Abstract
Enterohemorrhagic Escherichia coli O157:H7 has a natural reservoir in the intestinal tracts of cattle. Colonization is asymptomatic and transient, but it is not clear if protective immunity is induced. This study demonstrates that prior colonization induces humoral immune responses to bacterial antigens and reduces bacterial shedding after experimental challenge with the homologous strain.
Enterohemorrhagic Escherichia coli has emerged in developed countries over the past 20 years as an important cause of human intestinal disease. In addition to bloody diarrhea, intestinal infection can lead to potentially fatal systemic sequelae resulting from the activity of Shiga toxins. The majority of these infections in the United States, Canada, the United Kingdom, and Japan are caused by E. coli O157:H7 (10). This serotype has frequently been isolated from cattle feces, and most human E. coli O157:H7 infections originate, either directly or indirectly, from this source (1). The ability of this serotype to colonize cattle experimentally has been confirmed by a number of groups (2, 5, 7, 14, 18). Consistent features of this colonization include the necessity of the LEE pathogenicity island (4, 12, 16); the lack of overt pathological responses (2, 11); the colonization of mucosal surfaces within the large intestine, in particular the terminal rectum (11); and the ability to maintain relatively high shedding levels for a sustained but not indefinite period (2, 5, 7, 11). A key question relevant to the development of vaccines for the control of E. coli O157:H7 infection is whether the previous exposure of cattle and, more specifically, a period of colonization result in a protective response reducing subsequent colonization and bacterial shedding. Three studies have previously attempted to answer this question (8, 9, 14). The first study (8) demonstrated no effect of colonization 3 weeks previously on fecal shedding, despite the observation of antilipopolysaccharide (anti-LPS) responses in sera. The second study (9) demonstrated no effect on subsequent shedding of the homologous strain, but the times between challenges were 22 and 33 weeks (two calves for each interval were examined). The authors of the third study (14) observed a small reduction in the shedding duration following rechallenge, but the results concerning a difference among successive challenges were unclear as the number of calves used was small (n = 4), there was no naïve control group, and the calves underwent considerable physiological development between the two challenges, changing from milk-fed preruminants to weaned ruminants. Evidence that cattle develop immunity to this organism is provided by epidemiological data that suggest a lower prevalence in adult cattle than weaned calves (13, 15). However, it is not clear if this phenomenon is a consequence of specific responses to prior E. coli O157:H7 infection.
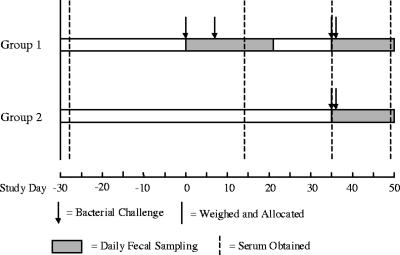
In the present study, the effects of prior colonization with E. coli O157:H7 in experimentally challenged calves were assessed. A total of 25 calves were assigned to treatment groups based on initial body weights (mean weights at day −30: group 1, 76.7 kg, and group 2, 77.1 kg). All calves were confirmed to be negative for E. coli O157:H7 upon arrival at the Moredun Research Institute, Penicuik, United Kingdom, as determined by standard fecal enrichment-immunomagnetic separation procedures described previously (11). Eight calves received two initial oral challenges, as described previously (11), of 4 × 109 CFU (day 0) and 1 × 1010 CFU (day 7) to serve as the previously exposed group (group 1). The 17 naïve calves that had no prior exposure to the challenge strain were housed in three pens but were combined into a single control group (group 2) for the purpose of assessing the effects of previous colonization. Four weeks following the second initial challenge, all calves received the test challenge (2 × 1010 CFU administered orally on two consecutive days, days 35 and 36), and the resulting bacterial fecal shedding was measured daily to assess the effects of prior colonization. The degree of colonization of each calf was summarized by calculating the area under the curve (AUC) of bacterial shedding, as estimated by the trapezoidal method, over the 15-day period following challenge. The timing of all these events is summarized in Fig. 1.
FIG. 1.
Timeline illustrating the chronology of the study. The timing of bacterial challenges, weighing, fecal sampling, and blood sampling is indicated.
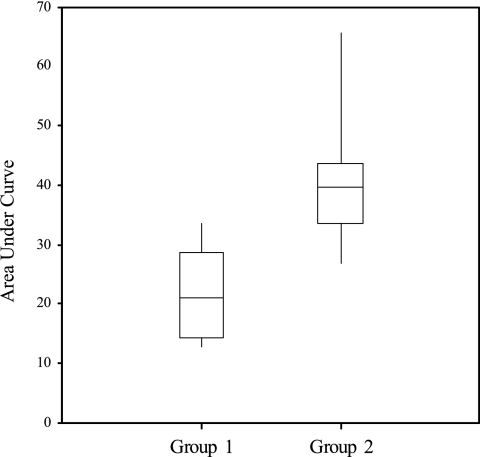
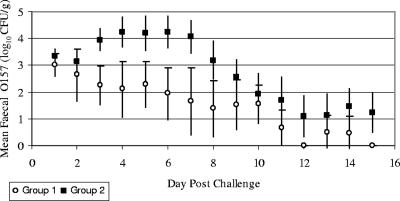
The estimated AUCs for groups 1 and 2 following the test challenge (Fig. 2) were compared by one-way analysis of variance, and the analysis confirmed statistically significantly higher levels of shedding in group 2 (F < 0.001), with means of 22.0 and 40.7, respectively, and a standard error of difference of 4.3. For illustrative purposes, these figures would respectively equate to levels of fecal shedding of approximately 103 CFU/g for 7 consecutive days and 104 CFU/g for 10 consecutive days. Group mean daily fecal E. coli O157:H7 concentrations are compared in Fig. 3. The difference between groups was apparent from day 3 postchallenge. The group 1 mean steadily decreased following challenge, while the group 2 mean increased from day 3 postchallenge. High levels for group 2 were sustained for several days and decreased towards the end of the sampling period to levels similar to those for group 1. Thus, there is strong evidence, over the time scale observed, that prior colonization reduced subsequent E. coli O157:H7 fecal shedding and presumably mucosal colonization after a secondary challenge with the homologous strain. The shedding data resulting from the initial challenges of group 1 calves are not statistically comparable with the subsequent data, as calves were challenged on two occasions, 1 week apart, to maximize the number of successfully colonized animals. This meant that the 15-day periods after these challenges overlapped and therefore would potentially skew any AUC calculations.
FIG. 2.
Box and whisker plots of the estimated areas under the shedding curves for groups 1 and 2 following the simultaneous test challenge. The boxes indicate the interquartile ranges, the horizontal lines within the boxes represent the median values, and whiskers extend to the minimum and maximum values.
FIG. 3.
Daily group mean fecal E. coli O157:H7 concentrations (expressed as log10 numbers of CFU per gram plus one) throughout the sampling period. Days postchallenge are the number of days after the first test challenge was administered (day 35). Error bars indicate 95% confidence intervals.
In order to measure humoral antibody responses to colonization, levels of immunoglobulins G and A (IgG and IgA) specific for O157 LPS and H7 flagellin in sera were quantified by an enzyme-linked immunosorbent assay (ELISA) (Table 1). Serum samples were collected on days −28, 14, 35, and 49. Briefly, purified O157 LPS (List Biological Laboratories) was conjugated to polymyxin B as previously described (6) and used as a coating antigen for an indirect ELISA. H7 flagellin was purified by the method described by Sherman et al. (17) and used as an ELISA coating antigen. Twofold-dilution series of test and standard sera were applied to coated plates for 1 h. Sheep anti-bovine IgG or IgA conjugated with horseradish peroxidase (Serotec) at 1 in 800 was used as a secondary antibody. Sigma-Fast o-phenylenediamine dihydrochloride (Sigma-Aldrich) was used as the substrate. Plates were read in a Dynex Revelation 3.04 ELISA plate reader at 492 nm, and end point titers were determined.
TABLE 1.
Serum anti-LPS and anti-H7 IgG and IgA titers in the two experimental groups, as determined by ELISA
| Day | Titera (SE) of indicated antibody in samples from:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Group 1
|
Group 2
|
|||||||
| Anti-LPS IgG | Anti-LPS IgA | Anti-H7 IgG | Anti-H7 IgA | Anti-LPS IgG | Anti-LPS IgA | Anti-H7 IgG | Anti-H7 IgA | |
| −28 | 0.4 (0.3) | 0.0 (0.0) | 1.4 (0.2) | 0.0 (0.0) | NS | NS | NS | NS |
| 14 | 2.1 (0.4) | 4.3 (0.6) | 1.3 (0.3) | 2.4 (0.6) | NS | NS | NS | NS |
| 35 | 2.6 (0.2) | 2.6 (0.5) | 2.3 (0.5) | 1.3 (0.3) | 1.1 (0.2) | 0.4 (0.2) | 1.0 (0.3) | 0.0 (0.0) |
| 49 | 5.0 (0.5) | 3.3 (0.5) | 2.9 (0.2) | 1.8 (0.7) | 4.7 (0.3) | 4.9 (0.3) | 3.8 (0.3) | 2.4 (0.4) |
Numbers are mean dilutions in serum, expressed as log2 values, corresponding to end point titers determined relative to the negative standard, as measured by ELISA. NS, no sample taken at this time.
There were antibody responses to colonization, as demonstrated by group means (Table 1), although the magnitudes of these responses varied within groups. Of particular significance is the first demonstration of a circulating-IgA response to O157 and H7 antigens following the E. coli O157:H7 colonization of cattle. For each animal, end point titers of anti-H7 and anti-LPS antibodies were compared, as were the AUC and the response of each antigen-Ig combination. All correlations yielded very low and insignificant r values, and it cannot be concluded that the antibodies produced against these specific antigens contributed to the protective effect observed in group 1. However, the mean titers of all antigen-Ig combinations at the time of the test challenge (day 35) were higher in group 1 than in the naïve group 2 calves (Table 1), and it is possible that these or similarly induced antibodies to other bacterial factors mediated or contributed to the observed protection. It is an established phenomenon that IgA responses are typically shorter lived than IgG responses (3), and interestingly, within group 1 calves, the serum IgA but not IgG titers fell substantially between the end of the initial colonization period (day 14) and the test challenge (day 35). Thus, it is possible that the lack of a longer protective effect from prior colonization observed by Johnson et al. (9) can be attributed to the further waning of protective antibodies. One interesting observation is the relatively weak IgA response of group 1 to the test challenge (between days 35 and 49) compared with that of group 2. This finding may be simply a consequence of the inferior bacterial colonization of group 1, or it may be related to the active migration from the circulation of specific IgA-producing B lymphocytes to mucosal sites of antigen exposure. In order to support the latter possibility, it would have been useful to quantitate mucosal IgA from relevant sites.
The colonization of cattle by E. coli O157:H7 is generally viewed as clinically inapparent. However, we have found a microscopic inflammatory infiltration of the colonized rectal mucosa (our unpublished results), and this paper and previous work (9) have shown that colonized calves develop serological responses to specific antigens. This paper also illustrates that calves are partially protected, at least in the short term, against subsequent challenge with the homologous strain. It is thus feasible that an enhancement of the protective response could offer a realistic method for the control of the pathogen. Following experimental challenge with this bacterium, there is typically a period of fecal shedding, followed by a reduction or elimination of the shedding (references 2, 5, 7, 11, 14, and 18 and this study). It is likely that this reduction is mediated by the same mechanisms responsible for the reduction in shedding resulting from previous colonization as described in this paper. The mechanisms may be attributable to the development of an acquired immunity or may result from short-lived protection influenced by innate responses, such as the mobilization of polymorphonuclear granulocytes or the secretion of antimicrobial peptides. The data presented here clearly demonstrate that protection is induced by colonization, but more extensive study is necessary to obtain an estimate of the likely degree and duration of such protection, to determine if it mediates protection from challenge with heterologous strains, and to clarify the immune mechanisms involved. In the future, it is recommended that mucosally secreted antibodies at the site of colonization be measured for correlation with the degree of protection from challenge.
Acknowledgments
We thank Moredun Research Institute clinical and farm staff for the care of calves used in this study and appreciate the assistance of Moredun Scientific Ltd. in conducting this experiment. Thanks to Ian Nevison of Biomathematics and Statistics Scotland for his advice regarding the analysis of the bacterial shedding data.
We acknowledge financial support from Novartis Animal Vaccines Ltd. and note that the Scottish Agricultural College receives funding from the Scottish Executive Environment and Rural Affairs Department.
Footnotes
Published ahead of print on 20 April 2007.
REFERENCES
- 1.Besser, R. E., P. M. Griffin, and L. Slutsker. 1999. Escherichia coli O157:H7 gastroenteritis and the hemolytic uremic syndrome: an emerging infectious disease. Annu. Rev. Med. 50:355-367. [DOI] [PubMed] [Google Scholar]
- 2.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler, J. E. 1983. Bovine immunoglobulins: an augmented review. Vet. Immunol. Immunopathol. 4(102):43-152. [DOI] [PubMed] [Google Scholar]
- 4.Cornick, N. A., S. L. Booher, and H. W. Moon. 2002. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infect. Immun. 70:2704-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Currie, C. G., and I. R. Poxton. 1999. The lipopolysaccharide core type of Escherichia coli O157:H7 and other non-O157 verotoxin-producing E. coli. FEMS Immunol. Med. Microbiol. 24:57-62. [DOI] [PubMed] [Google Scholar]
- 7.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffman, M. A., C. Menge, T. A. Casey, W. Laegreid, B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 13:1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson, R. P., W. C. Cray, Jr., and S. T. Johnson. 1996. Serum antibody responses of cattle following experimental infection with Escherichia coli O157:H7. Infect. Immun. 64:1879-1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. E. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. E. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonisation requires the LEE4 operon. Microbiology 151:2773-2781. [DOI] [PubMed] [Google Scholar]
- 13.Renter, D. G., J. M. Sargeant, and L. L. Hungerford. 2004. Distribution of Escherichia coli O157:H7 within and among cattle operations in pasture-based agricultural areas. Am. J. Vet. Res. 65:1367-1376. [DOI] [PubMed] [Google Scholar]
- 14.Sanderson, M. W., T. E. Besser, J. M. Gay, C. C. Gay, and D. D. Hancock. 1999. Fecal Escherichia coli O157:H7 shedding patterns of orally inoculated calves. Vet. Microbiol. 69:199-205. [DOI] [PubMed] [Google Scholar]
- 15.Sandhu, K. S., R. C. Clarke, K. McFadden, A. Brouwer, M. Louie, J. Wilson, H. Lior, and C. L. Gyles. 1996. Prevalence of the eaeA gene in verotoxigenic Escherichia coli strains from dairy cattle in Southwest Ontario. Epidemiol. Infect. 116:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng, H., J. Y. Lim, H. J. Knecht, J. Li, and C. J. Hovde. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 74:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman, P., R. Soni, and H. Yeger. 1988. Characterization of flagella purified from enterohemorrhagic, vero-cytotoxin-producing Escherichia coli serotype O157:H7. J. Clin. Microbiol. 26:1367-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wray, C., I. M. McLaren, L. P. Randall, and G. R. Pearson. 2000. Natural and experimental infection of normal cattle with Escherichia coli O157:H7. Vet. Rec. 147:65-68. [DOI] [PubMed] [Google Scholar]