Abstract
The kinetics and the metabolism of Bifidobacterium adolescentis MB 239 growing on galactooligosaccharides (GOS), lactose, galactose, and glucose were investigated. An unstructured unsegregated model for growth in batch cultures was developed, and kinetic parameters were calculated with a recursive algorithm. The growth rate and cellular yield were highest on galactose, followed by lactose and GOS, and were lowest on glucose. Lactate, acetate, and ethanol yields allowed the calculation of carbon fluxes toward fermentation products. Distributions between two- and three-carbon products were similar on all the carbohydrates (55 and 45%, respectively), but ethanol yields were different on glucose, GOS, lactose, and galactose, in decreasing order of production. Based on the stoichiometry of the fructose-6-phosphate shunt and on the carbon distribution among the products, the ATP yield was calculated. The highest yield was obtained on galactose, while the yields were 5, 8, and 25% lower on lactose, GOS, and glucose, respectively. Therefore, a correspondence among ethanol production, low ATP yields, and low biomass production was established, demonstrating that carbohydrate preferences may result from different distributions of carbon fluxes through the fermentative pathway. During the fermentation of a GOS mixture, substrate selectivity based on the degree of polymerization was exhibited, since lactose and the trisaccharide were the first to be consumed, while a delay was observed until longer oligosaccharides were utilized. Throughout the growth on both lactose and GOS, galactose accumulated in the cultural broth, suggesting that β(1-4) galactosides can be hydrolyzed before they are taken up.
Many nondigestible oligosaccharides, such as fructooligosaccharides, isomaltooligosaccharides, galactooligosaccharides (GOS), and xylooligosaccharides, have been reported to beneficially affect human health. They are defined as prebiotics and are increasingly being used as functional food ingredients (18). β-GOS are manufactured from highly concentrated lactose solutions by the action of β-galactosidases (β-Gals) which have transgalactosylation activity. In standardized large-scale production, trisaccharides to hexasaccharides are the main products; hence, commercial GOS occur as mixtures of various degrees of polymerization (DP) and glycosidic linkages and contain large amounts of glucose and unreacted lactose. The linkage between galactose moieties (mainly β1-4, β1-6, and β1-3), the efficiency of transgalactosylation, and the components in the final product depend upon the enzymes and the reaction conditions (3, 7, 25, 30, 34, 39, 41).
As demonstrated in several in vitro and in vivo studies (5, 40), GOS resist hydrolysis by human digestive enzymes and are not absorbed on transit through the small intestine; therefore, they are available for fermentation by the colon-resident microflora. The utilization of GOS by the major intestinal bacteria has been investigated (25, 34, 46). It was shown that β1-4-linked oligosaccharides were selectively utilized by all Bifidobacterium and some Lactobacillus and Bacteroides species, whereas they were not fermented by Clostridium, Eubacterium, Peptostreptococcus, Enterococcus, and Fusobacterium spp. and Escherichia coli. Both in vitro fecal cultures and in vivo nutritional studies demonstrated that GOS led to a significant and selective increase in the concentration of bifidobacteria in human and animal colonic flora (5, 16, 17, 38, 40) and modified the intestinal fermentative activity, increasing lactate, acetate, and total short-chain fatty acid production (5, 40). As a consequence of such bifidogenic effects and the increase in short-chain fatty acids, in the last few decades GOS have been increasingly introduced as prebiotic ingredients for dietary or pharmaceutical applications. In particular, the addition of fructooligosaccharide and GOS mixtures to infant formulas stimulated Bifidobacterium growth and metabolic activity in the colon, thus reproducing the Bifidobacterium-dominant microflora of breast-fed infants (14, 15, 27).
The ability of bifidobacteria to utilize GOS has been extensively demonstrated (10, 12, 22, 25, 46). Bifidobacteria produce several β-Gals, which can have different properties, for hydrolysis of galactans and GOS (11, 20, 28, 36, 45). Three intracellular β-Gals and one extracellular endo-β-Gal with a transmembrane domain were predicted to occur in the Bifidobacterium longum genome (36); one extracellular and two intracellular β-Gals were cloned and characterized from Bifidobacterium bifidum (20); two intracellular enzymes were cloned and characterized from both Bifidobacterium infantis (20) and Bifidobacterium adolescentis (11, 45). In this latter species, a β-Gal was induced only by GOS (DP, >2) and preferred β(1-4)-galactosides over lactose, while the other was induced by lactose (11, 45). Interestingly, β-Gal from bifidobacteria can be exploited for glycosyl transfer reactions to synthesize oligosaccharides from lactose with the very attractive possibility of producing a prebiotic tailored towards specific Bifidobacterium strains (25, 30, 41).
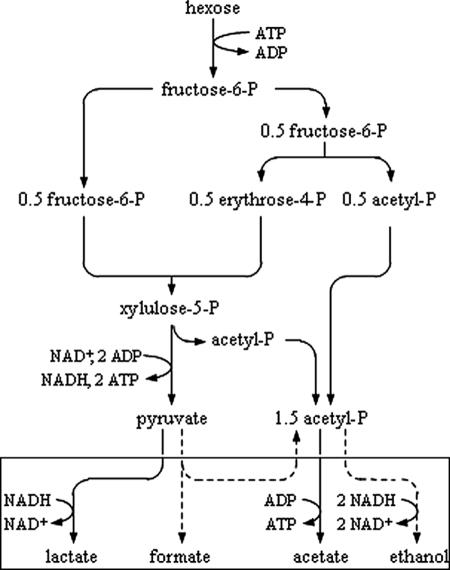
Although bifidobacteria have been proven to grow on GOS and some Bifidobacterium β-Gals are biochemically well characterized, little metabolic and kinetic information on GOS fermentation by bifidobacteria is available. The purpose of the present work was to study in depth the growth and the physiology of Bifidobacterium adolescentis MB 239 fermenting GOS and their components lactose, galactose, and glucose. These carbohydrates were used as the sole carbon source in batch experiments. β-Gal activity and location were determined. A mathematical model for growth of B. adolescentis MB 239 in batch cultures was developed, and kinetic parameters were calculated by means of a Matlab algorithm in order to provide the basis for more detailed comparison of the different carbon sources. The relative amounts of fermentation end products reflect cellular metabolic conditions. In order to give an explanation of the carbohydrate preferences of B. adolescentis MB 239, carbon fluxes and ATP yields were calculated on the basis of a fructose-6-phosphate shunt (Fig. 1), the peculiar pattern of hexose catabolism in the genus Bifidobacterium (2, 35, 42).
FIG. 1.
Fructose-6-phosphate shunt in Bifidobacterium. Hexose is broken down to 1 pyruvate and 1.5 acetylphosphate, which can give alternative end products (in rectangle). Minor pathways are shown with dashed lines.
MATERIALS AND METHODS
Organism.
Bifidobacterium adolescentis MB 239 was obtained from the Collection of the Ex-Institute of Agricultural Microbiology (at present the Department of Agroenvironmental Sciences) of the University of Bologna (V. Scardovi collection). B. adolescentis MB 239 was subcultured anaerobically at 37°C for 24 h in lactobacillus DeMan, Rogosa, Sharpe broth (Difco Laboratories, Sparks, MD) containing 0.5 g liter−1 cysteine-HCl.
Chemicals and media.
Glucose, galactose, and lactose were purchased from Sigma-Aldrich (Steinheim, Germany). A GOS syrup (Vivinal GOS) was obtained from Borculo Domo (Zwolle, The Netherlands). The GOS mixture contained 18.8% glucose, 3.5% galactose, 19.4% lactose, and 58.3% GOS with DP ranging between 3 and 9. A DP 3 oligomer accounted for 37% of total carbohydrates, and it was the most highly represented oligosaccharide. The concentration of the oligomers decreased with the increase in DP (Table 1). All other chemicals were obtained from Sigma-Aldrich (Steinheim, Germany) unless otherwise stated.
TABLE 1.
Relative sugar composition (percentage of C atoms) of Vivinal GOS
| DP or sugar | % of C atoms |
|---|---|
| DP 9 | 1.5 |
| DP 8 | 1.4 |
| DP 7 | 1.5 |
| DP 6 | 3.5 |
| DP 5 | 6.0 |
| DP 4 | 6.3 |
| DP 3 | 37.1 |
| Total GOS | 58.3 |
| Lactose | 19.4 |
| Galactose | 3.5 |
| Glucose | 18.8 |
Fermentation experiments on the above carbohydrates were carried out in a semisynthetic medium (SM) that contained a surplus of amino acids and all the vitamins and the salts required for the growth of B. adolescentis MB 239 (1). In cultures without pH regulation, 10 g liter−1 sodium acetate was used as a buffering salt to prevent pH from dropping down and so inhibiting growth of B. adolescentis MB 239. If pH regulation was applied, sodium acetate was not included in the SM composition. Carbohydrate solutions were filtered through a 0.22-μm-pore-size filter and added to the sterile basal medium as appropriate to obtain 600 mM C atoms (600 C-mM; 100 mM total hexose units).
Fermentation experiments.
Controlled-pH batch cultures were carried out in a BM-PPS3 bioreactor (Solaris Biotech, Porto Mantovano, Italy) with a working volume of 2 liters. The temperature was kept at 37°C, and constant stirring (300 rpm) was applied. Anaerobic conditions were maintained by sparging filter-sterilized nitrogen (Millex filter type GS, 33 mm) into the culture at a rate of 0.05 volume/volume/minute. No antifoaming agents were used. The fermentor was inoculated (5%, vol/vol) with exponential-phase precultures grown in the same medium buffered with 10 g liter−1 sodium acetate. The culture pH was continuously measured (Mettler Toledo InPro 3030/325) and regulated by automatic addition of 4 M NaOH. Growth was monitored by following changes in biomass dry weight (29). Samples were periodically collected for analysis of carbohydrates, fermentation products, and β-Gal activity.
All fermentations were carried out in triplicate. The results presented below are representative of the three fermentations.
Analysis of substrates and products.
The samples collected from B. adolescentis MB 239 cultures were immediately chilled at 0°C, centrifuged, and filtered (cellulose acetate syringe filter, 0.22 μm; Albet Filalbet, Barcelona, Spain) to remove cells and to avoid changes in analyte concentrations resulting from active metabolism. Supernatants were frozen at −20°C until analyzed.
Carbohydrates were analyzed in supernatants utilizing overpressure layer chromatography (OPLC) and modern instrumental high-performance thin-layer chromatography with automated multiple development (HPTLC-AMD) techniques. In all the fermentation experiments, glucose, galactose, and lactose were analyzed with OPLC, whereas oligosaccharides were analyzed with HPTLC-AMD. OPLC was carried out on HTSorb fine silica gel layers with an aluminum backing (Bionisis, Le Plessis Robinson, France). Elution was performed with 10 ml of an acetonitrile-water (Carlo Erba Reagenti) solution (85:15) at the flow rate of 300 μl min−1. HPTLC-AMD (Camag, Muttenz, Switzerland) step-gradient elution was performed on 10- × 20-cm Kieselgel 60 F254s HPTLC plates (Merck), using mixtures of ultrapure water and acetonitrile at different percentages (from 32 to 24% [vol/vol] water with a linear gradient). OPLC and HPTLC-AMD layers were derivatized with lead(IV) acetate and 2,7-dichlorofluorescein, according to the method of Funk et al. (8). The derivatized plates were scanned at 366 nm. The evaluation software Camag-WinCATS was used for quantitative determinations. Triplicate samples were analyzed.
Lactic acid, acetic acid, ethanol, and formic acid were analyzed using high-pressure liquid chromatography with a refractive index detector (Erma Inc., Tokyo, Japan). An Aminex HPX-87H ion exclusion column was used at room temperature. Isocratic elution was carried out with 0.005 M H2SO4 at 0.6 ml min−1. Triplicate samples were analyzed.
Analysis of β-Gal activity.
β-Gal activity was assayed on permeabilized cells, nonpermeabilized cells, and dialyzed supernatants. The culture was centrifuged at 6,000 × g for 10 min at 0°C. The supernatant was filtered at 0.22 μm and dialyzed against 50 mM pH 7 phosphate buffer in a 14-kDa-cutoff membrane at 4°C. Biomass was washed twice in Z buffer (0.1 M phosphate buffer, pH 7; 10 mM MgSO4 · 7H2O; 1 mM CaCl2) and concentrated 10-fold. Permeabilization was achieved by mixing 0.5 ml suspension with 0.5 ml of Triton X-100 (5% [vol/vol] in Z buffer) and incubating the mixture at 37°C for 10 min. All samples were examined using the o-nitrophenyl-β-d-galactopyranoside assay (1). One unit of β-Gal was defined as the amount of enzyme required to release 1 μmol of nitrophenol per minute under the assay conditions. Specific activity was expressed as units per milligram of dry biomass.
Modeling and parameter estimation.
An unstructured unsegregated model was used to describe B. adolescentis MB 239 fermentations. The model was based on the mass balance equations reported in Table 2. Growth was considered carbon limited, according to the Monod model. Production of acetate, lactate, formate, and ethanol was considered growth associated. Biomass and substrate balance equations took account of cellular death and maintenance terms, respectively.
TABLE 2.
Mathematical model for batch fermentations of B. adolescentis MB 239
| Component | Mass balance equation |
|---|---|
| Biomass carbon concn, X (C-mM) | 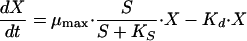 |
| Carbohydrate carbon concn, S (C-mM) | 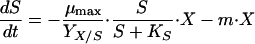 |
| Product carbon concn, PL,A,E,F (C-mM)a | 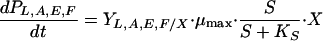 |
Distinct mass balance equations for lactate (L), acetate (A), ethanol (E), and formate (F) were used.
The experimental data obtained from batch fermentations were used to determine the parameter values in the kinetic equations. For this purpose, nonlinear least-square analysis was used to obtain best-fit values of the model parameters by solving numerically the system of ordinary differential equations for all the stated variables. This procedure was carried out using Matlab software (version 6.5, release 13; MathWorks), specifically the stiff differential equation solver ODE15S, in conjunction with the nonlinear regression analysis program LSQNONLIN. These programs required initial estimation of the parameters and iteratively sought the values that minimized the sum of squared residuals (RSS): RSS = RSSX + RSSS + RSSP, where RSSX, RSSS, and RSSP correspond to the sum of the squared residuals between experimental and modeled values for biomass, substrate, and products, respectively. The NLPARCI function was used to estimate the 95% confidence intervals of the calculated parameters.
Experimental carbon concentrations (C-mM) of carbohydrate, biomass, and products were input. The biomass molar concentration was calculated on the basis of Lactococcus lactis elemental composition, C1H1.95O0.63N0.22P0.02S0.01, corresponding to a molecular mass of 27.8 g C mol−1 (21).
RESULTS
Comparative growth on glucose, galactose, lactose, and GOS.
Controlled-pH batch culturing of Bifidobacterium adolescentis MB 239 was carried out in the carbon-limited SM that contained 600 C-mM glucose in order to determine the pH optimum for growth. The pH of batch cultures was kept at 4.5, 5.0, 5.5, 6.0, and 6.5 (Table 3). The maximum biomass yield and specific growth rate were both observed at pH 5.5. Acid production was determined by monitoring the addition of pH corrector. With the exception of pH 4.5, which did not lead to any growth, higher biomass production was associated with lower base addition, although this was not a linear correlation. Accordingly, the lowest level of production of organic acids from fermentative metabolism was observed at pH 5.5.
TABLE 3.
pH effects on μmax, YX/S, biomass production, and base addition in batch cultures of B. adolescentis MB 239 in SM containing 600 C-mM glucose
| pH | μmax (h−1) | YX/S (C-mol C-mol−1) | Biomass (C-mM) | Base addition (mM) |
|---|---|---|---|---|
| 4.5 | 0.02 | 0.004 | 2.4 | 0 |
| 5.0 | 0.04 | 0.143 | 86.0 | 139 |
| 5.5 | 0.21 | 0.148 | 89.0 | 124 |
| 6.0 | 0.15 | 0.136 | 81.6 | 187 |
| 6.5 | 0.14 | 0.052 | 31.2 | 226 |
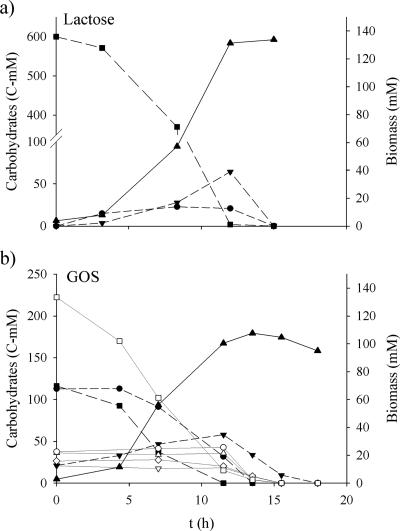
Comparative batch experiments on 600 C-mM glucose, galactose, lactose, or GOS as sole carbon source were carried out. The pH was kept at 5.5, specifically the optimal pH value for growth of B. adolescentis MB 239. The strain was incapable of growing in SM without addition of carbohydrate. It grew on glucose, galactose, and lactose with a continuous exponential growth phase, and stationary phase occurred when the carbon sources were depleted after 17, 9, and 12 h, respectively. On GOS, the specific growth rate progressively decreased, suggesting different consumption kinetics for the components of the mixture (Fig. 2). The specific growth rate was calculated from the exponential-phase biomass concentrations (for GOS, the initial growth phase was considered); the biomass yield coefficient was determined at the entrance into stationary phase (Table 4). B. adolescentis MB 239 grew with the highest specific growth rate and biomass production on galactose and with the lowest values on glucose. On both lactose and GOS, the specific growth rate and biomass production were higher than those on glucose but lower than those on galactose. Fermentation products were monitored throughout batch fermentations; their C-mM concentrations at the stationary phase are reported in Table 4. Lactic and acetic acids were the major products on all the carbon sources, even if different concentrations and relative amounts were observed. Acetic acid was more abundant than lactic acid on galactose, lactose, and GOS. In contrast, lactic acid was the most abundant fermentation product on glucose. Ethanol was a minor product on all the carbohydrates. The highest ethanol concentration was obtained on glucose (89 C-mM), and the lowest was obtained on galactose (1.3 C-mM). Formic acid production was never observed on any of the carbon sources. Lactic acid, acetic acid, and ethanol were all growth-associated products since their accumulation ended at the entrance into stationary phase, in agreement with the trends for base addition (data not shown).
FIG. 2.
Batch fermentation of B. adolescentis MB 239 in SM that contained 600 C-mM glucose (a), galactose (b), lactose (c), and GOS (d). Symbols: ▴, biomass; □, total carbohydrates; solid line, intracellular β-Gal specific activity; dashed line, surface β-Gal specific activity.
TABLE 4.
μmax, YX/S, and concentrations of biomass and fermentation products at the entrance into stationary phase in batch fermentations of B. adolescentis MB 239 in SM which contained 600 C-mM glucose, galactose, lactose, and GOS
| Sugar | μmax (h−1) | YX/S (C-mol C-mol−1) | Concn (C-mM)
|
||||
|---|---|---|---|---|---|---|---|
| Biomass | Lactic acid | Acetic acid | Ethanol | Formic acid | |||
| Glucose | 0.22 | 0.16 | 94.2 | 226.7 | 182.3 | 89.0 | 0 |
| Galactose | 0.51 | 0.32 | 196.0 | 195.7 | 218.6 | 1.3 | 0 |
| Lactose | 0.32 | 0.22 | 133.8 | 174.1 | 202.8 | 13.1 | 0 |
| GOS | 0.41 | 0.18 | 107.6 | 246.1 | 280.6 | 25.1 | 0 |
Mathematical modeling and flux estimation.
C-mM concentrations of biomass, total carbohydrates, ethanol, acetic, lactic, and formic acids were used to estimate the parameters of the unstructured unsegregated kinetic model on glucose, galactose, lactose, and GOS. Values for the best-fit kinetic parameters (μmax, KS, m, Kd, YX/S, YL/X, YL/X, YE/X, and YF/X) are reported in Table 5, which also reports the 95% confidence interval for each parameter and the squared root of RSS values to assess the degree of fit of the model to each experimental data set.
TABLE 5.
Best-fit kinetic parameters calculated from experimental data and statistical analysis of the nonlinear regression results
| Sugar | Parametera | Value | 95% confidence interval
|
|
|---|---|---|---|---|
| Lower value | Upper value | |||
| Glucose | μmax, h−1 | 0.22 | 0.17 | 0.27 |
| KS, C-mM (g liter−1) | 52.4 (1.57) | 47.0 (1.41) | 57.7 (1.73) | |
| YX/S | 0.15 | 0.13 | 0.17 | |
| YL/X | 2.46 | 2.26 | 2.66 | |
| YA/X | 1.96 | 1.83 | 2.09 | |
| YE/X | 1.10 | 0.92 | 1.23 | |
| YF/X | ||||
| m, h−1 | 0.01 | 0.00 | 0.02 | |
| Kd, h−1 | 0.01 | 0.00 | 0.02 | |
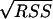 , C-mM , C-mM |
133.5 | |||
| Galactose | μmax, h−1 | 0.55 | 0.51 | 0.59 |
| KS, C-mM (g liter−1) | 32.0 (0.96) | 31.6 (0.95) | 32.4 (0.97) | |
| YX/S | 0.29 | 0.26 | 0.32 | |
| YL/X | 1.14 | 1.08 | 1.20 | |
| YA/X | 1.27 | 1.15 | 1.39 | |
| YE/X | 0.01 | 0.00 | 0.00 | |
| YF/X | ||||
| m, h−1 | 0.00 | 0.00 | 0.01 | |
| Kd, h−1 | 0.01 | 0.00 | 0.02 | |
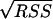 , C-mM , C-mM |
93.0 | |||
| Lactose | μmax, h−1 | 0.32 | 0.28 | 0.36 |
| KS, C-mM (g liter−1) | 38.4 (1.15) | 34.5 (1.04) | 42.3 (1.27) | |
| YX/S | 0.22 | 0.19 | 0.25 | |
| YL/X | 1.29 | 1.19 | 1.39 | |
| YA/X | 1.55 | 1.41 | 1.69 | |
| YE/X | 0.08 | 0.01 | 0.15 | |
| YF/X | ||||
| m, h−1 | 0.02 | 0.00 | 0.04 | |
| Kd, h−1 | 0.01 | 0.00 | 0.02 | |
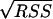 , C-mM , C-mM |
46.7 | |||
| GOS | μmax, h−1 | 0.38 | 0.37 | 0.39 |
| KS, C-mM (g liter−1) | 112.2 (3.37) | 101.3 (3.04) | 123.1 (3.69) | |
| YX/S | 0.18 | 0.13 | 0.23 | |
| YL/X | 2.22 | 2.07 | 2.37 | |
| YA/X | 2.45 | 2.36 | 2.54 | |
| YE/X | 0.26 | 0.21 | 0.31 | |
| YF/X | ||||
| m, h−1 | 0.01 | 0.00 | 0.02 | |
| Kd, h−1 | 0.03 | 0.01 | 0.05 | |
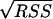 , C-mM , C-mM |
156.0 | |||
Yield coefficients are expressed as C-mol C-mol−1.
The highest maximum specific growth rate (μmax) was obtained on galactose, followed by GOS, lactose, and glucose (0.55, 0.38, 0.32, and 0.22 h−1, respectively). The biomass/substrate yield coefficient (YX/S) was higher on galactose (0.29) than on lactose (0.22), GOS (0.18), and glucose (0.15). Best-fit μmax and YX/S from nonlinear regression were in good agreement with experimental values reported in Table 4. The highest half-saturation constant (KS) was obtained on GOS, followed by glucose, lactose, and galactose in decreasing order (112.2, 52.4, 38.1, and 32.0 C-mM, respectively). No significant differences were observed among both maintenance and cellular death terms.
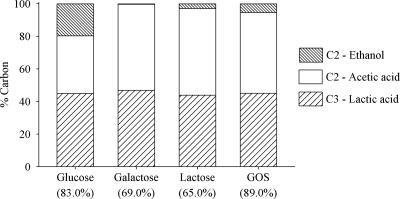
Best-fit product/biomass yield coefficients (YL/X, YA/X, YE/X, and YF/X) were multiplied by the biomass/substrate coefficient (YX/S) to calculate the respective product/substrate coefficients (YL/S, YA/S, YE/S, and YF/S). Since all yield coefficients were expressed as C-mol C-mol−1, product/substrate coefficients corresponded to the carbon yields of the fermentation products. The carbon fluxes giving acetate, lactate, ethanol, and formate were calculated as the ratios between the carbon yields (YL/S, YA/S, YE/S, and YF/S) and the total carbon yield of the fermentation products (YL/S + YA/S + YE/S + YF/S) and expressed as percentages (Fig. 3). Distributions between C3 (lactic acid) and C2 (acetic acid plus ethanol) products were similar on all the carbon sources. In fact, the flux toward lactic acid always accounted for 44.0 to 47.0% of the total flux toward the fermentation products, whereas the flux toward the C2 products ranged between 53.0 and 56.0%. Nevertheless, differences were observed in flux distribution between acetic acid and ethanol; in fact the flux toward ethanol was 0.2, 2.7, 5.4, and 19.6% on galactose, lactose, GOS, and glucose, respectively. The flux toward formic acid was 0% on all the carbon sources.
FIG. 3.
Percent carbon fluxes to lactic acid, acetic acid, and ethanol in batch fermentations of B. adolescentis MB 239 on glucose, galactose, lactose, and GOS. Total carbon yields into fermentation products are reported in parentheses.
The stoichiometry of the fermentative pathway and the knowledge of the carbon distribution among the end products allowed the calculation of ATP yields on the different carbohydrates. One ATP is consumed for each fermented hexose, two are generated in the reactions that lead to pyruvate, and one is generated from each acetylphosphate that is converted to acetate (Fig. 1). Therefore, galactose, lactose, GOS, and glucose yielded 2.4, 2.34, 2.28, and 1.86 mol of ATP per mol of fermented hexose, respectively.
Lactose and GOS consumption in batch cultures of B. adolescentis MB 239.
Carbohydrates were monitored with HPTLC-AMD and OPLC techniques during batch cultures on lactose and on the GOS mixture. When lactose was used as the sole carbon source, the production of glucose and galactose was observed in the first 12 h. Monosaccharides generated from lactose hydrolysis were consumed when lactose ran out (Fig. 4).
FIG. 4.
Batch fermentation of B. adolescentis MB 239 in SM that contained 600 C-mM lactose (a) or GOS (b) as the sole carbon source. Symbols: ▴, biomass; •, glucose; ▾, galactose; ▪, lactose; □, DP 3 ○, DP 4; ▵, DP 5; ▿, DP 6; ⋄, DP 7 to 9.
When the GOS mixture was used as the carbon source, lactose and the trisaccharide were consumed first, whereas glucose was not initially utilized (Fig. 4). Glucose, lactose, and the trisaccharide were simultaneously used after 5 h of culture and were depleted after 12, 14, and 15 h, respectively. None of the oligomers with DPs ranging between 4 and 9 were initially utilized, but all were all rapidly consumed when lactose was depleted (14 h). Throughout the consumption of the oligosaccharides, galactose accumulated in the cultural broth and was consumed when lactose ran out.
β-Gal activity.
Surface and intracellular β-Gal specific activities were monitored during batch cultures growing on glucose, galactose, lactose, and GOS. Surface activity was determined in intact cells; intracellular activity was calculated as the difference between activities in permeabilized and intact cells. On glucose, both intracellular and surface activities were never over 0.9 U mg−1 (Fig. 2). On galactose intracellular β-Gal decreased from 5.3 to 0.8 U mg−1 during the exponential phase; then it increased again during the stationary phase up to 3.0 U mg−1. About 2.0 U mg−1 surface activity was found in the first 5 h of fermentation; a rapid decrease was then observed in the late exponential phase and was followed by a slight increase during the stationary phase (Fig. 2). During growth on lactose, intracellular β-Gal continuously increased throughout the exponential phase up to 10 U mg−1, whereas surface activity was never over 1.1 U mg−1 (Fig. 2). B. adolescentis MB 239 increasingly produced intracellular β-Gal up to 7.2 U mg−1 when the strain was exponentially growing on GOS. As observed in galactose fermentation, surface hydrolytic activity was about 2.0 U mg−1 in the first 5 h, and then it decreased in the late exponential phase and increased again at the stationary phase (Fig. 2).
Extracellular β-Gal levels, analyzed in dialyzed supernatants at the late exponential phase, were always very low: 0.0093, 0.0078, 0.0045, and 0.0034 U mg−1 were obtained on galactose, lactose, glucose, and GOS, respectively (standard deviations in triplicate experiments were 0.0004, 0.0012, 0.0008, and 0.0008 U mg−1, respectively).
DISCUSSION
This study aimed to investigate the fermentative metabolism of Bifidobacterium adolescentis MB 239 on glucose, galactose, and lactose and on a mixture of GOS composed of oligosaccharides with DP ranging between 3 and 9 (58.3%), lactose (19.4%), galactose (3.5%), and glucose (18.8%). An unstructured unsegregated mathematical model that accurately described the kinetics for batch cultures was successfully developed. The kinetic parameters were calculated by means of a Matlab algorithm using experimental data from batch cultures as proposed by Boonmee et al. (4).
Based on the 95% confidence limits, accurate values were obtained for all the calculated parameters. Carbohydrate preferences were established by comparing maximum specific growth rates, cellular yields, and saturation constants. Galactose led to the highest growth rate and cellular yield, whereas glucose was the poorest carbon source for growth. GOS and lactose were both worse growth substrates than galactose but better than glucose, in agreement with a study demonstrating that B. adolescentis MB 239 preferred oligosaccharides over glucose (1).
Previous papers dealing with fermentation of mono- and oligosaccharides by bifidobacteria report wide differences in sugar preferences among oligosaccharides and their monomeric constituents. In fact, monosaccharides were preferred over oligosaccharides in a few cases (19, 24, 44), while growth rates and cell yields were higher on disaccharides and oligosaccharides than on their monomeric moieties in many others (1, 9, 12, 13, 22, 26, 43).
Even if the half-saturation constant cannot be used as a direct measure of microbial affinity for substrates, KS values confirmed that galactose was preferred over lactose and lactose over glucose. The KS value was significantly higher on GOS, since the mixture components were consumed with different kinetics and carbohydrates with lower affinity, which were used at the end of the growth phase, negatively affected the KS estimation. In fact the mathematical model regarded the GOS mixture as a single chemical species, and total carbohydrate concentration (C-mM) was used by the calculation algorithm.
B. adolescentis MB 239 exhibited a stringent selectivity based on the DP since the oligomers with DP 2 (lactose) and 3 were the first to be consumed, and longer oligosaccharides were simultaneously utilized after lactose depletion. The different consumption kinetics of the mixture components are consistent with a decreasing specific growth rate. Polyauxic consumption can be explained by the activity of different enzymes and/or transporters or by the different affinities of enzymes and/or transporters toward the oligomers with different DPs. Unlike the vast majority of documented cases in which monosaccharides are the substrates preferred by microorganisms in a mixed-carbon source environment (6, 32, 33), B. adolescentis MB 239 did not show any preference for glucose that was contained in the commercial mixture, in agreement with a previous study concerning its behavior on mixtures of mono- and oligosaccharides (1). The biochemical effects of lactose on galactose uptake and/or metabolism by bifidobacteria have never been investigated, but a study demonstrated that lactose led to the repression of a glucose-H+ symporter gene, glcP, in B. longum NCC2705, thus explaining the lactose-over-glucose preference in that strain (23) and possibly in B. adolescentis MB 239.
Throughout the batch fermentations on both lactose and GOS, galactose accumulated in the cultural broth, suggesting that β(1-4) galactosides can be hydrolyzed before they are taken up by B. adolescentis MB 239 and that the rate of galactose production was higher than the rate of galactose uptake, leading to a net accumulation into medium. Even if galactose supported the fastest growth, it was not used up before more complex sugars were, in agreement with previous chemostat and batch experiments on single and mixed carbohydrates which also demonstrated that similar extracellular hydrolysis by B. adolescentis MB 239 can occur for raffinose and fructooligosaccharides as well (1). In order to assess activity and location of β-Gal, intracellular, surface, and extracellular specific activities were assayed during batch cultures on GOS, lactose, galactose, and glucose. On all the carbohydrates, very low β-Gal activity was found in the supernatant. On glucose, intracellular β-Gal was scarce. On galactose, the intracellular β-Gal activity that was found in the early hours of cultivation probably came from the corresponding preculture; then it was not produced during exponential phase, or it was produced at a lower rate than biomass was. At the end of growth, when galactose ran out, β-Gal was restored. As expected, lactose was the best inducer of intracellular β-Gal and high levels were also found during growth on GOS. It is remarkable that β-Gal production was not strongly repressed during growth on GOS even if the commercial mixture contained a high glucose concentration. β-Gal activity was found on the surface of B. adolescentis MB 239 during growth on galactose, lactose, and GOS, confirming that both intracellular and extracellular hydrolysis are involved in the utilization of β(1-4) galactosides.
In bifidobacteria, oligosaccharides and polysaccharides are depolymerized down to their monomeric constituents, which are incorporated into the fructose-6-phosphate shunt, giving lactic and acetic acids as the major products and ethanol and formic acid as the minor ones (2, 35, 42). Hexoses are broken down throughout the shunt so that the carbon flux is equally divided into two-carbon and three-carbon molecules. In response to different ATP or NAD+ cellular requirements, pyruvate can be diverted from forming lactate to forming acetylphosphate and formate by the phosphoroclastic reaction, and acetylphosphate can be reduced to ethanol at the expense of acetate production (Fig. 1) (2, 35, 42). Moreover, part of the carbon flux is subtracted to fermentation and is directed to anabolic processes for biomass production. The relative amounts of fermentation products reflect cellular metabolic conditions that are anything but invariable, and it should not be expected that the theoretical lactate/acetate ratio of 1:1.5 is generally obtained. Wide differences in end product formation were actually reported in literature, depending on the strain, the carbon source, the cultural medium, and the growth conditions (22, 24, 31, 37, 42, 44).
In the present study, the yields of fermentation products on glucose, galactose, lactose, and GOS are discussed with respect to ATP gains. Two-carbon molecules (acetate plus ethanol) always accounted for about the 55% of the carbon flux toward products on all the carbon sources, whereas lactate accounted for the remaining 45%. No formate production was observed, suggesting that the phosphoroclastic reaction did not occur in B. adolescentis MB 239; hence, the imbalance between two-carbon and three-carbon products could be due to the efflux of metabolic intermediates from the fermentative shunt toward the anabolic pathways. The enzymes involved in the phosphoroclastic reaction in bifidobacteria are still not biochemically characterized. It is likely that they are formate acetyltransferase (EC 2.3.1.54) and phosphate acetyltransferase (EC 2.3.1.8). The putative genes coding for both the enzymes are present in the genomes of B. adolescentis ATCC 15703 and B. adolescentis L2-32. Nevertheless, this does not ensure that they are expressed by B. adolescentis MB 239 in the experimental conditions of the present study. It is remarkable that different ethanol yields were obtained: no ethanol was produced on galactose and low yields were obtained on lactose and GOS, whereas glucose led to the highest ethanol yield. Ethanol production caused a lower amount of acetylphosphate to be converted into acetate; thus, ATP production was lower, too. Based on the shunt stoichiometry and on the end product yields, ATP production was 25, 8, and 5% lower on glucose, GOS, and lactose, respectively, than on galactose. Sugar uptake mechanisms in B. adolescentis are not known; thus, energetic expenses for transport of glucose, galactose, lactose, and β-galactosides could not be included in calculations. Nevertheless, there was a correspondence among ethanol production, low ATP yields, low maximum specific growth rate, and low biomass yield.
This study extends the knowledge of Bifidobacterium interactions with such important prebiotic carbohydrates as GOS. Moreover it demonstrates that carbohydrate preferences in Bifidobacterium result not only from the efficiency of substrate transport systems as previously suggested (1, 12) but also from different distributions of carbon fluxes through the fermentative pathway.
Footnotes
Published ahead of print on 13 April 2007.
REFERENCES
- 1.Amaretti, A., E. Tamburini, T. Bernardi, A. Pompei, S. Zanoni, G. Vaccari, D. Matteuzzi, and M. Rossi. 2006. Substrate preference of Bifidobacterium adolescentis MB 239: compared growth on single and mixed carbohydrates. Appl. Microbiol. Biotechnol. 73:654-662. [DOI] [PubMed] [Google Scholar]
- 2.Bezkorovainy, A., and R. Miller-Catchpole. 1989. Nutrition and metabolism of bifidobacteria, p. 93-129. In A. Bezkorovainy and R. Miller-Catchpole (ed.), Biochemistry and physiology of bifidobacteria. CRC Press, Inc., Boca Raton, FL.
- 3.Boon, M. A., K. van't Riet, and A. E. Janssen. 2000. Enzymatic synthesis of oligosaccharides: product removal during a kinetically controlled reaction. Biotechnol. Bioeng. 70:411-420. [DOI] [PubMed] [Google Scholar]
- 4.Boonmee, M., N. Leksawasdi, W. Bridge, and P. L. Rogers. 2003. Batch and continuous culture of Lactococcus lactis NZ133: experimental data and model development. Biochem. Eng. J. 14:127-135. [Google Scholar]
- 5.Bouhnik, Y., B. Flouré, L. D'Agay-Abensour, P. Pochart, G. Gramet, M. Durand, and J. C. Rambaud. 1997. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J. Nutr. 127:444-448. [DOI] [PubMed] [Google Scholar]
- 6.Bruckner, R., and F. Titgemeyer. 2002. Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol. Lett. 209:141-148. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, C. C., M. C. Yu, T. C. Cheng, D. C. Sheu, K. J. Duan, and W. L. Tai. 2006. Production of high-content galacto-oligosaccharide by enzyme catalysis and fermentation with Kluyveromyces marxianus. Biotechnol. Lett. 28:793-797. [DOI] [PubMed] [Google Scholar]
- 8.Funk, W., W. Fischer, and H. Wimmer. 1990. Lead(IV) acetate-dichlorofluorescein reagent, p. 325-328. In H. Jork (ed.), Thin-layer chromatography, reagents and detection methods, vol. 1. VCH, Weinheim, Germany. [Google Scholar]
- 9.Gibson, G. R., and X. Wang. 1994. Bifidogenic properties of different types of fructo-oligosaccharides. Food Microbiol. 11:491-498. [Google Scholar]
- 10.Gopal, P. K., P. A. Sullivan, and J. B. Smart. 2001. Utilisation of galacto-oligosaccharides as selective substrates for growth by lactic acid bacteria including Bifidobacterium lactis DR10 and Lactobacillus rhamnosus DR20. Int. Dairy J. 11:19-25. [Google Scholar]
- 11.Hinz, S. W., L. A. van den Broek, G. Beldman, J. P. Vincken, and A. G. Voragen. 2004. Beta-galactosidase from Bifidobacterium adolescentis DSM20083 prefers beta(1,4)-galactosides over lactose. Appl. Microbiol. Biotechnol. 66:276-284. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins, M. J., J. H. Cummings, and G. T. McFarlane. 1998. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 85:381-386. [Google Scholar]
- 13.Kim, T. B., S. H. Song, S. C. Kang, and D. K. Oh. 2003. Quantitative comparison of lactose and glucose utilization in Bifidobacterium longum cultures. Biotechnol. Prog. 19:672-675. [DOI] [PubMed] [Google Scholar]
- 14.Knol, J., P. Sholten, C. Kafka, J. Steenbakkers, S Groß, K. Helm, K. M. Klarczy, H. Shopfer, H. M. Bockler, and J. Well. 2005. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J. Pediatr. Gastroenterol. Nutr. 40:36-42. [DOI] [PubMed] [Google Scholar]
- 15.Kunz, C., S. Rudloff, W. Baier, N. Klein, and S. Strobel. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699-722. [DOI] [PubMed] [Google Scholar]
- 16.Ito, M., Y. Deguchi, K. Matsumoto, M. Kimura, N. Onodera, and T. Yajima. 1993. Influence of galactooligosaccharides on the human fecal microflora. J. Nutr. Sci. Vitaminol. 39:635-640. [DOI] [PubMed] [Google Scholar]
- 17.Ito, M., Y. Deguchi, A. Miyamori, K. Matsumoto, H. Kikuchi, K. Matsumoto, Y. Kobayashi, T. Yajima, and T. Kan. 1990. Effects of administration of galactooligosaccharides on the human fecal microflora, stool weight and abdominal sensation. Microb. Ecol. Health Dis. 3:285-292. [Google Scholar]
- 18.Macfarlane, S., G. T. Macfarlane, and J. H. Cummings. 2006. Review article: prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 24:701-714. [DOI] [PubMed] [Google Scholar]
- 19.Mlobeli, N. T., N. A. Gutierrez, and I. S. Maddox. 1998. Physiology and kinetics of Bifidobacterium bifidum during growth on different sugars. Appl. Microbiol. Biotechnol. 50:125-128. [Google Scholar]
- 20.Møller, P. L., F. Jørgensen, O. C. Hansen, S. M. Madsen, and P. Stougaard. 2001. Intra- and extracellular β-galactosidases from Bifidobacterium bifidum and B. infantis: molecular cloning, heterologous expression, and comparative characterization. Appl. Environ. Microbiol. 67:2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira, A. P., J. Nielsen, and J. Forster. 2005. Modeling Lactococcus lactis using a genome-scale flux model. BMC Microbiol. 5:39-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palframan, R. J., G. R. Gibson, and R. A. Rastall. 2003. Carbohydrate preferences of Bifidobacterium species isolated from the human gut. Curr. Issues Intest. Microbiol. 4:71-75. [PubMed] [Google Scholar]
- 23.Parche, S., M. Beleut, E. Rezzonico, D. Jacobs, F. Arigoni, F. Titgemeyer, and I. Jankovic. 2006. Lactose-over-glucose preference in Bifidobacterium longum NCC2705: glcP, encoding a glucose transporter, is subject to lactose repression. J. Bacteriol. 188:1260-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrin, S., M. Warchol, J. P. Grill, and F. Schneider. 2001. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J. Appl. Microbiol. 90:859-865. [DOI] [PubMed] [Google Scholar]
- 25.Rabiu, B. A., A. J. Jay, G. R. Gibson, and R. A. Rastall. 2001. Synthesis and fermentation properties of novel galacto-oligosaccharides by β-galactosidases from Bifidobacterium species. Appl. Environ. Microbiol. 67:2526-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rada, V., J. Bartonova, and E. Vlkova. 2002. Specific growth rate of bifidobacteria cultured on different sugars. Folia Microbiol. 47:477-480. [DOI] [PubMed] [Google Scholar]
- 27.Rinne, M. M., M. Gueimonde, M. Kalliomaki, U. Hoppu, S. J. Salminen, and E. Isolauri. 2005. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol. Med. Microbiol. 43:59-65. [DOI] [PubMed] [Google Scholar]
- 28.Rossi, M., A. Altomare, A. Gonzalez Vara y Rodriguez, P. Brigidi, and D. Matteuzzi. 2000. Nucleotide sequence, expression and trascriptional analysis of the Bifidobacterium longum MB 219 lacZ gene. Arch. Microbiol. 174:74-80. [DOI] [PubMed] [Google Scholar]
- 29.Rossi, M., C. Corradini, A. Amaretti, M. Nicolini, A. Pompei, S. Zanoni, and D. Matteuzzi. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study in pure and fecal cultures. Appl. Environ. Microbiol. 71:6150-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roy, D., L. Daoudi, and A. Azaola. 2002. Optimization of galacto-oligosaccharide production by Bifidobacterium infantis RW-8120 using response surface methodology. J. Ind. Microbiol. Biotechnol. 29:281-285. [DOI] [PubMed] [Google Scholar]
- 31.Ruas-Madiedo, P., A. Hernandez-Barranco, A. Margolles, and C. G. de los Reyes-Gavilan. 2005. A bile salt-resistant derivative of Bifidobacterium animalis has an altered fermentation pattern when grown on glucose and maltose. Appl. Environ. Microbiol. 11:6564-6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saier, M. H., Jr. 1997. Multiple mechanisms controlling carbon metabolism in bacteria. Biotechnol. Bioeng. 58:170-174. [DOI] [PubMed] [Google Scholar]
- 33.Saier, M. H., Jr., S. Chauvaux, G. M. Cook, J. Deutscher, I. T. Paulsen, J. Reizer, and J. Ye. 1996. Catabolite repression and inducer control in Gram-positive bacteria. Microbiology 142:217-230. [DOI] [PubMed] [Google Scholar]
- 34.Sako, T., K. Matsumoto, and R. Tanaka. 1999. Recent progress on research and applications of non digestible galacto-oligosaccharides. Int. Dairy J. 9:69-80. [Google Scholar]
- 35.Scardovi, V. 1986. Genus Bifidobacterium, p. 1418-1434. In H. Sneath, N. Mair, M. Sharpe, and J. Holt (ed.), Bergey's manual of systematic bacteriology, 9th ed., vol. 2. Williams & Wilkins, Baltimore, MD. [Google Scholar]
- 36.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shene, C., M. Mardones, P. Zamora, and S. Bravo. 2005. Kinetics of Bifidobacterium longum ATCC 15707 fermentations: effect of the dilution rate and carbon source. Appl. Microbiol. Biotechnol. 67:623-630. [DOI] [PubMed] [Google Scholar]
- 38.Smiricky-Tjardes, M. R., C. M. Grieshop, E. A. Flickinger, L. L. Bauer, and G. C. Fahey, Jr. 2003. Dietary galactooligosaccharides affect ileal and total-tract nutrient digestibility, ileal and fecal bacterial concentrations, and ileal fermentative characteristics of growing pigs. J. Anim. Sci. 81:2535-2545. [DOI] [PubMed] [Google Scholar]
- 39.Splechtna, B., T. H. Nguyen, M. Steinbock, K. D. Kulbe, W. Lorenz, and D. Haltrich. 2006. Production of prebiotic galacto-oligosaccharides from lactose using beta-galactosidases from Lactobacillus reuteri. J. Agric. Food Chem. 54:4999-5006. [DOI] [PubMed] [Google Scholar]
- 40.Tzortzis, G., A. K. Goulas, J. M. Gee, and G. R. Gibson. 2005. A novel galacto-oligosaccharide mixtures increases the bifidobacterial population in continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J. Nutr. 135:1726-1731.15987856 [Google Scholar]
- 41.Tzortzis, G., A. K. Goulas, and G. R. Gibson. 2005. Synthesis of prebiotic galactooligosaccharides using whole cells of a novel strain, Bifidobacterium bifidum NCIMB 41171. Appl. Microbiol. Biotechnol. 68:412-416. [DOI] [PubMed] [Google Scholar]
- 42.van der Meulen, R., T. Adriany, K. Verbrugghe, and L. de Vuyst. 2006. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl. Environ. Microbiol. 72:5204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Meulen, R., L. Avonts, and L. de Vuyst. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 70:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van der Meulen, R., L. Makras, K. Verbrugghe,T. Adriany, and L. de Vuyst. 2006. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl. Environ. Microbiol. 72:1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Laere, K. M. J., T. Abee, H. A. Schols, G. Beldman, and A. G. J. Voragen. 2000. Characterization of a novel β-galactosidase from Bifidobacterium adolescentis DSM 20083 active towards transgalactooligosaccharides. Appl. Environ. Microbiol. 66:1379-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vernazza, C. L., G. R. Gibson, and R. A. Rastall. 2006. Carbohydrate preference, acid tolerance and bile tolerance in five strains of Bifidobacterium. J. Appl. Microbiol. 100:846-853. [DOI] [PubMed] [Google Scholar]