Abstract
The anaerobic acetogenic bacterium Acetobacterium woodii can conserve energy by oxidation of various substrates coupled to either carbonate or caffeate respiration. We used a cell suspension system to study the regulation and kinetics of induction of caffeate respiration. After addition of caffeate to suspensions of fructose-grown cells, there was a lag phase of about 90 min before caffeate reduction commenced. However, in the presence of tetracycline caffeate was not reduced, indicating that de novo protein synthesis is required for the ability to respire caffeate. Induction also took place in the presence of CO2, and once a culture was induced, caffeate and CO2 were used simultaneously as electron acceptors. Induction of caffeate reduction was also observed with H2 plus CO2 as the substrate, but the lag phase was much longer. Again, caffeate and CO2 were used simultaneously as electron acceptors. In contrast, during oxidation of methyl groups derived from methanol or betaine, acetogenesis was the preferred energy-conserving pathway, and caffeate reduction started only after acetogenesis was completed. The differential flow of reductants was also observed with suspensions of resting cells in which caffeate reduction was induced prior to harvest of the cells. These cell suspensions utilized caffeate and CO2 simultaneously with fructose or hydrogen as electron donors, but CO2 was preferred over caffeate during methyl group oxidation. Caffeate-induced resting cells could reduce caffeate and also p-coumarate or ferulate with hydrogen as the electron donor. p-Coumarate or ferulate also served as an inducer for caffeate reduction. Interestingly, caffeate-induced cells reduced ferulate in the absence of an external reductant, indicating that caffeate also induces the enzymes required for oxidation of the methyl group of ferulate.
Acetogens are strictly anaerobic bacteria that are key players in the anaerobic food web. They are nutritionally very versatile and ferment compounds such as sugars, alcohols, or C1 compounds to acetate (and CO2), which is then converted to methane by methanogenic archaea in nonmarine ecosystems. Acetogenic bacteria are characterized by a unique pathway, the Wood-Ljungdahl pathway, that enables the use of CO2 as an electron acceptor during heterotrophic growth as well as during autotrophic growth. During heterotrophic growth, hexoses are oxidized to 2 mol pyruvate via the Embden-Meyerhof-Parnas pathway, and pyruvate is subsequently oxidized to acetyl-coenzyme A (acetyl-CoA), CO2, and reduced ferredoxin. Acetyl-CoA is converted to acetate by the enzymes phosphotransacetylase and acetate kinase, and the latter reaction is coupled to substrate level phosphorylation. The electrons are funneled to CO2, which is reduced to acetate in the Wood-Ljungdahl pathway according to:
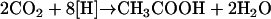 |
(1) |
In summary, 1 mol of sugar is converted to 3 mol of acetate. Due to the exclusive formation of acetate this fermentation is referred to as homoacetogenesis (14, 26, 33, 34, 37, 39, 40).
The Wood-Ljungdahl pathway not only uses electrons derived from organic compounds but also hydrogen and thus enables chemolithoautotrophic growth on H2 plus CO2 according to:
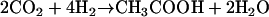 |
(2) |
The Wood-Ljungdahl pathway is coupled to a chemiosmotic mechanism of ATP synthesis, but the way that the ion gradients are established across the cytoplasmic membrane varies in acetogens (37). Some organisms, the so-called “proton organisms,” represented by the model organism Moorella thermoacetica, have a cytochrome-containing, proton motive electron transport chain with methylene tetrahydrofolate as the electron acceptor (4, 19, 23-25). The other class is the “sodium ion organisms,” which are strictly Na+ dependent. The model organism Acetobacterium woodii couples the Wood-Ljungdahl pathway to the generation of a primary sodium ion potential, which in turn drives ATP synthesis via an Na+-translocating ATP synthase of the F1F0 type (2, 21, 22, 38, 41). The Na+-translocating reaction of the pathway is unknown.
In recent years evidence has accumulated that some acetogens can use not only CO2 but also alternative electron acceptors, such as aromatic acrylate groups (the acrylate side chain of aromatic compounds), fumarate, dimethyl sulfoxide, or nitrate (3, 5, 10, 11, 17, 35, 43, 47). A. woodii is known to reduce caffeate, which is a degradation product of lignin and is readily available in its natural habitat. The electrons can be derived from various donors, such as fructose, methanol, or hydrogen, and are used to reduce the double bond in caffeate, yielding hydrocaffeate according to equation 3 (3):
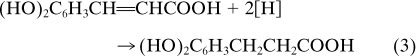 |
Experiments with cell suspensions of A. woodii revealed that caffeate reduction with hydrogen as the electron donor is coupled to the generation of a primary sodium ion potential across the cytoplasmic membrane that in turn drives ATP synthesis (28). Therefore, this process is also referred to as caffeate respiration. The enzymes involved in hydrogen-mediated caffeate reduction, as well as the Na+-translocating step, remain to be identified.
The ability of A. woodii to reduce at least two different electron acceptors poses a question about the regulation of the utilization of the electron acceptors. Earlier work based on growth experiments suggested that caffeate reduction in A. woodii is not constitutive but is induced in the presence of caffeate (9). However, despite its ecological impact little is known about the regulation of caffeate and CO2 reduction in A. woodii. Here, we addressed the regulation of the utilization of the two electron acceptors in A. woodii at the level of enzyme synthesis, as well as at the enzyme activity level.
MATERIALS AND METHODS
Organism and cultivation.
A. woodii DSMZ 1030 was cultivated at 30°C in 1.2-liter flasks (Müller-Krempel, Bülach, Switzerland). The medium was prepared using anaerobic techniques, as described previously (7, 27). The medium contained (under an N2-CO2 atmosphere [80:20, vol/vol]) (per liter) 1.76 g KH2PO4, 8.44 g K2HPO4, 1.0 g NH4Cl, 0.5 g cysteine hydrochloride, 0.33 g MgSO4, 2.9 g NaCl, 2.0 g yeast extract, 6.0 g KHCO3, 0.001 g resazurin, 1.0 ml trace element solution SL 9 (47), 1.0 ml selenite-tungstate solution (47), and 2.0 ml vitamin solution DSMZ 141. The pH was adjusted to 7.1 to 7.2 with HCl. Fructose, methanol, and betaine were used as carbon and energy sources at final concentrations of 20, 60, and 50 mM, respectively. Growth was monitored by measuring the optical density at 600 nm (OD600).
Preparation of cell suspensions.
All manipulations were done under strictly anaerobic conditions in an anaerobic chamber (Coy, Grass Lake, MI). For analyzing the induction of caffeate reduction cells were grown to an OD600 of 1.8 to 2.0. Cultures were harvested anaerobically by centrifugation (2,700 × g, 15 min, 4°C) and washed three times with imidazole-HCl buffer (50 mM imidazole-HCl, 20 mM MgSO4, 5 mM dithioerythritol, 1 mg/liter of resazurin; pH 7.0). Cells were resuspended in medium to a final protein concentration of 20 to 30 mg/ml under an N2-CO2 atmosphere (80:20, vol/vol). The suspension was kept on ice and used immediately.
For experiments with caffeate-induced cells cultures were grown to an OD600 of 0.3 to 0.4. Then caffeate was added from a 0.2 M stock solution to induce the ability of the cells to reduce caffeate. Cultures were harvested anaerobically at the end of the exponential growth phase (OD600, 1.8 to 2.0) by centrifugation (2,700 × g, 15 min, 4°C) and washed twice with imidazole-HCl buffer (see above). The cells were resuspended in the same buffer to a final protein concentration of 11 to 16 mg/ml. This suspension was kept on ice under an N2 atmosphere and used immediately for the experiments. The protein concentrations of cell suspensions were determined as described previously (42).
Induction of caffeate reduction in cell suspensions.
Experiments were carried out in 120-ml serum bottles containing medium (final volume, 50 ml). The medium was incubated at 30°C under an N2, H2, or H2-CO2 (80:20, vol/vol) atmosphere as specified below. When CO2 was used, the buffer contained 60 mM bicarbonate, unless stated otherwise. Cells were added from the concentrated cell suspension to a final protein concentration of 1 mg/ml. Fructose, methanol, betaine, and H2-CO2 were added as indicated below. After 15 min of incubation, caffeate was added (from a 1 M stock solution in dimethyl sulfoxide) as indicated below.
Experiments with caffeate-induced cell suspensions.
All experiments were performed in 58-ml bottles under an N2 or N2-CO2 (80:20, vol/vol) atmosphere. The mixtures (final volume, 10.5 ml) contained 9.5 ml of imidazole-HCl buffer (pH 7.0) and 1 ml of the concentrated cell suspension or 9 ml of buffer, 1 ml of the concentrated cell suspension, and 0.5 ml of caffeate, ferulate, or p-coumarate (from a 0.2 M stock solution). All suspensions were supplemented with 50 mM NaCl. After incubation of the bottles for 10 min at 30°C in a shaking water bath, the gas atmosphere was changed to H2 or H2-CO2 (80:20, vol/vol) by extensive gassing for 3 min. Then caffeate, ferulate, or p-coumarate was added.
Determination of caffeate concentration.
Samples (0.2 ml) were withdrawn with a syringe, and the cells were removed by centrifugation at 19,000 × g for 10 min. The caffeate concentration was determined photometrically at 312 nm using an extinction coefficient of 13.72 mM−1 cm−1.
Determination of acetate and fructose concentrations.
Samples (0.5 ml) were withdrawn with a syringe, and the cells were removed by centrifugation (19,000 × g, 10 min). Acetate and fructose concentrations were determined by coupled enzymatic assays using an acetic acid or fructose determination kit from R-biopharm according to the instructions of the manufacturer.
Electrophoresis and Western blotting.
Samples (0.8 ml) were withdrawn and centrifuged at 19,000 × g for 10 min. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed as described by Laemmli (32). Western blotting was performed as described previously (46) using an ECL detection reagent that was either purchased from PerkinElmer Life Sciences (Boston, MA) or made by using the following solutions: 4 ml of solution A (200 ml containing 0.1 M Tris-HCl [pH 6.8] and 50 mg luminol), 1.2 μl of H2O2 (30%), and 400 μl of solution B (10 ml of dimethyl sulfoxide containing 11 mg of para-hydroxycoumaric acid). Blot membranes were incubated in the solutions for 2 min before exposure to WICORex film (Typon Imaging AG, Burgdorf, Switzerland).
Statistics.
The data presented below are representative of at least three independent experiments, each performed using a different cell culture.
Chemicals and gases.
Chemicals were purchased from Roth (Karlsruhe, Germany), Merck (Ismaning, Germany), or Sigma (Taufkirchen, Germany). The purity of caffeate as determined by high-performance liquid chromatography was >99%. Gases were purchased from Air Liquide (Krefeld, Germany).
RESULTS
Experimental setup.
Cells of A. woodii grown on fructose in the presence of 60 mM bicarbonate were harvested and resuspended in medium containing 60 mM bicarbonate under an N2-CO2 (80:20, vol/vol) atmosphere to a final protein concentration of 1 mg/ml. These cell suspensions were capable of electron donor-mediated reduction of caffeate (see below), but caffeate concentrations above 5 mM were inhibitory and led to long lag phases and reduced rates of caffeate reduction. In contrast, when used at concentrations up to 3 mM, caffeate did not have an inhibitory effect (data not shown). Therefore, the subsequent experiments were performed with caffeate at concentrations up to 3 mM.
Caffeate respiration is induced in the presence of bicarbonate and fructose.
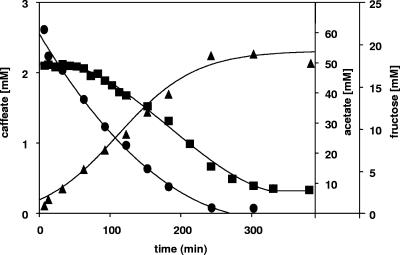
When fructose and caffeate were added to cell suspensions incubated in the presence of 60 mM bicarbonate, fructose consumption and acetate formation started immediately and continued for 240 min at a rate of 88 nmol fructose·min−1·mg protein−1 until the fructose was consumed. At the end of the experiment, a stoichiometry of fructose to acetate of 1:2.75 was obtained. In contrast to acetogenesis, the caffeate concentration did not decrease immediately but remained constant for about 60 min (Fig. 1). Thereafter, caffeate was reduced at a constant rate of 7.7 nmol caffeate·min−1·mg protein−1 for about 240 min.
FIG. 1.
Induction of caffeate respiration during acetogenesis from fructose by A. woodii. Cell suspensions of A. woodii (1.0 mg protein/ml) grown on fructose were incubated under an N2-CO2 atmosphere at 30°C in a shaking water bath in the presence of 20 mM fructose and 2 mM caffeate. At the times indicated, samples were withdrawn and analyzed to determine the caffeate (▪), fructose (•), and acetate (▴) concentrations as described in Materials and Methods.
The lag phase in caffeate reduction indicates that there is an induction process and may reflect the time required for induction of the enzymes catalyzing caffeate reduction. The fact that de novo synthesis of proteins is indeed required was shown by the inhibition of caffeate reduction in the presence of the antibiotic tetracycline (data not shown). At the same time, CO2 reduction, which is accomplished by the constitutive Wood-Ljungdahl pathway, was not affected by tetracycline. Furthermore, the amounts of the flavoproteins EtfA and EtfB, which are found only in caffeate-grown cells of A. woodii (F. Imkamp and V. Müller, unpublished data), increased from nondetectable to the maximal level within 60 min after caffeate addition (data not shown).
These data demonstrate that caffeate reduction is induced during fructose consumption and acetogenesis. After the induction process was completed, caffeate consumption and acetogenesis occurred simultaneously, indicating that the cells did not discriminate between CO2 and caffeate as electron acceptors. This can be concluded from the active acetogenesis during caffeate reduction and the rate of fructose consumption that exceeded by far the amount of fructose required to reduce the 2 mM caffeate present in the assay mixture. Furthermore, variation of the bicarbonate concentration from 0 to 60 mM had no effect on the length of time before caffeate reduction started or on the rates of caffeate reduction (data not shown).
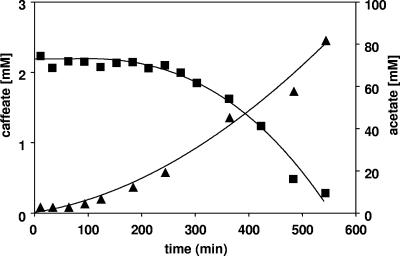
Caffeate respiration is induced in the presence of carbon dioxide and hydrogen.
Upon addition of H2 plus CO2 to cell suspensions prepared from fructose-grown cells, acetate formation started immediately (Fig. 2). As observed before with fructose as the electron donor, caffeate reduction was induced during acetogenesis from H2 plus CO2, again indicating that CO2 does not affect the cells' ability to induce caffeate respiration. This is also reflected by the fact that bicarbonate concentrations ranging from 10 to 60 mM had no influence on the length of time before caffeate reduction started (data not shown). After the ability to reduce caffeate was induced, caffeate was consumed at a rate of 5.1 nmol caffeate·min−1·mg protein−1, and CO2 and caffeate were used simultaneously as electron acceptors.
FIG. 2.
Induction of caffeate respiration during acetogenesis from H2 plus CO2 by cell suspensions of A. woodii. Cell suspensions of A. woodii (1.0 mg protein/ml) grown on fructose were incubated under an H2-CO2 atmosphere at 30°C in a shaking water bath. Caffeate was added from a stock solution. At the times indicated, samples were withdrawn and analyzed to determine the caffeate (▪) and acetate (▴) concentrations as described in Materials and Methods.
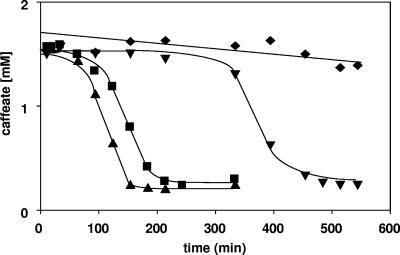
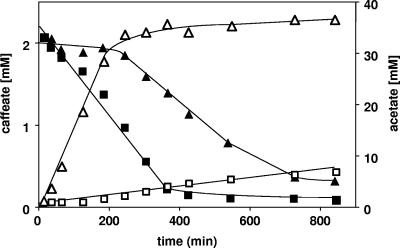
When cells were incubated in the presence of carbon dioxide and caffeate or in the presence of caffeate without an electron donor, neither acetogenesis nor caffeate reduction was observed at physiologically significant rates. However, addition of H2 led to both acetate formation and caffeate reduction. The time required for induction of caffeate reduction in the presence of H2 plus CO2 was much longer than the time required with fructose (Fig. 3). This could reflect the different energy status during homoacetogenesis from sugars (ΔG0′ = −311 kJ/mol) compared to acetogenesis from H2 plus CO2 (ΔG0′ = −105 kJ/mol). Interestingly, when cells were incubated in the absence of CO2 but in the presence of H2, caffeate reduction was impaired (Fig. 3). To analyze this effect, substrate combinations were used. A. woodii is known to grow mixotrophically on fructose and H2 plus CO2 (6). Under these conditions, caffeate respiration was induced, as observed previously (Fig. 4). When CO2 was omitted, fructose consumption and acetogenesis were largely inhibited. This was due to inhibition of fermentation by the presence of hydrogen (6, 8). However, the low rate of acetogenesis was apparently sufficient to induce caffeate respiration. Once caffeate reduction was induced, the rate was two times higher in the absence of CO2 than in the presence of CO2, again indicating that there is simultaneous use of CO2 and caffeate as electron acceptors and competition in both systems for electrons (Fig. 4).
FIG. 3.
Time course of caffeate reduction with fructose or hydrogen as the electron donor by cell suspensions of A. woodii incubated in the absence or presence of CO2. Cell suspensions of A. woodii (1.0 mg protein/ml) grown on fructose were preincubated either under an H2 atmosphere (⧫), under an H2-CO2 atmosphere (▾), under an N2-CO2 atmosphere with fructose (20 mM) (▪), or under an N2 atmosphere with fructose (▴) for 10 min. Caffeate (2 mM) was added from a stock solution. At the times indicated, samples were withdrawn and analyzed to determine the caffeate concentration as described in Materials and Methods.
FIG. 4.
Induction of caffeate respiration during acetogenesis with H2, CO2, and fructose or with H2 and fructose by cell suspensions of A. woodii. Cells were grown on fructose and harvested, and cell suspensions (1.0 mg protein/ml) were incubated with fructose, H2, and CO2 (solid symbols) or with fructose and H2 (open symbols) at 30°C in a shaking water bath for 10 min. Caffeate (2 mM) was added from a stock solution. At the times indicated, samples were withdrawn and analyzed to determine the caffeate (squares), acetate (triangles), and fructose (circles) concentrations as described in Materials and Methods.
CO2 is preferred over caffeate as an electron acceptor during methyl group oxidation.
A. woodii is able to grow on methyl group-containing compounds, such as methanol or betaine, according to:
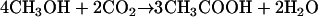 |
(4) |
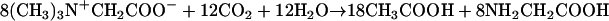 |
(5) |
The methyl group of methanol or betaine is disproportionated. One mole is oxidized to CO2, and the six electrons generated are used to reduce 3 mol of CO2 to CO that is combined with three additional methyl groups and HS-CoA to give rise to 3 mol of acetyl-CoA.
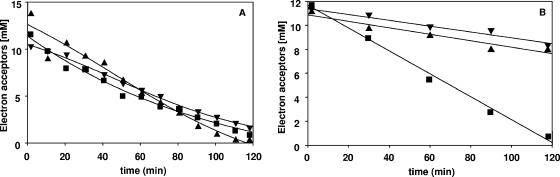
When methanol-grown cells were incubated in the presence of methanol and CO2, acetate formation started immediately and proceeded at a rate of 92.6 nmol·min−1·mg protein−1 for about 7 h. In contrast to what was observed previously with fructose or H2 (plus CO2) as an electron donor, caffeate consumption started only after acetogenesis was complete (Fig. 5A), indicating that CO2 is the preferred electron acceptor during methanol conversion and blocks the induction of caffeate reduction. The same phenomenon was observed with betaine as a substrate. Betaine was consumed much faster than methanol, but caffeate reduction started only after acetogenesis was complete (Fig. 5B).
FIG. 5.
Induction of caffeate respiration during acetogenesis from methanol (A) or betaine (B) by cell suspensions of A. woodii. Cell suspensions of A. woodii (1.0 mg protein/ml) grown on methanol or betaine were incubated at 30°C in a shaking water bath. Caffeate was added from a stock solution. At the times indicated, samples were withdrawn and analyzed to determine the caffeate (▪) and acetate (▴) concentrations as described in Materials and Methods.
Regulation of caffeate and CO2 reduction in resting cells.
The experiments described above demonstrated that there was inhibition of caffeate reduction in the presence of hydrogen or methanol. To determine whether this was due to regulation of enzyme synthesis or activity, we analyzed the effect of the electron donor on caffeate reduction in resting cells of A. woodii that had been pregrown on fructose plus caffeate to induce the caffeate reduction machinery. These cells were harvested and resuspended in buffer.
Upon addition of caffeate to resting cells incubated with H2, with H2 and CO2 (no bicarbonate in the buffer), or with H2, CO2 and bicarbonate, caffeate reduction started immediately in every case and proceeded at identical rates until caffeate was completely reduced (data not shown). At the same time that caffeate was reduced, cells produced acetate from H2 and CO2 or from H2, CO2, and bicarbonate. As expected, the rates of acetogenesis and the final yields were decreased in the presence of caffeate. These experiments clearly demonstrate that caffeate and bicarbonate are used simultaneously as electron acceptors during hydrogen oxidation.
When cells were pregrown on betaine plus caffeate, they produced acetate from betaine immediately after addition of betaine. In contrast, caffeate reduction started only after about 200 min, at a time when acetogenesis was complete. When CO2 (and bicarbonate) was omitted from the assay medium, caffeate reduction started immediately, while acetogenesis was largely impaired (Fig. 6). This experiment demonstrates that CO2 is preferred over caffeate with betaine as the electron donor and indicates that CO2 has a regulatory effect on enzymatic activities necessary for caffeate respiration.
FIG. 6.
Caffeate respiration with betaine as the reductant is impaired by CO2. A. woodii was grown with betaine plus caffeate to preinduce the caffeate reduction machinery and harvested. Cell suspensions (1.0 mg protein/ml) were incubated in medium containing 50 mM betaine in the absence (squares) or presence (triangles) of 60 mM bicarbonate at 30°C in a shaking water bath. After preincubation for 10 min, 2 mM caffeate was added from a stock solution. At the times indicated, samples were withdrawn and analyzed to determine the caffeate (solid symbols) and acetate (open symbols) concentrations.
Specificity of the caffeate-reducing system.
Next, we analyzed whether caffeate-induced cells were able to reduce p-coumarate or ferulate, which are analogues of caffeate and also have the acrylate side chain. As Fig. 7A shows, caffeate-induced cells incubated under a hydrogen atmosphere in buffer were able to reduce p-coumarate or ferulate without a lag phase, and the rates of reduction were comparable to the caffeate reduction rates. In addition, p-coumarate-induced cells reduced p-coumarate, ferulate, and caffeate, and ferulate-induced cells reduced ferulate, caffeate, or p-coumarate (data not shown). Control experiments demonstrated that hydrogen is absolutely required as a reductant for caffeate and p-coumarate. However, much to our surprise, cells pregrown on fructose plus caffeate reduced ferulate in the absence of hydrogen (Fig. 7B). The reduction rate was 40% higher than the rate observed with hydrogen as the reductant. Since the only source for reducing equivalents was the oxidation of the methyl group of ferulate to CO2, this experiment indicated that caffeate also induces the enzymes required for oxidation of the methyl group of ferulate. A small amount of caffeate reduction in the absence of added reductant was observed quite often, but at different (low) rates. This might have resulted from endogenous reducing equivalents.
FIG. 7.
Reduction of acrylate group-containing compounds by caffeate-preinduced resting cells of A. woodii in the presence of H2 (A) or N2 (B). Cell suspensions of A. woodii (1.35 mg/ml) were incubated in imidazole-HCl buffer at 30°C in a shaking water bath. Caffeate, p-coumarate, and ferulate were added from stock solutions to a final concentration of 10 mM. Samples were withdrawn at the times indicated and analyzed to determine the caffeate (▴), p-coumarate (▾), and ferulate (▪) concentrations.
DISCUSSION
Here, we demonstrated that in A. woodii de novo synthesis of proteins is required to induce the ability to reduce caffeate. In a strict sense, induction cannot be differentiated from derepression in our assays; this would require knowledge of genes and activators, as well as a detailed molecular analysis. However, it is clear that caffeate (directly or indirectly) activates expression of genes involved in caffeate reduction, such as a gene encoding a hypothetical caffeate reductase (and the electron transfer flavoprotein that probably mediates electron transfer from NADH to caffeate [Imkamp and Müller, unpublished]). Structural analogues of caffeate, such as ferulate and p-coumarate, also serve as inducers for caffeate reduction. This could be interpreted to result from isoenzymes that have limited catalytic specificity and which are induced by a cognate substrate (caffeate, ferulate, or p-coumarate). In contrast, it is also feasible that all inducers have the same effect. In addition to caffeate, ferulate, or p-coumarate, trimethoxycinnamate is able to act as an inducer for caffeate reduction in A. woodii (9).
As observed previously in A. woodii strain Nzva16, acetogenesis from H2 plus CO2 and H2-dependent caffeate reduction were catalyzed simultaneously by A. woodii DSM1030 (20). In addition, we show here that the induction of caffeate reduction is independent of the bicarbonate concentration. The same is true for induction of caffeate reduction during acetogenesis from fructose. Acetogens compete in their environment for CO2 as an electron sink with, for example, methanogens. Thermodynamically, methanogenesis (ΔGo′ = −135 kJ/mol) is preferred over acetogenesis (ΔGo′ = −105 kJ/mol), and therefore, methanogens would outcompete acetogens (12, 13). The simultaneous use of different electron acceptors enhances the competitiveness of acetogens in their anaerobic environment (14). The simultaneous use of different electron acceptors has been observed before in acetogens. Clostridium formicoaceticum uses fumarate and CO2 simultaneously in undefined medium as electron sinks (35). Ruminococcus productus is capable of growth with ferulate using the methyl group of ferulate as a sole carbon and energy source (36). The acrylate side chain of ferulate is used as a terminal electron acceptor. In the presence of CO2, acetogenesis and ferulate reduction occur simultaneously.
For energetic reasons, the use of the electron acceptor that allows maximal energy conservation should be preferred. This is seen in a number of prokaryotes, and the underlying regulatory mechanisms have been studied best for the ability of Escherichia coli to switch from fermentation to anaerobic respiration with nitrate, fumarate, or dimethyl sulfoxide (48). The best-studied example in the acetogens is M. thermoacetica, which can use CO2 or nitrate as an electron acceptor (17). Nitrate is the preferred electron acceptor for M. thermoacetica, and nitrate reduction is coupled to energy conservation (43, 44). The amount of energy conserved is larger during nitrate reduction than during acetogenesis with O-methyl groups. The redox potential of the CO2/acetate couple is about −0.34 V, whereas the redox potential of NO3−/NO2− is 0.42 V. Therefore, the Gibbs free energy change during acetogenesis from H2 plus CO2 is much smaller than the Gibbs free energy change for nitrate reduction (18, 43). Although the thermodynamic basis for the preference of nitrate over CO2 is clear, the regulatory events involved are far from being understood. One detailed study revealed that nitrate blocks the acetyl-CoA pathway in M. thermoacetica at the level of the electron transport pathway (17). The enzymes of the acetyl-CoA pathway were present and their activity was not regulated by nitrate, but a cytochrome b was missing in nitrate-grown cultures. A second study revealed an additional effect of the CO2 concentration. In CO2-sparged cultures grown in the presence of nitrate, methyltransferase and CO dehydrogenase activities were 5- to 10-fold lower, and molecular studies revealed that the regulation of enzymes, as well as electron transport proteins, such as cytochrome b, was on a transcriptional level (1). Nitrate induced the nitrate reductase (1, 17).
The redox potential of the caffeate/hydrocaffeate couple is not known, but based on the assumption that it is similar to the redox potential of the fumarate/succinate couple (E0′ = 0.032 V), caffeate reduction is energetically favored over CO2 reduction. This could lead to preferential use of caffeate over CO2, as indeed was observed previously for A. woodii Nzva16 (47). However, we did not observe preferential use of caffeate. Either caffeate and CO2 were used simultaneously (during hydrogen or fructose oxidation) or CO2 was preferred over caffeate during methyl group oxidation. The differences cannot be explained easily but may be due to the different physiologies of the two strains of A. woodii or the culture conditions used. Tschech and Pfenning (47) cultivated A. woodii Nzva16 with 60 mM bicarbonate and limiting amounts of methanol (2 mM), and caffeate was added at a concentration of 6 mM, giving a methanol/caffeate stoichiometry of 1:3. The oxidation of one methyl group yields six electrons, and Tschech and Pfenning observed that the electrons were funneled exclusively to caffeate under these conditions, with no acetate produced. We used 30 times more (60 mM methanol) or 75 times more (50 mM betaine) methyl groups than Tschech and Pfennig used, and it could be that the methyl group concentration plays a role in regulation. In line with this argument is the observation of Davies and Stephens (9), who observed an effect of the growth substrate and electron donor on caffeate reduction. Methanol-grown cells could not use fructose as an electron donor for caffeate reduction, which is surprising since the Embden-Meyerhof-Parnas pathway is constitutive. In addition, even small changes in the culture conditions can have a big impact on the regulation. For example, in undefined media, C. formicoaceticum uses CO2 and fumarate simultaneously, whereas CO2 is preferred in defined media (35).
The caffeate reductase system of A. woodii apparently does not discriminate between caffeate, ferulate, and p-coumarate. In addition, all three compounds were used as inducers for the system. A surprising finding, however, was that caffeate-induced cells were apparently able to demethylate ferulate. It is interesting that Davies and Stephens reported a similar phenomenon: cells grown with fructose plus caffeate were able to use methanol as a reductant for caffeate, and again, the methyltransferase system must have been induced by caffeate (9). O demethylation requires the concerted action of three proteins; methyltransferase I abstracts the methyl group and transfers it to the central cobalt atom in a corrinoid protein, from which it is transferred to tetrahydrofolate by the action of a second methyltransferase. The genes encoding these proteins are (often) organized in an operon, and the expression is induced by the O-methyl compound (15, 16, 29-31, 45). Here, the methyltransferase system was induced by caffeate, a compound that has no O-methyl groups. However, caffeate and O-methyl compounds are both derived from lignin and are present together in anaerobic soils that contain lignin. Therefore, the presence of caffeate might signal the presence of lignin and thus a O-methyl compound to the cells and induce a more global regulatory network involved in utilization of lignin derivatives.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft.
Footnotes
Published ahead of print on 6 April 2007.
REFERENCES
- 1.Arendsen, A. F., M. Q. Soliman, and S. W. Ragsdale. 1999. Nitrate-dependent regulation of acetate biosynthesis and nitrate respiration by Clostridium thermoaceticum. J. Bacteriol. 181:1489-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aufurth, S., H. Schägger, and V. Müller. 2000. Identification of subunits a, b, and c1 from Acetobacterium woodii Na+-F1F0-ATPase. Subunits c1, c2, and c3 constitute a mixed c-oligomer. J. Biol. Chem. 275:33297-33301. [DOI] [PubMed] [Google Scholar]
- 3.Bache, R., and N. Pfennig. 1981. Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch. Microbiol. 130:255-261. [Google Scholar]
- 4.Baronofsky, J. J., and W. J. A. Schreurs. 1984. Uncoupling by acetic acid limits growth of and acetogenesis by Clostridium thermoaceticum. Appl. Environ. Microbiol. 48:1134-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beaty, P. S., and L. G. Ljungdahl. 1991. Growth of Clostridium thermoaceticum on methanol, ethanol or dimethylsulfoxide, abstr. K-131, p. 236. Abstr. 91st Annu. Meet. Am. Soc. Microbiol. 1991. American Society for Microbiology, Washington, DC.
- 6.Braun, K., and G. Gottschalk. 1981. Effect of molecular hydrogen and carbon dioxide on chemo-organotrophic growth of Acetobacterium woodii and Clostridium aceticum. Arch. Microbiol. 128:294-298. [DOI] [PubMed] [Google Scholar]
- 7.Bryant, M. P. 1972. Commentary on the Hungate technique for culture of anaerobic bacteria. Am. J. Clin. Nutr. 25:1324-1328. [DOI] [PubMed] [Google Scholar]
- 8.Chung, K. T. 1976. Inhibitory effects of H2 on growth of Clostridium cellobioparum. Arch. Environ. Microbiol. 31:342-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies, E. T., and G. M. Stephens. 1998. Effect of growth substrate and electron donor on hydrogenation of carbon-carbon double bonds by Acetobacterium woodii. Enzyme Microb. Technol. 23:129-132. [Google Scholar]
- 10.Dorn, M., J. R. Andreesen, and G. Gottschalk. 1978. Fermentation of fumarate and l-malate by Clostridium formicoaceticum. J. Bacteriol. 133:26-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn, M., J. R. Andreesen, and G. Gottschalk. 1978. Fumarate reductase of Clostridium formicoaceticum. A peripheral membrane protein. Arch. Microbiol. 119:7-11. [DOI] [PubMed] [Google Scholar]
- 12.Drake, H. L., S. Daniel, K. Küsel, C. Matthies, C. Kuhner, and S. Braus-Strohmeyer. 1997. Acetogenic bacteria: what are the in situ consequences of their diverse metabolic diversities? Biofactors 1:13-24. [DOI] [PubMed] [Google Scholar]
- 13.Drake, H. L., S. L. Daniel, C. Matthies, and K. Küsel. 1994. Acetogenesis: reality in the laboratory, uncertainty elsewhere, p. 273-302. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, NY.
- 14.Drake, H. L., K. Küsel, and C. Matthies. 2006. Acetogenic prokaryotes, p. 354-420. In M. Dworkin, S. Falkow, E. Rosenberg, K.-H. Schleifer, and E. Stackebrandt (ed.), The prokaryotes, 3rd ed. Springer-Verlag, New York, NY.
- 15.el Kasmi, A., S. Rajasekharan, and S. W. Ragsdale. 1994. Anaerobic pathway for conversion of the methyl group of aromatic methyl ethers to acetic acid by Clostridium thermoaceticum. Biochemistry 33:11217-11224. [DOI] [PubMed] [Google Scholar]
- 16.Engelmann, T., F. Kaufmann, and G. Diekert. 2001. Isolation and characterization of a veratrol:corrinoid protein methyl transferase from Acetobacterium dehalogenans. Arch. Microbiol. 175:376-383. [DOI] [PubMed] [Google Scholar]
- 17.Fröstl, J. M., C. Seifritz, and H. L. Drake. 1996. Effect of nitrate on the autotrophic metabolism of the acetogens Clostridium thermoautotrophicum and Clostridium thermoaceticum. J. Bacteriol. 178:4597-4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fuchs, G. 1986. CO2 fixation in acetogenic bacteria: variations on a theme. FEMS Microbiol. Rev. 39:181-213. [Google Scholar]
- 19.Gottwald, M., J. R. Andreesen, J. LeGall, and L. G. Ljungdahl. 1975. Presence of cytochrome and menaquinone in Clostridium formicoaceticum and Clostridium thermoaceticum. J. Bacteriol. 122:325-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen, B., M. Bokranz, P. Schönheit, and A. Kröger. 1988. ATP formation coupled to caffeate reduction by H2 in Acetobacterium woodii NZva16. Arch. Microbiol. 150:447-451. [Google Scholar]
- 21.Heise, R., V. Müller, and G. Gottschalk. 1992. Presence of a sodium-translocating ATPase in membrane vesicles of the homoacetogenic bacterium Acetobacterium woodii. Eur. J. Biochem. 206:553-557. [DOI] [PubMed] [Google Scholar]
- 22.Heise, R., J. Reidlinger, V. Müller, and G. Gottschalk. 1991. A sodium-stimulated ATP synthase in the acetogenic bacterium Acetobacterium woodii. FEBS Lett. 295:119-122. [DOI] [PubMed] [Google Scholar]
- 23.Hugenholtz, J., D. M. Ivey, and L. G. Ljungdahl. 1987. Carbon monoxide-driven electron transport in Clostridium thermoautotrophicum membranes. J. Bacteriol. 169:5845-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hugenholtz, J., and L. G. Ljungdahl. 1990. Amino acid transport in membrane vesicles of Clostridium thermoautotrophicum. FEMS Microbiol. Lett. 69:117-122. [DOI] [PubMed] [Google Scholar]
- 25.Hugenholtz, J., and L. G. Ljungdahl. 1989. Electron transport and electrochemical proton gradient in membrane vesicles of Clostridium thermoaceticum. J. Bacteriol. 171:2873-2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hugenholtz, J., and L. G. Ljungdahl. 1990. Metabolism and energy generation in homoacetogenic clostridia. FEMS Microbiol. Rev. 87:383-389. [DOI] [PubMed] [Google Scholar]
- 27.Hungate, R. E. 1969. A roll tube method for cultivation of strict anaerobes. Methods Microbiol. 3b:117-132. [Google Scholar]
- 28.Imkamp, F., and V. Müller. 2002. Chemiosmotic energy conservation with Na+ as the coupling ion during hydrogen-dependent caffeate reduction by Acetobacterium woodii. J. Bacteriol. 184:1947-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufmann, F., G. Wohlfarth, and G. Diekert. 1997. Isolation of O-demethylase, an ether-cleaving enzyme system of the homoacetogenic strain MC. Arch. Microbiol. 168:136-142. [DOI] [PubMed] [Google Scholar]
- 30.Kaufmann, F., G. Wohlfarth, and G. Diekert. 1998. O-Demethylase from Acetobacterium dehalogenans—cloning, sequencing, and active expression of the genes encoding the corrinoid protein. Eur. J. Biochem. 257:515-521. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann, F., G. Wohlfarth, and G. Diekert. 1998. O-Demethylase from Acetobacterium dehalogenans—substrate specificity and function of the participating proteins. Eur. J. Biochem. 253:706-711. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 33.Ljungdahl, L. G. 1994. The acetyl-CoA pathway and the chemiosmotic generation of ATP during acetogenesis, p. 63-87. In H. L. Drake (ed.), Acetogenesis. Chapman & Hall, New York, NY.
- 34.Ljungdahl, L. G. 1986. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu. Rev. Microbiol. 40:415-450. [DOI] [PubMed] [Google Scholar]
- 35.Matthies, C., A. Freiberger, and H. L. Drake. 1993. Fumarate dissimilation and differential reductant flow by Costridium formicoaceticum and Clostridium aceticum. Arch. Microbiol. 160:273-278. [Google Scholar]
- 36.Misoph, M., and H. L. Drake. 1996. Effect of CO2 on the fermentation capacities of the acetogen Peptostreptococcus productus U-1. J. Bacteriol. 178:3140-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Müller, V. 2003. Energy conservation in acetogenic bacteria. Appl. Environ. Microbiol. 69:6345-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Müller, V., S. Aufurth, and S. Rahlfs. 2001. The Na+ cycle in Acetobacterium woodii: identification and characterization of a Na+ translocating F1F0-ATPase with a mixed oligomer of 8 and 16 kDa proteolipids. Biochim. Biophys. Acta 1505:108-120. [DOI] [PubMed] [Google Scholar]
- 39.Ragsdale, S. W. 1997. The eastern and the western branch of the Wood/Ljungdahl pathway: how the east and west were won. Biofactors 6:3-11. [DOI] [PubMed] [Google Scholar]
- 40.Ragsdale, S. W. 1991. Enzymology of the acetyl-CoA pathway of autotrophic CO2 fixation. Crit. Rev. Biochem. Mol. Biol. 26:261-300. [DOI] [PubMed] [Google Scholar]
- 41.Reidlinger, J., and V. Müller. 1994. Purification of ATP synthase from Acetobacterium woodii and identification as a Na+-translocating F1F0-type enzyme. Eur. J. Biochem. 223:275-283. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt, K., S. Liaanen-Jensen, and H. G. Schlegel. 1963. Die Carotinoide der Thiorodaceae. Arch. Mikrobiol. 46:117-126. [PubMed] [Google Scholar]
- 43.Seifritz, C., S. L. Daniel, A. Gossner, and H. L. Drake. 1993. Nitrate as a preferred electron sink for the acetogen Clostridium thermoaceticum. J. Bacteriol. 175:8008-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seifritz, C., H. L. Drake, and S. L. Daniel. 2003. Nitrite as an energy-conserving electron sink for the acetogenic bacterium Moorella thermoacetica. Curr. Microbiol. 46:329-333. [DOI] [PubMed] [Google Scholar]
- 45.Stupperich, E., and R. Konle. 1993. Corrinoid-dependent methyl transfer reactions are involved in methanol and 3,4-dimethoxybenzoate metabolism by Sporomusa ovata. Appl. Environ. Microbiol. 59:3110-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tschech, A., and N. Pfennig. 1984. Growth yield increase linked to caffeate reduction in Acetobacterium woodii. Arch. Microbiol. 137:163-167. [Google Scholar]
- 48.Unden, G., and B. Schink. 1997. Alternative respiratory pathways of Escherichia coli: energetics and transcriptional regulation in response to electron acceptors. Biochim. Biophys. Acta 1320:217-234. [DOI] [PubMed] [Google Scholar]