Abstract
Burkholderia glumae strain PG1 produces a lipase of biotechnological relevance. Lipase production by this strain and its derivative LU8093, which was obtained through classical strain improvement, was investigated under different conditions. When 10% hexadecane was included in the growth medium, lipolytic activity in both strains could be increased ∼7-fold after 24 h of growth. Hexadecane also stimulated lipase production in a strain containing the lipase gene fused to the tac promoter, indicating that hexadecane did not affect lipase gene expression at the transcriptional level, which was confirmed using lipA-gfp reporter constructs. Instead, hexadecane appeared to enhance lipase secretion, since the amounts of lipase in the culture supernatant increased in the presence of hexadecane, with a concomitant decrease in the cells, even when protein synthesis was inhibited with chloramphenicol. In the presence of olive oil as a carbon source, nonionic detergents, such as Tween 80, increased extracellular lipase activity twofold. Like hexadecane, Tween 80 appeared to stimulate lipase secretion, although in a more disruptive manner, since other, normally nonsecreted proteins were found in the culture supernatant. Additionally, like olive oil, Tween 80 was found to induce lipase gene expression in strain PG1 in medium containing sucrose as a carbon source but not in glucose-containing medium, suggesting that lipase gene expression is prone to catabolite repression. In contrast, lipase production in the lipase-overproducing strain LU8093 was independent of the presence of an inducer and was not inhibited by glucose. In conclusion, hexadecane and Tween 80 enhance lipase production in B. glumae, and they act via different mechanisms.
Many bacterial species produce lipases, which catalyze the hydrolysis and synthesis of esters of glycerol with, preferably, long-chain fatty acids (EC 3.1.1.3). Since lipases remain active in organic solvents, usually do not require a cofactor, and display exquisite chemo-, regio-, and enantioselectivity, they have become important biocatalysts in various industrial sectors, such as the agrochemical, pharmaceutical, detergent, and food industries (13). In particular, lipases of microbial origin find immense application, since they can catalyze a variety of hydrolytic or synthetic reactions (15, 34). Therefore, many bacterial lipases, particularly those from members of the genera Acinetobacter, Pseudomonas, and Burkholderia, have been cloned and characterized (16).
A drawback in the production of lipases for industrial application is the inability to produce them in an enzymatically active form in the heterologous host Escherichia coli. This inability relates to the fact that E. coli generally does not secrete proteins into the extracellular medium and that heterologous overexpression is often accompanied by misfolding and intracellular deposition of the protein in the form of insoluble inclusion bodies (2). In particular, the heterologous overexpression of lipases originating from the genera Pseudomonas and Burkholderia is problematic (31). In these species, regulation of lipase gene expression and folding and secretion of the enzyme are complex processes involving a variety of proteins (30). After transcription and translation of the lipase structural gene lipA, the lipase precursor is first transported across the inner membrane via the Sec machinery. After the proteolytic removal of the signal sequence, the lipase folds in the periplasm into an enzymatically active conformation. Folding of lipase is facilitated by the specific intermolecular chaperone Lif (Lipase-specific foldase) (31), which is encoded by the gene designated lipB in the case of Burkholderia glumae (8) and by general folding catalysts, such as DsbA and DsbC (39), which catalyze the formation and isomerization of disulfide bonds. The single disulfide bond in lipase and a bound Ca2+ ion are not necessary for activity but provide stability to the protein under harsh conditions (6, 23). Enzymatically active lipase is then secreted across the outer membrane into the extracellular medium via the type II secretion pathway (38).
The mechanism of lipase production has been studied most extensively with Pseudomonas aeruginosa (31). However, P. aeruginosa is less suitable for bulk enzyme production, since it is an opportunistic human pathogen and is able to secrete potent proteases and toxins. In addition, lipase produced by B. glumae, a rice pathogen, was found to be superior in performance for several biotechnological applications (D. Thom, T. Swarthoff, and T. Maat, 1986, European patent applications 0205208 and 0206390 [Unilever]). However, in B. glumae, several aspects of lipase production, such as the regulation of lipase gene expression, still remain to be elucidated. Classical mutagenesis has been performed with the B. glumae wild-type strain PG1 to increase the production of lipase, yielding strain LU8093 (1; R. Braatz, R. Kurth, E. Menkerl-Conen, H. Rettenmaier, T. Friedrich, and T. Subkowski, 1992, patent application WO 9300924 A1). In this study, we investigated whether we could improve lipase production even further by including various additives, such as hexadecane and detergents, in the growth medium. Previously, such compounds have been found to stimulate lipase production in other bacteria (12, 17, 18, 24-26, 35), but which specific step in lipase production they stimulate is not exactly known. We demonstrate that these additives affect lipase production in B. glumae at various stages.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli K-12 strain DH5α (14) was used for routine cloning. Wild-type B. glumae strain PG1 (10), its lipA::Tcr mutant derivative PG3 (10), and its lipase-overproducing derivative LU8093 (1; Braatz et al., 1992, patent application WO 9300924 A1) were routinely grown in LB at 30°C. For expression of the lipase gene, strains were grown in PG medium (10) containing 1% (vol/vol) olive oil as a carbon source. When appropriate, kanamycin (100 μg/ml) or tetracycline (25 μg/ml) was added to the growth medium for plasmid maintenance. Expression from plasmids was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside to the growth medium.
Recombinant DNA techniques.
All recombinant DNA techniques were performed as described previously (33). Plasmids pUR6502 and pUR6524 contain the lipA or lipB gene, respectively, from B. glumae under control of the tac promoter (8). For stable expression of lipase and Lif in B. glumae, pLE25 was constructed by insertion of an EcoRI/HindIII (blunted) fragment containing lipAB from pUR6525 (9) into EcoRI/SmaI-digested pBBR1MCS-2 (20). Similarly, an EcoRI/HindIII (blunted) fragment from pUR6524 was cloned into pBBR1MCS-2 to generate the lipB expression plasmid pLE24. In pBBR1MCS-2, the cloned genes are under control of the tac promoter.
For the construction of plasmids containing lipA::gfp fusions, a 421-bp DNA fragment upstream of the lipAB operon containing the lipA promoter was PCR amplified from B. glumae strains PG1 and LU8093 with primers lipABup (5′-CGATGAATTCACCTTGAACGCAGGCG-3′) and lipABTF (5′-CGTCATATGTTTATCTCCATCGTTAAAGC-3′) in the presence of 1% dimethyl sulfoxide. Underlined nucleotides indicate restriction enzyme sites. The PCR fragments were cloned in front of the gfpmut3 gene in pSWgfp (S. Wilhelm, Ruhr-University Bochum, unpublished) with EcoRI/SmaI for transcriptional fusion. For translational fusion, the PCR fragments were digested with EcoRI/NdeI and cloned in front of the gfpmut3 gene in EcoRI/SphI-digested pSWgfp. The lipA::gfp fusions were cloned in the broad-host-range vector pBBR1MCS-2 with EcoRI/HindIII. In the case of the transcriptional fusions (pGPG1-TC and pGLU-TC), the Shine-Dalgarno sequence of gfpmut3 was maintained, while in the case of the translational fusions (pGPG1-TL and pGLU-TL), the gfpmut3 start codon was replaced by the lipA start codon. Plasmids pGPG1-TC and pGPG1-TL contain the lipA promoter of B. glumae PG1, while plasmids pGLU-TC and pGLU-TL contain the lipA promoter of B. glumae LU8093.
Electroporation.
Overnight cultures of B. glumae grown at 37°C were diluted 100-fold in prewarmed LB and grown further for 3 h at 37°C. Cells were cooled on ice, washed three times with an ice-cold 300 mM sucrose solution, and finally resuspended in 300 mM sucrose, 15% (vol/vol) glycerol (ice cold). Fifty-microliter aliquots of the cell suspension and up to 10 μg of DNA were added to precooled electroporation cuvettes (2 mm). Immediately after the pulse (25 μF, 600 Ω, and 2.5 kV), 500 μl of LB was added and the cells were incubated for 2 h at 30°C with shaking. Cells were plated on LB with the appropriate antibiotic.
Lipase activity assay.
Lipase activity was measured in 1 ml of Tris buffer (20 mM Tris-HCl, pH 8.0) containing appropriate dilutions of culture supernatant with either para-nitrophenyl caprylate or para-nitrophenyl palmitate (Sigma) as the substrate (37). The reactions were followed spectrophotometrically at 410 nm. The lipase activity (U/ml) was correlated to the cell density at 580 nm (OD580). One unit is the amount of lipase that liberates 1 mmol of para-nitrophenol per min. Statistical analysis was done with Student's t test.
Fluorescence measurements.
Cells of B. glumae grown for 24 h in PG medium containing different supplements were washed and resuspended in phosphate-buffered saline buffer (150 mM NaCl, 20 mM NaH2PO4, pH 7.2) and adjusted to an OD580 of 0.4. Cells were lysed by incubation with lysozyme (0.5 mg/ml) for 30 min on ice, followed by sonication (two times, 10 s). After centrifugation, green fluorescence of the supernatant was measured using the LS 50 B fluorescence spectrometer (Perkin-Elmer). The excitation wavelength was set at 485 nm (excitation slit, 7.5 nm), and the emission was detected between 460 and 560 nm (emission slit, 10 nm). Documentation of the spectra was performed using Fluorescence Data Manager software (Perkin-Elmer). The measured values of relative fluorescence units were correlated to the OD580 values.
Western blot analysis.
Proteins were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (11% polyacrylamide) according to the method of Laemmli (21) with 0.2% SDS in the running gel and Western blotting. Blots were incubated with polyclonal antilipase or anti-Lif antisera and alkaline phosphatase-coupled goat anti-rabbit immunoglobulin antiserum (1:10,000; Biosource).
Lipase-Lif interaction.
The lipase-Lif interaction was studied in vitro with purified lipase and His-tagged Lif by affinity chromatography (7). His-tagged Lif from B. glumae (12 μg) was coupled to Cu2+-charged chelating Sepharose beads (Amersham Pharmacia Biotech) by incubation for 30 min at room temperature on a rotating wheel. After washing of the beads with Tris buffer containing 0 and 25 mM imidazole, native B. glumae lipase (12 μg) was added and incubation was continued for 30 min. The beads were washed twice, once with Tris buffer and once with Tris buffer containing 25 mM imidazole, and bound proteins were eluted with Tris buffer containing 10 mM EDTA. Proteins from all fractions were precipitated with 5% trichloroacetic acid and analyzed by SDS-polyacrylamide gel electrophoresis. To test the effect of hexadecane on the lipase-Lif interaction, wash buffers contained 10% (vol/vol) hexadecane and were emulsified by sonication for 30 s.
RESULTS
Hexadecane increases extracellular lipase production.
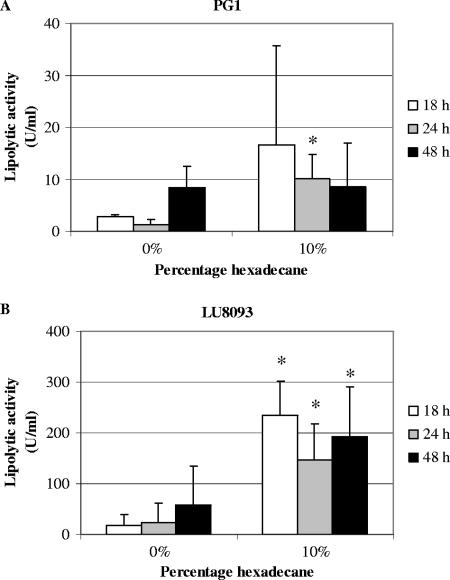
Previously it was shown that the production of lipase in Pseudomonas species strain G6 was optimal when hexadecane was used as a carbon source (17). However, hexadecane did not support the growth of B. glumae strains PG1 and LU8093 when used as a carbon source in PG medium, and it did not affect the growth of these strains on PG medium supplemented with other carbon sources, such as olive oil (data not shown). To determine whether the addition of hexadecane to the growth medium increases lipase production in B. glumae, strains PG1 and LU8093 were grown in PG medium containing 1% olive oil as the carbon source, supplemented or not with hexadecane. Extracellular lipase activity in the medium was determined after 18 h (when an OD580 of ∼3 was reached), 24 h, and 48 h (when the OD580 was further increased to ∼6) of growth. Indeed, the presence of 10% hexadecane increased lipase production in PG1 7.6-fold (Fig. 1A) and in LU8093 6.2-fold (Fig. 1B) after 24 h. Hexadecane did not increase the specific activity or stability of the extracellular lipase, since the lipolytic activity in cell culture supernatant of cells grown without hexadecane did not increase upon addition of hexadecane but rather was slowly reduced to 44% after 48 h at 30°C. This result is in contrast to those obtained for the lipase of Acinetobacter calcoaceticus, which was reported to be protected against proteolysis in the presence of hexadecane (18). In conclusion, it appears that the presence of hexadecane in the growth medium stimulates the production of extracellular lipase in B. glumae.
FIG. 1.
Lipolytic activity in culture supernatant of B. glumae PG1 (A) or LU8093 (B) in the presence of 0% or 10% of hexadecane at different time points. The averages from at least three experiments are shown, with error bars indicating the standard deviations. Asterisks indicate significant differences from sample without hexadecane (P < 0.05).
Expression of the lipase operon.
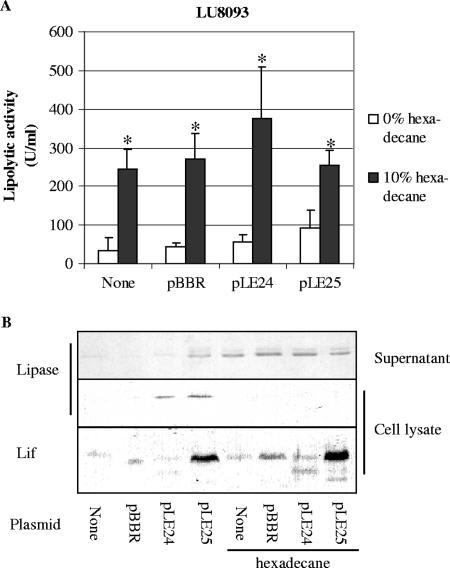
In an attempt to increase the production of lipase further, extra copies of the lipA and lipB genes together or of the lipB gene alone were introduced in LU8093 on a plasmid, i.e., pLE25 and pLE24, respectively, under the control of the tac promoter. Expression of lipase and Lif from pLE25 increased the lipolytic activity in the absence of hexadecane only twofold (Fig. 2A, white bars). In the presence of hexadecane, LU8093 produced significantly higher lipolytic activities (Fig. 2A, black bars), as before. However, there was no additive effect of hexadecane and the presence of the overexpression plasmids (Fig. 2A), suggesting that the expression of lipA and lipB is not limiting when hexadecane is present. Consistent with the increased lipolytic activity, Western blot analysis showed increased amounts of lipase in the culture supernatant of the cells grown in the presence of hexadecane (Fig. 2B). Interestingly, Western blot analysis showed that in the absence of hexadecane, lipase accumulated in the cells upon overproduction of lipase and/or Lif (Fig. 2B).
FIG. 2.
Influence of overexpression of lipase and/or Lif on lipolytic activity. (A) Lipolytic activity in culture supernatants of B. glumae LU8093 containing different plasmids or without plasmid (None). pBBR represents the empty vector pBBR1MCS-2, whereas pLE24 and pLE25 contain the lipB gene alone or the entire lipAB operon, respectively. Cells were grown for 24 h in medium with or without 10% hexadecane. The averages of at least three experiments are shown, with error bars indicating the standard deviations. Asterisks indicate significant difference from the sample without hexadecane (P < 0.05). (B) Western blots of culture supernatants and cell lysates of B. glumae LU8093 containing different plasmids were probed with antisera directed against lipase or Lif.
A similar experiment was performed using strain PG3, a derivative of PG1 lacking the chromosomal copy of the lipA gene. Lipase activity was not detectable in overnight cultures of B. glumae PG3 with or without vector plasmid pUR6500. Strain PG3 with pUR6502, containing the lipase gene under the control of the tac promoter, produced lipase at a low level, i.e., 1.0 U/ml, in PG medium supplemented with 1% olive oil and 1 mM isopropyl-β-d-thiogalactopyranoside. In the presence of 10% hexadecane, lipase production in this strain was increased up to 88.8 U/ml. Consistent with the increased lipolytic activity, Western blot analysis showed dramatically increased amounts of lipase in the culture supernatant of cells grown in the presence of hexadecane and accumulation of lipase within the cells without hexadecane (data not shown). Since in this experiment lipA was not expressed from its own promoter, hexadecane seems to exert its effect at a posttranscriptional level.
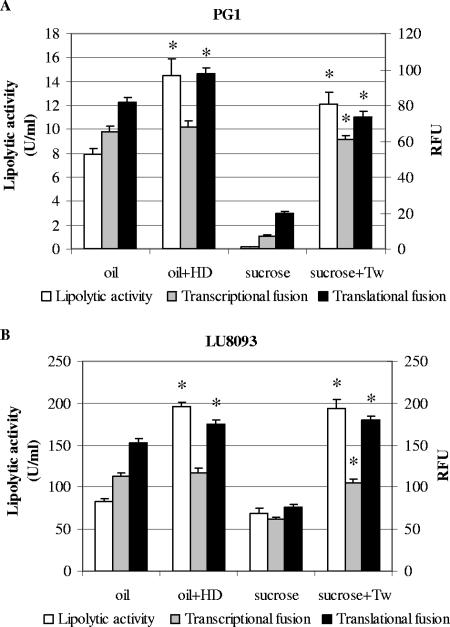
To corroborate these results, transcriptional and translational fusions of the lipA promoter from PG1 and LU8093 to the gfp reporter gene were constructed and introduced into the strain from which the promoter fragment originated. Fluorescence measurements in the strains carrying the transcriptional lipA::gfp fusions confirmed that hexadecane did not induce lipA transcription when the strains were grown in the presence of 1% olive oil (Fig. 3, gray bars). The analysis of the translational lipA::gfp fusions revealed a slight increase of 14 to 20% in relative fluorescence units when the PG medium with olive oil was supplemented with 10% hexadecane (Fig. 3, black bars). However, since the increase in lipolytic activity was much larger, i.e., 83 to 136% (Fig. 3, white bars), it is unlikely that this slight increase in translation fully explains the stimulatory effect of hexadecane on lipase production.
FIG. 3.
Lipolytic activity (left axis) in culture supernatants (white bars) and relative fluorescence measurements (relative fluorescence units [RFU]) (right axis) of transcriptional (pGPG1-TC and pGLU-TC; gray bars) or translational (pGPG1-TL and pGLU-TL; black bars) lipA::gfp fusions in B. glumae PG1 (A) or LU8093 (B). Strains were grown for 24 h in PG medium supplemented with 1% olive oil (oil), 1% olive oil and 10% hexadecane (oil+HD), 0.5% sucrose (sucrose), or 0.5% sucrose and 0.1% Tween 80 (sucrose+Tw). Averages from at least three experiments are shown, with error bars indicating standard deviations. Asterisks indicate significant difference from sample without hexadecane or Tween 80 (P < 0.05).
From these results, we conclude that hexadecane-induced lipase production is not mediated by increased lipA transcription and is mediated only marginally by increased lipA translation.
Hexadecane stimulates secretion of lipase.
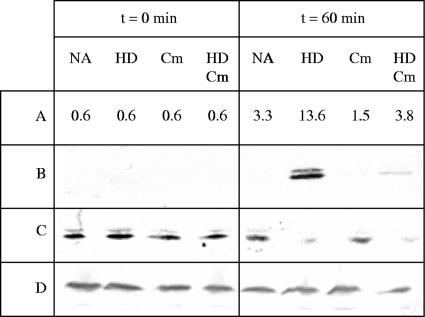
The experiments described above showed that hexadecane increases lipase production without affecting lipA expression. To confirm that hexadecane acts at a posttranslational level to increase extracellular lipase activity, we determined its effect on the lipolytic activity of metabolically inactive cells, which do not produce lipase de novo. An overnight culture of LU8093 in PG medium with olive oil was washed three times with a physiological salt solution and used to inoculate fresh medium with or without hexadecane and 25 μg/ml chloramphenicol to inhibit protein synthesis. After 60 min of incubation, samples were taken for analysis. Without chloramphenicol, lipase activity was fourfold higher when the cells were incubated with hexadecane. Incubation with chloramphenicol in the absence of hexadecane resulted in a twofold-lower activity (Fig. 4A). However, also in the presence of chloramphenicol, the presence of hexadecane in the medium clearly enhanced lipase activity (Fig. 4A). Western blot analysis of cells (Fig. 4C) and culture supernatants (Fig. 4B) showed that hexadecane stimulated the release of lipase from the cells both in the presence and in the absence of chloramphenicol. The levels of Lif in the cells were not affected (Fig. 4D). These results show that hexadecane stimulates the secretion of lipase.
FIG. 4.
Effect of hexadecane and chloramphenicol on lipase production in B. glumae LU8093. Cells were washed and incubated for 60 min in PG medium containing 1% olive oil, supplemented with nothing (NA), hexadecane (HD), and/or chloramphenicol (Cm). Samples were taken after 0 or 60 min. (A) Lipolytic activity in culture supernatants (U/ml). (B) Western blot of culture supernatants immunostained with lipase-specific antiserum. (C) Western blot of cell lysates probed with antisera directed against lipase. (D) Western blot of cell lysates probed with antisera directed against Lif.
The possibility that hexadecane stimulates secretion of lipase by accelerating its release from the membrane-bound chaperone was tested in vitro. His-tagged Lif was immobilized on Cu2+-charged chelating Sepharose beads. Subsequently, these beads were incubated with purified lipase. Independently of the presence of hexadecane during the wash steps, most of the lipase was retained on the beads and was subsequently eluted together with the His-tagged Lif by EDTA (data not shown). Thus, hexadecane did not release lipase from His-tagged Lif in vitro.
Effects of other compounds on lipase production.
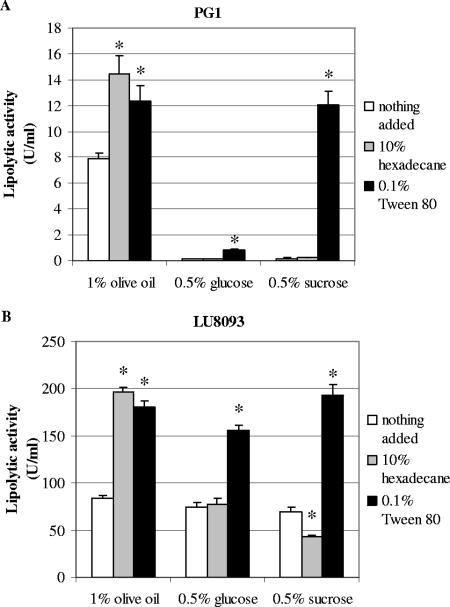
Other compounds, such as Tweens, have been shown to stimulate the production of lipase in Acinetobacter and Pseudomonas species (12, 24, 26). To determine whether such compounds stimulate lipase production in B. glumae as well, strains PG1 and LU8093 were grown with 10% hexadecane or tetradecane, 1% gum arabic, or 0.1% of the nonionic detergents Tween 80 and Triton X-100. When olive oil was used as the carbon source, all compounds tested stimulated lipase production to an extent similar to that for 10% hexadecane (Fig. 5 and data not shown). Culture supernatants of the cells incubated with the detergents contained Lif, in contrast to those of cells treated with hexadecane, as was revealed by Western blot analysis (data not shown). Apparently, the detergents, in contrast to hexadecane, destabilized the bacterial membranes, thereby releasing not only lipase but also Lif from the periplasm and membranes.
FIG. 5.
Lipolytic activity in culture supernatants of B. glumae PG1 (A) or LU8093 (B) after 24 h of growth. Cells were grown in the presence of 1% olive oil, 0.5% glucose, or 0.5% sucrose supplemented with nothing (white bars), 10% hexadecane (gray bars), or 0.1% Tween 80 (black bars). Averages from at least three experiments are shown, with error bars indicating standard deviations. Asterisks indicate significant difference from sample without hexadecane or Tween 80 (P < 0.05).
Tween 80 functions as an inducer of lipase gene expression.
In the experiments described so far, olive oil was present in the growth medium as a carbon source. Presumably, olive oil also functions as an inducer of the lipase operon. To test this possibility, lipase production was evaluated after growth in PG medium supplemented with alternative carbon sources, i.e., glucose and sucrose, which functioned as a good or poor carbon source, respectively (reaching an OD580 after 24 h of growth of ∼3.0 and ∼0.7, respectively, for both strains). Indeed, PG1 produced very low lipolytic activities in medium with these alternative carbon sources (Fig. 5A, white bars). Strain LU8093 has been obtained by classical mutagenesis and produces higher lipolytic activities than the wild-type strain, PG1 (Fig. 1), possibly due to mutations in regulatory genes involved in lipase gene expression. Consequently, lipase production in strain LU8093 might be less dependent on the presence of an inducer in the growth medium. Indeed, LU8093 produced lipase at similar levels in the presence of oil, glucose, or sucrose as a carbon source, in contrast to PG1 (Fig. 5, white bars). Hexadecane (Fig. 5, gray bars) and Triton X-100 (data not shown) did not increase extracellular lipase activity in either strain when sucrose or glucose was used as a carbon source, suggesting that under these conditions the secretion process is not limiting. In contrast, Tween 80 dramatically increased the extracellular lipase activity of strain PG1 in the presence of sucrose but much less in the presence of glucose as a carbon source (Fig. 5A, black bars). These results indicate that Tween 80, which, in contrast to Triton X-100, contains a fatty acyl ester bond, functions as an inducer of the lipase operon and, furthermore, that expression of this operon in strain PG1 in the presence of glucose is prone to catabolite repression. B. glumae could grow in PG medium with 0.1% Tween 80 as the sole carbon source, but only to an OD580 of ∼1.2 after 24 h of growth, whereas growth on 1% olive oil, 0.5% glucose, or 0.5% sucrose was only slightly increased by supplementing the medium with 0.1% Tween 80. Analysis of the lipA::gfp fusions confirmed that Tween 80 induced lipA gene expression in the presence of sucrose (Fig. 3A) but not of glucose (data not shown) as a carbon source. Also, in strain LU8093, Tween 80 stimulated lipase production when sucrose was the carbon source (Fig. 3B and 5B). However, even in the presence of glucose, Tween 80 stimulated lipase production in strain LU8093, suggesting that in this strain lipase expression is not prone to catabolite repression (Fig. 5B).
DISCUSSION
B. glumae strain PG1 produces a lipase of biotechnological relevance. We demonstrate here that the production of the enzyme in the wild-type strain depends on the presence of an inducer (olive oil or Tween 80) in the growth medium and is probably prone to catabolite repression, since induction by Tween 80 did not occur in the presence of glucose. A mutant strain, i.e., strain LU8093, which produces much higher levels of lipase, was previously obtained through classical strain improvement (1; Braatz et al., 1992, patent application WO 9300924 A1). We show in this study that strain LU8093 produces lipase independently of the presence of an inducer in the growth medium and that this strain is insensitive to catabolite repression. We investigated the possibilities for increasing the production of lipase in this strain even further by modifying the growth medium. Several compounds, including alkanes, gum arabic, spans, and Tweens, have been shown to increase lipase production in other bacteria, such as A. calcoaceticus and Pseudomonas species (12, 17, 18, 24-26, 35). Indeed, we found that additives, such as hexadecane, Triton X-100, and Tween 80, considerably improved productivity.
The mechanism by which such additives affect lipase production in various bacteria is largely unknown. Our results show that depending on the compound tested, different mechanisms are involved. It is important to note that hexadecane was not able to support normal growth of B. glumae when used as the sole carbon source in PG medium. In contrast, hexadecane could be used as the sole carbon source while stimulating the production of lipase for Pseudomonas sp. strain G6 and A. calcoaceticus BD413 (17, 19). In A. calcoaceticus BD413, hexadecane increased lipase production by stabilization of lipase in the supernatant and not by increasing the expression of lipA (18). By using lipA::gfp fusions, we also found that hexadecane did not affect lipA gene expression in B. glumae at the transcriptional level. Consistently, the stimulating effect of hexadecane was found also when lipA was expressed from a different (i.e., tac) promoter. However, we did not find extracellular lipase to be stabilized by hexadecane.
Our experiments showed that hexadecane increases lipase production mainly at the posttranslational level. Upon overexpression of lipase and Lif, lipase accumulated in the cells in the absence of hexadecane (Fig. 2), indicating that secretion is limiting under these conditions. However, in the presence of hexadecane, lipase no longer could be detected in the cells, suggesting that hexadecane enhances the secretion of lipase. This notion was confirmed with an experiment in which protein synthesis was inhibited with chloramphenicol. Even in the absence of de novo protein synthesis, hexadecane increased the lipolytic activity in the supernatant (Fig. 4), indicating that hexadecane indeed stimulates the secretion of intracellularly accumulated lipase. However, secretion of intracellularly accumulated lipase cannot solely be responsible for the drastically increased lipase activity observed. Somehow, the improved secretion leads to increased production of the enzyme without a concomitant increase in transcription of lipA. One possibility is that in the absence of hexadecane only a proportion of the total pool of lipase molecules synthesized is secreted, while the other part, which remains in the periplasm, is vulnerable to proteolysis. Thus, enhanced secretion in the presence of hexadecane may result in a smaller amount of lipase that is degraded and thus in a higher yield of lipase.
The mechanism underlying the stimulating effect of hexadecane on lipase secretion is unclear. Hexadecane is probably present only in small amounts in the outer membrane or in the periplasm. Alkanes can insert into lipid bilayers (5, 27), but their insertion into the bacterial outer membrane is probably hampered by lipopolysaccharide (LPS) (36). Nevertheless, bacteria, particularly members of the genus Pseudomonas, can modify their surface properties in response to the presence of hydrophobic solvents, such as hexadecane. Hydrophobic solvents can induce the release of membrane vesicles (22) and changes in LPS composition (29), resulting in an increase in the cell surface hydrophobicity (3, 29). Such alterations might stimulate the secretion of lipase in B. glumae. Of note, the structure of LPS has been shown to be important for the functioning of the secretory system in P. aeruginosa (28).
Alternatively, hexadecane might enhance the release of lipase from the secretin of the type II secretion system. Secretins form channels in the outer membrane for the transport of macromolecules. Hexadecane might solubilize lipase from the secretin, thereby freeing the way for the secretion of the next lipase molecule. Secretion has been shown to be a bottleneck in lipase production in Pseudomonas alcaligenes (11). Moreover, gum arabic, polysaccharides, alginate, and inert compounds can also affect the secretion of lipase in various bacteria (25, 26, 40, 41). Gum arabic is a high-molecular-weight (Mr, 340,000 to 560,000) polymer (4) and is probably unable to penetrate the outer membrane. Whether these compounds act by the same mechanism on lipase production as hexadecane does is presently unknown.
In the presence of olive oil as the carbon source and inducer, Triton X-100 also stimulated lipase production in B. glumae. In this case, increased lipase production was accompanied by the release of Lif into the growth medium, suggesting serious membrane damage. The disruption of the outer membrane may trigger periplasmic stress responses, including the expression of several chaperones (32), which may aid in the folding and subsequent secretion of lipase. In contrast to Triton X-100, Tween 80 also improved lipase production when sucrose was present as the carbon source. The drastic induction observed in strain PG1 suggested that Tween 80, like olive oil, could act as an inducer of the lipase operon, a hypothesis which was confirmed when the expression of a lipA::gfp transcriptional fusion was analyzed. Consistently, unlike Triton X-100, Tween 80 contains a fatty acyl ester bond and thus mimics natural lipase substrates. Taken together, the different compounds tested appear to affect lipase production in B. glumae via different mechanisms.
In conclusion, lipase production in B. glumae requires an inducer and is sensitive to catabolite repression. The mutant strain LU8093, which was obtained through classical strain improvement, displays a high level of lipase production, which was unresponsive to the presence of an inducer or to catabolite repression. The yield of lipase production could further be improved by including additives, such as hexadecane, Triton X-100, or Tween 80, in the growth medium. Hexadecane stimulates the production of lipase primarily at the posttranslational level, most likely by enhancing its secretion. Although Triton X-100 and Tween 80 increased lipase production to an extent similar to that of hexadecane, these compounds are less suitable for inclusion in large-scale production because of contamination of the culture supernatant with cellular proteins, which affects the downstream processing of lipase.
Footnotes
Published ahead of print on 27 April 2007.
REFERENCES
- 1.Balkenhohl, F., K. Ditrich, B. Hauer, and W. Ladner. 1997. Optisch aktive Amine durch Lipase-katalysierte Methoxyacetylierung. J. Prakt. Chem. 339:381-384. [Google Scholar]
- 2.Baneyx, F., and M. Mujacic. 2004. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22:1399-1408. [DOI] [PubMed] [Google Scholar]
- 3.Bredholt, H., P. Bruheim, M. Potocky, and K. Eimhjellen. 2002. Hydrophobicity development, alkane oxidation, and crude-oil emulsification in a Rhodococcus species. Can. J. Microbiol. 48:295-304. [DOI] [PubMed] [Google Scholar]
- 4.Churms, S. C., E. H. Merrifield, and A. M. Stephen. 1983. Some new aspects of the molecular structure of Acacia senegal gum (gum arabic). Carbohydr. Res. 123:267-279. [Google Scholar]
- 5.Coster, H. G. L., and D. R. Laver. 1986. The effect of temperature on lipid-n-alkane interactions in lipid bilayers. Biochim. Biophys. Acta 857:95-104. [DOI] [PubMed] [Google Scholar]
- 6.El Khattabi, M., P. Van Gelder, W. Bitter, and J. Tommassen. 2003. Role of the calcium ion and the disulfide bond in the Burkholderia glumae lipase. J. Mol. Catal. B Enzym. 22:329-338. [Google Scholar]
- 7.El Khattabi, M., P. Van Gelder, W. Bitter, and J. Tommassen. 2000. Role of the lipase-specific foldase of Burkholderia glumae as a steric chaperone. J. Biol. Chem. 275:26885-26891. [DOI] [PubMed] [Google Scholar]
- 8.Frenken, L. G. J., J. W. Bos, C. Visser, W. Muller, J. Tommassen, and C. T. Verrips. 1993. An accessory gene, lipB, required for the production of active Pseudomonas glumae lipase. Mol. Microbiol. 9:579-589. [DOI] [PubMed] [Google Scholar]
- 9.Frenken, L. G. J., A. de Groot, J. Tommassen, and C. T. Verrips. 1993. Role of the lipB gene product in the folding of the secreted lipase of Pseudomonas glumae. Mol. Microbiol. 9:591-599. [DOI] [PubMed] [Google Scholar]
- 10.Frenken, L. G. J., M. R. Egmond, A. M. Batenburg, J. W. Bos, C. Visser, and C. T. Verrips. 1992. Cloning of the Pseudomonas glumae lipase gene and determination of the active site residues. Appl. Environ. Microbiol. 58:3787-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerritse, G., R. Ure, F. Bizoullier, and W. J. Quax. 1998. The phenotype enhancement method identifies the Xcp outer membrane secretion machinery from Pseudomonas alcaligenes as a bottleneck for lipase production. J. Biotechnol. 64:23-28. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert, E. J., J. W. Drozd, and C. W. Jones. 1991. Physiological regulation and optimization of lipase activity in Pseudomonas aeruginosa EF2. J. Gen. Microbiol. 137:2215-2221. [DOI] [PubMed] [Google Scholar]
- 13.Gupta, R., N. Gupta, and P. Rathi. 2004. Bacterial lipases: an overview of production, purification and biochemical properties. Appl. Microbiol. Biotechnol. 64:763-781. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Jaeger, K.-E., and M. T. Reetz. 1998. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 16:396-403. [DOI] [PubMed] [Google Scholar]
- 16.Jaeger, K. E., B. W. Dijkstra, and M. T. Reetz. 1999. Bacterial biocatalysts: molecular biology, three-dimensional structures, and biotechnological applications of lipases. Annu. Rev. Microbiol. 53:315-351. [DOI] [PubMed] [Google Scholar]
- 17.Kanwar, L., B. K. Gogoi, and P. Goswami. 2002. Production of a Pseudomonas lipase in n-alkane substrate and its isolation using an improved ammonium sulfate precipitation technique. Bioresour. Technol. 84:207-211. [DOI] [PubMed] [Google Scholar]
- 18.Kok, R. G., C. B. Nudel, R. H. Gonzalez, I. M. Nugteren-Roodzant, and K. J. Hellingwerf. 1996. Physiological factors affecting production of extracellular lipase (LipA) in Acinetobacter calcoaceticus BD413: fatty acid repression of lipA expression and degradation of LipA. J. Bacteriol. 177:6025-6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kok, R. G., J. J. van Thor, I. M. Nugteren-Roodzant, M. B. Brouwer, M. R. Egmond, C. B. Nudel, B. Vosman, and K. J. Hellingwerf. 1995. Characterization of the extracellular lipase, LipA, of Acinetobacter calcoaceticus BD413 and sequence analysis of the cloned structural gene. Mol. Microbiol. 15:803-818. [DOI] [PubMed] [Google Scholar]
- 20.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop II, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Leahy, J. G., Z. M. Khalid, E. J. Quintero, J. M. Jones-Meehan, J. F. Heidelberg, P. J. Batchelor, and R. R. Colwell. 2003. The concentrations of hexadecane and inorganic nutrients modulate the production of extracellular membrane-bound vesicles, soluble protein, and bioemulsifier by Acinetobacter venetianus RAG-1 and Acinetobacter sp. strain HO1-N. Can. J. Microbiol. 49:569-575. [DOI] [PubMed] [Google Scholar]
- 23.Liebeton, K., A. Zacharias, and K.-E. Jaeger. 2001. Disulfide bond in Pseudomonas aeruginosa lipase stabilizes the structure but is not required for interaction with its foldase. J. Bacteriol. 183:597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, S.-F., C.-M. Chiou, and Y.-C. Tsai. 1995. Effect of triton X-100 on alkaline lipase production by Pseudomonas pseudoalcaligenes F-111. Biotechnol. Lett. 17:959-962. [Google Scholar]
- 25.Mahler, G. F., R. G. Kok, A. Cordenons, K. J. Hellingwerf, and B. C. Nudel. 2000. Effects of carbon sources on extracellular lipase production and lipA transcription in Acinetobacter calcoaceticus. J. Ind. Microbiol. Biotechnol. 24:25-30. [Google Scholar]
- 26.Martinez, D. A., and B. C. Nudel. 2002. The improvement of lipase secretion and stability by addition of inert compounds into Acinetobacter calcoaceticus cultures. Can. J. Microbiol. 48:1056-1061. [DOI] [PubMed] [Google Scholar]
- 27.McIntosh, T. J., S. A. Simon, and R. C. MacDonald. 1980. The organization of n-alkanes in lipid bilayers. Biochim. Biophys. Acta 597:445-463. [DOI] [PubMed] [Google Scholar]
- 28.Michel, G., G. Ball, J. B. Goldberg, and A. Lazdunski. 2000. Alteration of the lipopolysaccharide structure affects the functioning of the Xcp machinery in Pseudomonas aeruginosa. J. Bacteriol. 182:696-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neumann, G., S. Cornelissen, F. van Breukelen, S. Hunger, H. Lippold, N. Loffhagen, L. Y. Wick, and H. J. Heipieper. 2006. Energetics and surface properties of Pseudomonas putida DOT-T1E in a two-phase fermentation system with 1-decanol as second phase. Appl. Environ. Microbiol. 72:4232-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenau, F., and K.-E. Jaeger. 2000. Bacterial lipases from Pseudomonas: regulation of gene expression and mechanisms of secretion. Biochimie 82:1023-1032. [DOI] [PubMed] [Google Scholar]
- 31.Rosenau, F., J. Tommassen, and K.-E. Jaeger. 2004. Lipase-specific foldases. Chembiochem 5:152-161. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz, N., and T. J. Silhavy. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122-126. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook, J., T. Maniatis, and E. F. Fritsch. 1989. Molecular cloning: A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 34.Schmid, A., J. S. Dordick, B. Hauer, A. Kiener, M. Wubbolts, and B. Witholt. 2001. Industrial biocatalysis today and tomorrow. Nature 409:258-268. [DOI] [PubMed] [Google Scholar]
- 35.Shabtai, Y., and N. Daya-Mishne. 1992. Production, purification, and properties of a lipase from a bacterium (Pseudomonas aeruginosa YS-7) capable of growing in water-restricted environments. Appl. Environ. Microbiol. 58:174-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder, D. S., and T. J. McIntosh. 2000. The lipopolysaccharide barrier: correlation of antibiotic susceptibility with antibiotic permeability and fluorescent probe binding kinetics. Biochemistry 39:11777-11787. [DOI] [PubMed] [Google Scholar]
- 37.Staeudinger, M., O. von Deimling, C. Grossarth, and T. Wienker. 1973. Esterase. 8. Histochemical, electrophoretic and quantitative studies on phenobarbital influence on mouse liver esterase. Histochemie 34:107-116. [DOI] [PubMed] [Google Scholar]
- 38.Tommassen, J., A. Filloux, M. Bally, M. Murgier, and A. Lazdunski. 1992. Protein secretion in Pseudomonas aeruginosa. FEMS Microbiol. Rev. 103:73-90. [DOI] [PubMed] [Google Scholar]
- 39.Urban, A., M. Leipelt, T. Eggert, and K.-E. Jaeger. 2001. DsbA and DsbC affect extracellular enzyme formation in Pseudomonas aeruginosa. J. Bacteriol. 183:587-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingender, J., and U. K. Winkler. 1984. A novel biological function of alginate in Pseudomonas aeruginosa and its mucoid mutants: stimulation of exolipase. FEMS Microbiol. Lett. 21:63-69. [Google Scholar]
- 41.Winkler, U. K., and M. Stuckmann. 1979. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J. Bacteriol. 138:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]