Abstract
Recent molecular evidence suggests that different species and/or genotypes of Cryptosporidium display strong host specificity, altering our perceptions regarding the zoonotic potential of this parasite. Molecular forensic profiling of the small-subunit rRNA gene from oocysts enumerated on microscope slides by U.S. Environmental Protection Agency method 1623 was used to identify the range and prevalence of Cryptosporidium species and genotypes in the South Nation watershed in Ontario, Canada. Fourteen sites within the watershed were monitored weekly for 10 weeks to assess the occurrence, molecular composition, and host sources of Cryptosporidium parasites impacting water within the region. Cryptosporidium andersoni, Cryptosporidium muskrat genotype II, Cryptosporidium cervine genotype, C. baileyi, C. parvum, Cryptosporidium muskrat genotype I, the Cryptosporidium fox genotype, genotype W1, and genotype W12 were detected in the watershed. The molecular composition of the Cryptosporidium parasites, supported by general land use analysis, indicated that mature cattle were likely the main source of contamination of the watershed. Deer, muskrats, voles, birds, and other wildlife species, in addition to sewage (human or agricultural) may also potentially impact water quality within the study area. Source water protection studies that use land use analysis with molecular genotyping of Cryptosporidium parasites may provide a more robust source-tracking tool to characterize fecal impacts in a watershed. Moreover, the information is vital for assessing environmental and human health risks posed by water contaminated with zoonotic and/or anthroponotic forms of Cryptosporidium.
Cryptosporidiosis remains a public health concern, as demonstrated by continued outbreaks of this disease attributable to waterborne transmission. This has led to more stringent monitoring and treatment requirements for drinking water in the United Kingdom and the United States (4, 47). However, research on the molecular epidemiology of Cryptosporidium suggests that the genus is highly host specific and as a result has changed the perceptions regarding the zoonotic potential of the parasite (52, 54). The species and genotypes known to cause human infections (Cryptosporidium hominis, C. parvum, C. meleagridis, C. felis, C. canis, C. muris, C. suis, and the cervine genotype) represent only a portion of those which have been described, including 15 species and more than 40 genotypes isolated from specific hosts and environmental waters (8, 15, 38, 52).
Existing techniques commonly used for the identification of Cryptosporidium spp. in water, such as the United States Environmental Protection Agency's method 1623 (46), provide a quantitative assessment of the number of parasites present within a water sample. This method does not identify the species or genotypes present in the water and therefore does not provide an accurate assessment of the human health risk posed by these parasites when they are detected in a water sample. Although large numbers of Cryptosporidium oocysts could be detected in a water sample, the risk to human health may be low because the species and genotypes present may not be capable of infecting humans. Conversely, low numbers of human-adapted parasites (i.e., C. hominis) present in water may represent a significant human health threat. Molecular analysis carried out with Cryptosporidium oocysts recovered from water samples demonstrates that a diverse range of species and genotypes may be present in raw water samples (14, 15, 48, 51). Effective risk assessment requires the identification of Cryptosporidium species and/or genotypes that may be present in a water sample. Detection and subsequent molecular characterization of the oocysts in the water can also be used to infer potential host fecal sources impacting water quality within a watershed (i.e., microbial source tracking [MST]), with the goal of designing watershed protection strategies aimed at reducing environmental burdens of anthroponotic and zoonotic forms of the parasite.
Ruecker et al. previously described a method for molecular forensic profiling of Cryptosporidium oocysts from microscope slides prepared from water samples processed by method 1623 (34). In the present study, this method was used to identify Cryptosporidium species and genotypes in raw water samples collected at various geographic locations within the South Nation watershed in Ontario, Canada. Molecular profiling of Cryptosporidium parasites was integrated with land use analysis in an attempt to develop more robust source tracking tools to identify sources of fecal impact contaminating the South Nation watershed. The data were also used to assess the potential risk to human health based on the detection of zoonotic or anthroponotic forms of the parasite in the watershed.
MATERIALS AND METHODS
Site selection and land use survey.
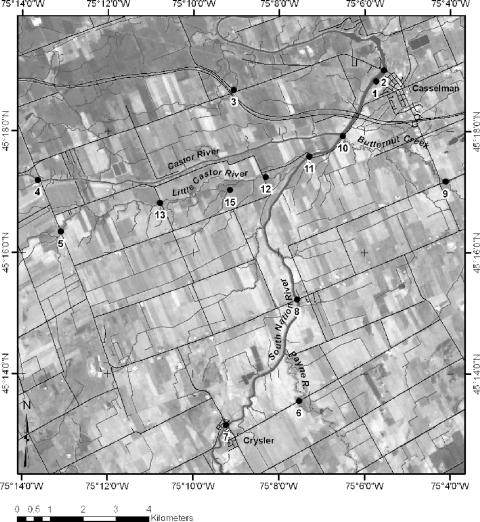
The water sampling sites were located within the South Nation watershed, Ontario, Canada. The total area of the watershed is 3,900 km2 with a river length of approximately 175 km. Peak monthly maximum discharges near the river mouth are just under 500 m3 s−1 for spring melt conditions (April). The sampling sites cover an area of approximately 200 km2 (Fig. 1) that has a generally flat topography where tile drainage and groundwater primarily contribute to flow.
FIG. 1.
Geographic location of the South Nation watershed and distribution of sampling locations within the watershed. Water flow occurs in a northerly direction, toward MST-2 (the most downstream site).
Land use surveys were carried out in the fall of 2005. Geographic Information Systems software ArcMap 9.1 (Environmental Systems Research Institute [ESRI], Redland, CA) and ArcPad 6.0.1 (ESRI) were interfaced with a Trimble AgGPS 122 differential global positioning system receiver (Trimble Navigation Limited, Sunnyvale, CA) to collect, store, and analyze spatial information. Land use characteristics such as cropland, pasture, forage, exposed rock, shrubland, wetland, forest, and development intensity were generated. A combination of hydrography, road network, and aerial photography data served as a base map to locate the points of land use. Land use observations were made progressively upstream of the water sampling sites by means of a roadside survey based on the predetermined categories of land use. Within the class of agricultural land, attributes such as type of on-farm structures, crop type, pasturing or forage type, crop development stage, tillage, soil moisture, pasture animal, access to water, fertilization, manure storage, migratory bird presence or absence, and building location and distance to water were recorded. After collection, the data were integrated into an ESRI Personal Geodatabase with ArcMap. Thiessen polygons were created from observed land use points to the limit of the land use observation area. Sample basin limits were determined using a digital elevation model with no integration of a vector stream layer. Both the point observations and the Thiessen polygon layers were used to generate general descriptions of the water sampling locations, associated river and stream characteristics, and predominant upstream land use (Tables 1 to 3).
TABLE 1.
Site descriptions and potential fecal impacts at the sample sites in the study area, with sites on individual waterways listed in upstream order
| Site | Description |
|---|---|
| South Nation River | |
| MST-2 | Stream buffering is minimal along the banks of the South Nation River. This site receives more overland flow from northern Casselman due to its proximity to this part of the community. |
| MST-1 | Water samples collected at the water intake for the community of Casselman. Drainage from southern Casselman is released about 1.7 km upstream of the water sampling site. |
| MST-11 | Dairy operations on river are near the site, with pasturing about 400 m upstream. Forage and cropland line the waterway with a relatively low bank slope. |
| MST-8 | Sample collected from bridge in town of St. Albert. Municipal lagoon is situated upstream (2.6 km). Little riparian buffering is present. The community of Crysler is roughly 4.7 km upstream, potentially providing fecal contamination due to faulty septic systems and related urban waste. |
| MST-7 | Sample collected from bridge in community of Crysler. Urban development high but abruptly grades to agricultural (cash and livestock cropping) within a few kilometers upstream. Natural riparian buffering is limited near the site. One dairy operation is 3 km upstream of the site, located on the bank of the river. A second operation lies 1.5 km from the sampling location but is farther from the river. |
| Little Castor River | |
| MST-12 | Bank slope is relatively high with natural vegetation along river banks. Municipal lagoon about 8 km upstream and a trailer park 6.5 km upstream. Tile drain outlets are located along river bank immediately upstream of sampling site. |
| MST-13 | Similar to MST-12 but less concentrated riparian buffering close to the sampling location. Several dairy operations (6 within 2 km of the site) located immediately upstream. Liquid manure applications farther upstream, while solid applications were observed closer to the site on cropland located to the southwest. Contains the highest level of bank slope in close proximity to site compared to other locations. |
| MST-5 | A dairy operation is about 70 m upstream of the sampling site with a second 700 m upstream. Much of the Little Castor bank is more vegetated; however, buffering decreases up the reach from the sampling site. Sewage lagoon within the contributing area. Bank slope close to the site is below average. |
| Butternut Creek | |
| MST-10 | Sample collected from bridge crossing creek very near outlet to South Nation River. There is a dairy operation immediately upstream of sample site with cattle access. A golf course is within several hundred meters of the sampling site. Some urban development in the basin serves as a potential sewage source. Riparian buffering is rather limited. Above-average bank slope is found upstream. |
| MST-9 | Pasturing activity adjacent to the creek, as well as forage activity. Large wetland area within 2 km of the site. Little vegetation (forest and shrubs) immediately at the sampling site. High concentrations of dairy operations (n = 8) fall within 2.5 km of the site. Bank slope is generally low upstream. Poultry operation found about 2 km from the site. Solid manure applications were observed close to the site, while liquid applications were found further upstream. |
| Castor River, MST-4 | Banks of river are relatively steep, limiting animal access to river. On the south bank of the sampling site is a dairy operation with pasturing. The waterways are buffered by vegetation (grass, shrubs, trees) along the river and tributaries. A road system and urban development close to this site do not provide suitable habitat for wildlife to access the river. Sewage lagoon 12 km upstream. Solid fertilizer applications were observed. |
| Payne River, MST-6 | Significant pastureland intermixed with shrubs along the banks. West about 800 m from this site are several dairy operations. Solid manure applications were observed at many locations adjacent to tributaries of the river. Bank slope is low close to the site. |
| Rural drainage ditch | |
| MST-3 | Sample collected from edge of drainage ditch. The site is adjacent to a 4-lane divided highway. Dairy operations are located about 1.8 and 2.4 km upstream from the sampling site. |
| MST-15 | Sample collected at edge of drainage ditch. The sample site is located in an open pasture that grades to a more natural wooded pasture system with cattle access to waterway. The sampling site has associated cropland and pasture and forage upstream where manures are applied in spring and fall. Bank slope is very low adjacent to the site. |
TABLE 3.
Agriculture-related fecal sources associated with the surveyed land
| Site | Dairy operationsa | Cattle barnsa | Poultry barnsa | Hog barnsa | Horse barnsa | Direct access of livestock to waterb |
|---|---|---|---|---|---|---|
| South Nation River | ||||||
| MST-2 | 126 | 61 | 5 | 1 | 12 | 58 |
| MST-1 | 126 | 61 | 5 | 1 | 12 | 58 |
| MST-11 | 94 | 45 | 4 | 1 | 7 | 46 |
| MST-8 | 55 | 28 | 4 | 1 | 3 | 39 |
| MST-7 | 20 | 7 | 2 | 0 | 0 | 11 |
| Little Castor River | ||||||
| MST-12 | 21 | 11 | 0 | 0 | 4 | 6 |
| MST-13 | 21 | 11 | 0 | 0 | 4 | 5 |
| MST-5 | 16 | 10 | 0 | 0 | 3 | 3 |
| Butternut Creek | ||||||
| MST-10 | 28 | 12 | 1 | 0 | 1 | 10 |
| MST-9 | 18 | 9 | 1 | 0 | 1 | 6 |
| Castor River, MST-4 | 5 | 2 | 0 | 0 | 1 | 1 |
| Payne River, MST-6 | 28 | 16 | 1 | 0 | 2 | 32 |
| Rural drainage ditch | ||||||
| MST-3 | 3 | 1 | 0 | 0 | 0 | 0 |
| MST-15 | 0 | 0 | 0 | 0 | 0 | 1 |
Numbers of observations within surveyed regions upstream of the MST site (see Table 2 for an explanation of what is considered a surveyed region). During the land use survey, a unique spatial location was recorded for every dairy operation (barn) or cattle, poultry, hog, or horse barn observed. The fields where cattle had access to water were attributed a point within their boundary and stored like all other observations. These spatial locations of dairy, cattle, poultry, hog, and horse barns and points of livestock access to water within the contributing area for each MST basin were summed for comparative analysis.
Direct access of livestock to water represents the number of locations within the observational distance which have the potential for livestock to directly access the waterway.
Sample collection and processing.
Sampling for Cryptosporidium was carried out in the South Nation watershed on a weekly basis from 4 October through 13 December 2004. Approximately 20 liters of water was collected at each sampling site and transported to Agriculture and Agri-Food Canada laboratories in Ottawa, Ontario. Samples were processed by filtration with Filta-max filters (IDEXX, Westbrook, ME). The filters and any residual water from the filter housing were transferred to plastic bags, sealed, and shipped on ice by overnight delivery to the Alberta Provincial Laboratory for Public Health (Microbiology) in Calgary, Alberta, Canada.
Upon arrival at the Provincial Laboratory, the samples were processed for parasite enumeration by method 1623 (46). Immunomagnetic separation was carried out with a Dynabeads-GC Combo Kit (Dynal Biotech, Brown Deer, WI). Oocysts were enumerated with immunofluorescent antibodies (IFA), whereby slides were first stained with 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma-Aldrich, Oakville, Ontario, Canada), followed by fluorescently labeled monoclonal antibodies (EasyStain; Biotechnology Frontiers, North Ryde, New South Wales, Australia). Parasites were enumerated by fluorescence microscopy (538 nm), and oocysts were confirmed to be Cryptosporidium spp. on the basis of DAPI staining characteristics and by their size, shape, and morphology by differential interference contrast microscopy. Only oocysts confirmed positive by DAPI or differential interference contrast microscopy were reported, and numbers were standardized to a volume of 1 liter.
Forensic profiling of Cryptosporidium spp. from microscope slides.
Cryptosporidium oocysts were removed from slides within 48 h of completion of microscopy, and DNA was extracted as described by Ruecker et al. (34). Molecular genotyping of Cryptosporidium spp. was carried out by nested PCR-restriction fragment length polymorphism (RFLP) analysis on the basis of the small-subunit (18S) rRNA gene. Multiple nested PCRs (five replicates) were carried out with each DNA extract (i.e., water sample) by using the PCR conditions and primers described by Xiao et al. (51, 53). For the nested PCRs, 5 μl of DNA extract was added to the primary PCR (50-μl total volume), followed by 1 μl of primary product transferred to the secondary PCR (50-μl total volume). Electrophoresis of secondary PCR products was carried out with 2% agarose gels and visualized by ethidium bromide staining. A series of positive and negative controls were included for all nested PCR analyses to verify the validity of the results (35). All reaction products positive by nested PCR were digested with the restriction enzymes SspI, VspI, and DdeI (34), and the digested products were fractionated by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
Because of the weak banding of some reaction products on electrophoresis gels, some primary PCR products were reamplified by secondary PCR by using 100-μl reaction product volumes and increasing the number of cycles on the GeneAmp 2700 thermal cycler (Applied Biosystems, Foster City, CA) to 30 cycles. PCR products that appeared to contain only single species and genotypes, as indicated by the banding patterns of the three restriction enzymes, were selected for DNA sequence analysis. Secondary PCR products were excised from the electrophoresis gel, purified, and sequenced with a Prism 3100-Avant Genetic analyzer (Applied Biosystems). Accuracy of the sequences was confirmed by bidirectional sequencing of multiple PCR products.
Base calling was performed by using Seqscape version 2.1.1 (Applied Biosystems) with the KB base caller, and a consensus sequence was then constructed. The base calling and sequence assembly were confirmed with the Staden Package (43) at the Sun Center of Excellence for Visual Genomics (COE) at the University of Calgary.
DNA sequence analysis.
Reference sequences were selected on the basis of a detailed examination of GenBank and a previously published phylogenetic analysis (15, 52). The 14 sequences isolated from the South Nation watershed were initially aligned with the reference sequences with ClustalW (45) at the COE. The relationship between sequences from the South Nation watershed and the reference sequences was assessed by phylogenetic analysis carried out with the PHYLIP package (9) where evolutionary distances were calculated by DNADIST with the Kimura two-parameter model. Subsequently, the NEIGHBOR program (9) was used to construct a phylogenetic tree with Eimeria tenella as the outgroup. Trees constructed with PHYLIP were viewed with TreeView (27) and rooted at the outgroup.
On the basis of an examination of the tree, the number of reference sequences was reduced to include one sequence from each of the recognized Cryptosporidium species or genotypes and also any sequences which were similar to those obtained from the South Nation watershed. These were once again aligned at the COE with T-Coffee, a more accurate pairwise alignment tool (25). This multiple alignment is available upon request. The evolutionary distances were recalculated (as described above), and the tree was constructed. The reliability of the branches was evaluated by bootstrap analysis with SEQBOOT (PHYLIP program) with 1,000 subsamples as the initial input.
Nucleotide sequence accession numbers.
Unique partial 18S rRNA gene sequences obtained from the watershed during this study have been deposited in GenBank under accession numbers EF061288 (Cry_190), EF061289 (Cry_147), EF061290 (Cry_148), EF061291 (Cry_158), EF061292 (Cry_168), and EF061293(Cry_186).
RESULTS
Land use survey.
Land uses associated with individual sampling sites are summarized in Table 2. Because of the large spatial extent of the catchment areas associated with some sample sites, land uses were qualified on the basis of partial coverage of the catchment area starting from the sample site locale to a variable distance upstream. Agricultural activity (cropland, forage, and pasture) was the predominant observed land usage. The area devoted to agricultural activities varied from 49% (MST-3) to 94% (MST-15) for the sample catchment area. Remaining land use was split between developed lands (i.e., urban and rural housing) and natural lands (i.e., forest, shrubs and wetlands), with developed land ranging from 2% (MST-15) to 32% (MST-4) while natural lands varied from 4% (MST-15) to 27% (MST-3) (noting that observational areas varied between sampling sites).
TABLE 2.
General land use information for sites within the South Nation watershed
| Site | Observational distance upstream of sample site (km)a | % of land surveyedb | % of land use typec
|
||
|---|---|---|---|---|---|
| Agriculture | Developed land | Natural lands | |||
| South Nation River | |||||
| MST-2 | 49.2 | 16 | 67 | 16 | 15 |
| MST-1 | 49.2 | 16 | 67 | 16 | 15 |
| MST-11 | 45.1 | 18 | 69 | 15 | 15 |
| MST-8 | 39.7 | 14 | 67 | 15 | 17 |
| MST-7 | 22.0 | 4 | 82 | 8 | 9 |
| Little Castor River | |||||
| MST-12 | 31.8 | 48 | 74 | 14 | 11 |
| MST-13 | 27.7 | 44 | 73 | 15 | 11 |
| MST-5 | 23.4 | 38 | 73 | 14 | 11 |
| Butternut Creek | |||||
| MST-10 | 20.1 | 75 | 72 | 11 | 16 |
| MST-9 | 14.5 | 70 | 72 | 10 | 18 |
| Castor River, MST-4 | 11.8 | 2 | 53 | 32 | 14 |
| Payne River, MST-6 | 35.6 | 75 | 61 | 17 | 23 |
| Rural drainage ditch | |||||
| MST-3 | 2.6 | 73 | 49 | 24 | 27 |
| MST-15 | 6.6 | 100 | 94 | 2 | 4 |
Distance of land use survey, beginning at the sample site location to a variable distance upstream.
Surveyed land as a percentage of the total catchment area upstream of the sample site.
Observations of land use were recorded within a limited area contributing to the MST sites as points. Land use observations were made progressively upstream of the MST sites but not for the entire MST region due to the large extent of the contributing area (one exception was MST-15). These areas are subregions of the total MST basin(s). Within the extent of this subregion, the land use observations stored as points in the Geographic Information Systems software ArcMap 9.1 were converted to Thiessen polygons (areas). Percentages of agriculture, developed, and natural lands were then summed in these subregions. Agricultural land is land cropped, under pasture, forage, abandoned farmland, farm headquarters, and/or other structures associated with farming. Developed land is inhabited land not devoted to agriculture (commercial, residential). Natural lands are forested, shrubland, exposed rock, and wetlands.
Table 3 shows the number of livestock operations observed for cattle, poultry, hogs, and horses that occurred in the surveyed area for each site. Cattle were the major livestock present in the study area. Sample sites MST-2, MST-1, MST-11, MST-8, and MST-7 (upstream order) are located on the South Nation River and represent the waterway with the highest number of cattle-based land uses or impacts (i.e., 187 cumulative cattle operations at MST-1). The Little Castor River (MST-5, MST-12, and MST-13), the Castor River (MST-4), the Payne River (MST-6), Butternut Creek (MST-10 and MST-9), and the rural drainage ditch (MST-3) had 32, 7, 44, 40, and 4 cattle operations observed, respectively, within the surveyed portion of each respective catchment area. There were few horse operations compared to cattle operations, while horse operations outnumbered poultry and hog operations. The majority of the observations where livestock had direct access to waterways were highest on the South Nation River, with 58 observed for the cumulative observational distance for MST-1 and MST-2 (Table 3). Sites MST-15 and MST-4 had only one observed location where livestock had direct access to water, while MST-3 had no direct-access observations.
Prevalence of Cryptosporidium oocysts in the watershed.
A total of 120 water samples were collected over a 10-week period from the 14 sampling sites (Fig. 1). The number of Cryptosporidium oocysts reported on a weekly basis per site is summarized in Table 4. The frequency of Cryptosporidium oocysts found in the samples collected was 77%, with numbers ranging from 0.04 to 1.50 oocysts per liter of water. Every sampling site was positive for Cryptosporidium at some point during the sampling period, while some sites (MST-6, MST-9, and MST-15) had Cryptosporidium oocysts detected in all samples. The frequency of Cryptosporidium detection was lowest at MST-1, with 3 of 10 samples positive for Cryptosporidium. MST-9 was the site with the most Cryptosporidium contamination, with an average of 0.61 oocyst per liter detected weekly. The maximum number of oocysts observed in the samples was at MST-9, where 1.50 oocysts per liter were detected in week 3.
TABLE 4.
Concentration, prevalence, and molecular characterization of Cryptosporidium parasites impacting study sites within the South Nation watershed for the 10-week study period
| Site | No. of oocysts/litera at sampling wk:
|
Species and/or genotype by sequence analysis (accession no. of GenBank match) [frequency of detection of species or genotype] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| South Nation River | |||||||||||
| MST-2 | 0.20 | ND | ND | ND | 0.14 | 0.05 | ND | 0.54 | 0.18 | — | C. andersoni (AB089285) [1.0] |
| MST-1 | ND | 0.13 | ND | ND | ND | ND | ND | 0.63 | 0.13 | ND | C. andersoni (AB089285) [0.5] |
| C. parvum (AF308600) [0.5] | |||||||||||
| Genotype W12 (AY007254) [0.5] | |||||||||||
| MST-11 | 0.13 | ND | 0.07 | 0.07 | 0.07 | 0.13 | 0.09 | 0.88 | — | — | C. andersoni (AB089285) [1.0] |
| Genotype W12 (see Fig. 4) [1.0] | |||||||||||
| MST-8 | — | 0.05 | 0.05 | ND | 0.23 | 0.09 | 0.14 | 0.34 | 0.05 | — | C. andersoni (AB089285) [1.0] |
| MST-7 | ND | 0.15 | ND | 0.09 | 0.47 | 0.14 | 0.59 | 0.40 | 0.48 | — | C. andersoni (AB089285) [1.0] |
| Little Castor River | |||||||||||
| MST-12 | ND | ND | 0.36 | 0.26 | 0.14 | 0.07 | 0.04 | 0.45 | 0.26 | — | C. andersoni (AB089285) [0.5] |
| Genotype W12 (see Fig. 4) [0.5] | |||||||||||
| MST-13 | ND | 0.38 | 0.09 | 0.07 | 0.17 | 0.14 | ND | 0.48 | 0.20 | — | Cryptosporidium muskrat genotype II (AY545546) [0.7] |
| Genotype W12 (see Fig. 4) [0.3] | |||||||||||
| MST-5 | 0.04 | 0.08 | ND | 0.22 | 0.33 | 0.28 | 0.09 | 0.56 | 0.11 | — | Cryptosporidium muskrat genotype II (AY545546) [0.7] |
| C. baileyi (AF093495) [0.3] | |||||||||||
| Genotype W12 (AY007254) [0.3] | |||||||||||
| Butternut Creek | |||||||||||
| MST-10 | ND | 0.09 | ND | 0.20 | 0.36 | 0.07 | 0.13 | 0.88 | 0.27 | — | C. andersoni (AB089285) [1.0] |
| MST-9 | 0.35 | 0.60 | 1.50 | 1.03 | 0.78 | 0.36 | 0.53 | 0.18 | 0.19 | — | Genotype W12 (AY007254) [0.5] |
| C. andersoni (AB089285) [0.2] | |||||||||||
| Cryptosporidium cervine genotype (AY737593) [0.2] | |||||||||||
| Genotype W1 (AF262330) [0.2] | |||||||||||
| Castor River, MST-4 | ND | ND | ND | ND | ND | 0.21 | 0.04 | 0.67 | 0.14 | — | Cryptosporidium muskrat genotype II (AY737567) [0.5] |
| Cryptosporidium fox genotype (see Fig. 4) [0.5] | |||||||||||
| Payne River, MST-6 | 0.26 | 0.09 | 0.18 | 0.93 | 0.31 | 0.18 | 0.14 | 0.65 | — | — | C. andersoni (AB089285) [0.7] |
| Cryptosporidium muskrat genotype II (AY545546) [0.3] | |||||||||||
| Rural drainage ditch | |||||||||||
| MST-3 | 0.26 | 0.05 | ND | 0.05 | 0.09 | — | 0.18 | 0.09 | — | — | C. andersoni (AB089285) [0.5] |
| Cryptosporidium cervine genotype (AY737593) [0.5] | |||||||||||
| Genotype W12 (AY007254) [0.3] | |||||||||||
| MST-15 | — | — | 0.05 | 0.35 | 0.22 | 0.09 | 0.14 | 0.13 | 0.29 | — | C. andersoni (AB089285) [0.5] |
| Cryptosporidium muskrat genotype II (AY737567) [0.2] | |||||||||||
| Cryptosporidium muskrat genotype I (see Fig. 4) [0.2] | |||||||||||
| Avg | 0.10 | 0.12 | 0.16 | 0.23 | 0.24 | 0.14 | 0.15 | 0.49 | 0.21 | ND | |
A dash indicates that no sample was collected, and ND indicates that no Cryptosporidium oocysts were detected by IFA.
The average number of Cryptosporidium oocysts detected in the entire study area varied weekly, ranging from 0.10 to 0.49 oocyst per liter of water, with the highest concentration detected during week 8, corresponding to a major precipitation or discharge event in the watershed (data not shown). At 10 of the 14 sites, the highest oocyst numbers recorded corresponded to this event.
Cryptosporidium characterized by nested PCR-RFLP in water samples.
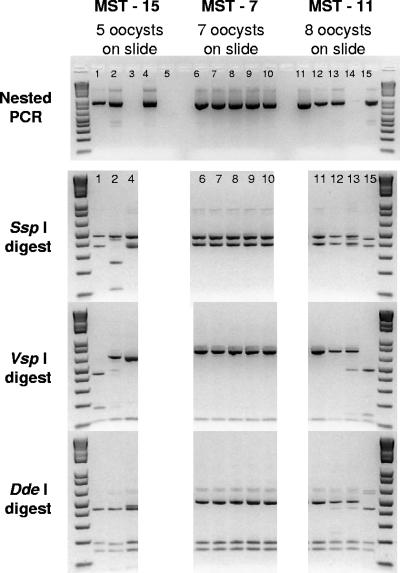
DNA was extracted from all of the slides processed according to method 1623 (both positive and negative by IFA) and subjected to repetitive nested PCR-RFLP analysis. Examples of repetitive nested PCR results are shown in the top panel of Fig. 2. PCR products were obtained from 31 of 92 water samples positive for Cryptosporidium by IFA. Although this appears to represent poor method sensitivity for PCR from DNA extracted from microscope slides, 57 of the 92 Cryptosporidium-positive microscope slides had three or fewer oocysts, suggesting that levels of sensitivity may be challenged when few oocysts are present on the microscope slide. In addition, on two occasions where no oocysts were seen by IFA, PCR products were obtained, while positive and negative controls proved the assay valid.
FIG. 2.
Molecular forensic profiling of Cryptosporidium parasites by repetitive nested PCR-RFLP analysis with microscope slides containing three water samples processed by method 1623. The top panel shows repetitive nested PCR results for three water samples in which five oocysts (lanes 1 to 5, MST-15), seven oocysts (lanes 6 to 10, MST-7), and eight oocysts (lanes 11 to 15, MST-11) were enumerated from the microscope slide. The lower panels show results of RFLP analysis with restriction enzymes SspI, VspI, and DdeI for positive PCRs. RFLP patterns in lanes 1 and 4 for site MST-15 represent two variants of Cryptosporidium muskrat genotype I verified by sequence analysis. Lane 2 represents a mixture of genotypes with the predominant genotype in the reaction product suspected to be the W1 genotype on the basis of RFLP comparison. RFLP patterns for the five positive reaction mixtures of repetitive PCR for sample MST-7 (lanes 6 to 10) indicate that all of the reaction products are composed of C. andersoni. DNA sequence analysis verified two sequences (differing by a single T addition; see Fig. 5) of C. andersoni in this water sample. RFLP results for the sample from MST-11 indicate two genotypes in this sample; lanes 11 and 12 show the single C. andersoni species, while lane 15 was a variant of the W12 genotype (determined by DNA sequence analysis). Lane 13 shows a mixture of these two genotypes.
All PCR products from the repetitive nested PCRs (73 reaction products in total from the 33 positive water samples) were digested with restriction enzymes SspI, VspI, and DdeI in an attempt to differentiate Cryptosporidium species within the water samples. Examples of the restriction enzyme digests carried out with repetitive reaction products from three water samples are shown in Fig. 2. SspI and VspI digestions from DNA amplified from all repetitive reaction products for MST-7 (lanes 6 through 10) resulted in the same banding pattern with all three enzymes, indicating that one genotype was predominant in the sample. Restriction enzyme digests from DNA amplified from five oocysts in a water sample from MST-15 suggested that at least three distinct Cryptosporidium species and genotypes were present in the water sample (Fig. 2). Lanes 1 and 4 represented a single genotype, while faint bands of an underlying genotype were mixed with the predominant banding pattern in lane 2. Similarly, restriction enzyme patterns for a water sample containing eight oocysts collected from MST-11 also indicated that more than one Cryptosporidium species or genotype was present in the sample (Fig. 2). Amplified DNAs from the reaction products shown in lanes 11, 12, and 15 could be further analyzed by DNA sequence analysis to identify and verify the two species and genotypes of Cryptosporidium observed by RFLP. For all of the reaction products positive by PCR, only 5 of the 73 reaction products showed mixed genotypes by RFLP while the other 68 reaction products were verified as containing single genotypes by sequence analysis. Repetitive nested PCR resolved different species and genotypes into individual reaction product tubes for 9 of the 33 water samples that tested positive by PCR, allowing confirmation of the species or genotype by DNA sequence analysis. There were only three water samples where a mixed genotype could not be resolved to allow species or genotype verification by sequence analysis. These results demonstrate the utility of repetitive nested PCR-RFLP to resolve mixed genotypes from water samples.
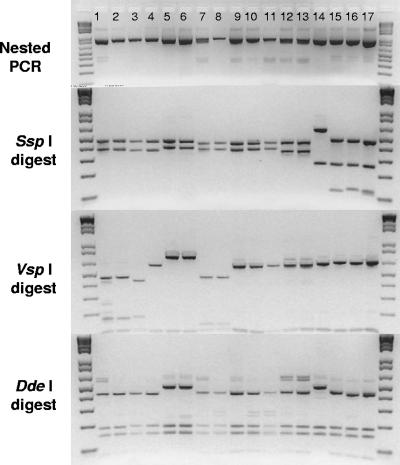
Initial observations of RFLP patterns obtained from positive repetitive nested PCRs from all water samples indicated that at least eight different species or genotypes of Cryptosporidium were found in the study region. A number of restriction enzyme digests were repeated, and bands were separated on a single agarose gel, revealing that as many as 11 unique RFLP patterns could be resolved (Fig. 3). Subtle differences in RFLP patterns were not obvious unless they occurred in adjacent lanes of the same gel (Fig. 3). By comparing our data to published patterns, the occurrence of at least three species was predicted on the basis of RFLP analysis, i.e., C. andersoni, C. baileyi, and C. parvum.
FIG. 3.
Differentiation of Cryptosporidium species and genotypes by RFLP analysis. Nested PCR products are displayed in the top panel. Secondary PCR products were digested with restriction enzymes SspI, VspI, and DdeI, and the results of the digestions are shown (bottom three panels). Lanes 1 and 2 represent two isolates with 100% identity match to the previously described cervine genotype (accession no. AY262328) and the W4 genotype (accession no. AY737592). Lanes 3 and 4 represent two genetically different isolates which were not an exact match to sequences in GenBank but clustered as variants of Cryptosporidium muskrat genotype I (see Fig. 4, isolates 186 and 190). Lanes 5 and 6 represent the two sequences of C. andersoni. The sequence from lane 8 was a 100% identity match to W12 (accession no. AY007254), while lane 7 showed variation with respect to the sequence under accession no. AY007254 but clustered with W12 by phylogenetic analysis (see Fig. 4 and 5, isolate 168). Lanes 9 (isolate 148) and 10 (isolate 147) display the RFLP profiles of two sequences with no GenBank match. Phylogenetic analysis placed these and two GenBank sequences into a cluster with the Cryptosporidium fox genotype (accession no. AY120907) (Fig. 4). The sample displayed in lane 11 was a 100% identity match to the sequence with accession no. AY737567 (muskrat genotype II), while the samples in lanes 12 and 13 matched the sequence with accession no. AY545548 (muskrat genotype II) and lane 14 matched C. baileyi (accession no. AF093495). The DNA sequence from the sample in lane 15 was a 100% identity match to the W1 genotype (accession no. AF262330) and displayed minor RFLP variation with respect to that in lane 16, which was a 100% identity match to the C. parvum KSU-1 isolate (accession no. AF308600). Lane 17 is the laboratory C. parvum control.
Phylogenetic characterization of Cryptosporidium DNA sequences.
DNA sequence analysis was carried out with the 68 nested PCR products which displayed single banding patterns by RFLP. Several RFLP patterns that appeared similar were subsequently shown to have some sequence variation. Fourteen different DNA sequences were isolated from water samples collected in the study area. These results demonstrate that RFLP could not reveal the complete spectrum of species and genotypes present in a water sample.
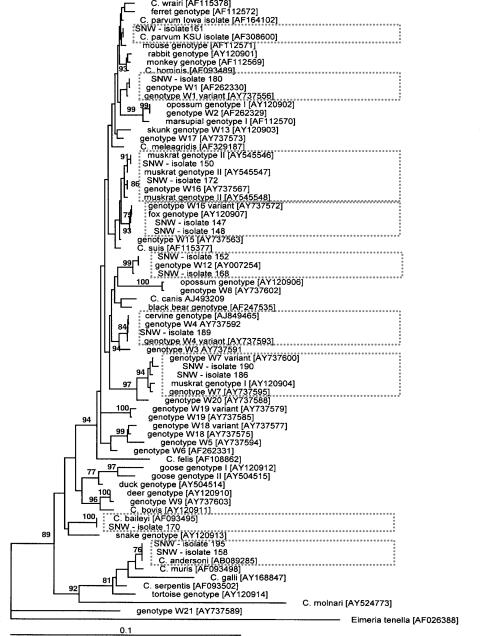
Phylogenetic analysis and DNA sequence alignments were used to identify the Cryptosporidium species and genotypes isolated from the study area. The 14 sequences were aligned with 59 Cryptosporidium sequences from GenBank, and a phylogenetic tree was constructed (Fig. 4). Three sequences had 100% identity matches to recognized Cryptosporidium species, i.e., isolate 195 (C. andersoni, accession no. AB089285), isolate 161 (C. parvum KSU isolate, accession no. AF308600), and isolate 170 (C. baileyi, accession no. AF093495). Two sequences were identical to Cryptosporidium genotypes isolated from mammalian hosts, isolate 150 (muskrat genotype II, accession no. AY545548) and isolate 189 (W4 genotype, accession no. AY737592), which matches the sequence with accession no. AF262328 previously described as a cervine genotype (37, 54). Three sequences were 100% identical to previously described environmental isolates, isolate 180 (genotype W1, AF262330), isolate 152 (genotype W12, accession no. AY007254), and isolate 172 (genotype W16, accession no. AY737567), which was previously shown to be a variation of muskrat genotype II (15).
FIG. 4.
Phylogenetic relationships among Cryptosporidium isolates from the South Nation watershed and known sequences in GenBank. Distances were calculated with the Kimura two-parameter model, and the phylogenetic tree was inferred by neighbor-joining analysis. The outgroup (E. tenella) was used to root the tree. Numbers on the branches are percent bootstrap values (>75%) obtained by using 1,000 resamples. GenBank accession numbers are in brackets, and boxes with dotted borders indicate the branches where isolates from the South Nation watershed clustered.
The remaining six unique DNA sequences observed in the area displayed some sequence variation with respect to GenBank sequences. On the basis of phylogenetic analysis (Fig. 4), isolate 158 clustered with C. andersoni while three other sequences clustered with previously described genotypes (15); isolate 168 clustered with the W12 genotype, while isolates 186 and 190 clustered with muskrat genotype I. Two sequences, those of isolates 147 and 148, formed a cluster with a genotype isolated from a fox (accession no. AY120907). The amounts of sequence variation observed among the five variant sequences in relation to their nearest neighbors are shown in Fig. 5. These variations include insertions, deletions, and substitutions, all located in the highly polymorphic region of the 18S rRNA gene.
FIG. 5.
Sequence variation for isolates from the South Nation watershed that do not have 100% identity matches to GenBank sequences. Dots indicate nucleotides identical to the first sequence. Dashes were inserted to accommodate insertions or deletions in the aligned sequences. Nucleotide positions are in relation to the specified GenBank sequence.
Distribution of Cryptosporidium species and genotypes in the South Nation watershed and correlations with land use information.
C. andersoni was the species most frequently identified at the sample sites; it was detected in 50% of the water samples positive by PCR (data not shown) and was present at 11 of the 14 sampling sites (Table 4). The second most commonly identified Cryptosporidium species and genotype was the W12 genotype, with a frequency of 21%, and was present at 7 of the 14 sites during the study period. Cryptosporidium muskrat genotype II was also present at a frequency of 21% but present at five sites within the study area. The cervine genotype of Cryptosporidium was detected in two water samples and distributed to two locations (MST-3 and MST-9), and the muskrat I genotype was detected in two water samples collected in different weeks from the same site (MST-7). C. parvum, C. baileyi, the fox genotype, and the W1 genotype occurred in a single water sample each. The largest number of Cryptosporidium genotypes isolated from any one site during the sampling period was four, recovered from MST-9, suggesting at least four host sources of fecal contamination contributed to the number of Cryptosporidium parasites observed at this site. It is not surprising that the distribution of Cryptosporidium species and genotypes was also affected temporally, with different species and genotypes being detected at different times at the same site during the relatively short study period. This is demonstrated by the differences in the frequencies of occurrence of the various species and genotypes at each site (Table 4). The significant precipitation event associated with the week 8 sampling also correlated with the highest incidence of detection of C. andersoni at all MST sites. C. andersoni was detected at 8 of the 14 sites in this sampling period (data not shown). Together, the data reinforce the ideas that multiple host source impacts occur within the watershed and that the patterns of contamination are dynamic and variable in both space and time.
The observation that C. andersoni predominates in water samples collected throughout the watershed (Table 4) is supported by land use information that highlights the intense cattle agricultural activity in the study region (Tables 2 and 3). All of the sites located on the South Nation River (MST-2, MST-1, MST-11, MST-8, and MST-7) had C. andersoni (presumably from cattle) detected at some point during the study. Cattle having direct access to river banks and tributary waters could be a potential C. andersoni source for these sites, as supported by land use observations (Table 3). Sites along the Little Castor River (MST-5, MST-13, and MST-12) had a reduced occurrence of C. andersoni (Table 4), which potentially correlates with lower overall cattle numbers in the catchment area and fewer opportunities for direct cattle access to the river (Table 3). The Castor River (MST-4) had no occurrences of C. andersoni, which can be supported by the observation of steep river banks (Table 1), which limit the cattle's access to streams (Table 3), and a more urban context relative to the other sites (Tables 1 and 2).
Large land bases devoted to agriculture but which also have a significant proportion of natural lands (forest, shrubs, and wetlands) are known to support a diverse range of wildlife species. Deer were commonly found in these areas, reinforcing the finding that oocysts of the Cryptosporidium cervine genotype were detected in water samples collected at sites MST-3 and MST-9 (Table 4). The detection of Cryptosporidium muskrat genotype II at five sites within the watershed (MST-4, MST-5, MST-6, MST-13, and MST-15) implies that muskrats or possibly voles (55) could be contributors of Cryptosporidium contamination of the watershed at specific sites (Table 4). This is supported by the facts that muskrats are indigenous to the region and that they were observed within the study area. That C. baileyi was observed on one occasion in the watershed suggests that avian fecal sources such as poultry (Table 3) or migratory birds (often observed within the watershed) may also impact the South Nation watershed.
The rural drainage ditches studied, MST-3 and MST-15, are excavated ditches designed to receive artificial subsurface drainage from nearby agricultural lands (Table 3). The profiling of Cryptosporidium parasites demonstrated that a number of different animal fecal sources impacted these sites (Table 4). In contrast, at sites MST-1 and MST-2 (Fig. 1), which represent the accumulation of impacts for the entire watershed, only 3 of the 14 Cryptosporidium species and genotypes detected throughout the watershed were observed at these downstream sites (Table 4). These results suggest that other factors may affect the occurrence of Cryptosporidium within the watershed.
DISCUSSION
This study examined the prevalence and distribution of Cryptosporidium species and genotypes within the South Nation watershed, with the intent of identifying host sources of Cryptosporidium that contribute to contamination events and the risks they pose to human health. Although oocysts were widely detected, with 77% of all samples showing some Cryptosporidium contamination, molecular forensic profiling demonstrated that anthroponotic or zoonotic species were rare. C. hominis was not detected in any of the waters from the study area, while C. parvum and the cervine genotype, both known to have zoonotic potential (26), were detected at low frequencies.
The majority of the cryptosporidia found in the South Nation watershed appear to be derived from mature cattle, a conclusion based on the high frequency of molecular typing of C. andersoni in the watershed and substantiated by land use observations related to widespread cattle (dairy and beef herds) agricultural activity. Contamination of the watershed presumably occurred either as a result of direct fecal inputs or through manure application with subsequent transportation of fecal contamination to waterways via tile drainage and overland flow. C. parvum, C. bovis, C. andersoni, and the deer-like genotype have been found to infect cattle (5, 7, 17, 18, 29, 39), yet the data show widespread occurrence of C. andersoni with a limited occurrence of C. parvum and no observed occurrence of the other two. This may be explained by the sequential infection of cattle with various species and genotypes as they increase in age. C. parvum is predominant in calves less than 3 weeks of age, with rates of infection declining as the calves age and until weaning, when calves appear to resolve infections (39). Infections in older calves and juvenile cattle (3 to 24 months old) tend to be the result of C. bovis, the deer-like genotype, or C. andersoni in various proportions. C. andersoni first infects calves after 60 days (18, 39) and becomes the predominant species after 12 months (7). On the basis of the age-related distribution of Cryptosporidium in cattle, it was concluded that mature cattle were the predominant host source contributing to parasite occurrence in the South Nation watershed.
C. parvum is not specific to calves but is generally considered a parasite of ruminants and humans (52), implying that the occurrence of the species in the study area is a result of agriculture, wildlife, or human sewage. The observation of C. parvum at MST-1, in combination with the upstream land uses related to cattle, deer habitat, and urban impacts (i.e., towns and sewage lagoons), indicates that either source is a possibility, yet the limited occurrence suggests that C. parvum is not a ubiquitous species in the watershed at this time of year.
Other host sources such as birds, deer, muskrats, voles, and other wildlife species were also important parasite contributors to contamination of the watershed (i.e., the Cryptosporidium cervine genotype, the Cryptosporidium muskrat II genotype, the Cryptosporidium muskrat I genotype, C. baileyi, the Cryptosporidium fox genotype, the W12 genotype, and the W1 genotype). These animals were frequently observed in the study area residing in the natural-vegetation buffer zones or intermeshed directly within agricultural and urban settings. The second most commonly detected Cryptosporidium species and genotype within the South Nation watershed was the genotype designated W12, which is suspected to be of wildlife origin (15). Spatial and temporal variations in this genotype were observed within the watershed. Phylogenetic analysis of the W12 sequence variants observed in this study demonstrated that they clustered with the mammalian branch of Cryptosporidium species and genotypes. However, these isolates do not cluster with any known domestic or livestock animal sources (Fig. 4), consistent with a putative wildlife host.
C. baileyi, a parasite of birds, was also detected in the watershed. Host sources of C. baileyi include chickens, turkeys, ducks, cockatiels, brown quail, ostriches, and geese (20, 23, 52). The wide diversity of bird species known to harbor C. baileyi infections precludes our ability to extrapolate host sources of these parasites within the watershed. However, both poultry operations and migratory waterfowl populations were observed within the South Nation watershed and therefore may represent potential host sources of C. baileyi.
Molecular profiling of Cryptosporidium parasites suggests diversity in the parasite host sources that impact a watershed. The diversity of Cryptosporidium in the watershed could not be accounted for in that the full repertoire of species and genotypes was not detected from the cumulative impact site (MST-1). A number of factors may impact the findings at the downstream site. Weekly sampling and the limited amount of water collected and processed may have been insufficient to capture all of the species and genotype diversity within the watershed. Alternatively, parasites from upstream sites may have been progressively diluted as they traveled downstream, resulting in lower concentrations of parasites and/or few positive samples (Table 4). Another possible explanation could be the settling of Cryptosporidium oocysts, an effect previously shown to reduce rates of occurrence (2). The data reinforce the complexity associated with contamination events within a watershed and the challenges associated with capturing and accounting for these dynamic spatial and temporal variations by grab sampling methods. Sampling strategies for surface waters and potential fecal sources within a watershed are a key determining factor in the efficacy of MST studies (21).
The occurrence of Cryptosporidium oocysts in surface waters has been reported at 11 to 97%, with oocyst concentrations ranging from 0.002 to 484/liter (1, 16, 19, 28, 31-33, 42). The maximum oocyst concentration detected over the course of this study was 1.50/liter, indicating that the watershed is only moderately contaminated. Averages over the sampling period for the sites along the South Nation River (MST-2, MST-1, MST-11, MST-8, and MST-7) range from 0.09 to 0.26 oocyst per liter, placing this source water in the Bin 2 category according to the Long Term 2 Enhanced Surface Water Treatment Rule (LT2ESWTR), which requires a minimal level of treatment for Cryptosporidium removal prior to human consumption (47). The absence of C. hominis, along with the infrequent and sporadic occurrence of C. parvum in the South Nation watershed, brings into question the human health risk associated with this level of oocyst contamination and further questions the validity of the risk assessment framework of the LT2ESWTR. Thus, models of risk assessment based solely on quantitative data on Cryptosporidium parasite occurrence in raw water (i.e., method 1623 for compliance under the proposed LT2ESWTR) may do little to protect public health or prioritize the financial needs associated with enhancing water treatment at drinking water facilities known to be at risk of contamination with zoonotic or anthroponotic forms of Cryptosporidium (regardless of the concentration of the parasite observed in the raw water).
Various MST tools have been developed in an attempt to identify sources of fecal pollution in the environment, but to date no single method has emerged as the perfect tool (22, 24, 40, 41, 44). The ideal tool would be a highly polymorphic gene locus that displays absolute restriction and correlation with host specificity at the species level. As no such tool is available, a variety of different MST tools have been used. Bacterium-based MST methods have been shown to resolve fecal contamination impacts at various levels of host resolution (3, 6, 10, 12, 24, 44, 49, 50). In a blind survey done by Stoeckel et al. (44), only pulsed-field gel electrophoresis was shown to resolve host specificity to the species level. The concept of host specificity within the genus Cryptosporidium, along with the 18S rRNA gene sequence data for more than 30 animal hosts, predisposes this organism to become a valuable, library-independent MST tool.
Caution needs to be used when extrapolating molecular data from water samples to concretely determine host sources of contamination. In studies that examine the host specificity of Cryptosporidium parasites, there is a direct association between the host being studied and the fecal material used for typing parasites (30, 37, 55). This direct association does not exist for isolates from water samples. Therefore, the host source can only be inferred from available host-parasite information and molecular phylogeny, which can then be further supported by land use information. For example, the host source of C. parvum cannot be inferred from the single occurrence detected; however, when the known host sources of C. parvum (36, 52) are combined with land use analysis, the data suggest that cattle, deer, or humans are the most probable source within the watershed.
Previous studies have tried to link species and genotypes of Cryptosporidium to human, livestock, and wildlife contamination by directly characterizing potential sources of contamination (i.e., sampling animals or sewage [13, 36]) rather than characterizing parasites present in natural watercourses in a watershed context. Some studies have focused on identifying species and genotypes of Cryptosporidium in the watersheds (11, 14) but failed to evaluate the spatial and temporal patterns of contamination. Another study noted differences based on ecological settings (15) with a limited number of sampling locations (one or two) in each watershed, and all sampling was carried out as a result of storm events. For future studies to provide a more complete source water protection evaluation, it would be necessary to routinely monitor sites over a longer period of time in order to obtain a composite picture of the various host sources that impact a watershed throughout the year and the risks they pose to human health. Coupled with land use analysis, sophisticated source water protection plans could be developed and implemented to protect drinking water sources within an entire watershed. For instance, diffuse and point source contamination of C. andersoni from agricultural animals could be reduced by incorporating best management practices such as (i) eliminating direct access of cattle to waterways, (ii) increasing manure or biosolid application setback distances, (iii) increasing the width of vegetation buffer strips, and (iv) controlling tile drainage to stream systems (especially for fields where manures are spread).
Linking the molecular epidemiological host data with land use information, animal density surveys, and environmental factors (i.e., topography, precipitation, etc.) within a watershed may provide a greater degree of resolution to track host source impacts. The present study shows the value of extending the capability of samples collected and processed according to method 1623 to include the molecular forensic profiling of Cryptosporidium oocysts and land use analysis to track host sources of fecal contamination in a watershed and determine the human health risks posed by these parasites when they are detected.
Acknowledgments
Special thanks to J. Matsune, B. Villeneuve, M. Vejdani, M. Andersen (Alberta Provincial Laboratory for Public Health), and M. Edwards (Eastern Cereal and Oilseed Research Centre, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada) for technical support.
This research was funded by the Agriculture Policy Framework's National Water Quality Surveillance Research Initiative, the National Agri-Environmental Standards Initiative (D. Lapen, T. Edge, N. Neumann, W. Robertson, and E. Topp), a strategic grant through the National Science and Engineering Research Council of Canada, and a Canadian Institutes of Health research grant (N. Neumann). C. Sensen is supported by Genome Canada through Genome Alberta's Integrated and Distributed Bioinformatics Platform Project and by the iCORE/Sun Microsystems Industrial Research Chair program. Support for N. Ruecker was provided in the form of a research affiliate work term sponsored by Agriculture and Agri-Food Canada through the Federal Student Work Experience Program.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Boutros, S. N. 1989. Sampling and analysis for Cryptosporidium in PA public surface water supply sources. Pennsylvania Division of Water Supply, Harrisburg, PA.
- 2.Brookes, J. D., M. R. Hipsey, M. D. Burch, R. H. Regel, L. G. Linden, C. M. Ferguson, and J. P. Antenucci. 2005. Relative value of surrogate indicators for detecting pathogens in lakes and reservoirs. Environ. Sci. Technol. 39:8614-8621. [DOI] [PubMed] [Google Scholar]
- 3.Carson, C. A., B. L. Shear, M. R. Ellersieck, and A. Asfaw. 2001. Identification of fecal Escherichia coli from humans and animals by ribotyping. Appl. Environ. Microbiol. 67:1503-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drinking Water Inspectorate. 2000. The water supply (water quality) regulations 2000. http://www.dwi.gov.uk/regs/si3184/3184.htm#273184. [Online.]
- 5.Enemark, H. L., P. Ahrens, C. J. Lowery, S. M. Thamsborg, J. M. Enemark, V. Bille-Hansen, and P. Lind. 2002. Cryptosporidium andersoni from a Danish cattle herd: identification and preliminary characterisation. Vet. Parasitol. 107:37-49. [DOI] [PubMed] [Google Scholar]
- 6.Evenson, C. J., and K. A. Strevett. 2006. Discriminant analysis of fecal bacterial species composition for use as a phenotypic microbial source tracking method. Res. Microbiol. 157:437-444. [DOI] [PubMed] [Google Scholar]
- 7.Fayer, R., M. Santin, J. M. Trout, and E. Greiner. 2006. Prevalence of species and genotypes of Cryptosporidium found in 1-2-year-old dairy cattle in the eastern United States. Vet. Parasitol. 135:105-112. [DOI] [PubMed] [Google Scholar]
- 8.Fayer, R., M. Santin, and L. Xiao. 2005. Cryptosporidium bovis n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus). J. Parasitol. 91:624-629. [DOI] [PubMed] [Google Scholar]
- 9.Felsenstein, J. 1989. PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164-166. [Google Scholar]
- 10.Hagedorn, C., S. L. Robinson, J. R. Filtz, S. M. Grubbs, T. A. Angier, and R. B. Reneau, Jr. 1999. Determining sources of fecal pollution in a rural Virginia watershed with antibiotic resistance patterns in fecal streptococci. Appl. Environ. Microbiol. 65:5522-5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hänninen, M. L., A. Horman, R. Rimhanen-Finne, H. Vahtera, S. Malmberg, S. Herve, and K. Lahti. 2005. Monitoring of Cryptosporidium and Giardia in the Vantaa river basin, southern Finland. Int. J. Hyg. Environ. Health 208:163-171. [DOI] [PubMed] [Google Scholar]
- 12.Harwood, V. J., B. Wiggins, C. Hagedorn, R. D. Ellender, J. Gooch, J. Kern, M. Samadpour, A. C. Chapman, B. J. Robinson, and B. C. Thompson. 2003. Phenotypic library-based microbial source tracking methods: efficacy in the California collaborative study. J. Water Health 1:153-166. [PubMed] [Google Scholar]
- 13.Heitman, T. L., L. M. Frederick, J. R. Viste, N. J. Guselle, U. M. Morgan, R. C. Thompson, and M. E. Olson. 2002. Prevalence of Giardia and Cryptosporidium and characterization of Cryptosporidium spp. isolated from wildlife, human, and agricultural sources in the North Saskatchewan River Basin in Alberta, Canada. Can. J. Microbiol. 48:530-541. [DOI] [PubMed] [Google Scholar]
- 14.Jellison, K. L., H. F. Hemond, and D. B. Schauer. 2002. Sources and species of Cryptosporidium oocysts in the Wachusett Reservoir watershed. Appl. Environ. Microbiol. 68:569-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang, J., K. A. Alderisio, and L. Xiao. 2005. Distribution of Cryptosporidium genotypes in storm event water samples from three watersheds in New York. Appl. Environ. Microbiol. 71:4446-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karanis, P., C. Papadopoulou, A. Kimura, E. Economou, C. Kourenti, and H. Sakkas. 2002. Cryptosporidium and Giardia in natural, drinking and recreational water of northwestern Greece. Acta Hydrochim. Hydrobiol. 30:49-58. [Google Scholar]
- 17.Kvác, M., M. Kouba, and J. Vitovec. 2006. Age-related and housing-dependence of Cryptosporidium infection of calves from dairy and beef herds in South Bohemia, Czech Republic. Vet. Parasitol. 137:202-209. [DOI] [PubMed] [Google Scholar]
- 18.Kvác, M., and J. Vitovec. 2003. Prevalence and pathogenicity of Cryptosporidium andersoni in one herd of beef cattle. J. Vet. Med. B Infect. Dis. Vet. Public Health 50:451-457. [DOI] [PubMed] [Google Scholar]
- 19.LeChevallier, M. W., W. D. Norton, and R. G. Lee. 1991. Giardia and Cryptosporidium spp. in filtered drinking water supplies. Appl. Environ. Microbiol. 57:2617-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay, D. S., B. L. Blagburn, and F. J. Hoerr. 1990. Small intestinal cryptosporidiosis in cockatiels associated with Cryptosporidium baileyi-like oocysts. Avian Dis. 34:791-793. [PubMed] [Google Scholar]
- 21.Lu, Z., D. Lapen, A. Scott, A. Dang, and E. Topp. 2005. Identifying host sources of fecal pollution: diversity of Escherichia coli in confined dairy and swine production systems. Appl. Environ. Microbiol. 71:5992-5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meays, C. L., K. Broersma, R. Nordin, and A. Mazumder. 2004. Source tracking fecal bacteria in water: a critical review of current methods. J. Environ. Manag. 73:71-79. [DOI] [PubMed] [Google Scholar]
- 23.Morgan, U. M., P. T. Monis, L. Xiao, J. Limor, I. Sulaiman, S. Raidal, P. O'Donoghue, R. Gasser, A. Murray, R. Fayer, B. L. Blagburn, A. A. Lal, and R. C. Thompson. 2001. Molecular and phylogenetic characterisation of Cryptosporidium from birds. Int. J. Parasitol. 31:289-296. [DOI] [PubMed] [Google Scholar]
- 24.Myoda, S. P., C. A. Carson, J. J. Fuhrmann, B. K. Hahm, P. G. Hartel, H. Yampara-Lquise, L. Johnson, R. L. Kuntz, C. H. Nakatsu, M. J. Sadowsky, and M. Samadpour. 2003. Comparison of genotypic-based microbial source tracking methods requiring a host origin database. J. Water Health 1:167-180. [PubMed] [Google Scholar]
- 25.Notredame, C., D. G. Higgins, and J. Heringa. 2000. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302:205-217. [DOI] [PubMed] [Google Scholar]
- 26.Ong, C. S., D. L. Eisler, A. Alikhani, V. W. Fung, J. Tomblin, W. R. Bowie, and J. L. Isaac-Renton. 2002. Novel Cryptosporidium genotypes in sporadic cryptosporidiosis cases: first report of human infections with a cervine genotype. Emerg. Infect. Dis. 8:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 28.Payment, P., A. Berte, M. Prevost, B. Menard, and B. Barbeau. 2000. Occurrence of pathogenic microorganisms in the Saint Lawrence River (Canada) and comparison of health risks for populations using it as their source of drinking water. Can. J. Microbiol. 46:565-576. [PubMed] [Google Scholar]
- 29.Peng, M. M., M. L. Wilson, R. E. Holland, S. R. Meshnick, A. A. Lal, and L. Xiao. 2003. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: implications for understanding the transmission dynamics. Parasitol. Res. 90:175-180. [DOI] [PubMed] [Google Scholar]
- 30.Perz, J. F., and S. M. Le Blancq. 2001. Cryptosporidium parvum infection involving novel genotypes in wildlife from lower New York State. Appl. Environ. Microbiol. 67:1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson, L. J., and B. Gjerde. 2001. Occurrence of Cryptosporidium oocysts and Giardia cysts in raw waters in Norway. Scand. J. Public Health 29:200-207. [PubMed] [Google Scholar]
- 32.Rose, J. B. 1988. Occurrence and significance of Cryptosporidium in water. J. Am. Water Works Assoc. 80:53-58. [Google Scholar]
- 33.Rose, J. B., D. Kayed, M. S. Madore, C. P. Gerba, M. J. Arrowood, C. R. Sterling, and J. L. Rigg. 1988. Methods for the recovery of Giardia and Cryptosporidium from environmental waters and the comparative occurrence. U.S. Environmental Protection Agency, Cincinnati, OH.
- 34.Ruecker, N. J., N. Bounsombath, P. Wallis, C. S. Ong, J. L. Isaac-Renton, and N. F. Neumann. 2005. Molecular forensic profiling of Cryptosporidium species and genotypes in raw water. Appl. Environ. Microbiol. 71:8991-8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruecker, N. J., and N. F. Neumann. 2005. Identifying host adapted Cryptosporidium species and genotypes in water, abstr. Mon 18_1. In Proceedings of the American Water Works Association Water Quality Technology Conference, Quebec City, Quebec, Canada, 6 to 10 November 2005. American Water Works Association, Denver, CO.
- 36.Ryan, U., C. Read, P. Hawkins, M. Warnecke, P. Swanson, M. Griffith, D. Deere, M. Cunningham, and P. Cox. 2005. Genotypes of Cryptosporidium from Sydney water catchment areas. J. Appl. Microbiol. 98:1221-1229. [DOI] [PubMed] [Google Scholar]
- 37.Ryan, U., L. Xiao, C. Read, L. Zhou, A. A. Lal, and I. Pavlasek. 2003. Identification of novel Cryptosporidium genotypes from the Czech Republic. Appl. Environ. Microbiol. 69:4302-4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryan, U. M., P. Monis, H. L. Enemark, I. Sulaiman, B. Samarasinghe, C. Read, R. Buddle, I. Robertson, L. Zhou, R. C. Thompson, and L. Xiao. 2004. Cryptosporidium suis n. sp. (Apicomplexa: Cryptosporidiidae) in pigs (Sus scrofa). J. Parasitol. 90:769-773. [DOI] [PubMed] [Google Scholar]
- 39.Santín, M., J. M. Trout, L. Xiao, L. Zhou, E. Greiner, and R. Fayer. 2004. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet. Parasitol. 122:103-117. [DOI] [PubMed] [Google Scholar]
- 40.Scott, T. M., J. B. Rose, T. M. Jenkins, S. R. Farrah, and J. Lukasik. 2002. Microbial source tracking: current methodology and future directions. Appl. Environ. Microbiol. 68:5796-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson, J. M., J. W. Santo Domingo, and D. J. Reasoner. 2002. Microbial source tracking: state of the science. Environ. Sci. Technol. 36:5279-5288. [DOI] [PubMed] [Google Scholar]
- 42.Smith, H. V., B. M. Campbell, C. A. Paton, and R. A. Nichols. 2002. Significance of enhanced morphological detection of Cryptosporidium sp. oocysts in water concentrates determined by using 4′,6′-diamidino-2-phenylindole and immunofluorescence microscopy. Appl. Environ. Microbiol. 68:5198-5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Staden, R. 1996. The Staden sequence analysis package. Mol. Biotechnol. 5:233-241. [DOI] [PubMed] [Google Scholar]
- 44.Stoeckel, D. M., M. V. Mathes, K. E. Hyer, C. Hagedorn, H. Kator, J. Lukasik, T. L. O'Brien, T. W. Fenger, M. Samadpour, K. M. Strickler, and B. A. Wiggins. 2004. Comparison of seven protocols to identify fecal contamination sources using Escherichia coli. Environ. Sci. Technol. 38:6109-6117. [DOI] [PubMed] [Google Scholar]
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.U.S. Environmental Protection Agency. 2003. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA 815-R-03-XXX. Office of Ground Water and Drinking Water Technical Support Center, U.S. Environmental Protection Agency, Washington, DC.
- 47.U.S. Environmental Protection Agency. 2006. National primary drinking water regulations: Long term 2 enhanced surface water treatment rule; final rule. Fed. Register 71:654-786. [PubMed] [Google Scholar]
- 48.Ward, P. I., P. Deplazes, W. Regli, H. Rinder, and A. Mathis. 2002. Detection of eight Cryptosporidium genotypes in surface and waste waters in Europe. Parasitology 124:359-368. [DOI] [PubMed] [Google Scholar]
- 49.Wheeler, A. L., P. G. Hartel, D. G. Godfrey, J. L. Hill, and W. I. Segars. 2002. Potential of Enterococcus faecalis as a human fecal indicator for microbial source tracking. J. Environ. Qual. 31:1286-1293. [DOI] [PubMed] [Google Scholar]
- 50.Wiggins, B. A., P. W. Cash, W. S. Creamer, S. E. Dart, P. P. Garcia, T. M. Gerecke, J. Han, B. L. Henry, K. B. Hoover, E. L. Johnson, K. C. Jones, J. G. McCarthy, J. A. McDonough, S. A. Mercer, M. J. Noto, H. Park, M. S. Phillips, S. M. Purner, B. M. Smith, E. N. Stevens, and A. K. Varner. 2003. Use of antibiotic resistance analysis for representativeness testing of multiwatershed libraries. Appl. Environ. Microbiol. 69:3399-3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao, L., K. Alderisio, J. Limor, M. Royer, and A. A. Lal. 2000. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl. Environ. Microbiol. 66:5492-5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao, L., R. Fayer, U. Ryan, and S. J. Upton. 2004. Cryptosporidium taxonomy: recent advances and implications for public health. Clin. Microbiol. Rev. 17:72-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiao, L., U. M. Morgan, J. Limor, A. Escalante, M. Arrowood, W. Shulaw, R. C. Thompson, R. Fayer, and A. A. Lal. 1999. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 65:3386-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao, L., and U. M. Ryan. 2004. Cryptosporidiosis: an update in molecular epidemiology. Curr. Opin. Infect. Dis. 17:483-490. [DOI] [PubMed] [Google Scholar]
- 55.Zhou, L., R. Fayer, J. M. Trout, U. M. Ryan, F. W. Schaefer III, and L. Xiao. 2004. Genotypes of Cryptosporidium species infecting fur-bearing mammals differ from those of species infecting humans. Appl. Environ. Microbiol. 70:7574-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]