Abstract
Vibrio cholerae lives in aquatic environments and causes cholera. Here, we show that quorum sensing enhances V. cholerae viability under certain stress conditions by upregulating the expression of RpoS, and this regulation acts through HapR, suggesting that a quorum-sensing-enhanced stress response plays a role in V. cholerae environmental survival.
Vibrio cholerae is the gram-negative bacterium responsible for the endemic diarrheal disease cholera. It is a facultative pathogen residing predominantly in a variety of aqueous environments, including coastal and estuarine areas, in close proximity to dense human populations (4). One key aspect of V. cholerae's ability to adapt to certain stresses is the expression of the RNA polymerase sigma factor RpoS (21). It has been shown that RpoS is important in V. cholerae resistance to oxidative and nutritional stresses, both of which V. cholerae may encounter in its marine habitat (1, 2, 21). However, the specific role played by RpoS during infections is not clear (13, 21). RpoS has also been shown to increase the expression of HapR, the central regulatory protein in V. cholerae's quorum-sensing system (20), which regulates a number of physiological functions related to V. cholerae pathogenesis and environmental survival.
Quorum sensing enables bacterial cells to monitor their density by responding to the accumulation of autoinducers secreted constitutively by the bacterial community (5, 19). The quorum-signaling cascade in V. cholerae is initiated by the membrane-associated receptors LuxPQ and CqsS, which act through LuxO, which in its active phosphorylated state works to indirectly repress the transcription of HapR. As cell density increases, autoinducer molecules produced by the synthases CqsA and LuxS accumulate and work through LuxPQ and CqsS, resulting in the dephosphorylation of LuxO, which leads to a derepression of hapR (10, 15). The V. cholerae quorum-sensing system can downregulate virulence gene expression and biofilm synthesis (10, 15, 22, 23) but also positively affects protease production (17, 18), chitin-induced competence (12), and resistance to protozoan grazing (11). In this study, we show that V. cholerae's quorum-sensing system enhances the upregulation of rpoS via HapR and positively affects the bacterium's response to oxidative and nutritional stresses.
We have previously reported that quorum sensing enhances the survival rate of bacteria under certain stress conditions (7). This relationship between quorum sensing and stress response led us to investigate further what role this system plays in V. cholerae's response to other stressors. We first tested wild-type El Tor C6706 (7) and a number of quorum-sensing mutants for their ability to respond to oxidative stress via exposure to H2O2. This strategy was employed to identify quorum-sensing components necessary for viability under this stress condition. H2O2 was chosen as an oxidative stressor because of its presence and importance in two of V. cholerae's reservoirs as an antimicrobial factor in the human intestine and its occurrence in V. cholerae's aquatic reservoirs (1).
V. cholerae strains were grown to late log phase in LB, H2O2 was then added directly to the culture to a final concentration of 15 mM, as established previously (21), and percent survival was calculated based on time zero and the indicated time point CFU counts. Wild-type strains displayed resistance to H2O2 at 30 min postexposure (Fig. 1A), consistent with previous reports that V. cholerae strains are relatively resistant to this type of stress at late stages of growth (1, 21). The luxO and luxS mutants were also unaffected by the addition of H2O2, while the remaining strains showed a much higher sensitivity to this stressor within 30 min of exposure (Fig. 1A). The luxO constitutive mutants and cqsA mutants, all showing high sensitivity to H2O2, have been shown to be deficient in quorum-sensing regulation because of a tight repression of this system (18). Conversely, the luxO-null mutant, which survived as well as the wild-type strain in this assay, exhibits a constitutive upregulated quorum-sensing system (15). The luxS mutant also showed resistance to H2O2, and this gene has been shown to be dispensable in the regulation of the quorum-sensing cascade (22). We also tested the oxidative stress response of the cqsA mutant grown in spent medium of wild-type bacteria (containing cholerae autoinducer 1). Oxidative stress resistance was completely rescued to wild-type levels when the cqsA mutant was grown under this condition (data not shown).
FIG. 1.
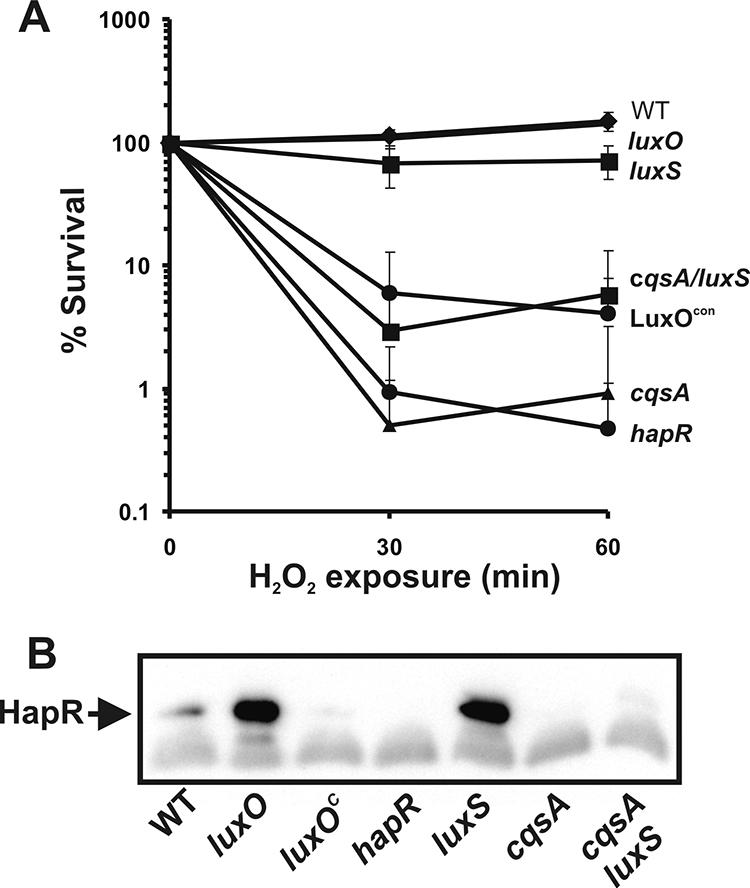
Quorum sensing in V. cholerae enhances the oxidative stress response. (A) Oxidative stress survival of the indicated quorum-sensing mutants. Error bars indicate standard deviations for three experiments. (B) Western blot showing late-log-phase expression patterns of HapR in the indicated strains. WT, wild type.
Because the quorum-sensing mutants sensitive to the oxidative stress assay have been documented as being deficient in HapR production, we performed a Western blot analysis to examine the production patterns of this protein at late-log-phase (immediately before the addition of H2O2 in the H2O2 stress response assay) growth in these strains (Fig. 1B). Equal amounts of total protein (10 μg) of late-log cultures were size fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and detected with affinity-purified polyclonal anti-HapR rabbit antiserum (produced by Proteintech, Inc.). The results confirm that the strains exhibiting a deficiency in the response to H2O2 had a negative HapR phenotype under the conditions of the stress response assay. These results strongly suggest that HapR plays an important role in enhancing the V. cholerae response to oxidative stress.
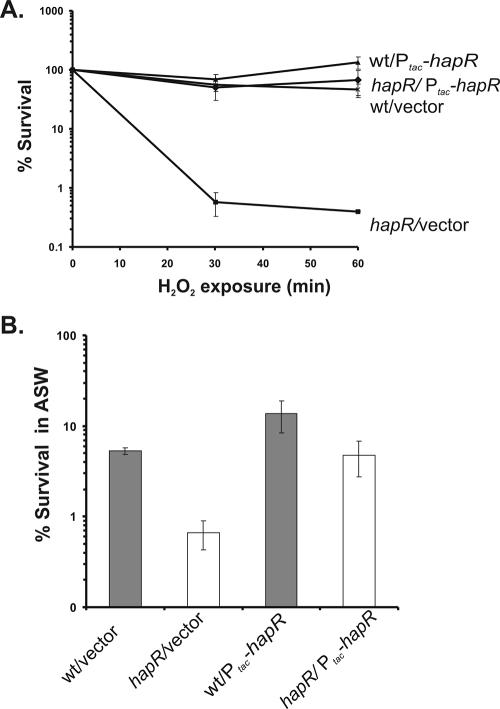
We repeated the oxidative stress response experiment with the wild-type and hapR mutant strains to confirm HapR's role in stress response. We also introduced each strain with either a plasmid containing Ptac-hapR or the vector control pBBR1-MCS2 (8). As expected, the hapR mutant strain was significantly more sensitive to H2O2, showing a 3-log decrease in viability within 30 min (Fig. 2A). The protective effect of HapR was complemented by the Ptac-hapR plasmid in the hapR mutant strain, indicating that HapR mediates this response.
FIG. 2.
HapR is important for the stress response in V. cholerae. (A) Oxidative stress survival of wild-type (wt) and hapR mutant strains. Error bars indicate standard deviations for three experiments. (B) Artificial seawater (ASW) survival of wild-type and hapR mutant strains. Error bars indicate standard deviations for three experiments.
To further characterize the role of quorum sensing in the stress response, we tested the viability of hapR wild-type and mutant strains under a low-nutrient stress condition in artificial seawater (2). After growth to late log phase, we resuspended bacteria in artificial seawater and assayed for survival after 18 h at 37°C. At this time point, we consistently observed a statistically significant 1-log difference (Student's t test P value of 0.01) in viability between wild-type and hapR vector strains (Fig. 2B). The protective effect of HapR was again complemented in trans via the Ptac-hapR plasmid. These data indicate that HapR, and thus quorum sensing, plays a role in conferring a protective effect on a range of environmental stresses. Interestingly, in our previous report (7), hapR mutants in fact survived slightly better than wild-type strains after being incubated in seawater at 4°C for 5 days. A different mechanism may be involved in survival in this extreme environment.
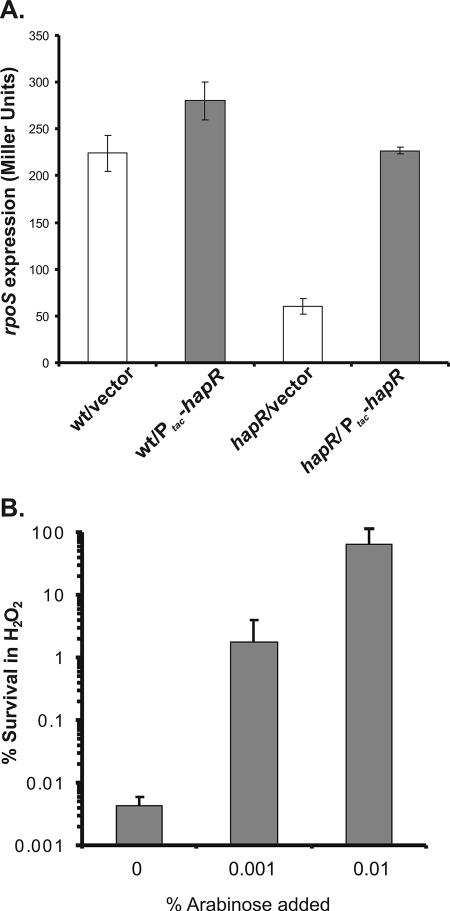
We compared the transcriptional profiles of the planktonic late log growth phase of wild-type and hapR mutant V. cholerae strains to elucidate the genetic reasons for the observed HapR-mediated protection. The microarray analysis was performed as previously described (23). We found that the expression of the stress response regulator RpoS in the wild type was threefold more than that of the hapR mutant (data not shown) under these conditions. RpoS has been shown to be critical in V. cholerae for survival under oxidative and nutritional stresses (21), and thus, we hypothesized that quorum sensing enhanced stress response acts through the regulation of rpoS. To test this hypothesis, we constructed rpoS-lacZ chromosomal fusions in wild-type and hapR mutant backgrounds to directly assay for transcriptional levels. The rpoS-lacZ transcriptional reporter was constructed by cloning the rpoS upstream region into a suicide plasmid containing a promoterless lacZ gene (pJZ244) (7), and the construct was then introduced into the V. cholerae chromosome by homologous recombination. A Ptac-hapR plasmid and its corresponding vector were subsequently introduced into these chromosomal fusion strains. β-Galactosidase assays were performed after cultures were grown to late log phase, where hapR is normally expressed (15). Figure 3A shows that rpoS expression was threefold higher in the hapR+ strain than in the hapR mutant strain, indicating that HapR stimulated rpoS transcription. A plasmid that constitutively expressed hapR in the hapR mutant restored rpoS transcription. These results indicate that HapR can affect oxidative and nutritional stress responses via the global stress response regulator RpoS.
FIG. 3.
HapR-induced rpoS expression is important for the V. cholerae oxidative stress response. (A) β-Galactosidase production (14) from rpoS-lacZ chromosomal fusions. Error bars indicate standard deviations for three independent experiments. (B) Overexpression of rpoS restores survival under oxidative stress conditions in a hapR mutant. Error bars indicate standard deviations for three experiments. wt, wild type.
To confirm that quorum sensing enhances the stress response in V. cholerae through the regulation of rpoS, we constructed an rpoS insertional mutation in wild-type and hapR mutant strains, along with either a Ptac-hapR plasmid or a corresponding vector, and exposed these strains to the oxidative stress assay. The rpoS mutant strains showed a much higher sensitivity to H2O2 than their RpoS wild-type counterparts, as has been previously reported (21), regardless of whether HapR was present (data not shown). This indicates that HapR alone does not mediate this response directly but through the action of RpoS. To further confirm this, we cloned the rpoS coding region into pBAD24 (6), which has an arabinose-inducible PBAD promoter. The resulting plasmid was then introduced into a hapR mutant strain and was grown to late log phase in the presence of various concentrations of arabinose. Upon the induction of rpoS with arabinose, the hapR mutant's ability to resist H2O2 was restored to wild-type levels (Fig. 3B). These data confirm that RpoS is critical for the oxidative stress response in V. cholerae and that HapR may enhance this response directly through RpoS.
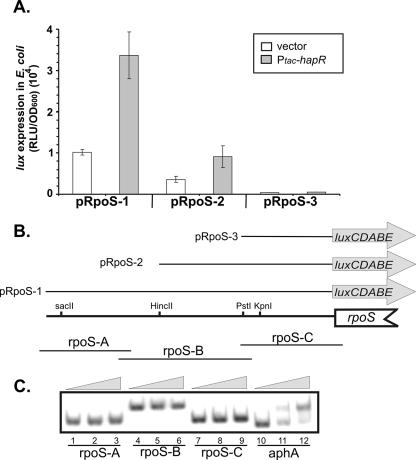
We then investigated whether the quorum-sensing regulator HapR regulates rpoS expression directly or indirectly. We first constructed three separate PrpoS-luxCDABE transcriptional reporter fusions on plasmids (3) by using three different-length segments of the 5′ upstream region of rpoS (Fig. 4B) and tested light production in the absence or in the presence of HapR in Escherichia coli. After overnight growth to stationary phase, the strains containing pRpoS-1 and pRpoS-2 and HapR produced threefold more luminescence than the vector control strain (Fig. 4A), confirming that HapR acts to upregulate rpoS expression. pRpoS-3, however, showed no luminescence in either strain, regardless of the presence of HapR, indicating that this region does not contain rpoS promoter sequences and is not recognized by HapR in E. coli. The greater production of luminescence in the strain harboring pRpoS-1 than in the strain harboring pRpoS-2 indicates that the extended 5′ region of pRpoS-1 is important in rpoS activation.
FIG. 4.
HapR activates the rpoS promoter directly. (A) Light production of PrpoS-lux. Error bars indicate the standard deviations for three experiments. (B) Representative diagram of 5′ rpoS regions cloned into three separate lux reporter plasmids and regions amplified for the gel shift assay. Representative restriction enzyme sites are shown. (C) Results of HapR gel shift assays. aphA promoter DNA was used as previously described (17). RLU, relative luciferase units; OD600, optical density at 600 nm.
To examine the specific binding region where HapR interacts with this promoter, we performed a gel mobility shift analysis of the region represented in the PrpoS-luxCDABE reporter plasmids. To purify HapR proteins, we cloned the hapR coding sequence into pET32a (Novagen), producing a construct encoding HapR with a His tag at the C terminus. The expressed recombinant HapR was then purified according to the manufacturer's instructions. The gel mobility shift assay was performed by using various lengths of overlapped radiolabeled rpoS promoter region segments (Fig. 4B). The promoter regions of rpoS and aphA were exposed to 0, 15, or 30 ng of purified C-terminal six-His-tagged HapR protein and resolved on a 5% polyacrylamide gel, as described previously (9). The results show that HapR did not bind directly to these fragments while, as a control, HapR bound to aphA promoter DNA, established as being repressed directly by HapR (Fig. 4C) (9). We also purified HapR with an N-terminal His tag (17) and performed the same gel shift assays but observed no binding of HapR to the rpoS promoter region (data not shown). These data, taken together with the PrpoS-lux expression in E. coli, suggest that HapR may require a cofactor conserved between E. coli and V. cholerae to bind to this region. The need for an unidentified cofactor has also been established for the HapR regulation of the hapA protease gene, where direct upregulation by HapR has been confirmed, but binding of HapR to this promoter region was not observed by a gel shift assay (17).
We have shown in this study that in V. cholerae, the quorum-sensing signaling system acting through HapR enhances the expression of rpoS and resistance to various stresses. The fact that RpoS can also activate hapR expression (20) highlights the possible importance of a HapR/RpoS autoregulation loop in the face of environmental stressors. A possible outcome of this is that this interaction of quorum sensing and the stress response may play a survival role in biofilm-associated cells in V. cholerae's natural environment.
Although hapR is not critical for the infection of the mouse cholera model (23), it could also be viewed as a virulence gene in that it serves an accessory role in processes important for pathogenesis and the spread of disease. A key example of this is the dispersal of V. cholerae from biofilms and the mucosal surface, an important event in the infectious cycle. It has been shown that HapR aids in the escape from biofilms and that RpoS also serves a role in the escape from the epithelial surface of the small intestine (16, 22). Together, these two elements may function in a regulatory loop enhancing each other's expression, leading to a shift in genetic expression (for example, upregulation of extracellular proteases and downregulation of biofilm formation genes) that aids in the stress response to host factors, dispersal within the lumen, and the exit of V. cholerae from the host, where the cycle of infection repeats from a pool of environmental bacteria.
Acknowledgments
We are grateful to Jorge Benitez for providing the PBAD-H6-hapR strains used in this study. We thank our lab members for helpful discussions and critical review of the manuscript.
This study was supported by the NIH/NIAID K22 (AI060715) and R01 (AI072479) awards (to J.Z.).
Footnotes
Published ahead of print on 13 April 2007.
REFERENCES
- 1.Arana, I., A. Muela, J. Iriberri, L. Egea, and I. Barcina. 1992. Role of hydrogen peroxide in loss of culturability mediated by visible light in Escherichia coli in a freshwater ecosystem. Appl. Environ. Microbiol. 58:3903-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker, R. M., F. L. Singleton, and M. A. Hood. 1983. Effects of nutrient deprivation on Vibrio cholerae. Appl. Environ. Microbiol. 46:930-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29:948-950, 952. [DOI] [PubMed] [Google Scholar]
- 4.Colwell, R. R., and A. Huq. 1994. Environmental reservoir of Vibrio cholerae. The causative agent of cholera. Ann. N. Y. Acad. Sci. 740:44-54. [DOI] [PubMed] [Google Scholar]
- 5.Fuqua, W. C., S. C. Winans, and E. P. Greenberg. 1994. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guzman, L.-M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joelsson, A., Z. Liu, and J. Zhu. 2006. Genetic and phenotypic diversity of quorum-sensing systems in clinical and environmental isolates of Vibrio cholerae. Infect. Immun. 74:1141-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 9.Kovacikova, G., and K. Skorupski. 2002. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol. Microbiol. 46:1135-1147. [DOI] [PubMed] [Google Scholar]
- 10.Lenz, D. H., K. C. Mok, B. N. Lilley, R. V. Kulkarni, N. S. Wingreen, and B. L. Bassler. 2004. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 118:69-82. [DOI] [PubMed] [Google Scholar]
- 11.Matz, C., D. McDougald, A. M. Moreno, P. Y. Yung, F. H. Yildiz, and S. Kjelleberg. 2005. Biofilm formation and phenotypic variation enhance predation-driven persistence of Vibrio cholerae. Proc. Natl. Acad. Sci. USA 102:16819-16824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meibom, K. L., M. Blokesch, N. A. Dolganov, C. Y. Wu, and G. K. Schoolnik. 2005. Chitin induces natural competence in Vibrio cholerae. Science 310:1824-1827. [DOI] [PubMed] [Google Scholar]
- 13.Merrell, D. S., and A. Camilli. 2000. Regulation of Vibrio cholerae genes required for acid tolerance by a member of the “ToxR-like” family of transcriptional regulators. J. Bacteriol. 182:5342-5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 15.Miller, M. B., K. Skorupski, D. H. Lenz, R. K. Taylor, and B. L. Bassler. 2002. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 110:303-314. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen, A. T., N. A. Dolganov, G. Otto, M. C. Miller, C. Y. Wu, and G. K. Schoolnik. 2006. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathogens 2:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva, A. J., K. Pham, and J. A. Benitez. 2003. Haemagglutinin/protease expression and mucin gel penetration in El Tor biotype Vibrio cholerae. Microbiology 149:1883-1891. [DOI] [PubMed] [Google Scholar]
- 18.Vance, R. E., J. Zhu, and J. J. Mekalanos. 2003. A constitutively active variant of the quorum-sensing regulator LuxO affects protease production and biofilm formation in Vibrio cholerae. Infect. Immun. 71:2571-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters, C. M., and B. L. Bassler. 2005. Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21:319-346. [DOI] [PubMed] [Google Scholar]
- 20.Yildiz, F. H., X. S. Liu, A. Heydorn, and G. K. Schoolnik. 2004. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol. Microbiol. 53:497-515. [DOI] [PubMed] [Google Scholar]
- 21.Yildiz, F. H., and G. K. Schoolnik. 1998. Role of rpoS in stress survival and virulence of Vibrio cholerae. J. Bacteriol. 180:773-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu, J., and J. J. Mekalanos. 2003. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev. Cell 5:647-656. [DOI] [PubMed] [Google Scholar]
- 23.Zhu, J., M. B. Miller, R. E. Vance, M. Dziejman, B. L. Bassler, and J. J. Mekalanos. 2002. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 99:3129-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]