Abstract
Bacillus sphaericus cannot metabolize sugar since it lacks several of the enzymes necessary for glycolysis. Our results confirmed the presence of a glucokinase-encoding gene, glcK, and a phosphofructokinase-encoding gene, pfk, on the bacterial chromosome and expression of glucokinase during vegetative growth of B. sphaericus strains. However, no phosphoglucose isomerase gene (pgi) or phosphoglucose isomerase enzyme activity was detected in these strains. Furthermore, one glcK open reading frame was cloned from B. sphaericus strain C3-41 and then expressed in Escherichia coli. Biochemical analysis revealed that this gene encoded a protein with a molecular mass of 33 kDa and that the purified recombinant glucokinase had Km values of 0.52 and 0.31 mM for ATP and glucose, respectively. It has been proved that this ATP-dependent glucokinase can also phosphorylate fructose and mannose, and sequence alignment of the glcK gene indicated that it belongs to the ROK protein family. It is postulated that the absence of the phosphoglucose isomerase-encoding gene pgi in B. sphaericus might be one of the reasons for the inability of this bacterium to metabolize carbohydrates. Our findings provide additional data that further elucidate the specific metabolic pathway and could be used for genetic improvement of B. sphaericus.
Bacillus sphaericus is an aerobic, mesophilic, spore-forming bacterium with terminal swollen sporangia and spherical spores. As a consequence of the specific toxicity to mosquito larvae of binary toxin (Bin) and mosquitocidal toxins (Mtxs) produced during the sporulation and vegetative stages, respectively, some toxic strains have been widely used for many years as biopesticides in the field in mosquito control programs.
B. sphaericus can metabolize a wide variety of organic compounds and amino acids, but it is unable to use hexoses and pentoses as unique carbon sources (20). The specific metabolic limitation of B. sphaericus hampers its potential industrial development due to the high costs of the proteinaceous media used for toxin production compared to the costs of alternative media based on starch or molasses. Consequently, studies elucidating the whole metabolic potential of B. sphaericus are essential for genetic improvement of this bacterium.
Previous research indicated that the inability of B. sphaericus to metabolize carbohydrates could be attributed to its inability to transport glucose or sucrose into the cell (24) and the absence of key enzyme activities in the Embden-Meyerhof-Parnas (EMP) (glucokinase, glucose-6-phosphate isomerase, 6-phosphofructokinase), hexose monophosphate pathway (phosphogluconate dehydratase), and Entner-Doudoroff (6-phospho-2-keto-3-deoxyglyconate aldolase) pathways (20). Recent studies revealed that B. sphaericus strain 2362 has a glucose transport system (2) and that it also has 6-phosphofructokinase activity; thus, it could use N-acetylglucosamine as a sole carbon source (3). However, there are no data available about the presence of gene and enzyme activities of glucose kinase (ATP:α-d-glucose-6-phosphotransferases; EC 2.7.1.2), an enzyme catalyzing the ATP-dependent conversion of glucose to glucose 6-phosphate, in the EMP pathway of B. sphaericus.
The glucokinase of Bacillus belongs to the ROK family (repressor, open reading frame, and kinase) (11). Albano et al. demonstrated that the ROK protein was a repressor of a family of genes that encode membrane-localized and secreted proteins, including a number of genes that encode products with antibiotic activity (1). Aside from the kinase activity, glucokinase of Bacillus spp. has some regulatory functions for host cell growth and development.
B. sphaericus C3-41, belonging to flagellum serotype H5a5b like strains 2362 and 1593, is a highly active strain that was isolated from a mosquito-breeding site in China in 1987. This strain has various levels of toxicity against Culex sp., Anopheles sp., and Aedes sp. and has significantly higher activity against Culex spp. than commercialized strain 2362 (29); thus, it has been developed as a commercial larvicide (JianBao) and has been used for mosquito larva control in China during the last decade (27).
Considering the lack of information about the sugar metabolism behavior of B. sphaericus, it is important to investigate the genes involved in glycolysis or metabolism of sugar. In this paper, the glucokinase-encoding gene glcK, the phosphofructokinase-encoding gene pfk, and the phosphoglucose isomerase-encoding gene pgi were detected in wild-type B. sphaericus strains. Additionally, glucokinase and phosphofructokinase activities were also detected in these strains. Furthermore, the glcK gene was cloned from B. sphaericus strain C3-41, and the purified expressed glucokinase was characterized. To our knowledge, this is the first report on the characterization of GlcK from B. sphaericus. The results should help to elucidate the metabolic mechanisms of the organism, as well as provide a foundation for optimization of the genetic makeup of B. sphaericus for commercial applications.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The following B. sphaericus strains from our laboratory were used in this study: KellenQ (serotype H1a), LP1-G (serotype H3), IAB881 (serotype H3), Dak614 (serotype H4), NRS1184 (serotype H5), C3-41 (serotype H5a5b), 2362 (serotype H5a5b), IAB763 (serotype H6), IAB769 (serotype H6), IAB59 (serotype H6), COK31 (serotype H9), 2173 (serotype H26), 2317-2 (serotype H26), and IAB872 (serotype H48). All B. sphaericus strains were grown at 30°C in MBS broth [0.68% KH2PO4, 1% tryptone, 0.2% yeast extract, 0.03% MgSO4·7H2O, 0.002% CaCl2·2H2O, 0.002% MnSO4·H2O, 0.002% ZnSO4·7H2O, 0.002% Fe2(SO4)3; pH 7.2]. For comparison of the enzyme activity produced by B. sphaericus strains in a different medium, NYSM medium was used to grow the bacteria for the glucokinase activity assay (26). A glk mutant Escherichia coli strain, ZSC13 [λ− glk-3 relA1 rpsL150 (strR) bglR6], was obtained from the E. coli Genetic Stock Center of Yale University. E. coli ZSC13 and E. coli strains DH5α and BL21, as well as the ZSC13 recombinant E-pUC-glck and the BL21 recombinant E-pET-glck, were grown at 37°C in Luria-Bertani (LB) medium supplemented with 30 μg kanamycin ml−1or 50 μg ampicillin ml−1.
Detection of glcK, pfk, and pgi genes in B. sphaericus strains.
Based on the genomic sequence and the primary gene annotation results for B. sphaericus strain C3-41, two pairs of PCR primers, primers glck-1 (5′-GG GGA TCC ATG AGT CAT ATT TTA-3′) and glck-2 (5′-GG AAG CTT CAT TGC TGC ACC ATA-3′) and primers pfk-1 (5′-CG GGA TCC ATG AAA AAA ATT GCT-3′) and pfk-2 (5′-CG AAG CTT TTA TAT TGA TAG TTC-3′) (containing BamHI/HindIII restriction sites [underlined]), were designed and synthesized for glcK- and pfk-specific fragment detection. The presence of the pgi gene in B. sphaericus strains was detected by PCR by using primer pgi-1 (5′-GG GCA TGC ATG AGT ACA CAT GTA AG-3′) and primer pgi-2 (5′-CC AAG CTT TTA TTT TAA ACG CTC T-3′), which was designed based on the sequence of the pgi gene from Bacillus cereus E33L (GenBank accession no. YP_086203) and Bacillus subtilis subsp. subtilis 168 (GenBank accession no. NP 391013). The presence of the three genes in 14 B. sphaericus strains was detected by PCR with total bacterial genomic DNA as the template (5).
Glucokinase, phosphoglucose isomerase, and phosphofructokinase activity assay.
B. sphaericus strains were incubated in MBS and/or NYSM broth at 30°C with agitation (200 rpm) for 48 h. Cells in different growth stages were collected every 4 h and then lysed by sonication at 4°C in buffer (20 mM Tris-HCl [pH 7.5], 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride) and centrifuged at 4°C and 12,000 rpm for 15 min, and the supernatant was then drawn out and used as the crude enzyme preparation. B. subtilis subsp. subtilis 168 was used as the positive control.
Specific glucokinase activities of the crude enzyme samples of Bacillus spp. and the purified GlcK obtained from the recombinant strain were determined by a coupled enzyme assay using the protocol of Sigma (19, 22). Each sample was added to assay buffer (75 mM Tris-HCl [pH 9.0], 600 mM MgCl2, 120 mM ATP, 360 mM glucose, 27 mM NADP, 1 U glucose-6-phosphate dehydrogenase), and the mixture was left for 5 min at 30°C. The optical density at 340 nm was determined for each sample by using a Synergy HT multidetection microplate reader (BIO-TEK). One unit of glucokinase activity was defined as the activity phosphorylating 1.0 μmol of d-glucose to d-glucose 6-phosphate per min at pH 9.0 at 30°C. Measurements were duplicated for each sample, and tests were repeated on at least three different days.
The phosphoglucose isomerase activities and phosphofructokinase activities of the crude enzyme preparations of Bacillus spp. were assayed separately using the methods described by Mathur et al. (15) and Alice et al. (3), respectively.
Cloning procedures and sequencing analysis.
Plasmids were isolated from E. coli by a standard alkaline lysis procedure. Cloning experiments and restriction enzyme analysis were carried out as described previously (21). Total genomic DNA was prepared from B. sphaericus C3-41 by the method of Bourgouin et al. (5).
The glcK gene was amplified by PCR from B. sphaericus strain C3-41 chromosomal DNA by using PCR primers glck-1 and glck-2. The PCR mixture (total volume, 50 μl) comprised 0.5 μg chromosomal DNA, 100 pmol of each primer, and 1.25 U Pfu polymerase (Fermentas). The following PCR procedure was used: 94°C for 5 min, followed by 30 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 1 min and then a final extension at 72°C for 7 min. A ∼896-bp PCR fragment was cloned into the vector pUC18 in E. coli DH5α, giving recombinant plasmid pUC-glck. The amplified PCR fragment was digested by BamHI and HindIII and then introduced into BamHI- and HindIII-digested plasmid pET28a (Novagen), a His tag expression vector, resulting in recombinant plasmid pET-glck.
The cloned glcK gene was sequenced, the nucleotide and amino acid sequence homologies of glucokinases from B. sphaericus and other 13 bacteria were analyzed by using BLASTT and CLUSTALW, and the evolutionary distances and relationships were calculated by the neighbor-joining method.
Expression and purification of GlcK protein.
The recombinant plasmids pUC-glck and pET-glck were transformed into glk-deficient E. coli mutant strain ZSC13 and E. coli BL21, referred to below as recombinant E. coli strains E-pUC-glck and E-pET-glck, respectively. Overnight cultures of recombinant strains E-pUC-glck and E-pET-glck were transferred into 20 ml fresh LB medium containing kanamycin (30 μg ml−1), and then the cultures were incubated for 3 h at 37°C with shaking (200 rpm) and 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to induce the expression of GlcK. After 4 h of incubation at 30°C with shaking, each culture was collected and used for sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) analysis (14), and the GlcK activity was assayed by the method described above (8, 22).
For large-scale expression and purification of GlcK in E-pET-glck, the overnight culture of this strain was transferred into 500 ml of fresh LB medium and cultured under the conditions described above. Cells were harvested by centrifugation (10,000 × g, 4°C) and stored at −20°C. Recombinant GlcK was purified by using a His-Bind Resin chromatography kit following the manufacturer's procedure (Novagen). Cells were lysed by sonication at 4°C, and the cell debris was removed by centrifugation (14,000 × g, 20 min, 4°C). The supernatant was filtered with a 0.45-μm membrane, and the collected elutant was added to an equilibrated Ni-nitrilotriacetic acid resin column (Novagen) and left to stand at room temperature for elution. The collected eluted solution was dialyzed in buffer A (20 mM Tris-HCl [pH 8.0], 40 mM KCl, 0.1 mM EDTA) and then stored in buffer B (20 mM Tris-HCl [pH 8.0], 40 mM KCl, 0.1 mM EDTA, 10 mM mercaptoethanol, 50% [vol/vol] glycerol) at −70°C.
Properties and kinetic parameters of glucokinase.
The pH-dependent purified glucokinase activities were measured by using a continuous assay in Tris-HCl buffer at pH values ranging from 3.0 to 9.0 (pH 3 to 4, 4 to 5.5, 5.5 to 6.5, 6.5 to 7.5, 7.5 to 8.5, and 8 to 9) at 30°C. The effect of temperature on glucokinase activity was determined by using 25, 30, 35, 37, 40, 42, 45, 48, and 50°C. The activities of the purified protein were assayed as described above (12).
To determine whether GlcK is a hexokinase or a specific glucokinase, several hexoses were tested to determine the phosphorylating activity of the protein. Purified GlcK (5.0 μl, 0.4 mg/ml) was mixed separately with 50 mM glucose, fructose, and mannose in buffer containing 50 mM Tris-HCl (pH 7.65), 10 mM MgCl2, and 50 mM ATP. Each mixture was incubated at 30°C for 20 min (22), and then the resulting products were analyzed by silica-coated thin-layer chromatography by developing the plates with butanol-ethanol-water (5:3:2, vol/vol/vol), and the sample spots were visualized by dipping the plates in a mixture of aniline-diphenylamine-phosphate, followed by drying for 10 min at 85°C (16). A parallel mixture without ATP was used to investigate whether the GlcK from B. sphaericus C3-41 was ATP dependent.
Nucleotide sequence accession number.
The nucleotide sequence of the cloned glcK gene has been deposited in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession number EF065663.
RESULTS
glcK, pfk, and pgi gene detection and the corresponding enzyme activities in B. sphaericus.
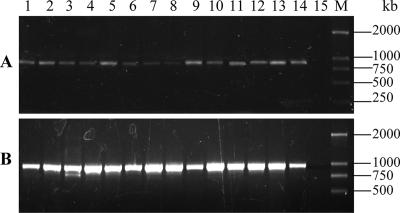
Using primers glck-1 and glck-2 and primers pfk-1 and pfk-2, ∼880-bp and ∼960-bp DNA fragments, respectively, were amplified from all B. sphaericus strains tested by PCR (Fig. 1A and B), indicating that the glcK and pfk genes were both present in wild-type B. sphaericus strains. No specific DNA fragment was amplified by PCR by using primers pgi-1 and pgi-2.
FIG. 1.
Detection of the glcK gene (A) and the pfk gene (B) in 14 B. sphaericus strains by PCR. Lanes 1 to 15 contained KellenQ (serotype H1a), LP1-G (serotype H3), IAB881 (serotype H3), Dak614 (serotype H4), NRS1184 (serotype H5), C3-41 (serotype H5a5b), IAB763 (serotype H6), IAB769 (serotype H6), IAB59 (serotype H6), 2173 (serotype H26), 2317-2 (serotype H26), IAB872 (serotype H48), Cok31 (serotype H9), 2362 (serotype H5a5b), and a negative control, respectively. Lane M contained the DL2000 DNA marker (TaKaRa).
A further glucokinase activity assay revealed that all B. sphaericus strains tested expressed glucokinase protein in the vegetative growth stage, with activities varying from 120 to 1.73 × 103 nmol/min/mg cell extract (Table 1). However, the glucokinase activities of different strains were about 10-fold lower on NYSM medium than on MBS medium (Table 1). The phosphofructokinase activities produced by B. sphaericus strains in MBS medium were comparable to the results of Alice et al. (3) (data not shown). As expected, no phosphoglucose isomerase activity was detected in any B. sphaericus strain.
TABLE 1.
Glucokinase activities in the vegetative growth stage of wild-type B. sphaericus strains
| Organism | Serotype | Virulence (activity with Culex spp.) | GlcK activities (nmol/min/mg cell extract) in:
|
|
|---|---|---|---|---|
| MBS medium | NYSM medium | |||
| Bacillus sphaericus strains | ||||
| 2173 | H26 | High | 434 ± 35 | 49.8 ± 7.1 |
| IAB769 | H6 | High | 370 ± 19 | 55.3 ± 4.3 |
| IAB872 | H48 | High | 203 ± 15 | 23.7 ± 5.8 |
| KellenQ | H1a | Low | 120 ± 13 | 19.9 ± 4.9 |
| LP1-G | H3 | Medium | 351 ± 24 | 32.7 ± 8.0 |
| Dak614 | H4 | None | 277 ± 7.3 | 16.4 ± 6.7 |
| NRS1184 | H5 | None | 277 ± 11 | 29.3 ± 3.6 |
| Cok31 | H9 | Medium | 305 ± 26 | 23.6 ± 8.2 |
| IAB59 | H6 | High | 250 ± 7.9 | 20.7 ± 3.3 |
| 2317-2 | H26 | High | 1,730 ± 150 | 75.3 ± 9.5 |
| IAB881 | H3 | High | 240 ± 14 | 28.6 ± 7.5 |
| IAB763 | H6 | High | 139 ± 10 | 26.9 ± 6.2 |
| 2362 | H5a5a | High | 166 ± 31 | 59.5 ± 7.8 |
| 2297 | H25 | High | 222 ± 29 | 54.7 ± 5.5 |
| Bacillus subtilis | 157 ± 20 | 29.7 ± 4.8 | ||
In B. sphaericus C3-41, glucokinase was continuously expressed during the all growth stages, and the peak value for GlcK activity occurred in the stationary growth phase before spores formed (Fig. 2); this value was 670 nmol/min/mg cell extract, corresponding to 1.85 mmol NADP reduced per min per mg cell extract.
FIG. 2.
Expressed GlcK activity in different growth stages of B. sphaericus C3-41. OD600, optical density at 600 nm.
Cloning and sequencing of B. sphaericus C3-41 glcK gene.
Sequence analysis of the ∼880-bp amplified fragment showed that the fragment sequence was composed of 876 bp encoding 292 amino acids with a predicted molecular mass of 32.6 kDa. This protein had 30% identity to the ROK family (regulators, open reading frames, and kinases), and a DVGGT motif (an ATP binding site) was found in its N terminus (Fig. 3A). Additionally, in the C terminus, an α-helix-turn-α-helix DNA binding motif was found. An alignment of glucokinase sequences from different bacteria and an associated phylogenetic tree were constructed by using CLUSTALW and the neighbor-joining method (Fig. 3B).
FIG. 3.
CLUSTALW multiple-sequence alignment of GlcK for 14 species. (A) The first box indicates the conserved ATP-binding site, and the second box indicates the conserved sequence of the ROK protein. (B) Neighbor-joining tree showing the phylogenetic relationships according to the GlcK sequence.
Expression and purification of glucokinase.
The glcK gene was first transferred to glucokinase-deficient E. coli strain ZSC13 and recombinant strain E-pUC-glck. The latter strain exhibited a GlcK activity of 126 ± 28 nmol/min/mg cell extract, while GlcK-deficient strain ZSC13 had an activity of 3.01 ± 0.32 nmol/min/mg cell extract, suggesting that the expression of GlcK in E-pUC-glck could complement the GlcK activity of GlcK-deficient strain ZSC13.
Overproduction of the glucokinase was achieved in E. coli BL21 after IPTG induction in LB medium for at least 4 h. This enzyme produced a major band at about 33 kDa on an SDS-PAGE gel. The glucokinase was purified with an Ni-nitrilotriacetic acid resin column (Fig. 4), and the protein concentration was determined by the method of Bradford to be 0.4 mg/ml (stored buffer containing 50% glycerol). A 500-ml culture yielded about 1.29 mg of pure protein.
FIG. 4.
SDS-PAGE of purified glucokinase from recombinant E. coli E-pET-glck (BL21). Lane 1, crude cell extract from E-pET-glck; lane 2, purified GlcK; lane M, protein molecular marker (Fermentas).
Kinetic parameters of glucokinase.
GlcK had Km values of 0.52 and 0.31 mM for ATP and glucose, respectively. The activities of the purified glucokinase with different hexoses were diverse: 1.95 ± 0.93 μmol/min/mg GlcK for glucose, 0.786 ± 0.183 μmol/min/mg GlcK for fructose, and 0.941 ± 0.213 μmol/min/mg GlcK for mannose. The optimal pH was pH 8.5, and the optimum reaction temperature was 30°C.
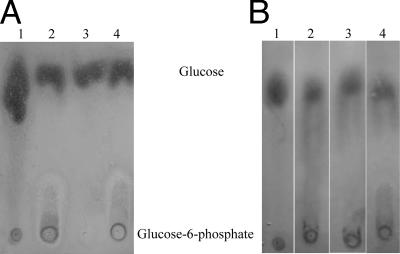
In a coupled enzyme assay and thin-layer chromatography assay, no significant glucokinase activity was detected without ATP (Fig. 5A). We concluded that the glucokinase from B. sphaericus is an ATP-dependent hexokinase (Fig. 5B).
FIG. 5.
Phosphorylation of glucose and other hexoses by purified glucokinase. Twenty-microliter samples of each reaction mixture were spotted onto a thin-layer chromatography plate. (A) Mixtures contained the buffer and 10 μl of 50 mM d-glucose (lane 1), glucose, ATP, and GlcK from B. sphaericus (lane 2), glucose and GlcK from B. sphaericus (lane 3), and glucose, ATP, and GlcK from B. subtilis cell extract (lane 4). (B) Mixtures contained the buffer and glucose as a control (lane 1), 50 mM glucose, ATP, and GlcK (lane 2), 50 mM fructose, ATP, and GlcK (lane 3), and 50 mM mannose, ATP, and GlcK (lane 4).
DISCUSSION
Previous reports revealed that the lack of several enzymes in B. sphaericus, including glucokinase, phosphoglucose isomerase, and phosphofructokinase in the glycolysis pathway, resulted in this bacterium being unable to catabolize glucose (20). In this study, we confirmed the presence of the glcK and pfk genes in all wild-type B. sphaericus strains and that all strains could exhibit high levels of glucokinase and phosphofructokinase activities during vegetative growth. However, no correlation was noticed between the enzyme activity and the toxicity of the bacterium for target mosquito larvae. Even if the B. sphaericus strains tested could produce GlcK activity in both MBS and NYSM media, the GlcK activity was 10-fold lower in low-salt NYSM medium than in high-salt MBS medium (Table 1). This difference might be caused by the different responses of bacterial cells to osmotic stress. No pgi gene fragment and no phosphoglucose isomerase activity were detected in these strains. Furthermore, a glcK gene from B. sphaericus C3-41 was cloned and sequenced, and the glucokinase activity of the expressed protein was verified. Our primary genomic sequence analysis of B. sphaericus C3-41 showed that a glcK-specific DNA fragment and a pfk-specific DNA fragment were present on the bacterial chromosome and that no specific sequence with a high level of similarity with pgi genes from other Bacillus spp. was found (data not shown). It is postulated that the absence of a pgi phosphoglucose isomerase-encoding gene in B. sphaericus might be one of the reasons for the inability of this bacterium to metabolize carbohydrates.
Sequence analysis revealed that the glcK gene from B. sphaericus C3-41 contained a conserved ATP binding motif (in the case of kinases) at the N terminus and a conserved helix-turn-helix DNA binding motif (in the case of repressors) at the C terminus, which were similar to the motifs of other bacteria (6). Comparison with glcK genes from other Bacillus spp. indicated that the glcK gene might be a conserved gene in the B. sphaericus genome and belongs to the B group of the HK (hexokinase) family (11). Additionally, its high levels of homology to other glucokinases and the specificity of the glcK-encoded enzyme allowed us to characterize this enzyme as a hexokinase.
B. sphaericus is an archaic organism, and its spores have even been found in a sample of amber determined to be 25 to 40 million years old (7). Interestingly, this bacterium has a unique approach to energy production in comparison with known family members. For some identified genes (such as the bin and mtx genes) the levels of similarity among different serotypes and isolates are extremely high (27, 28). However, our phylogenetic tree analysis indicated that the glucokinase from B. sphaericus is distantly related to other glucokinases from Bacillus spp. and had a higher level of similarity with E. coli glucokinase (11). This suggests that mutation or deletion of some genes related to the EMP pathway resulted in the inability to utilize glucose in B. sphaericus, while other EMP pathway-related genes were maintained. Thus, the sequence and distribution of genes in B. sphaericus perhaps could provide additional insight regarding this archaic bacterium. In our previous research, it was found that this mesophilic Bacillus had a kind of thermostable DNA polymerase I which had sequence similarity to the enzymes of thermophilic bacteria (4). Recent studies suggested that Bacillus pasteurii, Bacillus psychrophilus, and Bacillus globiporus, which are neighbors in the 16S rRNA phylogenetic tree, should be classified in the genus Sporosarcina (25), since the biological behavior and growth behavior are similar for all these species and are different from those of other bacilli (18). So far, only a few studies on the metabolism of these strains have been described. Thus, a clearer understanding of the metabolism of B. sphaericus will be important for further unraveling the metabolism and evolution of these bacteria.
The expressed purified GlcK has substrate ambiguity, phosphorylating not only glucose but also fructose and mannose. This ambiguity may continue to play a role in the functional diversification of proteins and metabolic pathways. Some researchers proved that the ambiguous activities of some enzymes could provide selective advantages to the host cells under conditions of extreme selective pressure and provide a fertile substrate for the natural evolution of a protein with new catalytic activities (9, 17). However, the role of the glcK gene in the evolution of B. sphaericus still needs to be investigated.
In B. sphaericus, glucose enters the cell in two forms; one form is glucose phosphorylated through the phosphoenolpyruvate-dependent phosphotransferase system before it enters the cell, and the other is driven by ATP hydrolysis (ABC transporters) or by ion gradients (H+ or Na+ symporters) and then phosphorylated by the glucokinase (2, 13). In some bacteria, such as E. coli, glucose kinase seems to be important for metabolic pathways because it phosphorylates only intracellular glucose, e.g., from disaccharide hydrolysis (10, 23). Despite the nonessential role of glucokinase, the enzymatic capacity of this enzyme is sufficient to support growth under conditions in which glucose enters the cell without phosphorylation. When B. sphaericus was cultured on different media, it showed high glucokinase activities on the high-salt MBS medium and low activities on the low-salt NYSM medium (Table 1). This may be the reason that Russell et al. did not detect GlcK activity in NYSM medium (20). We presume that expression of glucokinase was the observed behavior when the cell had a response to high osmotic stress. Our present data provided no evidence for any regulatory function or other functional roles of GlcK in B. sphaericus. This protein might phosphorylate some free glucose inside the cell or regulate cell activity to some extent. However, further studies are required to evaluate this possibility.
Acknowledgments
We are grateful to Simon Rayner for useful suggestions and critical reading of the manuscript and to Cai Quanxin for his technical assistance.
This project was supported by grants KSCX2-SW-301-10 and KSCX2-SW-315 from the Chinese Academy of Sciences, by 973 project 2003CB114201, and by grant 30470037 from NFSC, China.
Footnotes
Published ahead of print on 30 March 2007.
REFERENCES
- 1.Albano, M., W. K. Smits, L. T. Y. Ho, B. Kraigher, M. M. Ines, O. P. Kuipers, and D. Dubnau. 2005. The Rok protein of Bacillus subtilis represses genes for cell surface and extracellular functions. J. Bacteriol. 187:2010-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alice, A. F., P. M. Gaspar, and S. R. Carmen. 2003. Phosphoenolpyruvate phosphotransferase system and N-acetylglucosamine metabolism in Bacillus sphaericus. Microbiology 149:1687-1698. [DOI] [PubMed] [Google Scholar]
- 3.Alice, A. F., P. M. Gaspar, and S. R. Carmen. 2002. Existence of a true phosphofructokinase in Bacillus sphaericus: cloning and sequencing of the pfk gene. Appl. Environ. Microbiol. 68:6410-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bei, H., L. Haizhou, H. Xiaomin, and Y. Zhiming. 2006. Preliminary characterization of a thermostable DNA polymerase I from a mesophilic Bacillus sphaericus strain C3-41. Arch. Microbiol. 186:203-209. [DOI] [PubMed] [Google Scholar]
- 5.Bourgouin, C., A. Delecluse, F. de la Torre, and J. Szulmajster. 1990. Transfer of the toxin protein genes of Bacillus sphaericus into Bacillus thuringiensis subsp. israelensis and their expression. Appl. Environ. Microbiol. 56:340-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brigham, C. J., and M. H. Malamy. 2005. Characterization of the RokA and HexA broad-substrate-specificity hexokinases from Bacteroides fragilis and their role in hexose and N-acetylglucosamine utilization. J. Bacteriol. 187:890-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cano, R. J., and M. K. Borucki. 1995. Revival and identification of bacterial spores in 20- to 40-million-year-old Dominican amber. Science 268:1060-1064. [DOI] [PubMed] [Google Scholar]
- 8.Curtis, S. J., and W. Epstein. 1975. Phosphorylation of d-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J. Bacteriol. 122:1189-1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorr, C., M. Zaparty, B. Tjaden, H. Brinkmann, and B. Siebers. 2003. The hexokinase of the hyperthermophile Thermoproteus tenax: ATP-dependent hexokinases and ADP-dependent glucokinsase, two alternatives for glucose phosphorylation in archaea. J. Biol. Chem. 278:18744-18753. [DOI] [PubMed] [Google Scholar]
- 10.Hansen, T., B. Reichstein, R. Schmid, and P. Schonheit. 2002. The first archaeal ATP-dependent glucokinase, from the hyperthermophilic crenarchaeon Aeropyrum pernix, represents a monomeric, extremely thermophilic Rok glucokinase with broad hexose specificity. J. Bacteriol. 184:5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawai, S., T. Mukai, S. Mori, B. Mikami, and K. Murata. 2005. Structure, evolution and ancestor of glucose kinase in the hexokinase family. J. Biosci. Bioeng. 99:320-330. [DOI] [PubMed] [Google Scholar]
- 12.Labes, A., and P. Schönheit. 2003. ADP-dependent glucokinase from the hyperthermophilic sulfate-reducing archaeon Archaeoglobus fulgidus strain 7324. Arch. Microbiol. 180:69-75. [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., W. J. Mitchell, M. Tangney, and H. P. Blaschek. 2005. Evidence for the presence of an alternative glucose transport system in Clostridium beijerinckii NCIMB 8052 and the solvent-hyperproducing mutant BA101. Appl. Environ. Microbiol. 71:3384-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahr, K., G. P. van Wezel, C. Svensson, U. Krengel, M. J. Bibb, and F. Titgemeyer. 2000. Glucose kinase of Streptomyces coelicolor A3(2): large-scale purification and biochemical analysis. Antonie Leeuwenhoek 78:253-261. [DOI] [PubMed] [Google Scholar]
- 15.Mathur, S., Z. Ahsan, M. Tiwari, and L. C. Garg. 2005. Biochemical characterization of recombinant phosphoglucose isomerase of Mycobacterium tuberculosis. Biochem. Biophys. Res. Commun. 337:626-632. [DOI] [PubMed] [Google Scholar]
- 16.Meyer, D., C. Schneider-Fresenius, R. Horlacher, R. Peist, and W. Boos. 1997. Molecular characterization of glucokinase from Escherichia coli K-12. J. Bacteriol. 179:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, B. G., and R. T. Raines. 2005. Reconstitution of defunct glycolytic pathway via recruitment of ambiguous sugar kinase. Biochemistry 44:10776-10783. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura, L. K. 2000. Phylogeny of Bacillus sphaericus-like organisms. Int. J. Syst. Evol. Microbiol. 50:1715-1722. [DOI] [PubMed] [Google Scholar]
- 19.Park, S. Y., H. K. Kim, S. K. Yoo, T. K. Oh, and J. K. Lee. 2000. Characterization of glk, a gene coding for glucose kinase of Corynebacterium glutamicum. FEMS Microbiol. Lett. 188:209-215. [DOI] [PubMed] [Google Scholar]
- 20.Russell, B. L., S. A. Jelley, and A. A. Yousten. 1989. Carbohydrate metabolism in the mosquito pathogen Bacillus sphaericus 2362. Appl. Environ. Microbiol. 55:294-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., and J. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 22.Skarlatos, P., and M. K. Dahl. 1998. The glucokinase of Bacillus subtilis. J. Bacteriol. 180:3222-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Späth, C., A. Kraus, and W. Hillen. 1997. Contribution of glucose kinase to glucose repression of xylose utilization in Bacillus megaterium. J. Bacteriol. 179:7603-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White, P. J., and H. K. Lotay. 1980. Minimal nutritional requirements of Bacillus sphaericus NCTC9602 and 26 other species: the majority grow and sporulate with acetate as sole source of carbon. J. Gen. Microbiol. 118:13-19. [Google Scholar]
- 25.Yoon, J. H., K. C. Lee, N. Weiss, Y. H. Kho, K. H. Kang, and Y. H. Park. 2001. Sporosarcina aquimarina sp. nov., a bacterium isolated from seawater in Korea, and transfer of Bacillus globisporus (Larkin and Stokes 1967), Bacillus psychrophilus (Nakamura 1984) and Bacillus pasteurii (Chester 1898) to the genus Sporosarcina as Sporosarcina globispora comb. nov., Sporosarcina psychrophila comb. nov. and Sporosarcina pasteurii comb. nov., and emended description of the genus Sporosarcina. Int. J. Syst. Evol. Microbiol. 51:1079-1086. [DOI] [PubMed] [Google Scholar]
- 26.Yousten, A. A., and E. W. Davidson. 1982., Ultrastructural analysis of spores and parasporal crystals formed in Bacillus sphaericus 2297. Appl. Environ. Microbiol. 44:1449-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan, Z. M., C. Neilsen-LeRoux, N. Pasteur, A. Delecluse, J. F. Charles, and R. Frutos. 1999. Cloning and expression of the binary toxin genes of Bacillus sphaericus C3-41 in a crystal minus B. thuringiensis subsp. israelensis. Wei Sheng Wu Xue Bao 39:29-35. [PubMed] [Google Scholar]
- 28.Yuan, Z. M., C. Rang, R. C. Maroun, J. P. Victor, R. Frutos, N. Pasteur, C. Vendrely, J. F. Charles, and C. Neilsen-LeRoux. 2001. Identification and molecular structural prediction analysis of a toxicity determinant in the Bacillus sphaericus crystal larvicidal toxin. Eur. J. Biochem. 268:2751-2760. [DOI] [PubMed] [Google Scholar]
- 29.Yuan, Z. M., Y. M. Zhang, and E. Y. Liu. 2000. High-level resistance to Bacillus sphaericus C3-41 in field-collected Culex quinquefasciatus. Biocontrol Sci. Technol. 10:43-51. [Google Scholar]