. W. Boykin. 2005. Dications that target the DNA minor groove: compound design and preparation, DNA interactions, cellular distribution, and biological activity. Curr. Med. Chem. Anticancer Agents 5:389-408. [DOI] [PubMed] [Google Scholar]
- 35.Wilson, W. D., F. A. Tanious, M. Fernandez-Saiz, and C. T. Rigl. 1997. Evaluation of drug-nucleic acid interactions by thermal melting curves. Methods Mol. Biol. 90:219-240. [DOI] [PubMed] [Google Scholar]