Abstract
To explore the use of DNA microarrays for pathogen detection in food, we produced DNA oligonucleotide arrays to simultaneously determine the presence of Arcobacter and the presence of Campylobacter in retail chicken samples. Probes were selected that target housekeeping and virulence-associated genes in both Arcobacter butzleri and thermotolerant Campylobacter jejuni and Campylobacter coli. These microarrays showed a high level of probe specificity; the signal intensities detected for A. butzleri, C. coli, or C. jejuni probes were at least 10-fold higher than the background levels. Specific identification of A. butzleri, C. coli, and C. jejuni was achieved without the need for a PCR amplification step. By adapting an isolation method that employed membrane filtration and selective media, C. jejuni isolates were recovered from package liquid from whole chicken carcasses prior to enrichment. Increasing the time of enrichment resulted in the isolation of A. butzleri and increased the recovery of C. jejuni. C. jejuni isolates were further classified by using an additional subset of probes targeting the lipooligosaccharide (LOS) biosynthesis locus. Our results demonstrated that most of the C. jejuni isolates likely possess class B, C, or H LOS. Validation experiments demonstrated that the DNA microarray had a detection sensitivity threshold of approximately 10,000 C. jejuni cells. Interestingly, the use of C. jejuni sequence-specific primers to label genomic DNA improved the sensitivity of this DNA microarray for detection of C. jejuni in whole chicken carcass samples. C. jejuni was efficiently detected directly both in package liquid from whole chicken carcasses and in enrichment broths.
Bacterial contamination of food supplies during food harvesting, processing, and preparation is an area of increasing concern in food safety. Infections caused by bacterial food-borne pathogens continue to be a serious health issue, and the prevalence of food-borne illnesses is still substantial in the United States (5). The bacterial agent in the family Campylobacteraceae that is the most significant contributor to human gastrointestinal infections is Campylobacter; in laboratory-confirmed cases of infection in 2005, Campylobacter was second only to Salmonella (5). Although the causative agent of these human infections is determined often only at the genus level, the Campylobacter spp. that are associated primarily with human gastrointestinal disease are Campylobacter jejuni and Campylobacter coli (16, 56, 57). These species are often referred to as thermotolerant campylobacters because of their optimal growth at 42°C and are also associated prominently with poultry by colonizing preferentially the avian gastrointestinal tract (8). However, other important infection sources identified are pigs for C. coli (59) and untreated water and raw milk for C. jejuni (44). Among cases of campylobacteriosis, C. jejuni is predominant (16), and in rare instances, these infections may be followed by autoimmune neuropathies such as Guillain-Barré syndrome and Miller Fisher syndrome (24, 47). It has been postulated that sialylated lipooligosaccharides (LOS) on the cell surface of C. jejuni exhibit molecular mimicry with gangliosides on peripheral nerves, resulting in the development of these autoimmune neuropathies.
Arcobacter, another genus in the family Campylobacteraceae, also is of importance in human health and food safety (14, 54, 58, 64, 67) and is similar to Campylobacter (63). Although four Arcobacter species have been associated with animals and humans, Arcobacter butzleri is the species isolated mostly from human infections (14, 64). Similar to Campylobacter, Arcobacter has been isolated more frequently from poultry than from red meats (10, 25, 45), suggesting that poultry may be a major reservoir for this pathogen. However, Arcobacter species grow at temperatures much lower (15 to 37°C) than those required for Campylobacter growth and lower than the normal avian body temperature (∼42°C) (33). These observations suggest that the presence of Arcobacter in poultry may be due to contamination from the skin of the birds or through processing and not due to contamination from the birds' gastrointestinal contents (19, 22, 68). Given that contaminated food products are a potential source of Campylobacter and Arcobacter (14, 44), a key issue in food safety is the development of efficient detection and identification methods for these pathogens as a first step in the control of human infections.
Traditionally, food-borne pathogens have been identified by microbiological culture, followed by immunological methods (4, 38). Most methods for the isolation and identification of campylobacters in foods require enrichment culturing for several days, followed by subculturing on selective media for further phenotypic identification (9). Recently, nucleic acid amplification methods have been developed for specific and efficient detection of these food-borne pathogens (41). For example, multiplex PCR assays have been used to simultaneously identify and discriminate, in a single-step reaction, C. coli and C. jejuni (6, 7, 28, 42, 46) or Arcobacter spp. (3, 20, 23). Although these multiplex PCR assays are more efficient than traditional culturing methods, they are limited by the number of genes that can be detected in a single reaction (4). In addition, certain contaminants that may be present in crude DNA preparations can inhibit the PCRs or can result in nonspecificity (46).
The advent of whole-genome-based methods, such as DNA microarrays, offers another means to enhance the detection capabilities and to overcome the limitations of established procedures, such as culturing and PCR (4, 38). The advantage of microarray-based detection is that it can combine the amplification of nucleic acids with its massive screening capability, resulting in sensitivity, specificity, and high-throughput capacity. Previous reports have described the use of microarray-based identification of thermotolerant campylobacters (26, 27, 55, 65). However, these detection assays relied on PCR amplification of a specific region in a few target genes as a first step in pathogen detection.
In the present study, a DNA oligonucleotide array was designed to contain a comprehensive set of 70-mer oligonucleotide probes targeting genes implicated in metabolism or pathogenicity for A. butzleri, C. coli, and C. jejuni. This DNA oligonucleotide array allowed specific identification of A. butzleri, C. coli, and C. jejuni present in retail chicken samples without PCR amplification of a few target genes prior to pathogen detection, as well as genotypic classification of C. jejuni isolates based on LOS class. By using C. jejuni sequence-specific primers to label genomic DNA, C. jejuni was detected directly in package liquid from whole chicken carcasses or in enrichment broths.
MATERIALS AND METHODS
Bacterial reference strains and growth conditions.
The bacterial reference strains that were used in this study are listed in Table 1. C. jejuni and C. coli strains were grown routinely at 42°C under microaerobic conditions (8% CO2, 4% O2, 80% N2, 8% H2) on Mueller-Hinton (Becton Dickinson Co., Sparks, MD) solid medium supplemented with 0.025% (wt/vol) FeSO4·7H2O, 0.025% (wt/vol) sodium metabisulfite (anhydrous), and 0.025% (wt/vol) sodium pyruvate (anhydrous). A. butzleri strains were grown optimally at 28°C under microaerobic conditions on Oxoid anaerobe basal agar (ABA) (Remel Inc., Lenexa, KS) supplemented with 5% laked horse blood (Hema Resource and Supply, Inc., Aurora, OR).
TABLE 1.
Bacterial reference strains used in this study
| Species | Strain | Other designation | Penner heat-stable serotype/biotype | Clinical or environmental source | Provider (reference)a |
|---|---|---|---|---|---|
| Arcobacter butzleri | RM3790 | 29.19 | Human | A. Lastovica | |
| RM4018 | ATCC 49616 | Human | I. Wesley | ||
| RM4477 | NADC 4061 | Pig | I. Wesley | ||
| RM4481 | NADC 9565 | Turkey | I. Wesley | ||
| Arcobacter cibarius | RM5244 | KH2 | Chicken | K. Houf | |
| Arcobacter cryaerophilus | RM1582 | ATCC 43158 | 1A | Cow | I. Wesley |
| RM1583 | ATCC 49615 | 1B | Human | I. Wesley | |
| RM4603 | NADC 3144 | 1A | Pig | I. Wesley | |
| Campylobacter coli | RM1051 | ATCC 43479 | HS:30 | Human | P. Guerry |
| RM1166 | T21 | Chicken | D. Woodward | ||
| RM1505 | ATCC 49299 | HS:61 | W. Johnson (53) | ||
| RM1896 | 1921 | Swine | L. Stanker | ||
| RM2228 | 72664 | HS:34 | Chicken | D. Woodward | |
| Campylobacter jejuni | RM1045 | ATCC 43429 | HS:1 | Human | P. Guerry |
| RM1048 | ATCC 43432 | HS:4 | Human | P. Guerry | |
| RM1050 | ATCC 43449 | HS:23 | Human | P. Guerry | |
| RM1170 | HS:31 | Chicken | Laboratory collection | ||
| RM1221 | ATCC BAA-1062 | HS:53 | Chicken | Laboratory collection (15) | |
| RM1285 | HS:19 | Chicken | Laboratory collection | ||
| RM1503 | ATCC 43462 | HS:43 | Human | W. Johnson | |
| RM1862 | NCTC11168 | HS:2 | Human | R. Meinersmann (50) | |
| RM1864 | 81-176 | HS:23,36 | Human | R. Meinersmann | |
| RM3407 | HS:3 | D. Woodward | |||
| Campylobacter lari | RM1890 | ATCC 43675 | Human | L. Stanker | |
| Campylobacter upsaliensis | RM3195 | 300.94 | Human | A. Lastovica | |
| RM3777 | 3.97 | Human | A. Lastovica | ||
| Escherichia coli | RM2084 | ATCC 43895 | O157:H7 | Raw meat | T. Whittam |
| Salmonella enterica serovar Typhimurium | RM2967 | SARB65 | Human | M. Brandl |
Affiliations of providers: M. Brandl, USDA-ARS, Western Regional Research Center, Albany, CA; P. Guerry, Naval Medical Research Institute, Bethesda, MD; W. Johnson, Laboratory Centre for Disease Control, Winnipeg, Canada; K. Houf, Ghent University, Merelbeke, Belgium; A. Lastovica, University of Cape Town, Cape Town, South Africa; R. Meinersmann, USDA-ARS, R. B. Russell Agricultural Research Center, Athens, GA; L. Stanker, USDA-ARS, Western Regional Research Center, Albany, CA; I. Wesley, USDA-ARS, National Animal Disease Center, Ames, IA; T. Whittam, Michigan State University, East Lansing, MI; D. Woodward, National Microbiology Laboratory, Winnipeg, Canada.
Isolation of bacteria from retail chicken samples.
Ten whole carcasses of chickens that were raised either organically or conventionally were purchased from various local retailers. The liquid from each package was collected and kept at 4°C for 30 min. To promote the growth of Campylobacter or Arcobacter that may have been present in a food sample, an enrichment culture was prepared by adding 5 ml of the package liquid to 45 ml of Oxoid anaerobe basal broth (Remel Inc., Lenexa, KS) amended with 20 μg/ml amphotericin B and 10 μg/ml trimethoprim (Sigma-Aldrich, St. Louis, MO) in a sterile cell culture flask with a 0.2-μm vented cap, and the flask was incubated at 37°C with gentle shaking (30 rpm) under microaerobic conditions. The antibiotics were added at concentrations that do not inhibit the growth of campylobacteria on the selective media (32, 38a). After incubation of the enrichment broth for 24 h, bacterial isolates were recovered by plating 1 ml of the enrichment broth on ABA. The plates were incubated for 24 to 48 h at 37°C under microaerobic conditions.
A membrane filtration method was adapted (12, 36) for efficient recovery of bacterial isolates from package liquid or from enrichment broths containing package liquid that were incubated for 6, 12, or 24 h at 37°C. A 250-μl sample of either package liquid or enrichment broth was applied in small drops to a sterile mixed cellulose ester membrane filter that was 47 mm in diameter and had a pore size of 0.65 μm (Millipore Corporation, Billerica, MA), which was placed on the surface of either ABA or Oxoid modified CCDA-Preston (Remel Inc., Lenexa, KS) plates amended with 20 μg/ml amphotericin B and 10 μg/ml trimethoprim. Samples were filtered passively for 30 min at room temperature under the ambient atmosphere. After this incubation period, the filters were removed, and the plates were incubated further for 24 to 48 h at 37°C under microaerobic conditions. The samples were not spread or streaked after the filters were removed. The recovered isolates with a Campylobacter-like colony morphology (pale orange colonies on ABA or light gray colonies on modified CCDA-Preston agar) were inspected visually for a very small, curved (S-shaped) single-cell morphology and corkscrew motility by phase-contrast microscopy using a Leica DMR light microscope (Leica Microsystems Inc., Bannockburn, IL).
PCR.
PCR reagents were supplied by Epicenter (Madison, WI), and oligonucleotides were purchased from QIAGEN Operon (Alameda, CA) or Sigma-Genosys (The Woodlands, TX). The multiplex PCR assay for simultaneous identification of Campylobacter species was adapted from the assay of Klena et al. (28), with the following modifications: 10 pmol/μl forward primers complementary to the lpxA nucleotide sequence of C. coli and C. jejuni and 30 pmol/μl of the lpxARRK2m reverse primer were mixed in a 50-μl reaction mixture containing each deoxynucleoside triphosphate at a concentration of 250 μM, 1× MasterAmp Taq PCR buffer, 1× MasterAmp Taq enhancer, 2.5 mM MgCl2, and 1 U MasterAmp Taq DNA polymerase. Genomic DNA (50 ng) from bacterial isolates, prepared as described below, was then added, and the reaction mixture was placed into an MJ Research thermocycler (Bio-Rad Laboratories, Hercules, CA) with the following settings: 1 min at 94°C, 45 s at 50°C, and 1 min at 72°C for 30 cycles, followed by a final extension of 5 min at 72°C. The multiplex PCR assay for the simultaneous identification of Arcobacter species was performed according to the methods described by Houf et al. (23). Conventional PCR for LOS gene-specific amplification was performed as described previously (48). Products for each PCR mixture were examined by agarose gel electrophoresis through 1.5% agarose, and bands were visualized with UV light after ethidium bromide straining. Positive samples were identified based on the presence of bands of anticipated sizes. Images were captured with an AlphaImager gel documentation analysis system (Alpha Innotech, San Leandro, CA).
Construction of the multipathogen oligonucleotide microarray.
The 70-mer oligonucleotide probes specific for members of the Campylobacteraceae family (see Tables S1 and S2 in the supplemental material) were designed by using the Array Designer 3.0 software (Premier Biosoft International, Palo Alto, CA) with an average melting temperature of 73°C. For printing the DNA microarray, the oligonucleotide probes were dissolved in water to a concentration of 200 μM and were then further diluted in 0.3× saline sodium citrate (SSC)-50% dimethyl sulfoxide to a final DNA concentration of 50 μM (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). Each oligonucleotide probe was spotted in triplicate on UltraGaps glass slides (Corning Inc., Corning, NY) coated with amino propyl groups, using an OmniGrid Accent robot (GeneMachines, Ann Arbor, MI) with ChipMaker microspotting pins and printhead (TeleChem International, Inc. Sunnyvale, CA). Immediately after printing, the microarrays were UV cross-linked at 600 mJ by using a GS Gene linker UV chamber (Bio-Rad Laboratories, Hercules, CA) and were stored in a desiccator until further use.
Genomic DNA isolation and labeling.
Forty-five milliliters of the package liquid from a whole chicken carcass or 1 ml of an enrichment broth was centrifuged at 14,500 × g for 5 min. The pellet was resuspended in 1 ml of anaerobe basal broth, and a 100-μl sample was then centrifuged at 14,500 × g for 5 min. Genomic DNA was further isolated from the sample of package liquid, enrichment broth, or bacterial isolates by using a DNeasy Blood & Tissue kit (QIAGEN, Valencia, CA) and following the manufacturer's specifications. Labeling of genomic DNA from the bacterial isolates, reference strains, or 24-h enrichment broth samples was performed by mixing approximately 5 μg of genomic DNA with 5 μl of 10× random octadeoxyribonucleotides (New England Biolabs, Ipswich, MA) and water to obtain a final volume of 41 μl. Labeling of genomic DNA from package liquid or from each 6-, 12-, or 24-hr enrichment broth sample was performed by mixing 15 μg of genomic DNA with 2 μM each of the C. jejuni sequence-specific primers (see Table S3 in the supplemental material), 5 μl of 10× NEBuffer 2 (New England Biolabs), and water to obtain a final volume of 41 μl. All labeling reaction samples were heated to 95°C for 5 min, cooled for 5 min at 4°C, and then added to the remaining labeling reaction mixture consisting of 5 μl of 10× deoxynucleoside triphosphate labeling mixture (1.2 mM each of dATP, dGTP, dCTP, and 0.5 mM dTTP in 10 mM Tris [pH 8.0]-1 mM EDTA) (Promega Corporation, Madison, WI), 3 μl of Amersham CyDye fluorescent nucleotides, Cy3-dUTP or Cy5-dUTP (GE Healthcare Life Sciences Corp., Piscataway, NJ), and 5 U of Klenow fragment (3′→5′ exo−) (New England Biolabs). The mixtures were incubated overnight at 37°C, as previously described (49). Genomic DNA from C. jejuni reference strains NCTC11168 and RM1221 were fluorescently labeled with Cy5-dUTP. Genomic DNA from bacterial isolates, from package liquid, and from each enrichment broth were fluorescently labeled with Cy3-UTP. The labeled genomic DNA was purified from unincorporated CyDye fluorescent label by using a QIAquick PCR purification kit (QIAGEN, Valencia, CA) and was eluted finally in 100 μl of 10 mM Tris (pH 8.0). The CyDye-labeled genomic DNA from the tested food sample and from the reference strains were combined and vacuum dried using a Savant ISS110 SpeedVac concentrator (Thermo Electron Corp., Milford, MA).
Microarray hybridization.
The CyDye-labeled genomic DNA from the tested food sample and from the reference strains were resuspended in 20 μl of Pronto! Long Oligo/cDNA hybridization solution (Corning Inc., Corning, NY), heated to 95°C for 5 min, and immediately centrifuged at 14,500 × g for 2 min at room temperature. The hybridization mixture was then applied to each DNA microarray and sealed with a HybriSlip plastic coverslip (Sigma-Aldrich, St. Louis, MO). The microarray slide was placed in a hybridization chamber (Corning) and was incubated at 42°C for 18 h, as in previous studies (49). Following hybridization, the slides were transferred to a microarray wash station (TeleChem International, Inc., Sunnyvale, CA) and were washed twice in 2× SSC-0.1% sodium dodecyl sulfate at 42°C for 5 min, twice in 1× SSC at room temperature for 10 min, and finally twice in 0.2× SSC at room temperature for 10 min. The microarray slides were transferred to a slide-drying tray (Evergreen Scientific, Los Angeles, CA) and were dried by centrifugation at 300 × g for 10 min prior to scanning. At least three replicate hybridization reactions were performed for each food sample. Each replicate hybridization reaction mixture was applied to a microarray on a different slide to account for the slide-to-slide variation.
Microarray data analysis.
DNA microarrays were scanned using an Axon GenePix 4000B microarray laser scanner (Molecular Devices Corporation, Sunnyvale, CA) at excitation wavelengths of 532 nm (Cy3) and 635 nm (Cy5) with a 10-μm resolution, as previously described (49). For the analysis of the DNA microrarray data, the fluorescence signal intensities for each probe spotted in triplicate on each replicate microarray (a total of three replicates) were quantified after subtraction of the local background by using GenePix 4.0 software (Molecular Devices). No significant differences were observed between replicate microarrays. Probes were excluded from further analysis if they had an anomalous spot morphology or were within regions of nonspecific fluorescence. The microarray data were analyzed further with GeneSpring 7.2 software (Agilent Technologies, Santa Clara, CA) for averaging the background-corrected fluorescence signal for the probes on replicate microarrays, for fluorescence signal comparisons, or for average-linkage hierarchical clustering with the standard correlation (49). The average fluorescence signals for probes targeting a pathogen are shown in the figures below using a color scheme, where yellow corresponds to fluorescence signals of >2,000 to 3,000 U, hazel corresponds to fluorescence signals of 1,500 to 2,250 U, blue corresponds to fluorescence signals of 1,000 to 1,500 U, purple corresponds to fluorescence signals of 500 to 750 U, and red corresponds to background levels due to lack of hybridization (<200 to 300 U). Average fluorescence intensities that were at least threefold higher than the background fluorescence (areas on the microarray surrounding the probes) were considered positive signals, as in previous studies (66). A species was considered present in a food sample if positive signals were obtained for a majority of the probes (approximately 70% of the probes) targeting that pathogen.
RESULTS AND DISCUSSION
Development and validation of the DNA oligonucleotide array.
To develop DNA microarray technology for the simultaneous detection of multiple pathogens from food, DNA oligonucleotide arrays were designed to contain a comprehensive set of 70-mer oligonucleotide probes specific for members of the Campylobacteraceae family (see Table S1 in the supplemental material). The species-specific probes were selected to target genes in A. butzleri (W. G. Miller, C. T. Parker, M. Rubenfield, G. L. Mendz, M. M. S. M. Wösten, D. W. Ussery, J. F. Stolz, G. Wang, J. A. Malek, A. Rogosin, L. H. Stanker, and R. E. Mandrell, submitted for publication) and in thermotolerant C. jejuni and C. coli (15, 50) that encode proteins for housekeeping functions, including biosynthetic and cellular functions, and virulence factors (see Table S1 in the supplemental material). For example, the C. jejuni-specific probes targeted the ciaB, cadF, and peb2 genes involved in several aspects of bacterial virulence, such as host adherence and invasion (30, 31, 50, 52). As in previous microarray detection studies (26, 27, 65), probes for C. jejuni detection targeted hipO, a gene encoding hippuricase (37), and cdtB, a cytolethal distending toxin subunit gene (2, 13). The C. coli-specific probes were designed based on findings of a comparative genome analysis that revealed housekeeping and virulence genes in C. coli showing sequence similarity to genes of C. jejuni and other Campylobacter species (15). Additional probes included in this study targeted genes in C. jejuni and C. coli encoding novel hypothetical proteins that were identified in the genome sequence analysis of campylobacters to be species specific, having no match to other organisms in the database (15). The recent completion of the genome sequence of A. butzleri strain RM4018 provided the needed information for designing species-specific probes targeting genes in A. butzleri that showed sequence homology to virulence and housekeeping genes in Campylobacter species (Miller et al., submitted). Finally, the Ab0988, Ab2061, and Ab2302 probes were included to target A. butzleri genes that were identified to be unique in a pairwise comparative analysis of A. butzleri and characterized Campylobacter proteome sequences.
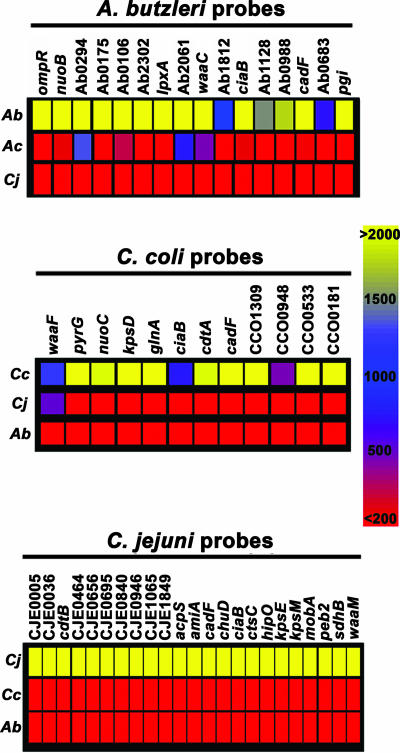
To confirm the specificity of the 70-mer oligonucleotide probes, genomic DNA from various reference strains (Table 1) were labeled separately with CyDye with Klenow polymerase and random primers (see Materials and Methods), a method that yields fragment sizes that hybridize efficiently to probes on the microarray (66). Results demonstrated that the fluorescence intensity values were specific for each species (Fig. 1). The fluorescence signal intensities for C. jejuni probes were at least 10-fold higher than the background levels when labeled genomic DNA from various C. jejuni reference strains was hybridized (Fig. 1). In contrast, the fluorescence signal intensities for the C. jejuni probes approached the background levels when genomic DNA from C. coli or A. butzleri was hybridized (Fig. 1). Fluorescence signals close to background levels were also obtained after testing of genomic DNA from other Campylobacter spp., such as Campylobacter lari and Campylobacter upsaliensis, or from other food-borne pathogens, such as Salmonella enterica serovar Typhimurium and Escherichia coli O157:H7 reference strains (data not shown). Similarly, a high level of specificity was also observed for probes targeting C. coli (Fig. 1). Analysis of the A. butzleri probe specificity demonstrated that high fluorescence signal intensities were obtained when several A. butzleri reference strains were tested (Fig. 1). In contrast, a lack of hybridization to most A. butzleri probes was observed after hybridization of genomic DNA from other closely related Arcobacter species, such as Arcobacter cryaerophilus (Fig. 1) or Arcobacter cibarius (data not shown). Regardless of the pathogen tested, the specific signal for each probe set had a similar range of fluorescence intensity values that were at least 10-fold higher than the local background levels.
FIG. 1.
Probe specificity of the DNA oligonucleotide array. Genomic DNA from A. butzleri (Ab), A. cryaerophilus (Ac), C. coli (Cc), and C. jejuni (Cj) reference strains (Table 1) was labeled separately with CyDye and hybridized to the microarray. The color bar shows the average fluorescence signal for each probe, where yellow corresponds to fluorescence signals of >2,000 U, hazel corresponds to fluorescence signals of 1,500 U, blue corresponds to fluorescence signals of 1,000 U, purple corresponds to fluorescence signals of 500 U, and red corresponds to background levels due to lack of hybridization (<200 U). Each row shows the signals for a probe set after labeling of the reference strains, indicated on the left.
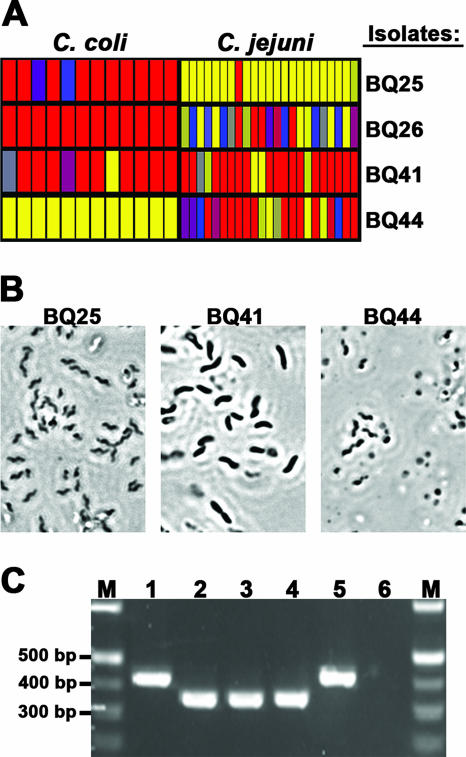
To validate this DNA microarray for the identification of pathogens from food, retail chickens were tested for the presence of Campylobacter spp. The package liquid from whole chicken carcasses was collected, added to an enrichment broth, and incubated for 24 h at 37°C (see Materials and Methods). Samples of the enrichment broth were plated on selective media, and the plates were incubated for an additional 48 h. Suspect bacterial colonies were selected, and their genomic DNA was fluorescently labeled and hybridized to the DNA microarray. All probes specific for either C. jejuni or C. coli yielded significant fluorescence, and the values were at least 10-fold higher than the background levels for isolates BQ25 and BQ44, respectively (Fig. 2A). Although analysis of isolate BQ26 showed that 7/23 of the C. jejuni probes (30%) had values that were close to the background values, a large proportion of the probes (70%) had significant fluorescence values. The average fluorescence signal values for 10/23 of the probes were 7.5- to 10-fold higher than the background values, while 6/23 of the probes had average fluorescence values that were 5-fold higher than the background values (Fig. 2A). In contrast, microarray analysis of isolate BQ41 resulted in no significant fluorescence values for most probes targeting either C. jejuni or C. coli (Fig. 2A). A lack of hybridization to all of the A. butzleri probes was observed when the isolates were analyzed (data not shown). The morphology of individual cells from these bacterial isolates was further assessed by phase-contrast microscopy (Fig. 2B). The cells of isolates BQ25 and BQ44, Campylobacter-positive isolates as determined by DNA microarray analysis, were small and spiral shaped (Fig. 2B), a typical single-cell morphology for Campylobacter species (62). A similar single-cell morphology was also observed for isolate BQ26 (data not shown). In contrast, isolate BQ41, a Campylobacter-negative isolate as determined by DNA microarray analysis, had a different single-cell morphology; the cells were longer and rod shaped (Fig. 2B). Finally, a multiplex PCR assay was conducted to confirm the species of poultry isolates identified by microarray analysis (Fig. 2C). As part of this PCR assay, C. coli and C. jejuni were discriminated by amplifying the lpxA gene using a common primer, LpxAKK2Rm, and species-specific primers (28). Similar to the results obtained with our DNA microarrays, the multiplex PCR assay identified isolates BQ25 and BQ26 as C. jejuni since amplification of lpxA from these isolates yielded a 330-bp fragment that comigrated with the fragment amplified from the reference strain C. jejuni RM1221 (Fig. 2C). Amplification of the isolate BQ44 sequence resulted in a 390-bp fragment that comigrated with the fragment amplified from reference strain C. coli RM1166, consistent with the results obtained with DNA microarrays. These results demonstrate that the DNA microarray was highly specific for these species and allowed accurate identification of bacterial isolates from food.
FIG. 2.
Validation of the DNA oligonucleotide array for identifying pathogens in food. (A) Genomic DNA from bacterial isolates recovered from a 24-h enrichment broth was labeled with CyDye and hybridized to the microarray. The average fluorescence signal for each C. jejuni and C. coli probe is color coded, as described in the legend to Fig. 1. (B) Morphology of individual cells as assessed by phase-contrast microscopy at a magnification of ×1,000. (C) Multiplex PCR assay (28) performed to corroborate the microarray data for species identification of Campylobacter isolates from retail chicken samples. Lanes M, 100-bp DNA ladder; lane 1, C. coli strain RM1166; lane 2, C. jejuni strain RM1221; lane 3, isolate BQ25; lane 4, isolate BQ26; lane 5, isolate BQ44; lane 6, isolate BQ41.
Identification of bacterial isolates from retail chicken samples.
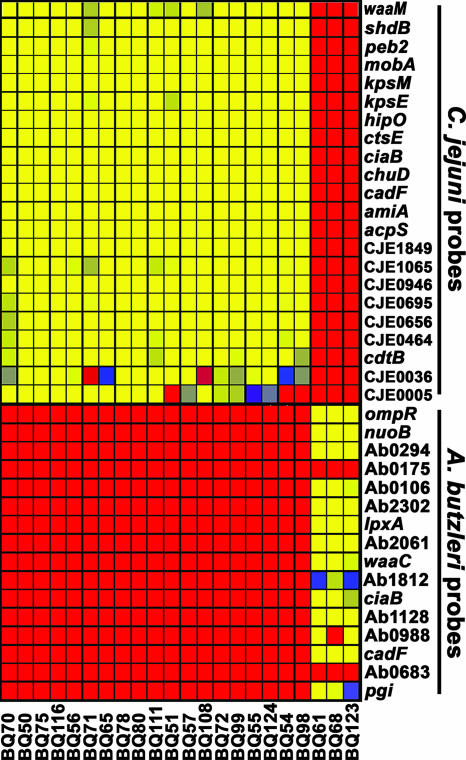
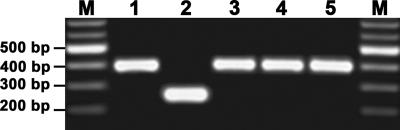
To better assess the presence of campylobacteria and related species that could be isolated from retail chicken samples, an efficient isolation protocol (see Materials and Methods) that employs a combination of both membrane filtration and selective media was adapted (12, 36). Samples of either package liquid from whole chicken carcasses or enrichment broths that were incubated for various times were applied to a membrane filter. Small motile bacteria, like most campylobacteria and arcobacteria, would be expected to pass through the membrane filter and be recovered on the selective growth medium after incubation under microaerobic conditions. By performing this isolation procedure, single colonies with phenotypic characteristics of campylobacter colonies were recovered directly from a package liquid sample prior to enrichment, and the estimated yield was approximately 4 to 10 CFU/ml (data not shown). Yields ranging from 12 to 28 CFU/ml were obtained from an enrichment broth that was incubated for 12 h, and similar results were obtained after incubation for 24 h (data not shown). Microarray analysis demonstrated that 80% of the bacterial isolates examined were C. jejuni. This conclusion was based on hybridization results indicating significant fluorescence values for all probes specific for this pathogen (Fig. 3). In addition, the microarray analysis demonstrated that three isolates, BQ61, BQ68, and BQ123, were A. butzleri since their hybridization signals resulted in significant fluorescence values for 80% of the A. butzleri probes, which targeted conserved genes encoding virulence factors and housekeeping functions, such as lpxA, nuoB, cadF, ciaB, and waaC. Although all of the probes were specific for the A. butzleri reference strains (Fig. 1), the lack of hybridization with some probes (Ab0175, Ab0988, and Ab0683) suggests that these genes are highly divergent or absent in these A. butzleri poultry isolates compared to the reference strains (Fig. 3). To confirm that these Arcobacter isolates from retail chicken samples were A. butzleri, a multiplex PCR assay that allows simultaneous identification of Arcobacter spp. was performed (23). As shown in Fig. 4, the multiplex PCR assay identified isolates BQ61, BQ68, and BQ123 all as A. butzleri; amplification with the primer set specific for A. butzleri resulted in a 400-bp fragment that comigrated with the fragment amplified from the reference strain A. butzleri RM4018. Only A. cryaerophilus strain RM1582 amplified a 260-bp fragment with the primer set specific for A. cryaerophilus (Fig. 4), an Arcobacter species that has been isolated previously from chicken carcasses (1, 21, 25). Isolates BQ61, BQ68, and BQ123 were analyzed further by matrix-assisted laser desorption ionization—time of flight mass spectrometry, an established technique for pathogen identification that detects ribosomal and highly expressed cytosolic proteins (40, 43). The matrix-assisted laser desorption ionization—time of flight mass spectra obtained for A. butzleri isolates BQ61, BQ68, and BQ123 were similar to the spectrum obtained for the reference strain A. butzleri RM4018, consistent with the species identification results obtained by microarray and PCR analyses (data not shown). No C. coli isolates were identified in this DNA microarray analysis. Possibly, C. coli was present in these samples but at much lower concentrations than C. jejuni; therefore, an increased number of isolates had to be examined to determine the presence of C. coli.
FIG. 3.
Identification of bacterial isolates from retail chicken samples recovered by membrane filtration. Genomic DNA from bacterial isolates recovered from retail chicken samples by membrane filtration (see Materials and Methods) was CyDye labeled and hybridized to the microarray. The average fluorescence signals for either C. jejuni or A. butzleri probes are color coded, where yellow corresponds to fluorescence signals of >3,000 U, hazel corresponds to fluorescence signals of 2,250 U, blue corresponds to fluorescence signals of 1,500 U, purple corresponds to fluorescence signals of 750 U, and red corresponds to background levels due to a lack of hybridization (<300 U). The bacterial isolates are indicated at the bottom.
FIG. 4.
Multiplex PCR for species identification of Arcobacter isolates. A multiplex PCR assay (23) was performed to validate the microarray data for species identification of Arcobacter isolates from retail chicken samples as determined by microarray analysis. Lanes M, 100-bp DNA ladder; lane 1, A. butzleri strain RM4018; lane 2, A. cryaerophilus strain RM1582; lane 3, isolate BQ61; lane 4, isolate BQ68; lane 5, isolate BQ123.
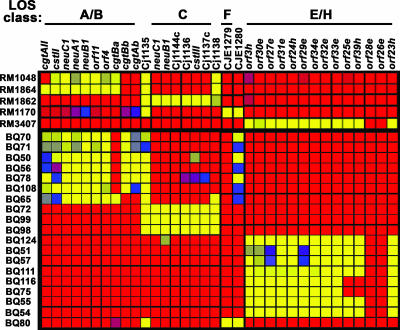
Genotypic classification of C. jejuni isolates based on their LOS classes.
The C. jejuni isolates that were recovered from either package liquid or enrichment broth were classified further using a subset of probes that target the LOS biosynthesis locus, one of the most diverse regions of the C. jejuni genome, as demonstrated in previous DNA microarray studies (11, 34, 35, 49, 51, 60). In the present study, probes were designed to target six LOS classes (see Table S2 in the supplemental material) that were shown previously to be represented among C. jejuni strains from various sources (48). The specificity of the probes targeting the LOS biosynthesis locus in C. jejuni was determined (Fig. 5). Fluorescence signal intensities that were at least 10-fold higher than the background levels were observed for most probes targeting a particular LOS class when reference strains representative of each LOS class were examined. Microarray analysis of C. jejuni strains RM1048 and RM1864, strains known to possess class A and B LOS, respectively, hybridized to probes targeting both classes. Only strain RM1864 showed significant fluorescence signals for cgtAII, a probe targeting only class B (Fig. 5). A high level of specificity was also observed when probes targeting LOS class C were tested with reference strain RM1862 (class C) or when a LOS class F probe targeting CJE1279 was tested with reference strain RM1170 (class F). C. jejuni strain RM3407, a class H reference strain, hybridized to the class H-specific probe orf39h and to probes targeting both LOS class E and H loci but failed to hybridize to orf28e and orf26e, probes targeting only class E loci (Fig. 5).
FIG. 5.
Genotypic classification of C. jejuni isolates based on their LOS classes. Genomic DNA from C. jejuni reference strains and from C. jejuni isolates recovered from retail chicken samples by membrane filtration (see Materials and Methods) was labeled with CyDye and hybridized to the microarray. The average fluorescence signals for probes targeting the various LOS classes in C. jejuni are color coded, as described in the legend to Fig. 3. The reference strains (designations beginning with RM) and bacterial isolates (designations beginning with BQ) are indicated on the left.
To identify differences among C. jejuni poultry isolates, the gene content of the LOS biosynthesis locus, a region that contributes to the C. jejuni genome diversity, was further examined. As shown in Fig. 5, the microarray analysis demonstrated that C. jejuni isolates with the same LOS class clustered together with a distinctive pattern, and most probes for each assigned LOS class had fluorescence signal intensities that were at least 10-fold higher than the background levels. Genomic DNA from seven C. jejuni isolates (BQ70, BQ71, BQ50, BQ56, BQ78, BQ108, and BQ65) hybridized to probes specific for LOS classes A and B. The fact that these isolates showed a positive signal for the cgtAII probe, a probe that is specific for only class B, indicates that all of these isolates may possess class B LOS (Fig. 5). Furthermore, isolates BQ70, BQ71, BQ50, BQ56, BQ78, and BQ108 also showed a positive signal for the cgtAb and cgtBb probes, which suggests that these isolates belong to subclass B2, a classification described previously (48). In contrast, C. jejuni isolate BQ65 hybridized to the cgtBa probe and not to the subclass B2 probes; therefore, this isolate may possess subclass B1 LOS. Another larger group of isolates (BQ124, BQ51, BQ57, BQ111, BQ116, BQ75, BQ55, and BQ54) showed significant fluorescence signals for probes specific for LOS classes E and H. However, the lack of hybridization to probes targeting orf26e and orf28e indicates that most of the isolates in this group may possess class H LOS. Further analysis of two isolates in this group (BQ75 and BQ116) showed that there was a lack of hybridization to probes targeting orf25e and orf39h, suggesting that these C. jejuni isolates may possess a new class of LOS that may be derived from class H (Fig. 5). Analysis of another small group of C. jejuni isolates (BQ72, BQ99, and BQ98) resulted in significant fluorescence signals for all probes specific for LOS class C, while only C. jejuni isolate BQ80 hybridized to the probe targeting the LOS class F gene, CJE1279 (Fig. 5). It should be noted that isolates with class B, C, or F LOS hybridized to probe Cj1135, while isolates with class B or F LOS hybridized to probe CJE1280 (Fig. 5; see Table S2 in the supplemental material). To verify the LOS class assignment by this DNA microarray analysis, PCR amplification of genes within the presumed LOS class was performed. Using LOS class-specific primer pairs (48), this PCR assay demonstrated that all C. jejuni isolates that were examined had patterns that were consistent with their class designations (Table 2), correlating with the results obtained with DNA microarrays. Therefore, the pathogen-specific DNA oligonucleotide array proved to be effective for genotypic classification of C. jejuni isolates from retail chicken samples.
TABLE 2.
PCR analysis of LOS classes for C. jejuni isolates from retail chicken samples
| Isolate | Putative LOS classa | Specific amplification of open reading frame:
|
|||||
|---|---|---|---|---|---|---|---|
| cgtAII | cstII | cstIII | CJE1279 | orf25e | orf27e | ||
| BQ50 | B2 | + | + | − | − | − | − |
| BQ51 | H | − | − | − | − | + | + |
| BQ54 | H | − | − | − | − | + | + |
| BQ55 | H | − | − | − | − | + | + |
| BQ56 | B2 | + | + | − | − | − | − |
| BQ57 | H | − | − | − | − | + | + |
| BQ65 | B1 | + | + | − | − | − | − |
| BQ70 | B2 | + | + | − | − | − | − |
| BQ71 | B2 | + | + | − | − | − | − |
| BQ72 | C | − | − | + | − | − | − |
| BQ75 | Unk | − | − | − | − | − | + |
| BQ78 | B2 | + | + | − | − | − | − |
| BQ80 | F | − | − | − | + | − | − |
| BQ98 | C | − | − | + | − | − | − |
| BQ99 | C | − | − | + | − | − | − |
| BQ108 | B2 | + | + | − | − | − | − |
| BQ111 | H | − | − | − | − | + | + |
| BQ116 | Unk | − | − | − | − | − | + |
| BQ124 | H | − | − | − | − | + | + |
Unk, unknown.
Efficient identification of C. jejuni in package liquid from retail chicken samples.
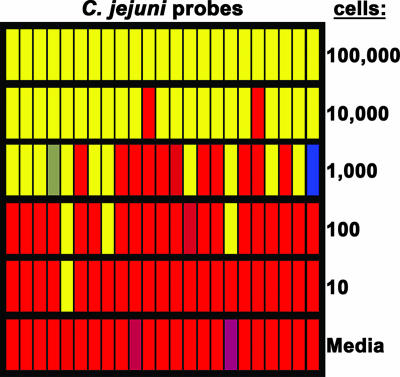
Given that C. jejuni is the most common Campylobacter species contributing to food-borne human illness and is associated prominently with poultry (44, 57), the sensitivity of our DNA microarray for detection of this species was assessed. Genomic DNA was isolated from C. jejuni strain NCTC11168 at various cell concentrations, CyDye labeled with Klenow polymerase and random primers, and hybridized to the microarray (Fig. 6). The results demonstrated that high fluorescence values were obtained for samples containing between 104 and 106 cells with a quantified signal for all C. jejuni probes that was at least 10-fold higher than the background levels (Fig. 6). The fluorescence intensity for the C. jejuni probes decreased with a decrease in cell concentration. In samples containing 1,000 cells, approximately 50% of the probes had fluorescence values that were 5- to 10-fold higher than the background values, but only 10% of the probes were significantly fluorescent after analysis of samples representing 100 to 1,000 cells (Fig. 6). No C. jejuni-specific signal was detected after microarray analysis of samples with less than 100 cells (Fig. 6). A similar sensitivity threshold was also observed with C. jejuni strain RM1221 or after labeling of genomic DNA with Klenow polymerase using C. jejuni sequence-specific primers (see Table S3 in the supplementary material) (data not shown). Thus, the detection limit of this DNA microarray for C. jejuni was estimated to be approximately 10,000 cells, a cell concentration that is within the range of C. jejuni's infective dose, estimated to be between 500 and 10,000 cells (57).
FIG. 6.
Threshold sensitivity of the DNA oligonucleotide array. Genomic DNA from various concentrations of C. jejuni strain NCTC11168 cells were isolated, CyDye labeled, and hybridized to the microarray. The average fluorescence signal for each C. jejuni probe is color coded, as described in the legend to Fig. 1.
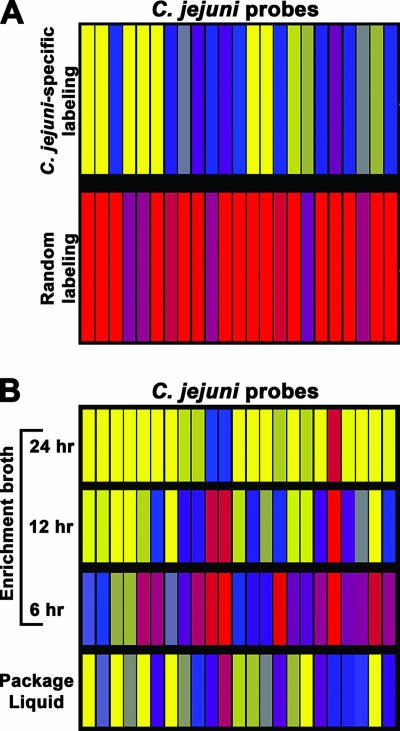
The feasibility of using our DNA microarrays for the identification of bacterial pathogens in a complex food sample was then examined (Fig. 7). Genomic DNA was isolated from mixed microbial communities from a 24-h enrichment broth and was fluorescently labeled by using Klenow polymerase with random primers. C. jejuni was not detected in the 24-h enrichment broth sample; the fluorescence signal intensities that corresponded to C. jejuni were the same as the background levels for most spots (Fig. 7A, bottom panel). However, a traditional culture method confirmed the presence of C. jejuni in the sample (data not shown). Since in the microbiota of poultry, other eubacteria, including Lactobacillus and Streptococcus (39, 69), that may interfere with the detection of Campylobacter spp. may be abundant, genomic DNA was labeled with CyDye by using C. jejuni sequence-specific primers (see Table S3 in the supplemental material). Interestingly, C. jejuni-specific labeling of genomic DNA resulted in identification of this pathogen in this sample (Fig. 7A, top panel). The average fluorescence signals for 8/23 of the C. jejuni probes (35%) were 5-fold higher than the background levels, and the average fluorescence signals for 12/23 of the probes (52%) were 7.5- to 10-fold higher than the background levels. Only 3/23 of the probes (13%) had values that approached background levels (Fig. 7A). Fluorescence values that were more than twofold greater than background levels were not detected in spots targeting other pathogens, such as C. coli or A. butzleri (data not shown). Therefore, these results demonstrate that the C. jejuni-specific labeling of total genomic DNA from a 24-h enrichment broth allowed detection of C. jejuni in this complex sample.
FIG. 7.
Detection of C. jejuni directly in package liquid from retail chicken samples before and after enrichment. (A) Total genomic DNA (5 μg) from a 24-h enrichment broth was CyDye labeled with either random primers or C. jejuni sequence-specific primers (see Materials and Methods). (B) Total genomic DNA (15 μg) from package liquid from whole chicken carcasses or from enrichment broths after various incubation times was CyDye labeled with C. jejuni sequence-specific primers (see Table S3 in the supplemental material). The CyDye-labeled samples were further hybridized to the microarray. The average fluorescence signal for each C. jejuni probe is color coded, as described in the legend to Fig. 1.
To assess further whether sequence-specific labeling can detect C. jejuni in a food sample without enrichment, total genomic DNA isolated from the microbiota in the package liquid from whole chicken carcasses was labeled and hybridized to the microarray (Fig. 7B). The results of this analysis demonstrated that C. jejuni was detectable directly in the poultry package liquid without enrichment (Fig. 7B). The fluorescence signal intensities for 12/23 of the C. jejuni probes (52%) were 7.5- to 10-fold higher than the background levels, while the values for 5/23 of the probes (22%) were 5-fold higher than the background levels (Fig. 7B). In contrast, the values for only 6/26 of the probes (26%) approached the background levels (Fig. 7B). Given that 74% of the C. jejuni probes had fluorescence values that were more than fivefold higher than the background levels, these results demonstrate that the C. jejuni-specific labeling of total genomic DNA from the package liquid allowed detection of C. jejuni in this complex sample. As expected, higher C. jejuni-specific signal intensities were obtained with increasing times of incubation of the enrichment broth. Less than 10% of the C. jejuni spots had significant fluorescence after a 6-h incubation, which resulted in a decrease in detection compared to package liquid without enrichment (Fig. 7B). A possible explanation for the reduced C. jejuni-specific hybridization after the 6-h enrichment is the 10-fold dilution of the package liquid in the enrichment broth. When the incubation time for enrichment was increased, at least 70% of the C. jejuni spots with fluorescence values that were 5- and 10-fold higher than the background levels were observed for the 12- and 24-h enrichment broth samples, respectively (Fig. 7B). Greater recovery of C. jejuni isolates on selective media was obtained with increasing times of incubation, demonstrating that the enrichment broth supported the growth of C. jejuni (data not shown). Thus, C. jejuni-specific labeling of genomic DNA increased the detection sensitivity of the microarray. Specific identification of C. jejuni present in a poultry package liquid sample was possible without an enrichment step.
Conclusions.
The present study demonstrated the use of DNA oligonucleotide arrays for the identification of members of the Campylobacteraceae family, specifically A. butzleri, C. coli, and C. jejuni. In the genus Campylobacter, C. jejuni is the primary cause of human gastrointestinal disease (16, 56, 57, 61). Although less prevalent, C. coli is also an important food-borne pathogen, accounting for a significant number of illnesses (61). Due to recent improvements in isolation techniques, Arcobacter infections of humans have been recognized to be important in human health and food safety (14, 54, 58, 64, 67). Epidemiological studies demonstrated that A. butzleri was the Arcobacter species most frequently associated with a persistent and watery diarrhea (64). The potential sources of Campylobacter and Arcobacter infections include contaminated food products (14, 44); therefore, a major concern in food safety is the development of efficient methods for pathogen detection, identification, and genotypic classification. PCR-based methods, such as multiplex PCR, have been developed to identify and discriminate these pathogens; however, two separate assays have to be performed to identify either A. butzleri (3, 20, 23) or C. jejuni and C. coli (6, 7, 28, 42, 46). The present study demonstrated that in a single assay DNA microarrays can simultaneously detect three species of the Campylobacteraceae family, C. jejuni, C. coli, and A. butzleri. Here we also provide the first evidence for the use of DNA microarrays for the detection of A. butzleri in food samples.
Results of validation experiments demonstrated that the 70-mer oligonucleotide probes on our DNA microarray were highly specific for each species. The signal intensities corresponding to each pathogen were at least 10-fold higher than the background levels. Previous reports on identification of campylobacters by DNA microarrays indicated that PCR amplification of a few target genes was performed as the first step in the detection of these pathogens (26, 27, 55, 65). The present study was the first study to demonstrate the use of DNA microarrays for identification of A. butzleri, C. coli, and C. jejuni without a PCR amplification step to examine a larger subset of genes, allowing further analysis of the genomic content and diversity of these pathogens.
By adapting a membrane filtration method that was developed initially for the efficient isolation of campylobacteria from human stool samples (12, 32), presumptive campylobacteria were successfully isolated from all retail chicken samples. This method also employed selective media with antibiotics at concentrations shown previously to support the growth of Campylobacter spp. (38a). Subsequent identification of these chicken isolates by our DNA microarray revealed that at least some C. jejuni isolates could be recovered from package liquid from whole chicken carcasses without enrichment. Nevertheless, increasing the time of enrichment resulted in more efficient recovery of C. jejuni and also resulted in the isolation of A. butzleri.
Validation experiments demonstrated that our DNA microarray had a detection sensitivity threshold of approximately 10,000 cells, which is within the range of C. jejuni's infectious dose, when genomic DNA from C. jejuni was labeled using Klenow polymerase with either random or C. jejuni sequence-specific primers. However, the use of random primers did not allow direct detection of low concentrations of C. jejuni in a 24-h enrichment broth sample, possibly due to the presence of other more prominent bacterial species that are part of the normal flora of chickens (39, 69). Interestingly, C. jejuni sequence-specific primers were adequate for labeling C. jejuni genomic DNA in a complex sample, thus increasing the sensitivity of the DNA microarray for detection of this pathogen. C. jejuni was detected efficiently both in package liquid from whole chicken carcasses and in enrichment broths.
An additional subset of probes was included in the microarray to target the LOS biosynthesis region, a region that contributes to the C. jejuni genome diversity (11, 34, 35, 49, 51, 60). The information obtained allowed us to further classify the gene content, to identify differences among the C. jejuni poultry isolates, and, potentially, to determine contamination sources. It is noteworthy that a large percentage (53%) of the C. jejuni isolates that were examined possessed either class B LOS or class C LOS, classes known for their sialylated ganglioside mimics (17). Sialylated LOS on the cell surface of C. jejuni are thought to exhibit molecular mimicry with gangliosides on peripheral nerves, resulting in the development of autoimmune neuropathies, such as Guillain-Barré syndrome and Miller Fisher syndrome (24, 47), and C. jejuni infections are considered to be the most frequent antecedent to the development of these neuropathies (24, 47). The finding that the isolates possessed class B LOS is of some importance to food safety and human health since C. jejuni strains recovered from Miller Fisher syndrome patients all possessed class B LOS (18, 29). Future work will be aimed at further expanding the probes represented on the microarray to target additional hypervariable regions in C. jejuni (11, 34, 35, 49, 51, 60), such as capsule biosynthesis and flagellar modification regions, to better classify C. jejuni isolates. Additionally, the DNA microarray will be developed for identification of other members of the Campylobacteraceae family, in particular emerging campylobacteria and arcobacteria, and other food-borne pathogens belonging to different genera that are of significant importance to human health to assess the prevalence of these species in either food or environmental samples.
Supplementary Material
Acknowledgments
This work was supported by United States Department of Agriculture Agricultural Research Service CRIS project 5325-42000-041 and was part of a United States collaboration with the European Commission Fifth Framework Project QLK1-CT-2002-0220 “CAMPYCHECK.”
We thank Sharon T. Horn, Anna H. Bates, Rommel D. Alfonso, and Bradley Gibbs for technical assistance and Magalie R. Guilhabert for critical reading of the manuscript. We especially thank Clifton K. Fagerquist, Leslie A. Harden, and Brandon R. Garbus for the matrix-assisted laser desorption ionization—time of flight data analysis.
Footnotes
Published ahead of print on 6 April 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Atabay, H. I., J. E. Corry, and S. L. On. 1998. Diversity and prevalence of Arcobacter spp. in broiler chickens. J. Appl. Microbiol. 84:1007-1016. [DOI] [PubMed] [Google Scholar]
- 2.Bang, D. D., F. Scheutz, P. Ahrens, K. Pedersen, J. Blom, and M. Madsen. 2001. Prevalence of cytolethal distending toxin (cdt) genes and CDT production in Campylobacter spp. isolated from Danish broilers. J. Med. Microbiol. 50:1087-1094. [DOI] [PubMed] [Google Scholar]
- 3.Brightwell, G., E. Mowat, R. Clemens, J. Boerema, D. J. Pulford, and S. L. On. 2007. Development of a multiplex and real time PCR assay for the specific detection of Arcobacter butzleri and Arcobacter cryaerophilus. J. Microbiol. Methods 68:318-325. [DOI] [PubMed] [Google Scholar]
- 4.Call, D. R., M. K. Borucki, and F. J. Loge. 2003. Detection of bacterial pathogens in environmental samples using DNA microarrays. J. Microbiol. Methods 53:235-243. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2006. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 states, United States, 2005. Morb. Mortal. Wkly. Rep. 55:392-395. [PubMed] [Google Scholar]
- 6.Chuma, T., S. Hashimoto, and K. Okamoto. 2000. Detection of thermophilic Campylobacter from sparrows by multiplex PCR: the role of sparrows as a source of contamination of broilers with Campylobacter. J. Vet. Med. Sci. 62:1291-1295. [DOI] [PubMed] [Google Scholar]
- 7.Cloak, O. M., and P. M. Fratamico. 2002. A multiplex polymerase chain reaction for the differentiation of Campylobacter jejuni and Campylobacter coli from a swine processing facility and characterization of isolates by pulsed-field gel electrophoresis and antibiotic resistance profiles. J. Food Prot. 65:266-273. [DOI] [PubMed] [Google Scholar]
- 8.Corry, J. E., and H. I. Atabay. 2001. Poultry as a source of Campylobacter and related organisms. J. Appl. Microbiol. 90:96S-114S. [DOI] [PubMed] [Google Scholar]
- 9.Corry, J. E., D. E. Post, P. Colin, and M. J. Laisney. 1995. Culture media for the isolation of campylobacters. Int. J. Food Microbiol. 26:43-76. [DOI] [PubMed] [Google Scholar]
- 10.de Boer, E., J. J. Tilburg, D. L. Woodward, H. Lior, and W. M. Johnson. 1996. A selective medium for the isolation of Arcobacter from meats. Lett. Appl. Microbiol. 23:64-66. [DOI] [PubMed] [Google Scholar]
- 11.Dorrell, N., J. A. Mangan, K. G. Laing, J. Hinds, D. Linton, H. Al-Ghusein, B. G. Barrell, J. Parkhill, N. G. Stoker, A. V. Karlyshev, P. D. Butcher, and B. W. Wren. 2001. Whole genome comparison of Campylobacter jejuni human isolates using a low-cost microarray reveals extensive genetic diversity. Genome Res. 11:1706-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engberg, J., S. L. On, C. S. Harrington, and P. Gerner-Smidt. 2000. Prevalence of Campylobacter, Arcobacter, Helicobacter, and Sutterella spp. in human fecal samples as estimated by a reevaluation of isolation methods for campylobacters. J. Clin. Microbiol. 38:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eyigor, A., K. A. Dawson, B. E. Langlois, and C. L. Pickett. 1999. Cytolethal distending toxin genes in Campylobacter jejuni and Campylobacter coli isolates: detection and analysis by PCR. J. Clin. Microbiol. 37:1646-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsythe, S. J. 2006. Arcobacter, p. 181-221. In Y. Mortarjemi and M. Adams (ed.), Emerging foodborne pathogens. CRC Press, Boca Raton, FL.
- 15.Fouts, D. E., E. F. Mongodin, R. E. Mandrell, W. G. Miller, D. A. Rasko, J. Ravel, L. M. Brinkac, R. T. DeBoy, C. T. Parker, S. C. Daugherty, R. J. Dodson, A. S. Durkin, R. Madupu, S. A. Sullivan, J. U. Shetty, M. A. Ayodeji, A. Shvartsbeyn, M. C. Schatz, J. H. Badger, C. M. Fraser, and K. E. Nelson. 2005. Major structural differences and novel potential virulence mechanisms from the genomes of multiple Campylobacter species. PLoS Biol. 3:0072-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman, C. R., R. M. Hoekstra, M. Samuel, R. Marcus, J. Bender, B. Shiferaw, S. Reddy, S. D. Ahuja, D. L. Helfrick, F. Hardnett, M. Carter, B. Anderson, and R. V. Tauxe. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38 Suppl. 3:S285-296. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert, M., M. F. Karwaski, S. Bernatchez, N. M. Young, E. Taboada, J. Michniewicz, A. M. Cunningham, and W. W. Wakarchuk. 2002. The genetic basis for the variation in the lipo-oligosaccharide of the mucosal pathogen, Campylobacter jejuni. Biosynthesis of sialylated ganglioside mimics in the core oligosaccharide. J. Biol. Chem. 277:327-337. [DOI] [PubMed] [Google Scholar]
- 18.Godschalk, P. C., A. P. Heikema, M. Gilbert, T. Komagamine, C. W. Ang, J. Glerum, D. Brochu, J. Li, N. Yuki, B. C. Jacobs, A. van Belkum, and H. P. Endtz. 2004. The crucial role of Campylobacter jejuni genes in anti-ganglioside antibody induction in Guillain-Barré syndrome. J. Clin. Investig. 114:1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gude, A., T. J. Hillman, C. R. Helps, V. M. Allen, and J. E. Corry. 2005. Ecology of Arcobacter species in chicken rearing and processing. Lett. Appl. Microbiol. 41:82-87. [DOI] [PubMed] [Google Scholar]
- 20.Harmon, K. M., and I. V. Wesley. 1997. Multiplex PCR for the identification of Arcobacter and differentiation of Arcobacter butzleri from other arcobacters. Vet. Microbiol. 58:215-227. [DOI] [PubMed] [Google Scholar]
- 21.Houf, K., L. De Zutter, J. Van Hoof, and P. Vandamme. 2002. Assessment of the genetic diversity among arcobacters isolated from poultry products by using two PCR-based typing methods. Appl. Environ. Microbiol. 68:2172-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houf, K., L. De Zutter, J. Van Hoof, and P. Vandamme. 2002. Occurrence and distribution of Arcobacter species in poultry processing. J. Food Prot. 65:1233-1239. [DOI] [PubMed] [Google Scholar]
- 23.Houf, K., A. Tutenel, L. De Zutter, J. Van Hoof, and P. Vandamme. 2000. Development of a multiplex PCR assay for the simultaneous detection and identification of Arcobacter butzleri, Arcobacter cryaerophilus and Arcobacter skirrowii. FEMS Microbiol. Lett. 193:89-94. [DOI] [PubMed] [Google Scholar]
- 24.Hughes, R. A., and D. R. Cornblath. 2005. Guillain-Barré syndrome. Lancet 366:1653-1666. [DOI] [PubMed] [Google Scholar]
- 25.Kabeya, H., S. Maruyama, Y. Morita, T. Ohsuga, S. Ozawa, Y. Kobayashi, M. Abe, Y. Katsube, and T. Mikami. 2004. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int. J. Food Microbiol. 90:303-308. [DOI] [PubMed] [Google Scholar]
- 26.Keramas, G., D. D. Bang, M. Lund, M. Madsen, H. Bunkenborg, P. Telleman, and C. B. Christensen. 2004. Use of culture, PCR analysis, and DNA microarrays for detection of Campylobacter jejuni and Campylobacter coli from chicken feces. J. Clin. Microbiol. 42:3985-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keramas, G., D. D. Bang, M. Lund, M. Madsen, S. E. Rasmussen, H. Bunkenborg, P. Telleman, and C. B. Christensen. 2003. Development of a sensitive DNA microarray suitable for rapid detection of Campylobacter spp. Mol. Cell. Probes 17:187-196. [DOI] [PubMed] [Google Scholar]
- 28.Klena, J. D., C. T. Parker, K. Knibb, J. C. Ibbitt, P. M. Devane, S. T. Horn, W. G. Miller, and M. E. Konkel. 2004. Differentiation of Campylobacter coli, Campylobacter jejuni, Campylobacter lari, and Campylobacter upsaliensis by a multiplex PCR developed from the nucleotide sequence of the lipid A gene lpxA. J. Clin. Microbiol. 42:5549-5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koga, M., M. Gilbert, M. Takahashi, J. Li, S. Koike, K. Hirata, and N. Yuki. 2006. Comprehensive analysis of bacterial risk factors for the development of Guillain-Barré syndrome after Campylobacter jejuni enteritis. J. Infect. Dis. 193:547-555. [DOI] [PubMed] [Google Scholar]
- 30.Konkel, M. E., S. G. Garvis, S. L. Tipton, D. E. Anderson, Jr., and W. Cieplak, Jr. 1997. Identification and molecular cloning of a gene encoding a fibronectin-binding protein (CadF) from Campylobacter jejuni. Mol. Microbiol. 24:953-963. [DOI] [PubMed] [Google Scholar]
- 31.Konkel, M. E., B. J. Kim, V. Rivera-Amill, and S. G. Garvis. 1999. Bacterial secreted proteins are required for the internalization of Campylobacter jejuni into cultured mammalian cells. Mol. Microbiol. 32:691-701. [DOI] [PubMed] [Google Scholar]
- 32.Lastovica, A. J. 2006. Emerging Campylobacter spp.: the tip of the iceberg. Clin. Microbiol. Newsl. 28:49-55. [Google Scholar]
- 33.Lehner, A., T. Tasara, and R. Stephan. 2005. Relevant aspects of Arcobacter spp. as potential foodborne pathogen. Int. J. Food Microbiol. 102:127-135. [DOI] [PubMed] [Google Scholar]
- 34.Leonard, E. E., II, T. Takata, M. J. Blaser, S. Falkow, L. S. Tompkins, and E. C. Gaynor. 2003. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J. Infect. Dis. 187:691-694. [DOI] [PubMed] [Google Scholar]
- 35.Leonard, E. E., II, L. S. Tompkins, S. Falkow, and I. Nachamkin. 2004. Comparison of Campylobacter jejuni isolates implicated in Guillain-Barré syndrome and strains that cause enteritis by a DNA microarray. Infect. Immun. 72:1199-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.le Roux, E., and A. J. Lastovica. 1998. The Cape Town protocol: how to isolate the most campylobacters for your dollar, pound, franc, yen, etc., p. 30-33. In A. J. Lastovica, D. G. Newell, and E. E. Lastovica (ed.), Proceedings of the 9th International Workshop on Campylobacter, Helicobacter and Related Organisms. Institute of Child Health, Cape Town, South Africa.
- 37.Linton, D., A. J. Lawson, R. J. Owen, and J. Stanley. 1997. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J. Clin. Microbiol. 35:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu-Stratton, Y., S. Roy, and C. K. Sen. 2004. DNA microarray technology in nutraceutical and food safety. Toxicol. Lett. 150:29-42. [DOI] [PubMed] [Google Scholar]
- 38a.Loades, C. J., L. E. Reiman, and C. W. Keevil. 2005. Abstr. 105th Gen. Meet. Am. Soc. Microbiol., abstr. D-198.
- 39.Lu, J., U. Idris, B. Harmon, C. Hofacre, J. J. Maurer, and M. D. Lee. 2003. Diversity and succession of the intestinal bacterial community of the maturing broiler chicken. Appl. Environ. Microbiol. 69:6816-6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mandrell, R. E., L. A. Harden, A. Bates, W. G. Miller, W. F. Haddon, and C. K. Fagerquist. 2005. Speciation of Campylobacter coli, C. jejuni, C. helveticus, C. lari, C. sputorum, and C. upsaliensis by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 71:6292-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandrell, R. E., and M. R. Wachtel. 1999. Novel detection techniques for human pathogens that contaminate poultry. Curr. Opin. Biotechnol. 10:273-278. [DOI] [PubMed] [Google Scholar]
- 42.Manfreda, G., A. De Cesare, V. Bondioli, and A. Franchini. 2003. Comparison of the BAX system with a multiplex PCR method for simultaneous detection and identification of Campylobacter jejuni and Campylobacter coli in environmental samples. Int. J. Food Microbiol. 87:271-278. [DOI] [PubMed] [Google Scholar]
- 43.Mazzeo, M. F., A. Sorrentino, M. Gaita, G. Cacace, M. Di Stasio, A. Facchiano, G. Comi, A. Malorni, and R. A. Siciliano. 2006. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for the discrimination of food-borne microorganisms. Appl. Environ. Microbiol. 72:1180-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, W. G., and R. E. Mandrell. 2005. Prevalence of Campylobacter in the food and water supply: incidence, outbreaks, isolation and detection, p. 101-163. In J. Ketley and M. E. Konkel (ed.), Campylobacter: molecular and cellular biology. Horizon Bioscience, Norfolk, United Kingdom.
- 45.Morita, Y., S. Maruyama, H. Kabeya, S. Boonmar, B. Nimsuphan, A. Nagai, K. Kozawa, T. Nakajima, T. Mikami, and H. Kimura. 2004. Isolation and phylogenetic analysis of Arcobacter spp. in ground chicken meat and environmental water in Japan and Thailand. Microbiol. Immunol. 48:527-533. [DOI] [PubMed] [Google Scholar]
- 46.On, S. L., and P. J. Jordan. 2003. Evaluation of 11 PCR assays for species-level identification of Campylobacter jejuni and Campylobacter coli. J. Clin. Microbiol. 41:330-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Overell, J. R., and H. J. Willison. 2005. Recent developments in Miller Fisher syndrome and related disorders. Curr. Opin. Neurol. 18:562-566. [DOI] [PubMed] [Google Scholar]
- 48.Parker, C. T., S. T. Horn, M. Gilbert, W. G. Miller, D. L. Woodward, and R. E. Mandrell. 2005. Comparison of Campylobacter jejuni lipooligosaccharide biosynthesis loci from a variety of sources. J. Clin. Microbiol. 43:2771-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker, C. T., B. Quiñones, W. G. Miller, S. T. Horn, and R. E. Mandrell. 2006. Comparative genomic analysis of Campylobacter jejuni strains reveals diversity due to genomic elements similar to those present in C. jejuni strain RM1221. J. Clin. Microbiol. 44:4125-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parkhill, J., B. W. Wren, K. Mungall, J. M. Ketley, C. Churcher, D. Basham, T. Chillingworth, R. M. Davies, T. Feltwell, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Moule, M. J. Pallen, C. W. Penn, M. A. Quail, M. A. Rajandream, K. M. Rutherford, A. H. van Vliet, S. Whitehead, and B. G. Barrell. 2000. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature 403:665-668. [DOI] [PubMed] [Google Scholar]
- 51.Pearson, B. M., C. Pin, J. Wright, K. I'Anson, T. Humphrey, and J. M. Wells. 2003. Comparative genome analysis of Campylobacter jejuni using whole genome DNA microarrays. FEBS Lett. 554:224-230. [DOI] [PubMed] [Google Scholar]
- 52.Pei, Z. H., R. T. Ellison III, and M. J. Blaser. 1991. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J. Biol. Chem. 266:16363-16369. [PubMed] [Google Scholar]
- 53.Penner, J. L., J. N. Hennessy, and R. V. Congi. 1983. Serotyping of Campylobacter jejuni and Campylobacter coli on the basis of thermostable antigens. Eur. J. Clin. Microbiol. 2:378-383. [DOI] [PubMed] [Google Scholar]
- 54.Prouzet-Mauléon, V., L. Labadi, N. Bouges, A. Ménard, and F. Mégraud. 2006. Arcobacter butzleri: underestimated enteropathogen. Emerg. Infect. Dis. 12:307-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sergeev, N., M. Distler, S. Courtney, S. F. Al-Khaldi, D. Volokhov, V. Chizhikov, and A. Rasooly. 2004. Multipathogen oligonucleotide microarray for environmental and biodefense applications. Biosens. Bioelectron. 20:684-698. [DOI] [PubMed] [Google Scholar]
- 56.Skirrow, M. B. 1994. Diseases due to Campylobacter, Helicobacter and related bacteria. J. Comp. Pathol. 111:113-149. [DOI] [PubMed] [Google Scholar]
- 57.Snelling, W. J., M. Matsuda, J. E. Moore, and J. S. Dooley. 2005. Under the microscope: Campylobacter jejuni. Lett. Appl. Microbiol. 41:297-302. [DOI] [PubMed] [Google Scholar]
- 58.Snelling, W. J., M. Matsuda, J. E. Moore, and J. S. Dooley. 2006. Under the microscope: Arcobacter. Lett. Appl. Microbiol. 42:7-14. [DOI] [PubMed] [Google Scholar]
- 59.Stanley, J., D. Linton, K. Sutherland, C. Jones, and R. J. Owen. 1995. High-resolution genotyping of Campylobacter coli identifies clones of epidemiologic and evolutionary significance. J. Infect. Dis. 172:1130-1134. [DOI] [PubMed] [Google Scholar]
- 60.Taboada, E. N., R. R. Acedillo, C. D. Carrillo, W. A. Findlay, D. T. Medeiros, O. L. Mykytczuk, M. J. Roberts, C. A. Valencia, J. M. Farber, and J. H. Nash. 2004. Large-scale comparative genomics meta-analysis of Campylobacter jejuni isolates reveals low level of genome plasticity. J. Clin. Microbiol. 42:4566-4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tam, C. C., S. J. O'Brien, G. K. Adak, S. M. Meakins, and J. A. Frost. 2003. Campylobacter coli—an important foodborne pathogen. J. Infect. 47:28-32. [DOI] [PubMed] [Google Scholar]
- 62.Vandamme, P. 2000. Taxonomy of the family Campylobacteriaceae, p. 3-26. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter. ASM Press, Washington, DC.
- 63.Vandamme, P., M. Vancanneyt, B. Pot, L. Mels, B. Hoste, D. Dewettinck, L. Vlaes, C. van den Borre, R. Higgins, J. Hommez, et al. 1992. Polyphasic taxonomic study of the emended genus Arcobacter with Arcobacter butzleri comb. nov. and Arcobacter skirrowii sp. nov., an aerotolerant bacterium isolated from veterinary specimens. Int. J. Syst. Bacteriol. 42:344-356. [DOI] [PubMed] [Google Scholar]
- 64.Vandenberg, O., A. Dediste, K. Houf, S. Ibekwem, H. Souayah, S. Cadranel, N. Douat, G. Zissis, J. P. Butzler, and P. Vandamme. 2004. Arcobacter species in humans. Emerg. Infect. Dis. 10:1863-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volokhov, D., V. Chizhikov, K. Chumakov, and A. Rasooly. 2003. Microarray-based identification of thermophilic Campylobacter jejuni, C. coli, C. lari, and C. upsaliensis. J. Clin. Microbiol. 41:4071-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vora, G. J., C. E. Meador, D. A. Stenger, and J. D. Andreadis. 2004. Nucleic acid amplification strategies for DNA microarray-based pathogen detection. Appl. Environ Microbiol. 70:3047-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wesley, I. V. 1997. Helicobacter and Arcobacter: potential human foodborne pathogens? Trends Food Sci. Technol. 8:293-299. [Google Scholar]
- 68.Wesley, I. V., and A. L. Baetz. 1999. Natural and experimental infections of Arcobacter in poultry. Poult. Sci. 78:536-545. [DOI] [PubMed] [Google Scholar]
- 69.Zhu, X. Y., T. Zhong, Y. Pandya, and R. D. Joerger. 2002. 16S rRNA-based analysis of microbiota from the cecum of broiler chickens. Appl. Environ. Microbiol. 68:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.