One of the most severe and widespread problems facing the agricultural industry is the degradation of soil quality due to desiccation and salinity. In fact, almost 40% of the world's land surface is affected by salinity-related problems (131). These two harsh environmental conditions can have a dramatic impact on the endogenous soil bacteria (38, 48). Of particular importance to the agricultural industry is the impact of these harsh environmental conditions on the endogenous group of proteobacteria, the rhizobia. These bacteria induce formation of nodules on legumes, in which atmospheric nitrogen is fixed and supplied to the plant, enhancing growth under nitrogen-limiting conditions. Desiccation and salinity negatively affect such interactions by limiting nitrogen fixation (131).
The importance of nitrogen fixation for agriculture cannot be understated and is illustrated by the numerous studies of the impact of soil management on rhizobial populations in arid regions (52), as well as the isolation and characterization of desiccation- and salt-resistant strains (28, 56, 128). Furthermore, to enhance nodulation and nitrogen fixation efficiency, techniques that allow close contact between the bacteria and the host seed have been developed. Despite such methods, there has been a decline in the number of viable bacteria on plant seeds, soil, and carrier material, in part because of the stresses caused by fertilizer and chemical applications (110), desiccation (100, 101), temperature changes (63, 74, 116), salinity changes (63), light (68), and growth media employed (23, 35, 63). These factors are encountered during the manufacture, storage, and use of the coated seeds, with desiccation as the principal cause of reduced bacterial survival on the seed (33, 119).
Although improvement of long-term survival and seed inoculum storage time has been the focus of desiccation research (25, 33), relatively little work has focused primarily on the bacterial cell. Many questions remain regarding the physiological response of rhizobia to desiccation. In this review, we evaluate studies of the physiological responses of rhizobia to environmental stresses (osmotic, salt, temperature, and oxygen) that affect desiccation survival. Our discussion will focus on one species of rhizobia in particular, Sinorhizobium meliloti.
DESICCATION RESPONSES OF RHIZOBIA
Early rhizobial desiccation research.
Desiccation produces many stress responses in the bacterial cell. In 1932, Fred and coworkers reported loss of viability in rhizobia used as seed inocula (41) and suggested that the nature of the suspending medium, pH, and temperature are important factors in the survival of the inoculum in the dry state. This led to the recommendation for farmers to refrain from using rhizobia in dry form. Vincent et al. (119) showed that the decline in abundance of Rhizobium trifolii during drying on glass beads correlates with the extraction of water, which suggests that part of the decline in viable counts is caused by both “seed factors” and the drying itself. The negative effect of drying can be partially countered by the addition of maltose, indicating that the availability of nutrients, and perhaps other solutes as well, affects survival. Since these early observations, progress in understanding survival during desiccation stress of rhizobia has been slow and limited to testing the ability of selected strains to survive under controlled conditions. This slow progress is probably caused by the complexity of the response to desiccation stress.
Desiccation stress.
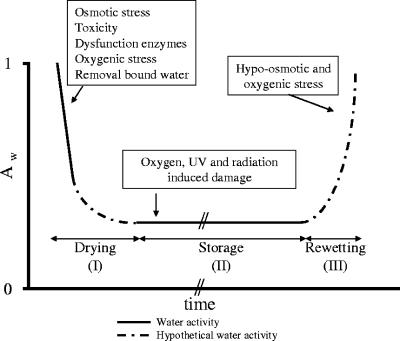
The ability of microorganisms, including rhizobia, to survive desiccation depends on their ability to cope with radiation stresses, reactive oxygen species (ROS), certain salts and solutes, and temperature extremes (6, 7, 33, 94, 96, 123). Desiccation stress can be differentiated into three main phases: drying (phase I), storage (phase II) and rewetting (stage III) (Fig. 1). These phases can be manipulated in several ways, namely, by the severity and the speed of drying and rewetting and by the duration of storage. The consequences of drying are fourfold: (i) the accumulation of salts and solutes, (ii) hyperosmotic stresses, (iii) the impairment of metabolism when a certain water activity has been reached, and (iv) the accumulation of damage when the aqueous monolayer is removed from macromolecules. The accumulation of damage during storage is comparable to that caused by ionizing and UV radiation and damage by ROS (79, 105) when organisms are not metabolically active and thus unable to repair any damage (94). Finally, during rewetting, hypoosmotic stresses and the appearance of ROS affect survival (111).
FIG. 1.
Relationship between water activity (Aw) and time during desiccation. Three main phases are recognized: drying (phase I), storage (phase II), and rewetting (phase III). The recognized variables are as follows: for drying, slow or fast and severe or mild; for storage, long or short; and for rewetting, slow or fast (after reference 94). The potential stresses that apply during these phases are as indicated.
(i) Drying (phase I).
Drying rates have profound effects on bacterial survival. Bushby and Marshall (21) and Antheunissen and Arkestein-Dijksman (1) observed a rapid decline and low survival of rhizobia when drying was rapid. Sleesman and Leben (107) showed that when bacteria are slowly dried, their survival is higher and is biphasic. Fast drying was achieved in force-dry ovens, while slow drying was achieved by exposure to air. These results were confirmed by Mary et al. (77, 78), who used S. meliloti RCR2011 to test survival after slow and fast drying with and without the addition of salts. Chao and Alexander (26) showed that fast drying leads to a decrease in survival in mineral soil. In conclusion, the increase in survival during slow drying suggests that physiological responses to dry conditions may take place during the drying process.
The extraction of water leads to the accumulation of salts and other compounds that cause osmotic and salt stress. These compounds can reach toxic levels, leading to a decrease in viability (e.g., with NaCl stress [112, 121]). Conversely, the accumulation of certain compounds, including osmoprotectants and compatible solutes, may increase desiccation survival (39, 44-47, 71). When water activity declines to below 0.53, RNA polymerase ceases to function and metabolism stalls (19). At this point, only a monolayer of water surrounds the molecules, and further extraction of water induces damages that accumulate until after rewetting (19).
(ii) Storage (phase II).
When the water phase in bacteria reaches equilibrium with that of the surrounding gas phase, further extraction of water halts, and the storage phase is initiated. The storage phase is characterized by a slow decline in viable counts in rhizobia after slow drying. Mary et al. (75, 78) observed better survival of Sinorhizobium during storage under desiccation conditions from 22% to 67% relative humidity (RH) than at 3% and 83.5% RH. The same patterns have been observed, but to a lesser extent, in Bradyrhizobium (14, 75) and also in Escherichia coli (4) and Azospirillum (89). The mechanisms causing these results remain unexplained. Dysfunction of intracellular enzymes has been proposed to be responsible for cell death at 83% RH. Antheunissen et al. (2) showed that when dried slowly, rhizobia can survive desiccation for up to 4 years. These long-term storage studies are rare, but they show that in the family Rhizobiaceae, sinorhizobia can survive desiccation for years. A decline in viable cells during long-term storage under desiccation conditions can be explained by the accumulation of oxygen- and radiation-induced damage (3, 68, 76, 79, 119).
(iii) Rewetting (phase III).
After rewetting, when bacterial metabolism restarts, accumulated damage is repaired. The rate of rewetting has important consequences for survival. Fast rewetting leads to disruption of the cell at the subpolar regions (supposedly where the flagella emerge through the cell envelope) and results in cell death (20, 101). Kosanke et al. (62) showed that slow rewetting results in higher survival rates of S. meliloti, Rhizobium leguminosarum, and Pseudomonas putida. Also, an oxygenic burst takes place in Campylobacter spp. upon rewetting (111), which has not been reported for rhizobia.
PHYSIOLOGICAL RESPONSES OF RHIZOBIA TO ENVIRONMENTAL CONDITIONS THAT AFFECT SURVIVAL DURING DESICCATION
Effect of osmotic and salt stress on the response of rhizobia to desiccation.
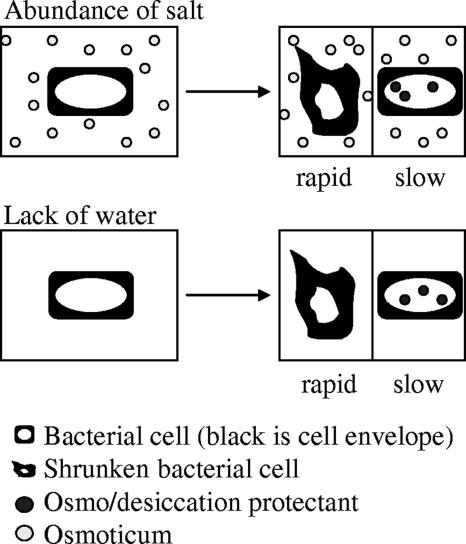
Osmotic and desiccation stress are very different. Osmotic stress is the abundance of solutes, while desiccation stress results from the lack of water (Fig. 2). However, existing data indicate a relationship between these two stresses. Chen and Alexander (27) related the growth of soil isolates at low water activity to their ability to survive desiccation. They found several soil isolates that, when pregrown in medium at low water activity, showed increased survival during desiccation. One such strain was a Rhizobium strain. These researchers used a mixture of NaCl, KCl, and Na2SO4 (5:3:2) to lower the water activity.
FIG. 2.
Hypothetical physiological responses to desiccation and to osmotic stress. An overlap in response to both stresses may exist in Sinorhizobium (after reference 94) but is unlikely to be limited to only the accumulation of desiccation protectants.
The presence of NaCl decreases survival during desiccation of rhizobia in soil, in seed inocula, and in vitro when cells are resuspended in water containing 400 mM NaCl prior to drying (63, 112, 121). However, data obtained from experiments performed with exposure to NaCl in medium indicate otherwise. Mary et al. (77), found a 5.5-fold increase in survival during desiccation of S. meliloti strain RCR2011 when it was grown in the presence of 530 mM NaCl in YMB medium. In the same experiment, such a response was not observed using S. meliloti strain 1.5. An increase in survival of S. meliloti RCR2011 was observed in the presence of LiCl. Furthermore, desiccation survival of Sinorhizobium meliloti 1021 during exposure to 400 mM NaCl or KCl in medium (YMB or PMM) increases compared to that in medium alone (121). These observations indicate that (i) chloride stress induces a response in combination with nutrients from the medium, (ii) the response is strain specific, and (iii) the increase in survival during NaCl-mediated desiccation is unlikely to be caused by external protection by solutes but is physiological in origin. Hence, the inducing stress leading to this increase is not clear and may be a combination of chloride, salt, osmotic, or water activity stress in combination with other compounds found in the medium used. This is illustrated by data presented by Vriezen et al. (121), who showed that the addition of sulfate salts prior to drying leads to an elevated survival compared to that after addition of chloride salts, despite a decrease in osmotic tension of the medium. These data imply that ionic strength and toxicity induce differential physiological responses to the several salts and are more important for survival than osmotic pressure or water activity. In summary, an overlap in the response to osmotic and salt stress and the ability to survive desiccation exists, as is depicted in Fig. 2.
General responses of rhizobia to NaCl.
Rhizobium strains differ in their ability to respond to an increase in osmotic pressure and salt stress. A generalized model can be derived from several studies and is similar to the response of enteric bacteria (81, 123-125). After an osmotic upshift, general metabolism slows (34). This is illustrated by the findings of Dominguez-Ferreras et al. (34), who reported that genes involved in the tricarboxylic acid cycle, in the uptake of a carbon source (they used mannitol), and in respiratory chains and ribosomal genes are repressed. Interestingly, 25% of all genes specifically downregulated by NaCl encode ribosomal proteins.
Rhizobia accumulate potassium ions (13, 129), for which no new protein synthesis is required. This suggests that K+ uptake is regulated biochemically and used as a secondary messenger. Nogales et al. (83) reported a high-affinity K+ uptake (Kup) system in Rhizobium tropici that has a homolog in S. meliloti 1021 (SMa1798), while a second, low-affinity Kup system can be identified (SMc00873), as well as the osmosensitive Kdp system (SMa2329, -2331, and -2333). BetS is a betaine/proline transporter also involved in the early response to osmotic stress. As with K+ uptake activity, BetS is regulated biochemically (11, 88, 93).
Under growth-limiting conditions, C sources accumulate in the form of glycogen, which may assist in restoring cell volume after osmotic shock (49). This is supported by the finding that glgA2, glgB2, and glgX, genes involved in glycogen metabolism (SMb20704, SMb21447, and SMb21446, respectively), are expressed at higher levels during exposure to osmotic stress, an indication that glycogen accumulates during osmotic stress. However, the accumulation of glycogen may also be a response to prevent starvation (34).
After these initial reactions, stressed cells accumulate compatible solutes, and uptake is preferred over synthesis. Compatible solutes include carbohydrates, disaccharides such as sucrose and trehalose (15, 17, 46, 81), maltose, cellobiose, turanose, gentiobiose, palatinose (46), and amino acids, of which mainly glutamate and proline accumulate, although many genetic mechanisms involved in amino acid uptake are downregulated (12, 13, 34, 53, 99). Furthermore, imino acids (e.g., pipecolate) (44), ectoin (114, 115), glycine betaine and stachydrin (9, 39, 91, 108), N-acetylglutaminylglutamine amide (109), and dimethylsulfoniopropionate (92) accumulate. Not all compounds are taken up from the medium when available, but some are synthesized de novo, for example, sucrose and trehalose (47, 81). It has been argued, however, that trehalose and glycine betaine are accumulated to prevent starvation rather than to function as osmotic stabilizers (85).
Finally, osmotically stressed cells alter macromolecular structures, including long-chain exopolysaccharides (EPS) (17, 29, 70) and lipopolysaccharides (LPS) (5, 24, 69).
The observation that the response to NaCl is more complex than the adaptations and responses mentioned above is a result of the studies performed by Wei et al. (122) and Miller-Williams et al. (82). Those authors identified Sinorhizobium mutants that are unable to grow at increased NaCl concentrations. The mutations could be traced to genes potentially involved in the central metabolism, such as elongation factors, DNA ligases, chaperones, and cell division proteins. Some of these loci were repressed at increased NaCl concentrations. These include the nuo operon (SMc01925), which is involved in cation efflux (34); tig (SMc02050), encoding a chaperone; and genes involved in cell division (ltsE). Furthermore, genes for DNA ligases were found to be expressed at a higher level, as were genes for a putative DNA polymerase, an invertase, and an RNase (34). This additional layer of complexity was only recently recognized, although it is not surprising considering that these genes are involved in DNA replication and cell division when growth resumes after an osmotic upshift.
One observation worth mentioning is the replicon bias of the osmotic response. Genomic analyses revealed that 64% of all upregulated genes are located on megaplasmids, and most of those genes are located on pSymB (34). This plasmid contains many genes that are otherwise found only in nonrelated microorganisms (127). Wong and Golding (127) have shown that approximately 13% of genes located on pSymB have been subject to lateral gene transfer, including some that are upregulated by NaCl stress.
Responses to salt stress potentially leading to an increase in survival during desiccation.
Although the response of S. meliloti to NaCl does increase its ability to survive desiccation, the question remains which responses to NaCl are involved in survival during desiccation. Vriezen et al. (121) showed that the response to NaCl-mediated survival during desiccation is more dependent on Cl− and SO42− than on Na+, K+, or Li+ (77, 121). A chloride- and sulfate-responsive gene has been identified (120); however, it is not involved in survival during desiccation. In the presence of Na+, survival is greater than in the presence of K+, indicating that Na+-responsive genes are involved, such as metH and the pha operon identified in Sinorhizobium fredii (58). This does not mean that K+-responsive genes are not involved in desiccation; however, these are not of major importance in NaCl-mediated desiccation survival.
The accumulation of osmoprotectants and compatible solutes may also increase survival during desiccation. For example, betaine increases survival of Rhizobium during desiccation in peat cultures (63) and reduces the negative effects of NaCl that are observed under certain conditions (65, 104). Sixty percent of the betaine accumulation during osmotic stress takes place through the betaine transporter BetS (11), making this locus especially interesting in understanding the early responses to NaCl and desiccation survival. Genetic mechanisms that support the accumulation of betaines have been identified, including a betaine/choline uptake or synthesis operon (SMc00093 to -00095 and SMc00127) as well as betP (SMb20333) and betB2 (SMa1731).
Trehalose accumulates in stressed bacteria (66, 94) and in particular in osmostressed rhizobia (15, 17, 113). Trehalose provides protection against desiccation by maintaining membrane integrity during drying and rewetting (66, 67), and its presence may explain the increase in desiccation survival during the stationary phase and when the cell is exposed to NaCl. Gouffi et al. (45-47) found that trehalose and sucrose are not accumulated from the medium but are synthesized de novo during early exponential growth. Furthermore, sucrose accumulation contributes indirectly to glutamate and N-acetylglutaminylglutamine amide accumulation. Uptake mechanisms have also been described; an agl operon for trehalose/maltose and sucrose uptake (SMb03060 to-03065) was identified by Willis and Walker (126), and Jensen et al. (57) identified an alternative trehalose/maltose/sucrose operon (thu) (SMb20324 to-20330). Using microarrays, it was shown that the thu operon is upregulated during an osmotic upshift (34) and may provide resistance during NaCl-mediated survival during desiccation.
Bushby and Marshall (20) have shown that S. meliloti SU47 leaks cell constituents upon rewetting, indicating that cell wall structure may be a weakness in survival during desiccation upon rewetting. This was emphasized by Salema et al. (101), who showed that the cell wall of Rhizobium leguminosarum broke upon rewetting at places where the flagella emerge, indicating a weak point in the cell wall, and Feng et al. (37) observed structural changes of the cell wall when cells were dried and stored in peat. However, Dominguez-Ferreras et al. (34) showed that the murACG operon, which is involved in cell wall synthesis, is downregulated when exposed to osmotic and NaCl stress. Thus, other loci involved in cell wall metabolism can be expected to be involved in desiccation survival. Interestingly, Vriezen (120) identified an S. meliloti 1021 Tn5luxAB transcriptional fusion that was sensitive to survival during desiccation. The Tn5luxAB transposon inserted in an NaCl-inducible putative open reading frame encoding a d-Ala-d-Ala ligase domain, which further supports a role for the cell wall in survival during desiccation.
Polysaccharides and NaCl-mediated survival during desiccation.
Polysaccharides are of interest with respect to desiccation, since adaptations of the polysaccharide composition have been observed for S. meliloti undergoing osmotic stress (17, 70) and are known to affect survival during dry conditions (30). However, EPS do not play a primary role in rhizobial survival during desiccation, since contradictory results have been obtained. For example, Mary et al. (77) showed a decrease in survival of rhizobia upon the addition of polysaccharides when dried at an RH of >3% but an increase in survival at 3% RH. When survivals of polysaccharide-producing variants of Rhizobium trifolii during desiccation in sandy soil and under fast-drying conditions were compared, no consistent improvement in survival was observed (22). Osa-Afiana and Alexander (86) showed that the production of EPS decreases survival during desiccation of Bradyrhizobium japonicum strains when they are dried slowly in Collamer silt loam. In contrast, Pena-Cabrialis and Alexander (90) showed that polysaccharides do increase survival of R. trifolii 412 slightly in a Lima silt loam.
The mechanisms by which polysaccharides provide protection are not known. However, specific properties of polysaccharides have different effects on a microorganism's ability to survive desiccation, of which we mention three (lists are presented by Potts [94] and Rinaudo [98]): (i) exclusion of toxic compounds, such as Cl− and O2 (for example, cyclic glucans sequester antibiotics [72], and the ability of polysaccharides to form glasses under dry conditions may limit O2 diffusion [94]); (ii) the final water content of polysaccharides under ambient conditions, which can expose cells to detrimental conditions of intermediate water content, leading to low survival; and (iii) the water retention isoterms of polysaccharides, where the effect of hysteresis will lead to a high water retention in the beginning stages of drying but will quickly lead to low water retention when drying proceeds. This leads to a short exposure to conditions of intermediate water activity when the water content is detrimental for the cells, leading to higher survival.
S. meliloti produces several polysaccharides which are important in the process of root colonization, host-microbe interaction, and nodule formation and have been well studied (18). In S. meliloti the cyclic glucans are produced by NdvA and NdvB (16, 54). However, production of these glucans decreases with increasing osmolarity, and ndvA and ndvB expression is downregulated when osmolarity increases (8, 34); these are unlikely to play a role in NaCl-mediated survival during desiccation.
EPS produced by S. meliloti can be divided into two groups: EPSI, which are succinoglycans, and EPSII, which are galactoglucans. Both classes of EPS can be divided into low-molecular-weight (LMW) and high-molecular-weight (HMW) EPS. EPSII is produced mainly under phosphate-limiting conditions (80, 132). However, the media used by Vriezen et al. (121) are not low in phosphates, and thus it is unlikely that EPSII production is the cause of the observed increase in survival during desiccation of S. meliloti when these media are used. An increase in osmotic pressure results in enhanced production of HMW succinoglycan over LMW succinoglycan (17). These observations suggest that NaCl-dependent production of EPS in S. meliloti will lead to the production of HMW succinoglycan and consequently to an increase in survival during desiccation. Ruberg et al. (99) showed that the expression of genes involved in EPSI production is upregulated during salt stress. Furthermore, Rinaudo (98) has shown that the structural conformation of succinoglycan is almost independent of the ionic concentration, which means that possible protection by succinoglycan during NaCl-mediated desiccation depends on the presence or absence of HMW succinoglycan but not on the external milieu, supporting the findings by Mary et al. (77).
Although Dominguez-Ferreras et al. (34) found that an initial response to NaCl resulted in the induction of genes producing LMW succinoglycan, we predict that genes involved in the production of HMW succinoglycan would positively affect survival, while enzymes involved in the production of LMW succinoglycan would have a negative affect. ExoP (ExoP1 [SMb20961] and ExoP2 [SMb21070]) was found to block polymerization, and ExoQ (SMb20944) is required for the production of HMW succinoglycan (43). Theoretically, null mutations in these two genes would disrupt HMW succinoglycan production and consequently lower survival. Interestingly, depolymerization of HMW leads to the production of LMW succinoglycan, which is ExoK (SMb20955) and ExsH (SMb20932) mediated (130). Null mutations in these genes would limit LMW succinoglycan production and accumulation of HMW succinoglucan.
LPS subunits fall within two groups: (i) complex macromolecules consisting of oligosaccharides called the O antigen and (ii) core oligosaccharide and lipid A. Structural changes under the influence of osmotic and salt stress have been reported by Bhattacharya and Das (5) and Llorett et al. (69). Interestingly, Llorett et al. (69) found different LPS contents in cells of strain EFB1 grown in different salts. Furthermore, polyethylene glycol 200, which causes only osmotic stress, does not induce such a change. This differential response to NaCl, KCl, and Na2SO4, and the lack of a response to osmotic stress, may correlate with the differences in survival during desiccation when cells are exposed to these salts (121) and argues for a potential role of LPS in survival during desiccation. Campbell et al. (24) found many loci involved in the synthesis of LPS; however, only rkpK (SMc02641) is responsive to an increase in NaCl (34). rkpK is induced by osmotic and NaCl stress, is involved in capsular polysaccharides, and is required for the formation of UDP-galacturonic acid, which is used in LPS synthesis. A second locus involved in cell surface characteristics is the stationary-phase regulator CbrA (SMc00776) (42). Although cbrA mutants overproduce LMW succinoglycan, they also display an increased expression of the exo genes, which may lead to an increased ability to survive desiccation.
Response to and impact of temperature on survival during desiccation.
Theoretically, temperature is involved in survival during desiccation through the phase change of membranes during drying and rewetting, leading to the loss of membrane integrity (66, 67). The logical consequences of this process would be that an increase in drying temperature prevents membrane transition, positively affecting survival during desiccation. Vriezen et al. (121) used strain S. meliloti USDA 1021 to test survival during desiccation under a temperature regimen. In these experiments, a positive correlation between survival and temperature was observed, with an optimum at 37°C. These results illuminate the importance not only of a physical factor, such as membrane integrity, but also of a physiological response, e.g., the production of heat shock proteins and chaperones, or the accumulation of compounds, that decrease the membrane midpoint transition temperature, such as trehalose (66, 67).
However, in situ conditions do not support the in vitro observations. Hartel and Alexander (50) found that under drying conditions (1% [wt/wt] moisture) bradyrhizobia survived desiccation to a lesser extent at 37°C than at 30°C. Similar results were found by multiple investigators (10, 26, 31, 32, 36, 40, 59, 63, 64, 107, 117, 118). These inconsistencies can be explained by the influence of the carrier material, the temperatures chosen, and the strains and drying methods employed. Thus, at least one additional factor must exist; for example, matrices (e.g., soil) apply an unknown yet overruling stress to dry cells. One potential cause of the observed differences is that dry seed inocula have a water activity of 0.45 to 0.6 (110) and thus still contain a relatively large amount of water. Additionally, isolated rhizobia show large differences in their ability to respond and adapt to life at high temperature; however, this is not linked to their ability to survive desiccation (116). Thus, identification of heat-tolerant strains may not enhance survival during desiccation, unless temperature, rather than drought, is the selective stress. These observations demonstrate the need to study the physiological response of the cell during desiccation, independent of the matrix (121). Although not necessarily relevant for the understanding of the effect of temperature on survival during desiccation in soil, peat, and seed inocula, being able to discriminate between the physiological conditions of the cell and the stresses imposed by matrices allows new hypotheses for testing those factors that are important in survival during desiccation in or on these matrices.
The identification by Rehman and Nautiyal (97) of a Rhizobium sesbania Tn5 mutant sensitive to drought and temperature reveals a genetic basis for this response. The question remains whether loci responsive to NaCl include loci responsive to an increase in temperature, which are also important in survival during desiccation. Dominguez-Ferreras et al. (34) identified several loci responsive to an increase in osmotic and salinity stress that were also associated with the temperature response. Genes groESL1 (SMc00912 to SMc00913) and groESL2 (SMa0744 to SMa0745) were repressed by osmotic and salinity stress, which can be explained by a reduction in protein synthesis during these conditions. Dominguez-Ferreras et al. (34) found that clpB (SMc02433), degP1 to -4 (degP1, SMc02365; degP2, SMc01438; degP3, SMc01280; and degP4, SMa1128), and ibpA (SMc04040) were upregulated during osmotic and salt stress. clpB encodes a protease dissolving inactive protein aggregates that accumulate during stress. Expression of clpB is also upregulated by NaCl and osmotic stress in Synechocystis (60). ibpA is a heat shock protein that functions as a chaperone in association with ibpB; however, S. meliloti does not contain ibpB.
DegP is a protease/chaperonin that confers resistance to oxygenic stress and elevated temperature (reviewed by Raivio [95]), two stresses involved in survival during desiccation of rhizobia. The DegP1 amino acid sequence is the most similar to DegP in E. coli K-12 (NP_414703.1) and thus would be an appropriate candidate to study NaCl-mediated, and consequently the importance in temperature-mediated, survival during desiccation.
Oxygen affects survival of rhizobia.
Radical oxygen species are by-products of the electron transport chain. The coordinated downregulation of metabolism can prevent the production of oxygen radicals and consequently damage caused by ROS. This coordinated response has been observed in desiccation-tolerant plants (e.g., Zea mays) (84) and in cyanobacteria (61). Data on NaCl-mediated gene expression using microarrays show that metabolism slows during the early phases of osmotic stress (34, 99). However, it is not known whether rhizobia apply such approaches to cope with oxygenic stress during the dry state.
Evidence shows that oxygen (or ROS)-induced damages affect survival of dried rhizobial cells, since rhizobia are sensitive to O2 during desiccation (76, 87, 119). According to Deaker et al. (33), oxygen becomes toxic to rhizobia when the RH drops below 70%. Oxygen also lowers survival of rhizobia (87) in lime silt loam when the water content drops below 40%. Mary et al. (76) showed a 10- to 100-fold decrease in viable counts of B. japonicum 3.2 SM and 3.32 SM in the presence of air compared to an N2 atmosphere in vitro at 3% RH. Thus, oxygen reduces survival of rhizobia in the dry state only when the RH has declined below a certain threshold. This suggests an active protective mechanism in dried cells at a water activity at which the metabolism is still functional.
Several strategies to prevent damage by ROS and oxygen during desiccation have been reported for bacteria. For example, glasses formed by polysaccharides exclude oxygen. Oxygen radical-scavenging enzymes such as catalase (KatA) and superoxide dismutase (SodA) in Deinococcus radiodurans are important in the reduction of damage caused by ionizing radiation (73). An Fe superoxide dismutase and sodF mRNA in desiccated Nostoc commune (105) remained active after years of desiccation. Interestingly, rhizobia dried in peat accumulate a manganese superoxide dismutase (37), illustrating a genetic basis for active mechanisms to protect against oxygenic damages in rhizobia in the dry state. sodA mutants are sensitive to the ROS-generating compound paraquat (a superoxide producer). The growth rate in minimal medium is significantly reduced, and they have an increased mutation rate (102).
Some data are available on oxygenic stress during infection and nodulation, the oxygenic burst in the plant defense against infection, and symbiosis (103). Besides sodA, the studied genes involved in the response to ROS are katA, katB, and katC (51, 106). The gene katA is induced during the exponential growth phase, katC is induced during stationary growth, and katB is constitutively expressed (55). katA is strongly induced by H2O2, while katC is strongly induced by heat, salt, ethanol, and paraquat. katC is a good candidate to study the importance of ROS in survival during desiccation, considering the many stresses in which it is involved, its expression during the stationary phase, and, mainly, its response to NaCl.
A MODEL FOR THE PHYSIOLOGICAL RESPONSE TO DESICCATION OF RHIZOBIA
Microorganisms are not able to physiologically respond to stress when they are desiccated. The consequence is that adaptation to desiccation conditions has to take place after drying can be sensed but before the water activity decreases to a level too low to respond to. Since drying leads to an increase in osmolites, an increase in toxic compounds and osmotic pressure, and a decrease in water activity, it is hard to imagine that cells respond to a decrease in water activity alone. It is reasonable to assume that if a physiological response to desiccation exists at all, this response will be induced by other means, such as very high osmotic or salt stress or loss of turgor.
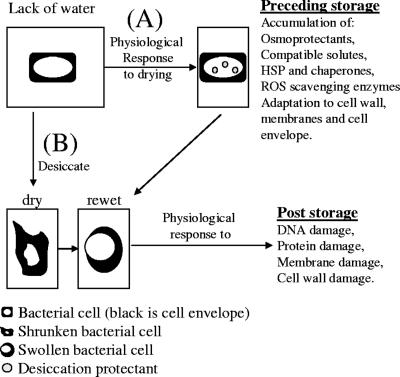
Only recently, such responses have been reported for cyanobacteria (61), in which differential responses between several metabolic pathways during drying take place. Interestingly, the photosynthetic apparatus is shut down, probably to prevent ROS from being formed, and osmotic stress-inducible genes are expressed. Indications that such a mechanism exists in rhizobia are as follows: (i) slow drying elevates survival rates over those with fast drying; (ii) conditions of elevated NaCl, osmotic stress, and/or a decrease in water activity lead to an increase in survival during desiccation; (iii) oxygen becomes toxic only when the water content decreases below a certain point, which indicates a response during the process of drying that allows ROS-scavenging proteins to be active; (iv) there is coordinated downregulation of the general metabolism during early osmotic shock; and (v) there is induction of genes important in NaCl, temperature, and oxygenic stress, including several genes potentially involved in survival during desiccation. We refer to this pathway as the “preceding-storage induction” pathway (Fig. 3). In this pathway, the lack of water (osmotic, salt, or water activity stress) induces a physiological response leading to an increase in survival during desiccation. This response is at least partly NaCl mediated in S. meliloti.
FIG. 3.
Model representing two hypothetical pathways for responses of rhizobia to desiccation stress and desiccation-induced damages. The “preceding-storage induction” pathway (A) implies a response to water, osmotic, or salt stress, and the “poststorage induction” pathway (B) implies a response to the desiccation-induced damages upon rewetting.
An alternative to this pathway is termed the “poststorage induction” pathway, in which damage upon rewetting is described (Fig. 3). Accumulated damages can be repaired only when a high enough water activity has been reached for the cells to detect and respond to damage (for example, extensive cell wall or DNA damage detected only upon rewetting and consequently inducing a response that takes place only after rewetting). Because the two pathways represent the two extremes in time of the response, they are not mutually exclusive, and thus a combination of the two pathways is the most likely scenario to take place in rhizobia.
SUMMARY AND FUTURE DIRECTIONS
Survival of bacteria during desiccation is the result of many different factors. Desiccation tolerance is an indirect result of coping with stresses, such as osmotic, temperature, and oxygenic stresses. Despite the uncertainties in understanding desiccation responses of rhizobia, we know that desiccation conditions influence survival and that responses to a decrease in water activity and an increase in osmotic or salt stress elevate the ability of some microorganisms to survive desiccation. This may lead to the expression of NaCl-responsive loci, leading to accumulation of osmoprotectants, desiccation protectants, ROS-scavenging compounds, heat shock proteins, and chaperones, increasing survival during desiccation of S. meliloti. Future research should include the identification and in-depth characterization of functional genes involved in the response to desiccation. A more detailed molecular and biochemical approach to NaCl-mediated survival during desiccation can directly be applied in the understanding and manipulation of soil microbial communities, the directed identification of desiccation-resistant microorganisms, and the production and development of dry seed inocula.
Acknowledgments
This work was partially funded by Liphatech, Inc. (now called Nitragin) grant 74576 to F.J.D.B. and USDA Hatch grant 1024 to K.N.
We thank two anonymous reviewers for their substantial contributions during the development of the manuscript.
Footnotes
Published ahead of print on 30 March 2007.
REFERENCES
- 1.Antheunisse, J., and L. Arkestein-Dijksman. 1979. Rate of drying and the survival of microorganisms. Antonie Leeuwenhoek 45:177-184. [DOI] [PubMed] [Google Scholar]
- 2.Antheunisse, J., J. W. de Bruijn-Tol, and M. E. VanderPol-VanSoest. 1981. Survival of microorganisms after drying and storage. Antonie Leeuwenhoek 47:539-545. [DOI] [PubMed] [Google Scholar]
- 3.Asada, S., M. Takano, and I. Shibasaki. 1979. Deoxyribonucleic acid strand breaks during drying of Escherichia coli on a hydrophobic filter membrane. Appl. Environ. Microbiol. 37:266-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bale, M. J., P. M. Bennett, J. M. Beringer, and M. Hinton. 1993. The survival of bacteria exposed to desiccation on surfaces associated with farm buildings. J. Appl. Bacteriol. 75:519-528. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya, I., and H. R. Das. 2003. Cell surface characteristics of two halotolerant strains of Sinorhizobium meliloti. Microbiol. Res. 158:187-194. [DOI] [PubMed] [Google Scholar]
- 6.Billi, D., and M. Potts. 2002. Life and death of dried prokaryotes. Res. Microbiol. 153:7-12. [DOI] [PubMed] [Google Scholar]
- 7.Billi, D., and M. Potts. 2000. Life without water: responses of prokaryotes to desiccation, p. 181-192. In K. B. Storey and J. M. Storey (ed.), Environmental stressors and gene responses. Elsevier Science BV, Amsterdam, The Netherlands.
- 8.Bohin, J. P. 2000. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiol. Lett. 186:11-19. [DOI] [PubMed] [Google Scholar]
- 9.Boncompagni, E., M. Osteras, M. C. Poggi, and D. le Rudulier. 1999. Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Appl. Environ. Microbiol. 65:2072-2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boonkerd, N., and R. W. Weaver. 1982. Survival of cowpea rhizobia in soil as affected by soil temperature and moisture. Appl. Environ. Microbiol. 43:585-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boscari, A., K. Mandon, L. Dupont, M. C. Poggi, and D. Le Rudulier. 2002. BetS is a major glycine betaine/proline betaine transporter required for early osmotic adjustment in Sinorhizobium meliloti. J. Bacteriol. 184:2654-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Botsford, J. L. 1984. Osmoregulation in Rhizobium meliloti: inhibition of growth by salts. Arch. Microbiol. 137:124-127. [Google Scholar]
- 13.Botsford, J. L., and T. A. Lewis. 1990. Osmoregulation in Rhizobium meliloti: production of glutamic acid in response to osmotic stress. Appl. Environ. Microbiol. 56:488-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boumahdi, M., P. Mary, and J. Hornez. 1999. Influence of growth phases and desiccation on the degrees of unsaturation of fatty acids and the survival rates of rhizobia. J. Appl. Microbiol. 87:611-619. [DOI] [PubMed] [Google Scholar]
- 15.Breedveld, M. W., C. Dijkema, L. P. T. M. Zevenhuizen, and A. J. B. Zehnder. 1993. Response of intracellular carbohydrates to a NaCl Shock in Rhizobium leguminosarum b.v. trifolii TA-1 and Rhizobium meliloti SU-47. J. Gen. Microbiol. 139:3157-3163. [Google Scholar]
- 16.Breedveld, M. W., J. A. Hadley, and K. J. Miller. 1995. A novel cyclic beta-1,2-glucan mutant of Rhizobium meliloti. J. Bacteriol. 177:6346-6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breedveld, M. W., L. P. T. M. Zevenhuizen, and A. J. B. Zehnder. 1991. Osmotically induced oligo and polysaccharide synthesis by Rhizobium meliloti SU-47. J. Gen. Microbiol. 136:2511-2519. [Google Scholar]
- 18.Brencic, A., and S. C. Winans. 2005. Detection of and response to signals involved in host-microbe interactions by plant-associated bacteria. Microbiol. Mol. Biol. Rev. 69:155-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown, A. D. 1990. Microbial water stress physiology, principles and perspectives. John Wiley & Sons, Inc., New York, NY.
- 20.Bushby, H. V. A., and K. C. Marshall. 1977. Desiccation induced damage to the cell envelope of root-nodule bacteria. Soil Biol. Biochem. 9:149-152. [Google Scholar]
- 21.Bushby, H. V. A., and K. C. Marshall. 1976. Some factors affecting the survival of root-nodule bacteria on desiccation. Soil Biol. Biochem. 9:143-147. [Google Scholar]
- 22.Bushby, H. V. A., and K. C. Marshall. 1977. Water status of rhizobia in relation to their susceptibility to desiccation and to their protection by montmorillonite. J. Gen. Microbiol. 99:19-27. [Google Scholar]
- 23.Caesar, A. J., and T. J. Burr. 1991. Effect of conditioning, betaine, and sucrose on survival of rhizobacteria in powdered formulations. Appl. Environ. Microbiol. 57:168-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell, G. R., L. A. Sharypova, H. Scheidle, K. M. Jones, K. Niehaus, A. Becker, and G. C. Walker. 2003. Striking complexity of lipopolysaccharide defects in a collection of Sinorhizobium meliloti mutants. J. Bacteriol. 185:3853-3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catroux, G., A. Hartmann, and C. Revellin. 2001. Trends in rhizobial inoculant production and use. Plant Soil 230:21-30. [Google Scholar]
- 26.Chao, W. L., and M. Alexander. 1984. Mineral soils as carriers for Rhizobium inoculants. Appl. Environ. Microbiol. 47:94-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen, M., and M. Alexander. 1973. Survival of soil bacteria during prolonged desiccation. Soil Biol. Biochem. 5:213-221. [Google Scholar]
- 28.Chen, W. M., T. M. Lee, C. C. Lan, and C. P. Cheng. 2000. Characterization of halotolerant rhizobia isolated from root nodules of Canavalia rosea from seaside areas. FEMS Microbiol. Ecol. 34:9-16. [DOI] [PubMed] [Google Scholar]
- 29.Cheng, H. P., and G. C. Walker. 1998. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J. Bacteriol. 180:20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chenu, C. 1993. Clay polysaccharide or sand polysaccharide associations as models for the interface between microorganisms and soil-water related properties and microstructure. Geoderma 56:143-156. [Google Scholar]
- 31.Danso, S. K. A., and M. Alexander. 1974. Survival of two strains of Rhizobium in soil. Soil Sci. Soc. Am. J. 38:86-89. [Google Scholar]
- 32.Davidson, F., and H. W. Reuszer. 1978. Persistance of Rhizobium japonicum on the soybean seed coat under controlled temperature and humidity. Appl. Environ. Microbiol. 35:94-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deaker, R., R. J. Roughley, and I. R. Kennedy. 2004. Legume seed inoculation technology—a review. Soil Biol. Biochem. 36:1275-1288. [Google Scholar]
- 34.Dominguez-Ferreras, A., R. Perez-Arnedo, A. Becker, J. Olivares, M. J. Soto, and J. Sanjuan. 2006. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J. Bacteriol. 188:7617-7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dye, M. 1982. A note on some factors affecting the survival of Rhizobium cultures during freeze drying and subsequent storage. J. Appl. Bacteriol. 52:461-464. [Google Scholar]
- 36.Evans, J., C. Wallace, and N. Dobrowolski. 1993. Interaction of soil type and temperature on the survival of Rhizobium leguminosarum bv viciae. Soil Biol. Biochem. 25:1153-1160. [Google Scholar]
- 37.Feng, L., R. J. Roughley, and L. Copeland. 2002. Morphological changes of rhizobia in peat cultures. Appl. Environ. Microbiol. 68:1064-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fierer, N., J. P. Schimel, and P. A. Holden. 2003. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 45:63-71. [DOI] [PubMed] [Google Scholar]
- 39.Fougere, F., and D. Le Rudulier. 1990. Uptake of glycine betaine and its analogues by bacteroids of Rhizobium meliloti. J. Gen. Microbiol. 136:157-163. [DOI] [PubMed] [Google Scholar]
- 40.Fraser, M. E. 1975. A method for culturing Rhizobium meliloti on porous granules to form a pre-inoculant for luzerne seeds. J. Appl. Bacteriol. 39:345-351. [Google Scholar]
- 41.Fred, E. B., I. L. Baldwin, and E. McCoy. 1932. Some factors which influence the growth and longevity of the nodule bacteria., p. 104-117. In I. L. Baldwin, E. McCoy, and E. B. Fred (ed.), Root nodule bacteria and leguminous plants., vol. 5. University of Wisconsin, Madison, WI. [Google Scholar]
- 42.Gibson, K. E., G. R. Campbell, J. Lloret, and G. C. Walker. 2006. CbrA is a stationary-phase regulator of cell surface physiology and legume symbiosis in Sinorhizobium meliloti. J. Bacteriol. 188:4508-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gonzalez, J. E., C. E. Semino, L. X. Wang, L. E. Castellano-Torres, and G. C. Walker. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 95:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gouffi, K., T. Bernard, and C. Blanco. 2000. Osmoprotection by pipecolic acid in Sinorhizobium meliloti: specific effects of d and l isomers. Appl. Environ. Microbiol. 66:2358-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gouffi, K., and C. Blanco. 2000. Is the accumulation of osmoprotectant the unique mechanism involved in bacterial osmoprotection? Int. J. Food Microbiol. 55:171-174. [DOI] [PubMed] [Google Scholar]
- 46.Gouffi, K., N. Pica, V. Pichereau, and C. Blanco. 1999. Disaccharides as a new class of nonaccumulated osmoprotectants for Sinorhizobium meliloti. Appl. Environ. Microbiol. 65:1491-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gouffi, K., V. Pichereau, J. P. Rolland, D. Thomas, T. Bernard, and C. Blanco. 1998. Sucrose is a nonaccumulated osmoprotectant in Sinorhizobium meliloti. J. Bacteriol. 180:5044-5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths, R. I., A. S. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2003. Physiological and community responses of established grassland bacterial populations to water stress. Appl. Environ. Microbiol. 69:6961-6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han, Y., D. Zhou, X. Pang, L. Zhang, Y. Song, Z. Tong, J. Bao, E. Dai, J. Wang, Z. Guo, J. Zhai, Z. Du, X. Wang, J. Wang, P. Huang, and R. Yang. 2005. Comparative transcriptome analysis of Yersinia pestis in response to hyperosmotic and high-salinity stress. Res. Microbiol. 156:403-415. [DOI] [PubMed] [Google Scholar]
- 50.Hartel, P. G., and M. Alexander. 1984. Temperature and desiccation tolerance of cowpea Rhizobia. Can. J. Microbiol. 30:820-823. [Google Scholar]
- 51.Herouart, D., S. Sigaud, S. Moreau, P. Frendo, D. Touati, and A. Puppo. 1996. Cloning and characterization of the katA gene of Rhizobium meliloti encoding a hydrogen peroxide-inducible catalase. J. Bacteriol. 178:6802-6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howieson, J., and R. Ballard. 2004. Optimising the legume symbiosis in stressful and competitive environments within southern Australia—some contemporary thoughts. Soil Biol. Biochem. 36:1261-1273. [Google Scholar]
- 53.Hua, S. T., V. Y. Tsai, G. M. Lichens, and A. T. Noma. 1982. Accumulation of amino acids in Rhizobium sp. strain WR1001 in response to sodium chloride salinity. Appl. Environ. Microbiol. 44:135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ingram-Smith, C., and K. J. Miller. 1998. Effects of ionic and osmotic strength on the glucosyltransferase of Rhizobium meliloti responsible for cyclic beta-(1,2)-glucan biosynthesis. Appl. Environ. Microbiol. 64:1290-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamet, A., S. Sigaud, G. Van de Sype, A. Puppo, and D. Herouart. 2003. Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol. Plant Microbe Interact. 16:217-225. [DOI] [PubMed] [Google Scholar]
- 56.Jenkins, M. B. 2003. Rhizobial and bradyrhizobial symbionts of mesquite from the Sonoran Desert: salt tolerance, facultative halophily and nitrate respiration. Soil Biol. Biochem. 35:1675-1682. [Google Scholar]
- 57.Jensen, J. B., N. K. Peters, and T. V. Bhuvaneswari. 2002. Redundancy in periplasmic binding protein-dependent transport systems for trehalose, sucrose, and maltose in Sinorhizobium meliloti. J. Bacteriol. 184:2978-2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang, J. Q., W. Wei, B. H. Du, X. H. Li, L. Wang, and S. S. Yang. 2004. Salt-tolerance genes involved in cation efflux and osmoregulation of Sinorhizobium fredii RT19 detected by isolation and characterization of Tn5 mutants. FEMS Microbiol. Lett. 239:139-146. [DOI] [PubMed] [Google Scholar]
- 59.Juwarkar, A., and R. B. Rewari. 1987. Synergistic effect of relative humidity and temperature on the survival of rhizobia in inoculant carrier. J. Appl. Bacteriol. 64:465-469. [Google Scholar]
- 60.Kanesaki, Y., I. Suzuki, S. I. Allakhverdiev, K. Mikami, and N. Murata. 2002. Salt stress and hyperosmotic stress regulate the expression of different sets of genes in Synechocystis sp. PCC 6803. Biochem. Biophys. Res. Commun. 290:339-348. [DOI] [PubMed] [Google Scholar]
- 61.Katoh, H., R. K. Asthana, and M. Ohmori. 2004. Gene expression in the Cyanobacterium anabaena sp PCC7120 under desiccation. Microb. Ecol. 47:164-174. [DOI] [PubMed] [Google Scholar]
- 62.Kosanke, J. W., R. M. Osburn, G. I. Shuppe, and R. S. Smith. 1991. Slow rehydration improves the recovery of dried bacterial populations. Can. J. Microbiol. 38:520-525. [DOI] [PubMed] [Google Scholar]
- 63.Kosanke, J. W., R. M. Osburn, R. S. Smith, and LiphaTech Inc. April 1999. Process for preparation of bacterial agricultural products. Canadian patent 2,073,507.
- 64.Kremer, R. J., and H. L. Peterson. 1983. Effect of carrier and temperature on survival of Rhizobium spp. in legume inocula: development of an improved type of inoculant. Appl. Environ. Microbiol. 45:1790-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Rudulier, D., and T. Bernard. 1986. Salt tolerance in Rhizobium: a possible role for betaines. FEMS Microbiol. Rev. 39:67-72. [Google Scholar]
- 66.Leslie, S. B., E. Israeli, B. Lighthart, J. H. Crowe, and L. M. Crowe. 1995. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 61:3592-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leslie, S. B., S. A. Teter, L. M. Crowe, and J. H. Crowe. 1994. Trehalose lowers membrane phase transitions in dry yeast cells. Biochim. Biophys. Acta 1192:7-13. [DOI] [PubMed] [Google Scholar]
- 68.Lindemann, W. C., and C. R. Glover. 1996. Inoculation of legumes. New Mexico State University, Cooperative Extension Service, College of Agriculture and Home Economics. http://cahe.nmsu.edu/pubs/-a/a-130.
- 69.Lloret, J., L. Bolanos, M. Mercedes Lucas, J. M. Peart, N. J. Brewin, I. Bonilla, and R. Rivilla. 1995. Ionic stress and osmotic pressure induce different alterations in the lipopolysaccharide of a Rhizobium meliloti strain. Appl. Environ. Microbiol. 61:3701-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lloret, J., B. B. Wulff, J. M. Rubio, J. A. Downie, I. Bonilla, and R. Rivilla. 1998. Exopolysaccharide II production is regulated by salt in the halotolerant strain Rhizobium meliloti EFB1. Appl. Environ. Microbiol. 64:1024-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Madkour, M. A., L. T. Smith, and G. M. Smith. 1990. Preferential osmolyte accumulation: a mechanism of osmotic stress adaptation in diazotrophic bacteria. Appl. Environ. Microbiol. 56:2876-2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mah, T. F., B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306-310. [DOI] [PubMed] [Google Scholar]
- 73.Markillie, L. M., S. M. Varnum, P. Hradecky, and K. K. Wong. 1999. Targeted mutagenesis by duplication insertion in the radioresistant bacterium Deinococcus radiodurans: radiation sensitivities of catalase (katA) and superoxide dismutase (sodA) mutants. J. Bacteriol. 181:666-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marshall, K. C. 1963. Survival of root nodule bacteria in dry soils exposed to high temperatures. Aust. J. Agric. Res. 15:273-281. [Google Scholar]
- 75.Mary, P., C. Dupuy, C. D. Biremon, C. Defives, and R. Tailliez. 1994. Differences among Rhizobium meliloti and Bradyrhizobium japonicum strains in tolerance to desiccation and storage at different relative humidities. Soil Biol. Biochem. 26:1125-1132. [Google Scholar]
- 76.Mary, P., N. Moschetto, and R. Tailliez. 1993. Production and survival during storage of spray dried Bradyrhizobium japonicum cell concentrates. J. Appl. Bacteriol. 74:340-344. [Google Scholar]
- 77.Mary, P., D. Ochin, and R. Tailliez. 1986. Growth status of rhizobia in relation to their tolerance to low water activities and desiccation stress. Soil Biol. Biochem. 18:179-184. [Google Scholar]
- 78.Mary, P., D. Ochin, and R. Tailliez. 1985. Rates of drying and survival of Rhizobium meliloti strains during storage at different relative humidities. Appl. Environ. Microbiol. 50:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mattimore, V., and J. R. Battista. 1996. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol. 178:633-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mendrygal, K. E., and J. E. Gonzalez. 2000. Environmental regulation of exopolysaccharide production in Sinorhizobium meliloti. J. Bacteriol. 182:599-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miller, K. J., and J. M. Wood. 1996. Osmoadaptation by rhizosphere bacteria. Annu. Rev. Microbiol. 50:101-136. [DOI] [PubMed] [Google Scholar]
- 82.Miller-Williams, M., P. C. Loewen, and I. J. Oresnik. 2006. Isolation of salt-sensitive mutants of Sinorhizobium meliloti strain Rm1021. Microbiology 152:2049-2059. [DOI] [PubMed] [Google Scholar]
- 83.Nogales, J., R. Campos, H. BenAbdelkhalek, J. Olivares, C. Lluch, and J. Sanjuan. 2002. Rhizobium tropici genes involved in free-living salt tolerance are required for the establishment of efficient nitrogen-fixing symbiosis with Phaseolus vulgaris. Mol. Plant Microbe. Interact. 15:225-232. [DOI] [PubMed] [Google Scholar]
- 84.Oliver, A. E., O. Leprince, W. F. Wolkers, D. K. Hincha, A. G. Heyer, and J. H. Crowe. 2001. Non-disaccharide-based mechanisms of protection during drying. Cryobiology 43:151-167. [DOI] [PubMed] [Google Scholar]
- 85.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Osa-Afiana, L. O., and M. Alexander. 1982. Differences among cowpea rhizobia in tolerance to high temperature and desiccation in soil. Appl. Environ. Microbiol. 43:435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Osa-Afiana, L. O., and M. Alexander. 1979. Effect of moisture on the survival of Rhizobium in soil. Soil Sci. Soc. Am. J. 43:925-930. [Google Scholar]
- 88.Osteras, M., E. Boncompagni, N. Vincent, M. C. Poggi, and D. Le Rudulier. 1998. Presence of a gene encoding choline sulfatase in Sinorhizobium meliloti bet operon: choline-O-sulfate is metabolized into glycine betaine. Proc. Natl. Acad. Sci. USA 95:11394-11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paul, E., J. Fages, P. Blanc, G. Goma, and A. Pareilleux. 1993. Survival of alginate-entrapped cells of Azospirillum lipoferum during dehydration and storage in relation to water properties. Appl. Microbiol. Biotechnol. 40:34-39. [Google Scholar]
- 90.Pena-Cabriales, J. J., and M. Alexander. 1979. Survival of Rhizobium in soils undergoing drying. Soil Sci. Soc. Am. J. 43:962-966. [Google Scholar]
- 91.Phillips, D. A., E. S. Sande, J. A. C. Vriezen, F. J. de Bruijn, D. Le Rudulier, and C. M. Joseph. 1998. A new genetic locus in Sinorhizobium meliloti is involved in stachydrine utilization. Appl. Environ. Microbio.l. 64:3954-3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pichereau, V., J. A. Pocard, J. Hamelin, C. Blanco, and T. Bernard. 1998. Differential effects of dimethylsulfoniopropionate, dimethylsulfonioacetate, and other S-methylated compounds on the growth of Sinorhizobium meliloti at low and high osmolarities. Appl. Environ. Microbiol. 64:1420-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pocard, J. A., N. Vincent, E. Boncompagni, L. T. Smith, M. C. Poggi, and D. Le Rudulier. 1997. Molecular characterization of the bet genes encoding glycine betaine synthesis in Sinorhizobium meliloti 102F34. Microbiol. 143:1369-1379. [DOI] [PubMed] [Google Scholar]
- 94.Potts, M. 1994. Desiccation tolerance of prokaryotes. Microbiol. Rev. 58:755-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raivio, T. L. 2005. Envelope stress responses and Gram-negative bacterial pathogenesis. Mol. Microbiol. 56:1119-1128. [DOI] [PubMed] [Google Scholar]
- 96.Ramos, J. L., M. T. Gallegos, S. Marques, M. I. Ramos-Gonzalez, M. Espinosa-Urgel, and A. Segura. 2001. Responses of Gram-negative bacteria to certain environmental stressors. Curr. Opin. Microbiol. 4:166-171. [DOI] [PubMed] [Google Scholar]
- 97.Rehman, A., and C. S. Nautiyal. 2002. Effect of drought on the growth and survival of the stress-tolerant bacterium Rhizobium sp NBR12505 sesbania and its drought-sensitive transposon Tn5 mutant. Curr. Microbiol. 45:368-377. [DOI] [PubMed] [Google Scholar]
- 98.Rinaudo, M. 2004. Role of substituents on the properties of some polysaccharides. Biomacromolecules 5:1155-1165. [DOI] [PubMed] [Google Scholar]
- 99.Ruberg, S., Z. X. Tian, E. Krol, B. Linke, F. Meyer, Y. P. Wang, A. Puhler, S. Weidner, and A. Becker. 2003. Construction and validation of a Sinorhizobium meliloti whole genome DNA microarray: genome-wide profiling of osmoadaptive gene expression. J. Biotechnol. 106:255-268. [DOI] [PubMed] [Google Scholar]
- 100.Salema, M. P., C. A. Parker, D. K. Kirby, and D. L. Chatel. 1982. Death of Rhizobia on inoculated seed. Soil Biol. Biochem. 14:13-14. [Google Scholar]
- 101.Salema, M. P., C. A. Parker, D. K. Kirby, D. L. Chatel, and T. M. Armitage. 1981. Rupture of nodule bacteria on drying and rehydration. Soil Biol. Biochem. 14:15-22. [Google Scholar]
- 102.Santos, R., S. Bocquet, A. Puppo, and D. Touati. 1999. Characterization of an atypical superoxide dismutase from Sinorhizobium meliloti. J. Bacteriol. 181:4509-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos, R., D. Herouart, A. Puppo, and D. Touati. 2000. Critical protective role of bacterial superoxide dismutase in Rhizobium-legume symbiosis. Mol. Microbiol. 38:750-759. [DOI] [PubMed] [Google Scholar]
- 104.Sauvage, D., J. Hamelin, and F. Larher. 1983. Glycine betaine and other structurally related compounds improve the salt tolerance of Rhizobium meliloti. Plant Sci. Lett. 31:291-302. [Google Scholar]
- 105.Shirkey, B., D. P. Kovarcik, D. J. Wright, G. Wilmoth, T. F. Prickett, R. F. Helm, E. M. Gregory, and M. Potts. 2000. Active Fe-containing superoxide dismutase and abundant SodF mRNA in Nostoc commune (cyanobacteria) after years of desiccation. J. Bacteriol. 182:189-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sigaud, S., V. Becquet, P. Frendo, A. Puppo, and D. Herouart. 1999. Differential regulation of two divergent Sinorhizobium meliloti genes for HPII-like catalases during free-living growth and protective role of both catalases during symbiosis. J. Bacteriol. 181:2634-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sleesman, J. P., and C. Leben. 1976. Bacteria desiccation: effect of temperature, relative humidity, and culture age on survival. Phytopathology 66:1334-1338. [Google Scholar]
- 108.Smith, L. T., J. A. Pocard, T. Bernard, and D. Le Rudulier. 1988. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J. Bacteriol. 170:3142-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith, L. T., and G. M. Smith. 1989. An osmoregulated dipeptide in stressed Rhizobium meliloti. J. Bacteriol. 171:4714-4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Smith, R. S. 1992. Legume inoculant formulation and application. Can. J. Microbiol. 38:485-492. [Google Scholar]
- 111.Stead, D., and S. F. Park. 2000. Roles of Fe superoxide dismutase and catalase in resistance of Campylobacter coli to freeze-thaw stress. Appl. Environ. Microbiol. 66:3110-3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steinborn, J., and R. J. Roughley. 1975. Toxicity of sodium and chloride ions to Rhizobium spp. in broth and peat culture. J. Appl. Bacteriol. 39:133-138. [DOI] [PubMed] [Google Scholar]
- 113.Streeter, J. G. 2003. Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J. Appl. Microbiol. 95:484-491. [DOI] [PubMed] [Google Scholar]
- 114.Talibart, R., M. Jebbar, G. Gouesbet, S. Himdi-Kabbab, H. Wroblewski, C. Blanco, and T. Bernard. 1994. Osmoadaptation in rhizobia: ectoine-induced salt tolerance. J. Bacteriol. 176:5210-5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Talibart, R., M. Jebbar, K. Gouffi, V. Pichereau, G. Gouesbet, C. Blanco, T. Bernard, and J. Pocard. 1997. Transient accumulation of glycine betaine and dynamics of endogenous osmolytes in salt-stressed cultures of Sinorhizobium meliloti. Appl. Environ. Microbiol. 63:4657-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trotman, A. P., and R. W. Weaver. 1995. Tolerance of clover rhizobia to heat and desication stresses in soil. Soil Sci. Soc. Am. J. 59:466-470. [Google Scholar]
- 117.van Rensburg, H., and B. W. Strijdom. 1979. Survival of fast and slowgrowing Rhizobium spp. under conditions of relative mild desiccation. Soil Biol. Biochem. 12:353-356. [Google Scholar]
- 118.van Schreven, D. A. 1970. Some factors affecting growth and survival of Rhizobium spp in soil-peat cultures. Plant Soil 32:113. [Google Scholar]
- 119.Vincent, J. M., J. A. Thompson, and K. O. Donovan. 1961. Death of root nodule bacteria on drying. Aust. J. Agric. Res. 13:258-270. [Google Scholar]
- 120.Vriezen, J. A. C. 2005. Responses of Sinorhizobium meliloti 1021 to water stress. Ph.d. thesis. University of Massachusetts, Amherst, MA.
- 121.Vriezen, J. A. C., F. J. de Bruijn, and K. Nüsslein. 2006. Desiccation responses and survival of Sinorhizobium meliloti USDA 1021 in relation to growth-phase, temperature, chloride and sulfate availability. Lett. Appl. Microbiol. 42:172-178. [DOI] [PubMed] [Google Scholar]
- 122.Wei, W., J. Jiang, X. Li, L. Wang, and S. S. Yang. 2004. Isolation of salt-sensitive mutants from Sinorhizobium meliloti and characterization of genes involved in salt tolerance. Lett. Appl. Microbiol. 39:278-283. [DOI] [PubMed] [Google Scholar]
- 123.Welsh, D. T. 2000. Ecological significance of compatible solute accumulation by micro-organisms: from single cells to global climate. FEMS Microbiol. Rev. 24:263-290. [DOI] [PubMed] [Google Scholar]
- 124.Welsh, D. T., and R. A. Herbert. 1999. Osmotically induced intracellular trehalose, but not glycine betaine accumulation promotes desiccation tolerance in Escherichia coli. FEMS Microbiol. Lett. 174:57-63. [DOI] [PubMed] [Google Scholar]
- 125.Welsh, D. T., R. H. Reed, and R. A. Herbert. 1991. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J. Gen. Microbiol. 137:745-750. [DOI] [PubMed] [Google Scholar]
- 126.Willis, L. B., and G. C. Walker. 1999. A novel Sinorhizobium meliloti operon encodes an alpha-glucosidase and a periplasmic-binding-protein-dependent transport system for alpha-glucosides. J. Bacteriol. 181:4176-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wong, K., and G. B. Golding. 2003. A phylogenetic analysis of the pSymB replicon from the Sinorhizobium meliloti genome reveals a complex evolutionary history. Can. J. Microbiol. 49:269-280. [DOI] [PubMed] [Google Scholar]
- 128.Yan, A. M., E. T. Wang, F. L. Kan, Z. Y. Tan, X. H. Sui, B. Reinhold-Hurek, and W. X. Chen. 2000. Sinorhizobium meliloti associated with Medicago sativa and Melilotus spp. in arid saline soils in Xinjiang, China. Int. J. Syst. Evol. Microbiol. 50:1887-1891. [DOI] [PubMed] [Google Scholar]
- 129.Yap, S. F., and S. T. Lim. 1983. Response of Rhizobium Sp UMKL-20 to sodium-chloride stress. Arch. Microbiol. 135:224-228. [Google Scholar]
- 130.York, G. M., and G. C. Walker. 1998. The succinyl and acetyl modifications of succinoglycan influence susceptibility of succinoglycan to cleavage by the Rhizobium meliloti glycanases ExoK and ExsH. J. Bacteriol. 180:4184-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zahran, H. H. 1999. Rhizobium-legume symbiosis and nitrogen fixation under severe conditions and in an arid climate. Microbiol. Mol. Biol. Rev. 63:968-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhan, H. J., C. C. Lee, and J. A. Leigh. 1991. Induction of the second exopolysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J. Bacteriol. 173:7391-7394. [DOI] [PMC free article] [PubMed] [Google Scholar]