Abstract
Pseudomonas syringae pv. tomato strain DC3000, a pathogen of tomato and Arabidopsis, occurs as an epiphyte. It produces N-acyl homoserine lactones (AHLs) which apparently function as quorum-sensing signals. A Tn5 insertion mutant of DC3000, designated PsrA− (Psr is for Pseudomonas sigma regulator), overexpresses psyR (a LuxR-type regulator of psyI) and psyI (the gene for AHL synthase), and it produces a ca. 8-fold-higher level of AHL than does DC3000. The mutant is impaired in its ability to elicit the hypersensitive reaction and is attenuated in its virulence in tomato. These phenotypes correlate with reduced expression of hrpL, the gene for an alternate sigma factor, as well as several hrp and hop genes during early stages of incubation in a Hrp-inducing medium. PsrA also positively controls rpoS, the gene for an alternate sigma factor known to control various stress responses. By contrast, PsrA negatively regulates rsmA1, an RNA-binding protein gene known to function as negative regulator, and aefR, a tetR-like gene known to control AHL production and epiphytic fitness in P. syringae pv. syringae. Gel mobility shift assays and other lines of evidence demonstrate a direct interaction of PsrA protein with rpoS promoter DNA and aefR operator DNA. In addition, PsrA negatively autoregulates and binds the psrA operator. In an AefR− mutant, the expression of psyR and psyI and AHL production are lower than those in DC3000, the AefR+ parent. In an RpoS− mutant, on the other hand, the levels of AHL and transcripts of psyR and psyI are much higher than those in the RpoS+ parent, DC3000. We present evidence, albeit indirect, that the RpoS effect occurs via psyR. Thus, AefR positively regulates AHL production, whereas RpoS has a strong negative effect. We show that AefR and RpoS do not regulate PsrA and that the PsrA effect on AHL production is exerted via its cumulative, but independent, effects on both AefR and RpoS.
Pseudomonas syringae pv. tomato sustains its life style as a pathogen, as an epiphyte, and as a commensal (18). The alternating lifestyles of these bacteria (i.e., switches from commensal to epiphyte and from epiphyte to pathogen) demand that genes required for their sustenance be regulated both tightly and rapidly. To meet these challenges, these bacteria have evolved to recognize environmental signals and to modulate gene expression accordingly. It is therefore no surprise that DC3000 produces or has the potential to produce a plethora of transcriptional factors, as is evident from the observation that over 12% of its genome (711 out of 5,763 total open reading frames [ORFs]) has been assigned to gene regulation, signal transduction, and transcription (4).
Alternate sigma factors play crucial roles in bacterial development as well as adaptations to various environmental niches, including associations with eukaryotic hosts. Both symbiotic and parasitic associations are controlled by several sigma factors. Since functions of such sigma factors are generally required under highly specialized and specific conditions, bacteria rigorously control their levels and activity. Such controls are accomplished by transcriptional and posttranscriptional regulation as well as protein-sigma factor interactions. In P. syringae pv. tomato strain DC3000, 17 ORFs are known or predicted to specify alternate sigma factors (4). At least three of those control genes required for plant interaction: sigma-L or HrpL (an alternate sigma factor), RpoN (RNA polymerase sigma-54 factor), and AlgT (= AlgU or SigE) (a member of the extracytoplasmic function family of sigma factors). Although RpoS in plant-pathogenic pseudomonads has received little or no attention, based upon studies with Pseudomonas aeruginosa and Escherichia coli, it is safe to conclude that this sigma factor may play an important role in secondary metabolite production and bacterial ability to cope with various stresses.
Most plant-pathogenic Pseudomonas species, including P. syringae pv. tomato strain DC3000, produce N-acyl homoserine lactone (AHL) (22, 30, 53, 54, 56), a putative quorum-sensing signal. Indeed, a search of the genome sequence databases for several plant-pathogenic Pseudomonas species revealed the existence of ORFs corresponding to LuxI-like proteins (PsyI of P. syringae pv. tomato [Pspto_3864], AhlI of P. syringae pv. syringae [Psyr_1621], AhlI of P. syringae pv. phaseolicola [Pspph_1614], and PsmI of P. syringae pv. maculicola [accession no. AF234628]) which function as AHL synthases and LuxR-like proteins (PsyR of P. syringae pv. tomato [Pspto_3863], AhlR of P. syringae pv. syringae [Psyr_1622], AhlR of P. syringae pv. phaseolicola [Pspph_1615], and PsmR of P. syringae pv. maculicola [accession no. AF234628]) predicted to specify a LuxR family AHL receptor. The production of a single AHL species by four strains of P. syringae pv. tomato (12) and the absence of paralogs of psyI and psyR in the DC3000 genome database strongly suggest a lack of redundancy in the production of the putative quorum-sensing signal in these strains, as noted for many other bacteria (51, 53, 56). Northern blot analysis of total RNAs also revealed the presence of a single psyI RNA and a single psyR RNA (each ca. 800 bases) in DC3000 (8). Although psyI and psyR sequences overlap in DC3000, they are expressed as independent transcriptional units.
Compared to those of P. aeruginosa (22, 36, 46, 55, 57), AHL-producing systems of P. syringae pathovars have received less attention. However, several regulators that control AHL production have been identified: GacS/GacA, members of a two component system (8, 38), as well as RNA-binding proteins and RNA regulators (7). Moreover, as in P. syringae pv. syringae (11) and P. aeruginosa (36), AHL production in DC3000 is autoregulated (A. Chatterjee et al., unpublished data). The presence of a LUX box in front of psyI of DC3000 also supports the notion that it is controlled by PsyR.
AHL production in P. syringae pv. syringae strain B728a is partially dependent on AefR (autoinducer and epiphytic fitness regulator), a member of the TetR family (38). This is an important finding as it demonstrates coregulation of AHL production with genes for the epiphytic state of this bacterium. Since a homolog of aefR is present in the DC3000 genome (Pspto_3549; 90% identity and 95% similarity), a similar regulatory effect is predicted to occur in P. syringae pv. tomato. P. aeruginosa strain PAO1 also has a putative ortholog of aefR (28% identity and 47% similarity), but its role in AHL production has not been assessed.
In P. aeruginosa and P. putida (3, 53, 58), RpoS has been found to control AHL production. By contrast, a similar regulatory effect of RpoS on AHL production in P. syringae has not yet been established. An RpoS− mutant of P. aeruginosa shows elevated levels of rhlI (the gene encoding RhlI, which is required for synthesis of N-butyryl homoserine lactone and RhlI-generated AHL production but, notably, no effect on transcripts of rhlR (encoding RhlR, a transcriptional activator that responds to N-butyryl-homoserine lactone) (58). An RpoS-deficient strain of P. putida WCS358 expressed higher levels of ppuI and produced more AHL than the RpoS+ strain (3). However, Girard et al. (15) reported that in a poor medium (MVB1), RpoS positively regulates N-hexanoyl-l-homoserine lactone production in Pseudomonas chlororaphis PCL1391. This observation raises the possibility that the effects of RpoS on AHL production may vary under different growth conditions, including medium, temperature, and pH.
Recent studies with P. aeruginosa, P. putida, and P. chlororaphis have disclosed the presence of PsrA (Pseudomonas sigma regulator), a transcriptional factor that controls rpoS expression as well as AHL production (10, 15, 25, 27). Examination of whole-genome sequences of three P. syringae pathovars revealed the presence of PsrA orthologs. We have extended this observation by examining the characteristics of PsrA of P. syringae pv. tomato strain DC3000. We document that DC3000 PsrA is a DNA-binding protein and that it positively controls RpoS production. Our findings for the first time document a negative effect of PsrA on expression of aefR and rsmA1, the gene for an RNA-binding protein, and positive effects on hrpL. A mutant deficient in PsrA produces reduced levels of hrpL transcript, fails to elicit a hypersensitive reaction (HR) at low cell concentration, and is attenuated in its virulence in tomato leaves. Moreover, we show that the PsrA effect on AHL production in DC3000 results from its cumulative effects on AefR and RpoS and that this response is different from those reported for P. aeruginosa, P. putida, and P. chlororaphis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
Bacterial strains and plasmids are described in Table 1. All the wild-type strains were maintained on KB or LB agar. The strains carrying antibiotic markers were maintained on KB or LB agar containing appropriate antibiotics.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains or plasmid | Relevant characteristics | Source (reference) |
|---|---|---|
| Strains | ||
| Pseudomonas syringae pv. tomato | ||
| DC3000 | Wild type | Tomato (8) |
| AC820 | PsrA− derivative of DC3000 | This work |
| AC821 | AefR− derivative of DC3000 | This work |
| AC822 | RpoS− derivative of DC3000 | This work |
| AC823 | PsyR− derivative of DC3000 | This work |
| AC824 | RpoS− PsyR− derivative of DC3000 | This work |
| Pseudomonas syringae pv. syringae | ||
| B3A | Wild type | Peach, D. Gross |
| B456 | Wild type | Tangelo (11) |
| B728a | Wild type | Bean (59) |
| 301D | Wild type | Pear, D. Gross |
| Pseudomonas syringae BR2R | Wild type | Bean, K. Willis |
| Pseudomonas syringae pv. maculicola ES4326 | Wild type | J. T. Greenberg |
| Pseudomonas syringae pv. glycinia | ||
| Race 1 | Wild type | Soybean (11) |
| Race 5 | Wild type | Soybean (11) |
| Pseudomonas syringae pv. tabaci Rif5 | Wild type | Tobacco, lab collection |
| Pseudomonas syringae pv. phaseolicola | ||
| 1448A | Wild type | Bean (20) |
| PDDC3019 | Wild type | Bean (11) |
| PM132 | Wild type | Bean (11) |
| Pseudomonas corrugata 0728-6 | Wild type | Potato (43) |
| Pseudomonas fluorescens Pf7-14 | Wild type | Rice (9) |
| Pseudomonas savastanoi 2009 | Wild type | Potato, T. C. Currier |
| Pseudomonas viridiflava | ||
| SF312A | Wild type | Squash, lab collection |
| MI-4 | Tn5 mutant of SF312A | Lab collection |
| Pseudomonas aeruginosa | ||
| PAO1 | Wild type | Lab collection |
| PAO2006 | Wild type | B. Holloway |
| Escherichia coli | ||
| DH5α | φ80lacZΔM15 Δ(lacZYA-argF)U169 hsdR17 recA1 endA1 thi-1 | Gibco BRL |
| MC4100 | araD139 Δ(lacIPOZYA)U169 recA1 thi-1 Strr | 29 |
| VJS533 | ara Δ(lac-proAB) rpsL φ80lacZΔM15 recA56 | 16 |
| Plasmids | ||
| pLARF5 | Tcr | 24 |
| pMAL-c2g | Apr, protein expression vector | New England Biolabs |
| pMMB66EH | Apr, vector | 14 |
| pMMB66EHΩ | Spr, Spr cassette inserted at PvuI site of pMMB66EH | 8 |
| pMP220 | Tcr, promoter-probe vector | 47 |
| pAKC1251 | Tcr, psrA+ in pLARF5 from DC3000 genomic library | This work |
| pAKC1252 | Tcr Kmr PsrA− derivative of pAKC1251 | This work |
| pAKC1253 | Apr, psrA coding region in pMAL-c2g | This study |
| pAKC1254 | Spr, psrA in pMMB66EHΩ | This work |
| pAKC1255 | Tcr, psyI-lacZ in pMP220 | This work |
| pAKC1256 | Tcr, psyR-lacZ in pMP220 | This work |
| pAKC1257 | Tcr, psrA-lacZ in pMP220 | This work |
| pAKC1258 | Tcr, aefR-lacZ in pMP220 | This work |
| pAKC1259 | Tcr, rpoS-lacZ in pMP220 | This work |
| pAKC1260 | Tcr, aefR+ in pLARF5 from DC3000 genomic library | This work |
| pAKC1261 | Tcr Kmr AefR− derivative of pAKC1260 | This work |
| pAKC1262 | Tcr, rpoS+ in pLARF5 from DC3000 genomic library | This work |
| pAKC1263 | Tcr Kmr RpoS− derivative of pAKC1262 | This work |
| pHV200 | Apr, 8.8-kb SalI fragment containing the lux operon | 16 |
| pHV200I | Apr, frameshift mutant of luxI in pHV200 | E. P. Greenberg |
The compositions of KB and LB media have been described in previous publications (6, 8). The Hrp-inducing medium was made as described by Huang et al. (19), and MGY medium was as described by Keane et al. (23). When required, antibiotics were added as follows: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; rifampin, 100 μg/ml; spectinomycin, 50 μg/ml; and tetracycline, 10 μg/ml. Media were solidified using 1.5% (wt/vol) agar.
Sequence alignment.
Sequence alignment was performed using ClustalW at www.expasy.ch, and default parameters were used. Domain searching was performed using rpsblast at www.ncbi.nlm.nlh.gov/Structure/ccd/wrpsb.cgi.
DNA techniques.
Standard procedures were used for the isolation of plasmid and chromosomal DNAs, gel electrophoresis, and DNA ligation (41). Restriction and modification enzymes were obtained from Promega Biotec (Madison, WI). The Prime-a-Gene DNA labeling system (Promega Biotec, Madison, WI) was used for labeling DNA probes. Southern blot analysis was carried out under high-stringency conditions (hybridization at 65°C in 6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's solution, 0.5% [wt/vol] sodium dodecyl sulfate [SDS], and 100 μg/ml denatured salmon sperm DNA and washing at 65°C with 2× SSC for 30 min, 1× SSC plus 0.1% [wt/vol] SDS for 30 min and 0.1× SSC plus 0.1% [wt/vol] SDS for 30 min).
Construction of PsrA−, AefR−, and RpoS− mutants.
psrA+, aefR+, and rpoS+ plasmids were obtained from a DC3000 genomic library by PCR using gene-specific primers which were designed based on nucleotide sequences of those genes in GenBank. The DNAs of the psrA+, aefR+, and rpoS+ plasmids were then mutagenized using the EZ:TN〈KAN-2〉 insertion kit (Epicenter Biotechnologies, Madison, WI). Mutants were constructed by marker exchange of DC3000 with inactivated psrA, aefR, and rpoS plasmids. The procedures for marker exchange have been described by Chatterjee et al. (6). Inactivation of psrA, aefR, and rpoS in mutants was confirmed by PCR and Northern blot analysis.
Construction of psrA+ plasmid pAKC1254 and of psyI-lacZ, psyR-lacZ, psrA-lacZ, aefR-lacZ, and rpoS-lacZ fusions.
A DNA fragment containing the entire ORF of psrA of DC3000 was PCR amplified from chromosomal DNA and cloned into pMMB66EHΩ behind ptac to yield pAKC1254. To construct psyI-lacZ, psyR-lacZ, psrA-lacZ, aefR-lacZ, and rpoS-lacZ fusions, PCR-amplified DNA fragments containing upstream DNAs of psyI (nucleotides [nt] −799 to +73), psyR (nt −645 to +47), psrA (nt −406 to +41), aefR (nt −401 to +49), and rpoS (nt −550 to +37) were cloned into pMP220 to yield pAKC1255, pAKC1256, pAKC1257, pAKC1258, and pAKC1259, respectively. The numbers correspond to the putative translational start site.
Northern blot analyses.
Bacterial cultures were grown at 28°C in KB medium to a Klett value of ca. 500 or to a Klett value of 100 and then switched into Hrp-inducing medium for an additional 2 h of incubation (for hrp and hop genes). Total RNA isolation and Northern blot analysis were performed as described by Liu et al. (29). Equal loading of RNA was checked by hybridization of the blot with a probe corresponding to the 16S rRNA gene.
Expression and purification of MBP-PsrA protein.
A DNA segment containing the coding region of psrA was PCR amplified and cloned into pMAL-c2g vector (New England Biolabs, Beverly, MA) to yield pAKC1253. E. coli strain DH5α carrying pAKC1253 was grown in LB medium supplemented with glucose (0.2%, wt/vol) and ampicillin at 37°C. IPTG (isopropyl-β-d-thiogalactopyranoside) was added to yield a final concentration of 1 mM when the culture reached an A600 of 0.7. Bacterial cells were harvested 3 hours after IPTG induction. Maltose-binding protein (MBP)-PsrA fusion protein was purified by amylose resin (New England Biolabs, Beverly, MA) affinity chromatography according to the protocol provided by the company. The protein concentration was determined by using the CB-X protein assay kit (Geno Technology, Inc., St. Louis, MO).
Gel mobility shift assays.
DNA fragments of rpoS, aefR, and psrA containing the putative PsrA-binding sequences were PCR amplified using primers rpoS-1 and rpoS-2, aefR-1 and aefR-2, and psrA-1 and psrA-2 (Table 2), respectively. The DNA fragments were purified using the Wizard SV gel and PCR clean-up system (Promega Biotec, Madison, WI) and end labeled with [α-32P]dATP and Klenow fragment. Protein-DNA interaction assays were performed in 20 μl of binding buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol, 0.1 mM EDTA, and 5% [wt/vol] glycerol) containing 1 μg of salmon sperm DNA, 2 μg of bovine serum albumin, and purified MBP-PsrA protein with or without competitors. The reaction mixtures were incubated at room temperature for 20 min and subjected to electrophoresis in 5% (wt/vol) polyacrylamide gels. The gels were dried and exposed to X-ray film.
TABLE 2.
Primers used in gel mobility shift assays and primer extension analysis
| Primer | Sequence (5′ to 3′) | Position (nt)a |
|---|---|---|
| rpoS-1 | CAAGTCTCCTACGGGTTGGACCTG | −117 to −94 |
| rpoS-2 | TGTGTTTGATGATGATCAAATCGC | +77 to +54 |
| rpoS-3 | ACTAACAACCTGCGATTATGACCG | +123 to +100 |
| aefR-1 | ATATGTAACAGTGTTTTACACTAC | −76 to −53 |
| aefR-2 | TGTTCTAGATGGCCGCCTGGACGATGGACTCG | +94 to +71 |
| psrA-1 | CCTTTGTCAGGTAGCTTGTATAAG | −122 to −99 |
| psrA-2 | TGTTCTAGAGCAGCATCAAGAATGCGTTCAACGG | +65 to +41 |
| psrA-3 | CGCAAGGATGTTTCAGCGAAACC | +110 to +88 |
Corresponds to base positions relative to the transcriptional start site.
Primer extension analysis.
Total RNA extracted from DC3000 grown in KB medium at 28°C to a Klett value of ca. 500 was utilized in the primer extension assays. The 32P-labeled rpoS-3, aefR-2, and psrA-3 oligonucleotide primers (Table 2) and the primer extension system from Promega Biotec (Madison, WI) were used for assays. The extension products were run in an 8% acrylamide-urea sequencing gel. The dried gel was developed using the FLA5000 phosphorimager system (Fuji Photo Film Co., Ltd., Kanagawa, Japan).
Bioluminescence assays for AHL production.
DC3000 and its PsrA−, AefR−, and RpoS− mutants were grown in KB medium to a Klett value of ca. 600. Culture supernatants were collected and assayed for AHL production using an E. coli-based bioassay system (6). There is a liner relationship between the quantity of AHL production and the emission of bioluminescence.
β-Galactosidase assays.
Bacterial constructs were grown at 28°C in KB or LB medium supplemented with appropriate antibiotics, and cultures were used for assays as described in the table footnotes. The β-galactosidase assays were performed as described by Miller (31).
HR and pathogenicity tests.
Bacteria were grown on KB agar overnight at 28°C, and cells were suspended in water. The previously published procedures (8) were followed. Young, fully expanded third and fourth leaves of ca. 8-week-old Nicotiana tabacum L. cv. Samsun were used for the HR test; 1 × 106 CFU/ml or 1 × 107 CFU/ml of bacterial cells were infiltrated. Pictures were taken 20 h after infiltration. Pathogenicity tests were performed by dipping 4-week-old tomato (Lycopersicon esculentum cv. moneymaker) plants into bacterial suspensions (2 × 108 CFU/ml in water containing 0.005% Silwet L-77 [Lehle Seeds, Round Rock, TX]) for 2 min. Pictures were taken 7 days after inoculation.
The experiments were performed at least two or three times, and the results were reproducible.
RESULTS AND DISCUSSION
PsrA occurs in P. syringae pv. tomato strain DC3000 and other P. syringae pathovars.
Examination of whole-genome sequences of P. syringae pathovars tomato, syringae, and phaseolicola disclosed the presence of ORFs corresponding to PsrA. Nucleotide and deduced amino acid sequences of DC3000 psrA (Pspto_3508), obtained from the whole genomic sequence, revealed that it encodes a protein of 238 amino acid residues with a theoretical pI of 9.47 and a molecular mass of 26.4 kDa. The helix-turn-helix motif (residues 10 to 56) of DC3000 PsrA shares high homology with the conserved region of the TetR family of bacterial regulatory proteins. DC3000 PsrA shares high homology with previously reported PsrA proteins of P. chlororaphis (accession number AAM52309; 95% similar and 92% identical), P. putida (accession number NP_744293; 96% similar and 92% identical), and P. aeruginosa (accession number AAG06394; 90% similar and 82% identical). The DC3000 PsrA protein also has very high homology with putative PsrA proteins of P. syringae pv. syringae B728a (accession number YP_236352; 99% similar and 98% identical), P. syringae pv. phaseolicola 1448A (accession number AAZ33298; 98% similar and 97% identical) and P. fluorescens Pf-5 (accession number AAY91237; 95% similar and 92% identical).
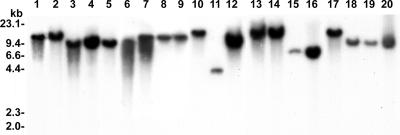
To assess the presence of psrA orthologs in other plant-pathogenic and plant-associated Pseudomonas species, we conducted Southern blot analysis of EcoRI-digested chromosomal DNAs with psrA DNA of DC3000 as the probe. The results in Fig. 1 show that psrA orthologs occur in strains of P. syringae pathovars maculicola, phaseolicola, glycinia, and syringae; P. corrugata; P. tabaci; P. viridiflava; P. savastanoi; P. aeruginosa; and P. fluorescens.
FIG. 1.
Southern blot analysis using the psrA+ DNA probe from P. syringae pv. tomato strain DC3000 under high-stringency conditions. Lane 1, P. syringae pv. tomato strain DC3000; lane 2, P. syringae pv. maculicola strain ES4326; lanes 3 to 6, P. syringae pv. syringae strains B3A, B456, B728a, and 301D, respectively; lane 7, P. syringae strain BR2R; lanes 8 to 10, P. syringae pv. phaseolicola strains 1448A, PDDC3019, and PM132, respectively; lane 11, P. corrugata strain 0782-6; lane 12, P. tabaci strain Rif5; lanes 13 and 14, P. syringae pv. glycinia strains Race 1 and Race 5, respectively; lanes 15 and 16, P. viridiflava strains SF312A and MI-4, respectively; lane 17, P. savastanoi strain 2009; lanes 18 and 19, P. aeruginosa strains PAO1 and PAO2006, respectively; and lane 20, P. fluorescens strain Pf7-14.
The high degree of genetic conservation raised the possibility that PsrA controls important biological functions in those plant pathogens, as in environmentally significant pseudomonads. To test this idea, we made a PsrA− mutant of DC3000. We discuss below the results of our comparative studies with PsrA+ DC3000 and its PsrA− derivative.
Effects of PsrA−deficiency on the capacity to elicit the HR and pathogenicity.
The results in Fig. 2A show that DC3000 elicited the typical HR symptom in tobacco leaf (site 1), whereas the PsrA− mutant (site 2) failed to elicit the HR when the leaf was infiltrated with 1 × 106 CFU/ml of bacterial cells. However, both the parent and the mutant induced the HR symptoms at a higher cell concentration (i.e., 1 × 107 CFU/ml) (Chatterjee et al., unpublished data). Northern blot analysis of total RNAs extracted from bacterial cells after 2 hours of incubation in Hrp-inducing medium revealed that transcript levels of hrpL as well as several hrp and hop genes, including hrpA, hrpZ, hopP to hopK, and hopP to hopJ, were lower in the PsrA− mutant than in DC3000 (Fig. 2B).
FIG. 2.
(A) Effect of disruption of PsrA on the elicitation of the HR by DC3000. Site 1, DC3000; site 2, PsrA− mutant AC820. Leaf panels were infiltrated with 1 × 106 CFU/ml of bacterial cells. (B) Northern blot analysis of hrpL, hrpZ, hrpA, hopP to hopK, and hopP to hopJ of DC3000 (lane 1) and AC820 (lane 2). (C) Disease symptoms caused by DC3000 (left) and AC820 (right) in tomato leaves. Leaves were dip inoculated in bacterial suspensions (2 × 108 CFU/ml). The pictures were taken 7 days after inoculation. (D) Northern blot analysis of rsmA1 of DC3000 (lane 1) and AC820 (lane 2). Each lane contained 10 μg of total RNA.
Previous studies on hrpL and the whole array of effector genes it controls (13, 28, 50) raised the possibility that the PsrA− mutant, due to its reduced level of hrpL expression, may affect virulence. To test this, we dip-inoculated leaves of 4-week-old tomato plants with cell suspensions (2 × 108 CFU/ml) of DC3000 and its PsrA− mutant. Four days after inoculation, disease symptoms appeared only on leaves inoculated with DC3000. The symptoms were visible after 5 days on leaves inoculated with the PsrA− mutant, but the severity was much reduced compared to that with DC3000. The results in Fig. 2C show that the symptoms were less severe than those of DC3000 at 7 days after inoculation. Moreover, the symptoms appeared on many more leaves inoculated with DC3000 (93 out of 153 leaflets [61%] had symptoms) than on those inoculated with the PsrA− mutant (31 out of 123 leaflets [25%] had symptoms).
The effects of PsrA on expression of hrpL are novel. Recent studies have established a major regulatory role of HrpL in the expression of many hrp, hrc, and hop genes and virulence effector genes (5, 13, 28, 50). The negative effects of PsrA mutation on expression of hrpA, hrpZ, hopP to hopK, and hopP to hopJ as well as the inhibition of the HR and pathogenicity are consistent with the lower transcript levels of hrpL. Shen et al. (45) also reported that PsrA is required for the full activation of transcription of the type III secretion system regulatory operon exsCEBA and effector exoS in Pseudomonas aeruginosa. These findings collectively suggest that PsrA is involved in full expression of effectors of the type III secretion system as well as the secretion pathway.
To determine if PsrA binds hrpL DNA to repress its expression, we performed a gel shift assay. We did not detect binding of purified MBP-PsrA protein with hrpL upstream DNA. The absence of a consensus PsrA-binding site upstream of hrpL was consistent with this observation. This suggested that the effect of PsrA on the hrpL gene is indirect. In this context, we have previously documented that rsmA1 (the gene encoding an RNA-binding protein which promotes mRNA decay) negatively regulates the expression of hrpL and other hrp and hop genes as well as elicitation of the HR (7). We therefore predicted that PsrA activated rsmA1, which, in turn, has a negative effect on expression of hrpL and other hrp and hop genes. Indeed, the results (Fig. 2D) show that the levels of the rsmA transcript are higher in the PsrA− mutant than in the parent. Thus, the effects of PsrA on expression of hrp, hop, and the HR may be at least partially due to overexpression of rsmA1 in the PsrA− mutant.
Expression of psrA is autoregulated in DC3000.
To test the expression of psrA, a transcriptional psrA-lacZ fusion plasmid was transferred into DC3000 and its PsrA− mutant. The β-galactosidase assay results (Table 3) revealed that expression of the psrA-lacZ fusion in the PsrA− mutant is higher than that in DC3000. Furthermore, E. coli strain MC4100 carrying the psrA-lacZ fusion along with a psrA+ plasmid produced much lower levels of β-galactosidase activities than MC4100 carrying the psrA-lacZ fusion and vector (Table 4). These data clearly demonstrate that (i) there is a high basal level of psrA expression and (ii) expression of psrA is negatively autoregulated in DC3000, as in P. chlororaphis, P. putida, and P. aeruginosa (10, 27).
TABLE 3.
Expression of psrA-lacZ, aefR-lacZ, and rpoS-lacZ fusions in DC3000 and its PsrA−, AefR−, and RpoS− mutants
| Bacterial constructa | Relevant characteristic | β-Galactosidase activity (mean ± SD)b |
|---|---|---|
| DC3000(pMP220) | Wild type (vector) | 31 ± 3 |
| AC820(pMP220) | PsrA− (vector) | 29 ± 4 |
| AC821(pMP220) | AefR− (vector) | 34 ± 6 |
| AC822(pMP220) | RpoS− (vector) | 32 ± 5 |
| DC3000(pAKC1257) | Wild type (psrA-lacZ) | 1,766 ± 70 |
| AC820(pAKC1257) | PsrA− (psrA-lacZ) | 5,391 ± 117 |
| AC821(pAKC1257) | AefR− (psrA-lacZ) | 1,713 ± 100 |
| AC822(pAKC1257) | RpoS− (psrA-lacZ) | 1,745 ± 111 |
| DC3000(pAKC1258) | Wild type (aefR-lacZ) | 800 ± 49 |
| AC820(pAKC1258) | PsrA− (aefR-lacZ) | 1,396 ± 47 |
| AC822(pAKC1258) | RpoS− (aefR-lacZ) | 848 ± 42 |
| DC3000(pAKC1259) | Wild type (rpoS-lacZ) | 1,430 ± 49 |
| AC820(pAKC1259) | PsrA− (rpoS-lacZ) | 263 ± 13 |
| AC821(pAKC1259) | AefR− (rpoS-lacZ) | 1,413 ± 61 |
Bacteria were grown in KB medium plus tetracycline to a Klett value of ca. 600, and the cultures were used for assays.
Expressed as Miller units.
TABLE 4.
Expression of psrA-lacZ, aefR-lacZ, and rpoS-lacZ fusions in E. coli strain MC4100 in the presence of psrA
| Bacterial constructa | Relevant characteristic | β-Galactosidase activity (mean ± SD)b |
|---|---|---|
| MC4100(pMMB66EHΩ, pMP220) | Vectors | 29 ± 6 |
| MC4100(pAKC1254, pMP220) | psrA + vector | 30 ± 3 |
| MC4100(pMMB66EHΩ, pAKC1257) | Vector + psrA-lacZ | 430 ± 17 |
| MC4100(pAKC1254, pAKC1257) | psrA + psrA-lacZ | 67 ± 8 |
| MC4100(pMMB66EHΩ, pAKC1258) | Vector + aefR-lacZ | 828 ± 33 |
| MC4100(pAKC1254, pAKC1258) | psrA + aefR-lacZ | 360 ± 12 |
| MC4100(pMMB66EHΩ, pAKC1259) | Vector + rpoS-lacZ | 101 ± 12 |
| MC4100(pAKC1254, pAKC1259) | psrA + rpoS-lacZ | 258 ± 21 |
Assays were performed with cultures grown in LB medium plus spectinomycin and tetracycline to a Klett value of ca. 500.
Expressed as Miller units.
Effects of PsrA on expression of aefR.
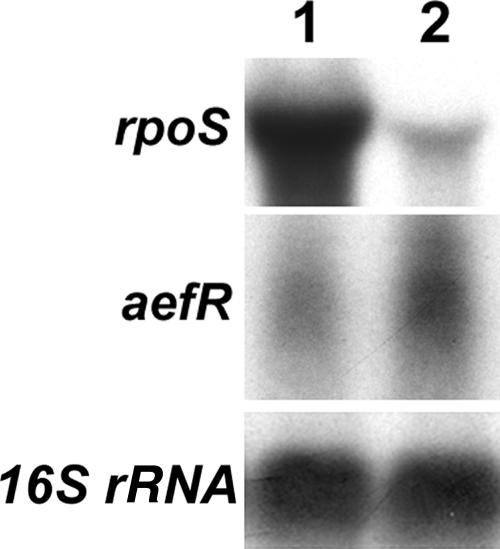
aefR is a tetR-like regulatory gene first identified in P. syringae pv. syringae, and it is known to control epiphytic fitness and AHL production (38). Northern blot analysis (Fig. 3) revealed that the PsrA− mutant of DC3000 produced higher levels of the transcript of aefR than the parent. In addition, β-galactosidase assay results (Table 3) revealed that the expression of the aefR-lacZ fusion in the PsrA− mutant was higher than that in DC3000. Moreover, the levels of β-galactosidase activity produced by MC4100 carrying the aefR-lacZ fusion and the psrA+ plasmid were lower than those produced by MC4100 carrying the aefR-lacZ fusion and the vector (Table 4).
FIG. 3.
Northern blot analysis of rpoS and aefR of DC3000 (lane 1) and its PsrA− mutant AC820 (lane 2). Each lane contained 10 μg of total RNA.
It is well established that P. syringae strains sustain alternating life styles as pathogens, epiphytes, and commensals. The epiphytic phase of P. syringae is considered one of the first steps in the infection process (1, 18). Studies with P. syringae pv. syringae strain B728a revealed that epiphytes can occur in aggregates comprising large populations of cells. Under desiccation stress, the aggregates have a greater ability to survive on leaves than solitary cells (32, 33, 34). An AefR− mutant of B728a produced reduced levels of AHL, and it was found to be less fit for stress survival than the wild-type parent B728a (38). Our studies revealed that AefR of DC3000 (Pspto_3549) shares high homology with that of P. syringae pv. syringae strain B728a (90% identity and 95% similarity) and possesses a helix-turn-helix motif (residues 11 to 57) which is highly similar to those found in the TetR family transcriptional regulators. We have established a negative effect of PsrA on expression of aefR. In addition, compared to DC3000, an AefR− mutant produced lower levels of psyI and psyR transcripts as well as AHL (see below). These data indicate a positive effect of AefR on AHL production in DC3000. How AefR controls the levels of psyI and psyR expression awaits clarification. While we do not have direct evidence for AefR controlling epiphytic fitness in DC3000, based on the high homology of the aefR products of B728a and DC3000 as well as the close relationship of those two strains, we predict that AefR of DC3000 also plays an important role in epiphytic fitness, possibly through its effects on AHL production.
Effects of PsrA on expression of rpoS.
Northern blot analysis (Fig. 3) revealed that the levels of rpoS transcripts are much reduced in the PsrA− mutant compared to DC3000. The β-galactosidase assay results (Table 3) for DC3000 and its PsrA− mutant carrying the rpoS-lacZ fusion plasmid also revealed that the expression of rpoS-lacZ in the mutant is much reduced compared to that in DC3000. Furthermore, E. coli strain MC4100 carrying the rpoS-lacZ fusion in the presence of the psrA+ plasmid produced higher levels of β-galactosidase activities than in the absence of the psrA+ plasmid (Table 4).
Our finding that PsrA positively controls rpoS expression in DC3000 was expected, since similar effects of PsrA on rpoS have been found in P. aeruginosa and P. putida (2, 25, 27, 52). RpoS is a stationary-phase alternative sigma factor, and it has been shown to be a global regulator in Pseudomonas species such as P. aeruginosa, P. putida, P. chlororaphis, and P. fluorescens (15, 40, 42, 49). Moreover, RpoS plays important roles as a regulator of virulence factors, secondary metabolites, or stress adaptation in those Pseudomonas species (3, 15, 17, 21, 35, 44, 48). However, Jorgensen et al. (21) reported that the requirement of RpoS for stress resistance, such as resistance to heat, high osmolarity, low pH, H2O2, and ethanol, is less pronounced in P. aeruginosa than in E. coli. It seems likely that in pseudomonads RpoS plays more specific roles related to virulence and colonization by modulating the overall cell-density-dependent expression of virulence determinants. This idea is supported by the observation that RpoS negatively regulates transcript levels of rhlI (the gene encoding RhlI for synthesis of N-butyryl-homoserine lactone) and RhlI-generated N-butyryl-homoserine lactone production in P. aeruginosa (58). Although RpoS function in P. syringae pathovars has yet to be understood, based upon studies with P. aeruginosa and E. coli as well as the data presented here, it is safe to conclude that RpoS may play an important role in secondary metabolite production and that this sigma factor may also affect bacterial ability to cope with various stresses.
Identification of putative PsrA-binding sites in the promoter regions of rpoS, aefR, and psrA.
The results of primer extension assays (Fig. 4A) revealed that the transcriptional start sites for rpoS, aefR, and psrA are located at 365, 47, and 24 bases upstream of their putative translational start sites. The consensus PsrA-binding sequence has been previously identified as C/GAAACN2-4GTTTG/C in P. putida (25). Sequence analysis results (Fig. 4B) revealed the presence of well-matched palindromic sequences CAAACGGCAGTTTG, spanning nt −58 to −45 corresponding to the transcriptional start site of DC3000 rpoS, and CAAACGTTCGTTTG, spanning nt −4 to +10 corresponding to the transcriptional start site of DC3000 psrA. In addition, the sequence TAAACGCACGTTTG (nt +12 to +25, corresponding to the transcriptional start site), with one base different from the consensus sequence, was found in the promoter region of DC3000 aefR.
FIG. 4.
(A) Primer extension analysis of rpoS, aefR, and psrA. Lane 1 represents the RNA sample from DC3000. For rpoS, 10 μg of total RNA was used, and for aefR and psrA, 40 μg of total RNA was used. The nucleotides on the left of each panel refer to the nucleotide sequence beyond the transcriptional start site. The asterisk indicates the residue at which transcription was initiated. (B) PsrA-binding sequences upstream of aefR, rpoS, and psrA based on sequence analysis. Numbers correspond to the nucleotide positions in relation to the transcriptional start sites.
Purified MBP-PsrA binds promoter regions of rpoS, aefR, and psrA.
To determine if PsrA binds to the promoter regions of aefR, rpoS and psrA, we first overexpressed and purified MBP-PsrA protein. The apparent molecular mass of overexpressed MBP-PsrA protein after IPTG induction is ca. 77.0 kDa, which matched the mass of 26.4 kDa of the polypeptide deduced from the psrA sequence plus the mass of the MBP-β-galactosidase α fragment made from the vector. The purified MBP-PsrA protein and α-32P-labeled DNA segments containing promoter regions of aefR, rpoS, and psrA were incubated in the DNA binding buffer and resolved on a nondenaturing 5% polyacrylamide gel. The results (Fig. 5) revealed that (i) MBP-PsrA binds aefR, rpoS, and psrA DNA fragments; (ii) the extents of band shift are proportional to the concentration of protein added; (iii) the shifts do not occur in the presence of excess unlabeled fragments, indicating specific binding; and (iv) the binding affinity of MBP-PsrA-psrA is greater than that of MBP-PsrA-aefR and MBP-PsrA-rpoS (i.e., the psrA DNA band was completely shifted by addition of 100 ng of MBP-PsrA, whereas the aefR and rpoS DNA bands were only partially shifted by addition of 100 ng or 200 ng of MBP-PsrA).
FIG. 5.
Gel mobility shift assays for binding of purified MBP-PsrA protein to aefR, rpoS, and psrA DNAs. DNA fragments were end labeled with [α-32P]dATP. Each reaction mixture contained 2 ng of labeled DNA probe. The amounts of protein and unlabeled DNA used in each reaction are indicated at the top.
Many of the TetR family regulators act as repressors of transcription (39). As reported above, in DC3000, PsrA negatively regulates (i) transcript levels of aefR and (ii) its own expression, like many other TetR-like transcriptional regulators. However, PsrA positively regulates transcription of rpoS in DC3000, as in P. putida and P. aeruginosa (25). In a subsequent study, Kojic et al. (26) provided additional evidence for both positive and negative regulation by PsrA in P. aeruginosa. Examination of the locations of the PsrA-binding sequences within aefR, psrA, and rpoS clearly indicates a relationship between the juxtaposition of the binding sequences to the start sites with the positive or negative regulation. The PsrA-binding sequences in the aefR and psrA operator regions are located at nt +12 to +25 and nt −4 to +10, respectively, corresponding to the cognate transcriptional start site. In these instances PsrA presumably blocks the transcription of those genes. By contrast, in rpoS the PsrA-binding sequences are located at nt −58 to −45 upstream of the transcriptional start site, which would be consistent with its activator function as clearly established by genetic data.
Effects of PsrA on transcript levels of psyI and psyR and AHL production.
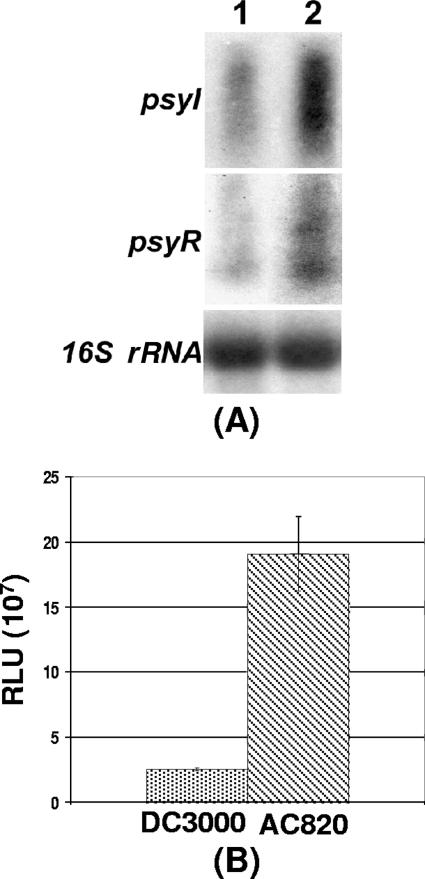
For the assay of AHL production, DC3000 and its PsrA− mutant were grown in KB medium. Cell samples were used for total RNA extraction and culture supernatants for AHL assays. Northern blot analysis (Fig. 6A) revealed that the PsrA− mutant overproduced psyI and psyR transcripts compared to the parent. Moreover, the β-galactosidase assay results (Table 5) revealed higher levels of β-galactosidase activity in the PsrA− mutant carrying the psyI-lacZ or psyR-lacZ fusion than in DC3000 carrying those two fusions. Consistent with overexpression of psyI and psyR, the PsrA− mutant produced a ca. 8-times-higher level of AHL than did DC3000 (Fig. 6B).
FIG. 6.
(A) Northern blot analysis of psyI and psyR of DC3000 (lane 1) and its PsrA− mutant AC820 (lane 2). Each lane contained 20 μg of total RNA. (B) Relative light units produced by spent cultures of DC3000 and AC820 in E. coli strain VJS533 harboring pHV200I. Bacterial cultures were grown in KB at 28°C to a Klett value of ca. 600 and used for assay. Error bars indicate standard deviations.
TABLE 5.
Expression of psyI-lacZ and psyR-lacZ in DC3000 and its PsrA−, AefR−, and RpoS− mutants
| Bacterial constructa | Relevant characteristic | β-Galactosidase activity (mean ± SD)b |
|---|---|---|
| DC3000(pMP220) | Wild type (vector) | 29 ± 3 |
| AC820(pMP220) | PsrA− (vector) | 27 ± 4 |
| AC821(pMP220) | AefR− (vector) | 32 ± 4 |
| AC822(pMP220) | RpoS− (vector) | 32 ± 4 |
| DC3000(pAKC1255) | Wild type (psyI-lacZ) | 789 ± 6 |
| AC820(pAKC1255) | PsrA− (psyI-lacZ) | 1,468 ± 14 |
| AC821(pAKC1255) | AefR− (psyI-lacZ) | 510 ± 12 |
| AC822(pAKC1255) | RpoS− (psyI-lacZ) | 1,213 ± 14 |
| DC3000(pAKC1256) | Wild type (psyR-lacZ) | 215 ± 12 |
| AC820(pAKC1256) | PsrA− (psyR-lacZ) | 398 ± 13 |
| AC821(pAKC1256) | AefR− (psyR-lacZ) | 148 ± 12 |
| AC822(pAKC1256) | RpoS− (psyR-lacZ) | 311 ± 13 |
Bacterial cultures used for assays were grown in KB medium plus tetracycline to a Klett value of ca. 600.
Expressed as Miller units.
To explain the negative effect of PsrA on AHL production we considered two possibilities: (i) PsrA acts as a repressor by directly binding psyI/psyR promoter/regulatory regions, or (ii) it acts indirectly via regulation of expression of aefR and rpoS. A direct repressor action of PsrA on psyI and psyR seemed unlikely due to the absence of the consensus PsrA-binding site within the promoter/regulatory region of the psyI/psyR genes. Moreover, we did not observe a band shift in gel mobility shift assays with purified MBP-PsrA protein and psyI/psyR upstream DNAs (Chatterjee et al., unpublished data).
Based on those observations and for the following reasons, we shifted our focus to AefR and RpoS: (i) it has been reported that AefR positively controls AHL production in P. syringae pv. syringae (38) and that RpoS negatively regulates AHL production in P. aeruginosa (58) and P. putida (3), and (ii) our results show that PsrA binds specifically to the aefR and rpoS promoter/operator and controls the expression of those two regulator genes. Therefore, it seemed likely that the overproduction of AHL in the PsrA− mutant of DC3000 resulted from PsrA effects on AefR and RpoS. To test this possibility, we first examined the effects of AefR and RpoS on expression of psyI and psyR as well as AHL production. Northern blot analysis (Fig. 7A) revealed that (i) the levels of psyI and psyR transcripts in the AefR− mutant (lane 2) were lower and (ii) the transcript levels of psyI and psyR in the RpoS− strain (lane 3) are higher than those in DC3000 (lane 1). In addition, expression of psyI-lacZ and psyR-lacZ fusions was lower in the AefR− mutant but higher in the RpoS− strain than in DC3000 (Table 5). The results in Fig. 7B show that the AefR− mutant produced a lower level of AHL, whereas the RpoS− strain produced ca. 7 times more AHL than DC3000.
FIG. 7.
(A) Northern blot analysis of psyI and psyR of DC3000 (lane 1), its AefR− mutant AC821 (lane 2), and RpoS− mutant AC822 (lane 3). Each lane contained 20 μg of total RNA. (B). Relative light units produced by spent cultures of DC3000, AC821, and AC822 in E. coli strain VJS533 harboring pHV200I. Bacterial cultures were grown in KB at 28°C to a Klett value of ca. 600 and used for assay. Error bars indicate standard deviations.
We then determined if rpoS and aefR regulate each other or psrA. The results of our Northern blot analysis (Fig. 8A and 8B) and β-galactosidase assays of aefR-lacZ and rpoS-lacZ fusions (Table 3) yielded similar results. These data clearly demonstrate that AefR and RpoS do not regulate each other. Moreover, transcript analysis of psrA in AefR− or RpoS− mutants (Fig. 8C) and β-galactosidase assays of the psrA-lacZ fusion in AefR− and RpoS− backgrounds (Table 3) revealed that expression of psrA is not regulated by AefR or RpoS. Thus, these data together with the observations mentioned above that PsrA controls the expression of aefR and rpoS and AefR and RpoS regulate production of AHL strongly suggested that overproduction of AHL in the PsrA− mutant of DC3000 is attributable to its cumulative, but independent, effects on AefR and RpoS (see model in Fig. 9).
FIG. 8.
Northern blot analysis of (A) rpoS of DC3000 (lane 1) and its AefR− mutant AC821 (lane 2), (B) aefR of DC3000 (lane 1) and its RpoS− mutant AC822 (lane 2), and (C) psrA of DC3000 (lane 1), its AefR− mutant AC821 (lane 2), and RpoS− mutant AC822 (lane 3).
FIG. 9.
A model depicting the regulatory effects of PsrA in P. syringae pv. tomato strain DC3000. PsrA positively controls transcription of rpoS and negatively regulates expression of aefR by binding to their promoter/operator. RpoS has a negative effect, whereas AefR has a positive effect, on AHL production and on transcript levels of psyI and psyR. RpoS and AefR do not regulate each other. Thus, the PsrA effect on AHL production is exerted via its cumulative, but independent, effects on both AefR and RpoS. PsrA effects on rpoS, aefR, psyI, and psyR transcript as well as AHL production are similar at different temperatures (i.e., 18°C and 28°C in KB medium) as well as in different media (i.e., in KB and MGY media). AHL production is autoregulated. The GacS/GacA system positively regulates the expression of rpoS and psyR as well as AHL production (8). The GacS/GacA system does not have a significant effect on psrA and aefR transcripts. PsrA negatively regulates expression of rsmA1, which, in turn, affects the transcript levels of hrpL (7). HrpL is required for expression of hrp, hop, and avr genes as well as virulence and elicitation of the HR (5, 13, 28, 50).
The following evidence, however, suggests that RpoS plays a dominant role over AefR in controlling AHL production in DC3000. The level of AHL produced by the RpoS− mutant is about 7 times higher and is very close to the level of AHL in the PsrA− mutant, which is ca. 8 times higher than that in DC3000 (Fig. 6B and 7B). Northern blot analysis (Fig. 3) revealed that the transcript levels of rpoS are much reduced in the PsrA− mutant compared to in DC3000, i.e., that expression of rpoS is tightly controlled by PsrA.
We have established for the first time that RpoS negatively controls the expression of psyI and psyR as well as production of AHL in P. syringae. Our recent observations (Table 6) revealed that the β-galactosidase level of psyI-lacZ in a PsyR− mutant is much lower than that in PsyR+ DC3000. Inactivation of rpoS in this PsyR− mutant (RpoS− PsyR−) did not restore the expression of psyI-lacZ, whereas RpoS deficiency in a PsyR+ background (RpoS− PsyR+) stimulated the psyI-lacZ expression. These results strongly suggest that RpoS regulates psyI expression via suppression of psyR. However, how RpoS regulates the expression of psyR remains unknown. This lack of understanding notwithstanding, our studies document a primary effect of RpoS on psyR, a gene for a LuxR-type regulator, and not on psyI, a gene for an AHL synthase. In fact, our observations indicate that psyI transcription in DC3000 requires PsyR as well as AHL, which is consistent with the LUX paradigm (30). Our results with RpoS contrasts with those reported for P. aeruginosa (58) and P. putida (3), where the negative effects of RpoS were found to be manifest at the level of genes for AHL synthases (rhlI for P. aeruginosa and ppuI for P. putida) and not with the genes for the cognate LuxR-type transcriptional regulators.
TABLE 6.
Expression of psyI-lacZ and psyR-lacZ in DC3000 and its RpoS−, PsyR−, and RpoS− PsyR− mutants
| Bacterial constructa | Relevant characteristic | β-Galactosidase activity (mean ± SD)b |
|---|---|---|
| DC3000(pMP220) | Wild type (vector) | 26 ± 4 |
| AC822(pMP220) | RpoS− PsyR+ (vector) | 29 ± 4 |
| AC823(pMP220) | RpoS+ PsyR− (vector) | 22 ± 3 |
| AC824(pMP220) | RpoS− PsyR− (vector) | 31 ± 6 |
| DC3000(pAKC1255) | Wild type (psyI-lacZ) | 1,131 ± 50 |
| AC822(pAKC1255) | RpoS− PsyR+ (psyI-lacZ) | 2,226 ± 106 |
| AC823(pAKC1255) | RpoS+ PsyR− (psyI-lacZ) | 149 ± 14 |
| AC824(pAKC1255) | RpoS− PsyR− (psyI-lacZ) | 162 ± 9 |
Cultures used for assays were grown to a Klett value of ca. 1,000 in KB medium plus tetracycline.
Expressed as Miller units.
In conclusion, we have documented that through its effects on AefR and RpoS, PsrA controls AHL levels as well as factors responsible for epiphytic fitness. Our data show that PsrA controls effector production by modulating the levels of HrpL. These findings, along with the evidence presented for P. syringae pv. syringae (37) that AHL controls bacterial movement, polysaccharide production, symptom development, and stress tolerance, allow the prediction that PsrA-mediated regulation has important ramifications in plant interaction, ecological fitness, or niche adaptation of P. syringae pathovars.
Acknowledgments
Our work was supported by the National Science Foundation (grant MCB-9728505) and grants from the Food for the 21st Century program and the Research Board of the University of Missouri.
We thank J. E. Schoelz for reviewing the manuscript.
Footnotes
Published ahead of print on 30 March 2007.
REFERENCES
- 1.Beattie, G. A., and S. E. Lindow. 1999. Bacterial colonization of leaves: a spectrum of strategies. Phytopathology 89:353-359. [DOI] [PubMed] [Google Scholar]
- 2.Bertani, I., M. Sevo, M. Kojic, and V. Venturi. 2003. Role of GacA, LasI, RhlI, Ppk, PsrA, Vfr and ClpXP in the regulation of the stationary-phase sigma factor rpoS/RpoS in Pseudomonas. Arch. Microbiol. 180:264-271. [DOI] [PubMed] [Google Scholar]
- 3.Bertani, I., and V. Venturi. 2004. Regulation of the N-acyl homoserine lactone-dependent quorum-sensing system in rhizosphere Pseudomonas putida WCS358 and cross-talk with the stationary-phase RpoS sigma factor and the global regulator GacA. Appl. Environ. Microbiol. 70:5493-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buell, R. C., V. Joardar, M. Lindeberg, J. Selengut, I. T. Paulsen, M. L. Gwinn, R. J. Dodson, R. T. Deboy, A. S. Durkin, J. F. Kolonay, R. Madupu, S. Daugherty, L. Brinkac, M. J. Beanan, D. H. Haft, W. C. Nelson, T. Davidsen, N. Zafar, L. Zhou, J. Liu, Q. Yuan, H. Khouri, N. Fedorova, B. Tran, D. Russell, K. Berry, T. Utterback, S. E. van Aken, T. V. Feldblyum, M. D'Ascenzo, W. L. Deng, A. R. Ramos, J. R. Alfano, S. Cartinhour, A. K. Chatterjee, T. P. Delaney, S. G. Lazarowitz, G. B. Martin, D. J. Schneider, X. Tang, C. L. Bender, O. White, C. M. Fraser, and A. Collmer. 2003. The complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. USA 100:10181-10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee, A., Y. Cui, and A. K. Chatterjee. 2002. Regulation of Erwinia carotovora hrpLEcc (sigma-LEcc), which encodes an extracytoplasmic function subfamily of sigma factor required for expression of the HRP regulon. Mol. Plant-Microbe Interact. 15:971-980. [DOI] [PubMed] [Google Scholar]
- 6.Chatterjee, A., Y. Cui, Y. Liu, C. K. Dumenyo, and A. K. Chatterjee. 1995. Inactivation of rsmA leads to overproduction of extracellular pectinases, cellulases, and proteases in Erwinia carotovora subsp. carotovora in the absence of the starvation/cell density-sensing signal, N-(3-oxohexanoyl)-l-homoserine lactone. Appl. Environ. Microbiol. 61:1959-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee, A., Y. Cui, G. Nong, S. Chaudhuri, A. Collmer, and A. K. Chatterjee. 2003. Post-transcriptional regulation in Pseudomonas syringae pv. tomato strain DC3000: characterization of regulatory RNA species and RNA-binding proteins, abstr. 134. Abstr. Int. Pseudomonas Meet. 2003.
- 8.Chatterjee, A., Y. Cui, H. Yang, A. Collmer, J. R. Alfano, and A. K. Chatterjee. 2003. GacA, the response regulator of a two-component system, acts as a master regulator in Pseudomonas syringae pv. tomato DC3000 by controlling regulatory RNA, transcriptional activators, and alternate sigma factors. Mol. Plant-Microbe Interact. 16:1106-1117. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee, A., R. Valasubramanian, W.-L. Ma, A. K. Vachhani, S. Gnanamanickam, and A. K. Chatterjee. 1996. Isolation of ant mutants of Pseudomonas fluorescens strain Pf7-14 altered in antibiotic production, cloning of the ant+ DNA, and evaluation of the role of antibiotic production in the control of blast and sheath blight of rice. Biol. Control 7:185-195. [Google Scholar]
- 10.Chin-A-Woeng, T. F., D. van den Broek, B. J. Lugtenberg, and G. V. Bloemberg. 2005. The Pseudomonas chlororaphis PCL1391 sigma regulator psrA represses the production of the antifungal metabolite phenazine-1-carboxamide. Mol. Plant-Microbe Interact. 18:244-253. [DOI] [PubMed] [Google Scholar]
- 11.Dumenyo, C. K., A. Mukherjee, W. Chun, and A. K. Chatterjee. 1998. Genetic and physiological evidence for the production of N-acyl homoserine lactones by Pseudomonas syringae pv. syringae and other fluorescent plant pathogenic Pseudomonas species. Eur. J. Plant Pathol. 104:569-582. [Google Scholar]
- 12.Elasri, M., S. Delorme, P. Lemancea, G. Stewart, B. Laue, E. Glickmann, P. M. Oger, and Y. Dessaux. 2001. Acyl-homoserine lactone production is more common among plant-associated Pseudomonas spp. than among soilborne Pseudomonas spp. Appl. Environ. Microbiol. 67:1198-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira, A. O., C. R. Myers, J. S. Gordon, G. B. Martin, M. Vencato, A. Collmer, M. D. Wehling, J. R. Alfano, G. Moreno-Hagelsieb, W. F. Lamboy, G. DeClerck, D. J. Schneider, and S. W. Cartinhour. 2006. Whole-genome expression profiling defines the HrpL regulon of Pseudomonas syringae pv. tomato DC3000, allows de novo reconstruction of the Hrp cis clement, and identifies novel coregulated genes. Mol. Plant-Microbe Interact. 19:1167-1179. [DOI] [PubMed] [Google Scholar]
- 14.Furste, J. P., W. Pansegrau, R. Frank, H. Blocker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tap expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 15.Girard, G., E. T. van Rij, B. J. J. Lugtenberg, and G. V. Bloemberg. 2006. Regulatory roles of psrA and rpoS in phenazine-1-carboxamide synthesis by Pseudomonas chlororaphis PCL1391. Microbiology 152:43-58. [DOI] [PubMed] [Google Scholar]
- 16.Gray, K. M., and E. P. Greenberg. 1992. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J. Bacteriol. 174:4348-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heeb, S., C. Valverde, C. Gigot-Bonnefoy, and D. Haas. 2005. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in Pseudomonas fluorescens CHA0. FEMS Microbiol. Lett. 243:251-258. [DOI] [PubMed] [Google Scholar]
- 18.Hirano, S. S., and C. D. Upper. 2000. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae: a pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 64:624-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, H.-C., R.-H. Lin, C.-J. Chang, A. Collmer, and W.-L. Deng. 1995. The complete hrp gene cluster of Pseudomonas syringae pv. syringae 61 includes two blocks of genes required for harpinPss secretion that are arranged collinearly with Yersinia ysc homologs. Mol. Plant-Microbe Interact. 8:733-746. [DOI] [PubMed] [Google Scholar]
- 20.Joardar, V., M. Lindeberg, R. W. Jackson, J. Selengut, R. Dodson, L. M. Brinkac, S. C. Daugherty, R. Deboy, A. S. Durkin, M. G. Giglio, R. Madupu, W. C. Nelson, M. J. Rosovitz, S. Sullivan, J. Crabtree, T. Creasy, T. Davidsen, D. H. Haft, N. Zafar, L. Zhou, R. Halpin, T. Holley, H. Khouri, T. Feldblyum, O. White, C. M. Fraser, A. K. Chatterjee, S. Cartinhour, D. J. Schneider, J. Mansfield, A. Collmer, and C. R. Buell. 2005. Whole-genome sequence analysis of Pseudomonas syringae pv. phaseolicola 1448A reveals divergence among pathovars in genes involved in virulence and transposition. J. Bacteriol. 187:6488-6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jorgensen, F., M. Bally, V. Chapon-Herve, G. Michel, A. Lazdunski, P. Williams, and G. S. Stewart. 1999. RpoS-dependent stress tolerance in Pseudomonas aeruginosa. Microbiology 145:835-844. [DOI] [PubMed] [Google Scholar]
- 22.Juhas, M., L. Eberl, and B. Tummler. 2005. Quorum sensing: the power of cooperation in the world of Pseudomonas. Environ. Microbiol. 7:459-471. [DOI] [PubMed] [Google Scholar]
- 23.Keane, P. J., A. Kerr, and P. B. New. 1970. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23:585-595. [Google Scholar]
- 24.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 25.Kojic, M., C. Aguilar, and V. Venturi. 2002. TetR family member psrA directly binds the Pseudomonas rpoS and psrA promoters. J. Bacteriol. 184:2324-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kojic, M., B. Jovcic, A. Vindigni, F. Odreman, and V. Venturi. 2005. Novel target genes of PsrA transcriptional regulator of Pseudomonas aeruginosa. FEMS Microbiol. Lett. 246:175-181. [DOI] [PubMed] [Google Scholar]
- 27.Kojic, M., and V. Venturi. 2001. Regulation of rpoS gene expression in Pseudomonas: involvement of a TetR family regulator. J. Bacteriol. 183:3712-3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindeberg, M., S. Cartinhour, C. R. Myers, L. M. Schechter, D. J. Schneider, and A. Collmer. 2006. Closing the circle on the discovery of genes encoding Hrp regulon members and type III secretion system effectors in the genomes of three model Pseudomonas syringae strains. Mol. Plant-Microbe Interact. 19:1151-1158. [DOI] [PubMed] [Google Scholar]
- 29.Liu, Y., A. Chatterjee, and A. K. Chatterjee. 1994. Nucleotide sequence and expression of a novel pectate lyase gene (pel-3) and a closely endopolygalacturonase gene (peh-1) of Erwinia carotovora subsp. carotovora 71. Appl. Environ. Microbiol. 60:2545-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loh, J., E. A. Pierson, L. S. Pierson III, G. Stacey, and A. K. Chatterjee. 2002. Quorum sensing in plant associated bacteria. Curr. Opin. Plant Biol. 5:285-290. [DOI] [PubMed] [Google Scholar]
- 31.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 32.Monier, J. M., and S. E. Lindow. 2003. Differential survival of solitary and aggregated bacterial cells promotes aggregate formation on leaf surfaces. Proc. Natl. Acad. Sci. USA 100:15977-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monier, J. M., and S. E. Lindow. 2004. Frequency, size, and localization of bacterial aggregates on bean leaf surfaces. Appl. Environ. Microbiol. 70:346-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris, C. E., J. M. Monier, and M. A. Jacques. 1998. A technique to quantify the population size and composition of the biofilm component in communities of bacteria in the phyllosphere. Appl. Environ. Microbiol. 64:4789-4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami, K., T. Ono, D. Viducic, S. Kayama, M. Mori, K. Hirota, K. Nemoto, and Y. Miyake. 2005. Role for rpoS gene of Pseudomonas aeruginosa in antibiotic tolerance. FEMS Microbiol. Lett. 242:161-167. [DOI] [PubMed] [Google Scholar]
- 36.Passador, L., J. M. Cook, M. J. Gambello, L. Rust, and B. H. Iglewski. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127-1130. [DOI] [PubMed] [Google Scholar]
- 37.Quinones, B., G. Dulla, and S. E. Lindow. 2005. Quorum sensing regulates exopolysaccharide production, motility, and virulence in Pseudomonas syringae. Mol. Plant-Microbe Interact. 18:682-693. [DOI] [PubMed] [Google Scholar]
- 38.Quinones, B., C. J. Pujol, and S. E. Lindow. 2004. Regulation of AHL production and its contribution to epiphytic fitness in Pseudomonas syringae. Mol. Plant-Microbe Interact. 17:521-531. [DOI] [PubMed] [Google Scholar]
- 39.Ramos, J. L., M. Martinez-Bueno, A. J. Molina-Henares, W. Teran, K. Watanabe, X. Zhang, M. T. Gallegos, R. Brennan, and R. Tobes. 2005. The TetR family of transcriptional repressors. Microbiol. Mol. Biol. Rev. 69:326-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos-Gonzalez, M. I., and S. Molin. 1998. Cloning, sequencing, and phenotypic characterization of the rpoS gene from Pseudomonas putida KT2440. J. Bacteriol. 180:3421-3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Sarniguet, A., J. Kraus, M. D. Henkels, A. M. Muehlchen, and J. E. Loper. 1995. The sigma factor sigma s affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc. Natl. Acad. Sci. USA 92:12255-12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schroeder, K. L., and W. Chun. 1995. Suppression of Clavibacter michiganensis subsp. sepedonicus in potato plants by using Pseudomonas corrugata. Phytopathology 85:1147. [Google Scholar]
- 44.Schuster, M., A. C. Hawkins, C. S. Harwood, and E. P. Greenberg. 2004. The Pseudomonas aeruginosa RpoS regulon and its relationship to quorum sensing. Mol. Microbiol. 51:973-985. [DOI] [PubMed] [Google Scholar]
- 45.Shen, D. K., D. Filopon, L. Kuhn, B. Polack, and B. Toussaint. 2006. PsrA is a positive transcriptional regulator of the type III secretion system in Pseudomonas aeruginosa. Infect. Immun. 74:1121-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, R. S., and B. H. Iglewski. 2003. P. aeruginosa quorum-sensing systems and virulence. Curr. Opin. Microbiol. 6:56-60. [DOI] [PubMed] [Google Scholar]
- 47.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. J. J. Lugtenberg. 1987. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol. Biol. 9:27-39. [DOI] [PubMed] [Google Scholar]
- 48.Stockwell, V. O., and J. E. Loper. 2005. The sigma factor RpoS is required for stress tolerance and environmental fitness of Pseudomonas fluorescens Pf-5. Microbiology 151:3001-3009. [DOI] [PubMed] [Google Scholar]
- 49.Suh, S. J., L. Silo-Suh, D. E. Woods, D. J. Hassett, S. E. West, and D. E. Ohman. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang, X., Y. Xiao, and J. M. Zhou. 2006. Regulation of the type III secretion system in phytopathogenic bacteria. Mol. Plant-Microbe Interact. 19:1159-1166. [DOI] [PubMed] [Google Scholar]
- 51.Toth, I. K., J. A. Newton, L. J. Hyman, A. K. Lees, M. Daykin, C. Ortori, P. Williams, and R. G. Fray. 2004. Potato plants genetically modified to produce N-acylhomoserine lactones increase susceptibility to soft rot erwiniae. Mol. Plant-Microbe. Interact. 17:880-887. [DOI] [PubMed] [Google Scholar]
- 52.Venturi, V. 2003. Control of rpoS transcription in Escherichia coli and Pseudomonas: why so different? Mol. Microbiol. 49:1-9. [DOI] [PubMed] [Google Scholar]
- 53.Venturi, V. 2006. Regulation of quorum sensing in Pseudomonas. FEMS Microbiol. Rev. 30:274-291. [DOI] [PubMed] [Google Scholar]
- 54.von Bodman, S. B., W. D. Bauer, and D. L. Coplin. 2003. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 41:455-482. [DOI] [PubMed] [Google Scholar]
- 55.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whitehead, N. A., A. M. Barnard, H. Slater, N. J. Simpson, and G. P. Salmond. 2001. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 57.Whiteley, M., K. M. Lee, and E. P. Greenberg. 1999. Identification of genes controlled by quorum sensing in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:13904-13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Willis, D. K., E. M. Hrabak, J. J. Rich, T. M. Barta, S. E. Lindow, and N. J. Panopoulos. 1990. Isolation and characterization of a Pseudomonas syringae pv. syringae mutant deficient in lesion formation on bean. Mol. Plant-Microbe Interact. 3:149-156. [Google Scholar]