Abstract
The objective of this study was to investigate the occurrence of sublethal injury after the pulsed-electric-field (PEF) treatment of two yeasts, Dekkera bruxellensis and Saccharomyces cerevisiae, as well as the relation of sublethal injury to the inactivating effect of the combination of PEF and sorbic acid. PEF caused sublethal injury in both yeasts: more than 90% of surviving D. bruxellensis cells and 99% of surviving S. cerevisiae cells were sublethally injured after 50 pulses at 12 kV/cm in buffer at pHs of both 7.0 and 4.0. The proportion of sublethally injured cells reached a maximum after 50 pulses at 12.0 kV/cm (S. cerevisiae) or 16.5 kV/cm (D. bruxellensis), and it kept constant or progressively decreased at greater electric field strengths and with longer PEF treatments. Sublethally PEF-injured cells showed sensitivity to the presence of sorbic acid at a concentration of 2,000 ppm. A synergistic inactivating effect of the combination of PEF and sorbic acid was observed. Survivors of the PEF treatment were progressively inactivated in the presence of 2,000 ppm of sorbic acid at pH 3.8, with the combined treatments achieving more than log10 5 cycles of dead cells under the conditions investigated. This study has demonstrated the occurrence of sublethal injury after exposure to PEF, so yeast inactivation by PEF is not an all-or-nothing event. The combination of PEF and sorbic acid has proven to be an effective method to achieve a higher level of yeast inactivation. This work contributes to the knowledge of the mechanism of microbial inactivation by PEF, and it may be useful for improving food preservation by PEF technology.
Among the nonthermal technologies dealing with the preservation of food, pulsed-electric-field (PEF) treatment is an effective method for inactivating microorganisms without altering the organoleptic and nutritional properties of liquid food (3). However, PEF technology is not being used for the moment to preserve food commercially since several aspects of PEF treatment are not yet clear. Among other information, knowledge of the way PEF treatments kill microorganisms is required for the design of effective PEF treatments that guarantee food safety and stability.
The mechanism of microbial inactivation by PEF is intimately related to the formation of pores in the membrane, as many authors have reported previously (2, 3, 13, 15, 16, 17, 22, 28, 29, 30). The pores can be reversible or irreversible, affecting the integrity and functionality of the membrane (2, 13, 29) and thus yielding sublethally injured cells under some treatment conditions (13).
An indirect way of evaluating sublethal injuries in the membrane is the use of the selective-medium plating technique (21). Since sublethal injury is supposed to be related to a higher sensitivity of survivors to stress conditions after treatment, from a practical point of view, the occurrence of sublethally injured cells highlights the optimal treatment conditions for combining PEF with additional stresses that may prevent cell repair and survival.
Recently, our research group has demonstrated the occurrence of sublethal injury in PEF-treated bacteria as a function of the treatment conditions (10, 11). It has also been demonstrated that sublethally PEF-injured cells lose viability during subsequent storage under acidic conditions. Consequently, the combination of PEF treatment applied to apple juice and subsequent storage under refrigeration for 48 h guarantees the inactivation of at least log10 5 cell cycles of the pathogen Escherichia coli O157:H7 (12).
In addition to ensuring food safety, PEF treatments have been proposed to prevent the development of spoiling microorganisms, thereby extending food shelf life. Saccharomyces cerevisiae and Dekkera bruxellensis are contaminating yeasts that lead to food deterioration and great economic losses. S. cerevisiae is the most common spoiling agent in many acidic foods, such as cider and fruit juices (27). D. bruxellensis is able to produce volatile phenols when yeast cells develop in wine, thereby impairing the wine's aroma (4). Although many authors have studied the inactivation of S. cerevisiae by PEF (1, 2, 8, 24, 26), to the best of our knowledge, the occurrence of sublethally injured yeast cells after PEF treatment and its utility in the development of combined processes have not been investigated previously.
Combined processes using PEF treatment must incorporate conditions that prevent the repair of cell envelopes (10, 12) by the addition of food preservatives (7, 9, 14, 18, 19, 23). Permeable membranes resulting from PEF treatment may facilitate the entry of antimicrobial compounds, such as organic acids, thereby increasing the antimicrobial action of the compounds (19). In this study, we opted for sorbic acid due to its neutral taste characteristics and common use in liquid food, such as fruit juices and soft drinks. Sorbic acid is a substance generally recognized as safe and permitted in most countries at a concentration of 1,000 to 2,000 ppm (20).
Given the relevance of the occurrence of sublethal injury to the development of appropriate combined processes, a study was carried out in order to determine whether sublethal injury occurs in PEF-treated yeast cells and how this circumstance may lead to the design of combined processes with a synergistic lethal effect.
The aim of this work was to investigate the occurrence of sublethal injury after the PEF treatment of two yeasts, D. bruxellensis and S. cerevisiae, as well as the relation of sublethal injury to the inactivating effect of the combination of PEF and sorbic acid.
MATERIALS AND METHODS
Microorganisms and growth conditions.
The strains of S. cerevisiae (STCC 11034) and D. bruxellensis (STCC 11045) used in this investigation were supplied by the Spanish Type Culture Collection. During this investigation, the strains were maintained on slants of potato dextrose agar (PDA; Biolife, Milan, Italy).
Broth subcultures were prepared by inoculating a test tube containing 10 ml of sterile Sabouraud broth (Biolife) with a single colony from a plate. After inoculation, the tubes were incubated at 30°C for 24 h. Next, 250-ml Erlenmeyer flasks containing 50 ml of Sabouraud broth were inoculated with these subcultures to a final concentration of 103 cells/ml. The flasks were incubated with agitation at 130 rpm on a Rotabit shaker (Selecta, Barcelona, Spain) at 30°C until the stationary growth phase was reached.
PEF equipment.
Strong electric field pulses were produced by discharging a set of 10 capacitors (6,800 pF; catalog no. C-20C682 [Behlke, Kronberg, Germany]) via a thyristor switch (Behlke; catalog no. HTS 160-500SCR). The set of capacitors was charged by using a high-voltage direct-current power supply (catalog no. HCK 2500 M 35000; FUG, Rosenhein, Germany). A function generator (catalog no. AFG 320; Tektronix, Wilsonville, OR) delivered the on-time signal to the switch. A cylindrical plastic tube closed with two polished stainless steel electrodes was used as a batch treatment chamber. The distance between electrodes was 0.25 cm, and the electrode area was 2.01 cm2. The circuit configuration generated exponential wave form pulses at different frequencies (1 to 60 Hz), pulse widths, and electric field strengths (1 to 40 kV/cm). The actual electric field strength and electrical intensity applied were measured in the treatment chamber with a high-voltage probe and a current probe, respectively, connected to an oscilloscope (catalog no. TDS 3012B; Tektronix, Wilsonville, OR). The PEF equipment included the necessary provisions for measuring sample temperature. Immediately after treatment, a pneumatically activated type K thermocouple of 0.9 mm in diameter entered the treatment chamber and the temperature was measured in the center of the chamber.
PEF treatments.
Before treatment, yeast cultures were centrifuged at 6,000 × g for 5 min and resuspended in citrate-phosphate McIlvaine buffer at pHs of 7.0, 6.0, 5.0, 4.0, and 3.0 (6), with the concentration adjusted to correspond to an electric conductivity of 2 ± 0.1 mS/cm, as well as in the same buffer at pHs of 7.0 and 4.0 with sorbic acid added to final concentrations of 20, 100, 200, 1,000, and 2,000 ppm. The addition of just 2,000 ppm of sorbic acid caused a significant reduction in pHs, to 4.6 and 3.8, respectively. Next, 0.5-ml samples of the microbial suspensions at a concentration of approximately 107 CFU/ml were placed in the batch treatment chamber with a sterile syringe, as previously described (25). Exponential wave form pulses at electric field strengths of 9.5, 12.0, 16.5, and 19.5 kV/cm and a pulse repetition rate of 1 Hz were used in this study. The specific energy inputs of each pulse were 0.6, 0.9, 1.7, and 2.4 kJ/kg, respectively. Experiments started at room temperature. In all cases, the temperatures of the samples after treatment were lower than 35°C.
Measurement of sensitivity to subsequent holding in the PEF treatment medium.
PEF-treated cells were held in the suspending medium, citrate-phosphate McIlvaine buffer at pHs of 7.0 and 4.0, as well as in the same buffer with sorbic acid added to final concentrations of 20, 200, and 2,000 ppm, at room temperature for 120 h. Samples were taken at preset intervals, and survivors were evaluated.
Measurement of sensitivity of native cells to the presence of sorbic acid.
By following the same protocol, native cells were held in citrate-phosphate McIlvaine buffer at pHs of 7.0 and 4.0, as well as in the same buffer with sorbic acid added to final concentrations of 20, 100, 200, 1,000, and 2,000 ppm, at room temperature for 120 h. Samples were taken at preset intervals, and survivors were evaluated.
Counts of viable cells.
After treatments, samples were adequately diluted, and 0.1-ml samples were pour plated onto PDA. Plates were incubated for 48 h (S. cerevisiae) or 72 h (D. bruxellensis) at 30°C. Previous experiments showed that longer incubation times did not influence the counts of surviving cells. After incubation, CFU were counted with an improved image analyzer automatic counter (Protos; Analytical Measuring Systems, Cambridge, United Kingdom) as previously described (5). The error bars in the figures indicate the mean standard deviations for the data obtained from at least three independent experiments.
Detection of sublethal injury.
In order to determine yeast cell injury, treated samples were also plated onto PDA with 7% (S. cerevisiae) and 1% (D. bruxellensis) sodium chloride (Probus, Barcelona, Spain) added. These levels of sodium chloride were previously determined to be the maximum noninhibitory concentrations for native cells (data not shown). Plates containing selective medium were incubated for 48 h longer than those containing nonselective medium. Previous experiments showed that longer incubation times did not influence counts of surviving cells.
The proportion of sublethally injured cells was estimated based on the difference in the log10 numbers of cycles of CFU obtained after plating PEF-treated cells onto the nonselective (PDA) and selective (PDA-sodium chloride) media.
Analyses of variance (P = 0.05) were calculated with SPSS software (SPSS, Chicago, IL).
RESULTS
Yeast inactivation by PEF: occurrence of sublethal injury.
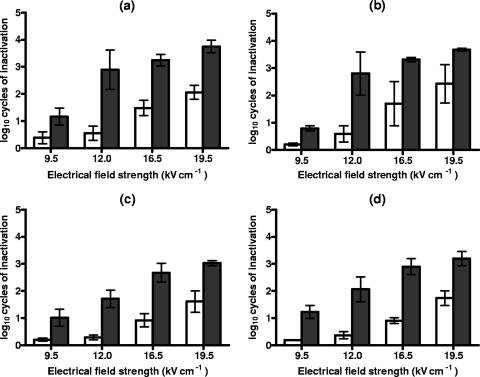
Figure 1 shows the log10 numbers of cycles of inactivation of S. cerevisiae and D. bruxellensis cells suspended in citrate-phosphate buffer at pHs of 7.0 and 4.0 after PEF treatments at different electric field strengths for 50 pulses and recovery on the nonselective and selective media.
FIG. 1.
Log10 numbers of cycles of inactivation of S. cerevisiae (a and b) and D. bruxellensis (c and d) cells after PEF treatments at different electric field strengths for 50 pulses in citrate-phosphate buffer at pH 7.0 (a and c) and pH 4.0 (b and d) and recovery on the nonselective (white bars) and the selective (dark bars) media. Data are means ± standard deviations (error bars).
As seen by comparing the data obtained with the nonselective medium, D. bruxellensis was slightly more resistant than S. cerevisiae, at pHs of both 7.0 and 4.0, to PEF treatment under any of the conditions examined. Nevertheless, in most cases, no statistically significant differences (P < 0.05) were observed. The levels of PEF resistance at pHs of 7.0 and 4.0 were the same (P < 0.05) for both microorganisms. Under the treatment conditions investigated, the greater the electric field strength, the higher the number of inactivated cells.
As shown by the differences between the counts obtained from the selective and the nonselective media, populations of both microorganisms showed great proportions of sublethally injured cells after PEF treatment when exposed to a range of electric field strengths. With the exception of the cells exposed to the weakest electric field strength studied, more than 90% of survivors of both yeast species were sublethally injured. The proportion of sublethally injured cells was maximum at 12.0 kV/cm (S. cerevisiae) and 16.5 kV/cm (D. bruxellensis) kV/cm, and it decreased at greater electric field strengths at both pHs. After 50 pulses at 12.0 kV/cm, the proportion of sublethally injured S. cerevisiae cells was higher than 99%.
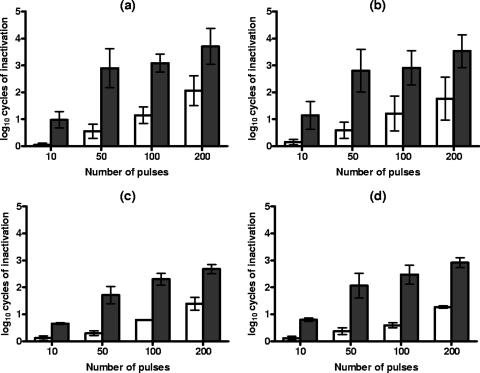
Figure 2 shows the influence of the treatment duration on PEF resistance and on the occurrence of sublethal injury in S. cerevisiae and D. bruxellensis cells at 12.0 kV/cm at pHs of 7.0 and 4.0. These results also show that, when recovering on nonselective medium, D. bruxellesis was slightly more PEF resistant after any treatment duration investigated.
FIG. 2.
Log10 numbers of cycles of inactivation of S. cerevisiae (a and b) and D. bruxellensis (c and d) cells after PEF treatments at 12.0 kV/cm in citrate-phosphate buffer at pH 7.0 (a and c) and pH 4.0 (b and d) for different time periods and recovery on the nonselective (white bars) and the selective (dark bars) media. Data are means ± standard deviations (error bars).
As shown in the figure, the number of dead cells increased with the duration of the PEF treatment, whereas the proportion of sublethally injured cells increased with durations of up to 50 pulses at 12.0 kV/cm and then kept constant (D. bruxellensis) or progressively decreased (S. cerevisiae) with longer PEF treatments.
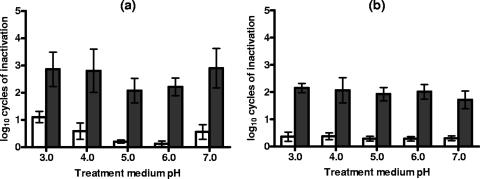
Figure 3 shows the log10 numbers of cycles of inactivation of S. cerevisiae and D. bruxellensis cells exposed to a range of treatment medium pHs after 50 pulses at 12.0 kV/cm and recovery on the nonselective and the selective media. The figure illustrates that, while the level of PEF resistance of S. cerevisiae varied slightly as a function of the treatment medium pH, that of D. bruxellensis kept constant (P < 0.05) within the range investigated. When recovering on either selective or nonselective medium, S. cerevisiae showed its maximum PEF resistance at pHs of 5.0 and 6.0. The proportion of sublethally injured cells of both species also kept constant under the range of treatment conditions investigated (P < 0.05).
FIG. 3.
Log10 numbers of cycles of inactivation of S. cerevisiae (a) and D. bruxellensis (b) cells after a PEF treatment at 12.0 kV/cm for 50 pulses in citrate-phosphate buffer at different pHs and recovery on the nonselective (white bars) and the selective (dark bars) media. Data are means ± standard deviations (error bars).
Yeast inactivation by sorbic acid: occurrence of sublethal injury.
Native cells of S. cerevisiae and D. bruxellensis at concentrations higher than 106 cells/ml were insensitive to incubation in citrate-phosphate buffer at pHs of 7.0 or 4.0, as well as incubation in the same medium with 20, 100, 200, and 1,000 ppm of sorbic acid added, for 120 h at room temperature (data not shown). The cell number kept constant (P < 0.05) on both nonselective and selective recovery media.
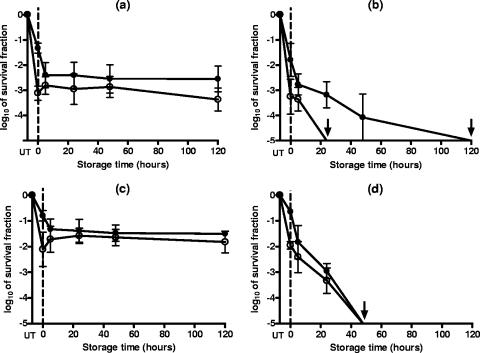
As mentioned in Materials and Methods, the addition of 2,000 ppm of sorbic acid to the buffer at pHs of 7.0 and 4.0 caused a reduction in pH to 4.6 and 3.8, respectively. Under these experimental conditions (Fig. 4), the evaluation of the number of survivors on the nonselective medium showed that both yeast species were insensitive to subsequent incubation at pH 4.6 in the presence of 2,000 ppm of sorbic acid (Fig. 4a and c), whereas approximately log10 2 and more than log10 5 cycles of S. cerevisiae and D. bruxellensis cells, respectively, died when incubated at pH 3.8 for 120 h at room temperature (Fig. 4b and d). The comparison of counts of survivors on the selective and nonselective media showed that sorbic acid at a concentration of 2,000 ppm in buffer at pH 3.8 caused sublethal injuries in the population of S. cerevisiae cells. The number of survivors recovered from the selective medium revealed that cells of more than log10 2 extra cell cycles were sublethally injured. The total populations of cells, both injured and dead, of both species affected by the presence of 2,000 ppm of sorbic acid during the 120-h incubation at room temperature corresponded to approximately log10 5 cycles.
FIG. 4.
Survival fractions of S. cerevisiae (a and b) and D. bruxellensis (c and d) cells suspended in citrate-phosphate buffers of different compositions with 2,000 ppm of sorbic acid added, giving final pHs of 4.6 (a and c) and 3.8 (b and d), for 120 h at room temperature. Survivors recovered on the nonselective (closed symbols) and the selective (opened symbols) media. Data are means ± standard deviations (error bars).
Yeast inactivation by combining sorbic acid and PEF.
By following the same procedure, native cells were resuspended in citrate-phosphate buffer at pHs of 7.0 and 4.0, either alone or in combination with 20, 200, and 2,000 ppm of sorbic acid, and treated at 12.0 kV/cm for 50 pulses. Next, PEF-treated cell suspensions were held for 120 h at room temperature, and survivors on the nonselective and selective media were assessed at preset intervals.
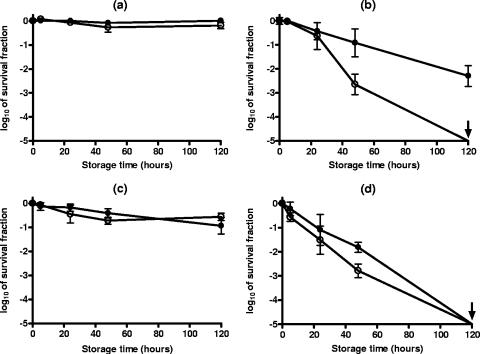
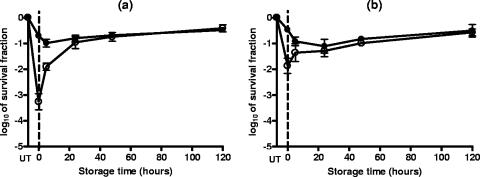
The comparison of the survival counts obtained immediately after PEF treatment reveals that the presence of 20 and 200 ppm of sorbic acid during the PEF treatment did not increase the level of inactivation or the proportion of sublethally injured cells and allowed the repair of the latter (data not shown). During the subsequent holding of the samples for 120 h at room temperature, the counts on the nonselective medium kept constant and those on the selective medium increased with the incubation time, revealing that sublethally injured cells were capable of repairing their damage. As an example, Fig. 5 has been included in order to show this behavior in S. cerevisiae and D. bruxellensis cells after a PEF treatment at 12.0 kV/cm for 50 pulses at pH 4.0.
FIG. 5.
Survival fractions of S. cerevisiae (a) and D. bruxellensis (b) cells after a PEF treatment at 12.0 kV/cm for 50 pulses in citrate-phosphate buffer at pH 4.0 and subsequent incubation in the same medium for 120 h at room temperature. Survivors recovered on the nonselective (closed symbols) and the selective (opened symbols) media. UT, untreated. Data are means ± standard deviations (error bars).
Results obtained when 2,000 ppm of sorbic acid was added to the treatment medium were quite different (Fig. 6). Counts of survivors on the nonselective medium immediately after PEF showed that in the presence of 2,000 ppm of sorbic acid, the degree of inactivation was barely higher than that caused by the PEF treatment in buffer alone (Fig. 5). Counts of survivors of both species on the selective medium after treatment at both pHs were also slightly lower. The subsequent incubation of the samples for 120 h showed that survivors of both yeast species were sensitive to the suspending medium. Nevertheless, the profile of the kinetics of cell injury and inactivation depended on the pH of the treatment medium. As shown by the counts obtained from the nonselective medium, after PEF treatment at pH 4.6, subsequent holding for 5 h in the presence of sorbic acid caused only a small additional decrease in numbers of viable cells (Fig. 6a and c). Thus, the survival counts obtained from both recovery media were very similar, which indicates that most PEF-damaged cells were sensitive to the presence of 2,000 ppm of sorbic acid. Note that, while most sublethally injured D. bruxellenis cells were inactivated by the presence of sorbic acid, a small proportion (about 10%) were able to repair their damage (Fig. 6c). The following holding step for up to 120 h did not injure and/or inactivate more cells, since the survival counts obtained from both recovery media kept constant. The final degree of inactivation of cells of both yeast species due to the combined process (log10 2 to log10 3 cycles, depending on the yeast) was much higher than that obtained by adding the effects of PEF treatment and those of incubation with 2,000 ppm of sorbic acid acting separately (lower than log10 1 cycles). Therefore, the two treatments acted synergistically when simultaneously applied.
FIG. 6.
Survival fractions of S. cerevisiae (a and b) and D. bruxellensis (c and d) cells after a PEF treatment at 12.0 kV/cm for 50 pulses in citrate-phosphate buffers of different compositions with 2,000 ppm of sorbic acid added, giving final pHs of 4.6 (a and c) and 3.8 (b and d), and subsequent incubation for 120 h at room temperature. Survivors recovered on the nonselective (closed symbols) and the selective (opened symbols) media. UT, untreated. Data are means ± standard deviations (error bars).
The synergistic effect was most pronounced at the lowest treatment medium pH assayed. As shown in Fig. 6b and d, survivors of the PEF treatment were progressively injured and inactivated for up to log10 1 to log10 3 extra cycles after 48 h. During the incubation for 120 h at room temperature, it was possible to inactivate more than log10 5 cycles of both yeast species.
DISCUSSION
As recently described for bacteria (10, 11), this work shows that PEF treatments also cause sublethal injury in yeasts. Therefore, yeast inactivation by PEF is not an all-or-nothing event. Sublethal injury measured by using a selective medium plating technique is supposed to be a consequence of the loss of membrane integrity and functionality (21). Therefore, the detection of sublethally injured cells after PEF confirms that membrane damage is also an important event in yeast inactivation by PEF.
Under the treatment conditions investigated, PEF caused the occurrence of sublethally injured cells at any pH investigated. These results differ from those obtained for bacteria. García et al. (11) observed characteristic behaviors as a function of the microorganism and the treatment medium pH. Thus, while Listeria monocytogenes and Bacillus subtilis were sublethally injured only when exposed to PEF at pH 7.0 but not at pH 4.0, the six gram-negative bacterium species studied were sublethally injured at pH 4.0 but not at pH 7.0. At the pHs associated with sublethal injury, the eight species of bacteria showed their maximum levels of PEF resistance. As shown in Fig. 1, 2, and 3, the levels of PEF resistance of the two yeasts investigated in this study were scarcely influenced by the treatment medium pH, and the occurrence of sublethal injury was detected at any pH investigated. These results suggest that the way PEF treatment kills yeasts and how the mechanism is influenced by environmental factors, such as the treatment medium pH, may be different from those observed for bacteria.
Nevertheless, the occurrence of sublethal injuries in the yeast membranes provides new useful data that contribute to the understanding of the mechanisms of microbial electroporation. Moreover, as previously described for PEF-treated bacteria (11), the detection of sublethal injury may help clarify the environmental circumstances under which PEF may act synergistically with other processes for food preservation. For instance, García et al. (10) observed that the maintenance of E. coli survivors of the PEF treatment in the same treatment medium at pH 4.0 for 48 h caused the inactivation of the sublethally injured cell population, thereby increasing the degree of inactivation exponentially with incubation time. However, as shown in Fig. 5, this strategy was not a valuable alternative for increasing the degree of yeast inactivation: both sublethally injured S. cerevisiae cells and D. bruxellensis cells were able to repair their damage when suspended in citrate-phosphate buffer at pH 4.0, regaining the ability to withstand the presence of the sodium chloride in the selective recovery medium.
Combined processes must incorporate conditions to prevent the repair of cell damage by inhibiting the necessary synthesis of new components and energy, etc., or to cause the final inactivation of the damaged cells through the augmentation of their sensitivity to other treatments. In this vein, PEF treatments have been combined with the addition of food preservatives in order to increase the degree of inactivation of some bacteria (7, 9, 14, 18, 19, 23). According to Liu et al. (19), membranes permeabilized under PEF may facilitate the entrance of antimicrobial compounds, such as organic acids, thus increasing their antimicrobial action. To the best of our knowledge, no attempts to evaluate the combined effect of PEF and organic acids on yeast inactivation have been carried out.
Sorbic acid and its salts are some of the most-used preservatives in the food industry due to the neutral taste characteristics and the effective inhibition of most molds and yeasts and some bacteria (20). Our results have shown that concentrations lower than 1,000 ppm did not sensitize S. cerevisiae and D. bruxellensis cells during 120 h of incubation at room temperature. The presence of 2,000 ppm at pH 4.6 did not sensitize both yeasts either, but this concentration injured and/or inactivated up to log10 5 cycles of yeast cells when the treatment medium pH was 3.8 (Fig. 4). This finding was consistent with the classical weak-acid theory, which suggests that the undissociated forms, present in a higher proportion as the pH decreases, are the molecules effective against microorganisms. D. bruxellesis, which had shown a higher level of PEF resistance at any pH investigated (Fig. 3), was more sensitive to the presence of sorbic acid than S. cerevisiae, showing a degree of inactivation of more than log10 5 cycles of yeast cells after 120 h of incubation (Fig. 4).
The use of food preservatives usually means to achieve the inhibition of microbial growth rather than the inactivation of microbial cells. However, under the experimental conditions assayed in this study, sorbic acid at a concentration of 2,000 ppm at pH 3.8 showed a killing effect on yeast cells. On the other hand, the fact that sorbic acid also caused the occurrence of sublethal injury in S. cerevisiae cells (Fig. 4b) is a relevant aspect never before described. The occurrence of sublethal injury means that sorbic acid affects the integrity and/or the functionality of the cytoplasmic membrane. These results support the hypothesis of Stratford et al. (27), which suggested that sorbic acid might act as a membrane-active substance rather than as a classical weak-acid preservative.
A synergistic killing effect of PEF and sorbic acid on both yeast species investigated has been observed: the inactivating effect of the combined treatment was greater than the sum of that of PEF and that of sorbic acid and the subsequent holding step. For instance, at pH 4.6, whereas PEF treatment at 12.0 kV/cm for 50 pulses caused the inactivation of fewer than log10 1 cycles and the incubation in the presence of sorbic acid (2,000 ppm) did not affect the survival of either microorganism, the combined treatment caused the inactivation of up to log10 3 cell cycles. As shown in Fig. 6a and c, most sublethally PEF-injured cells died in the presence of 2,000 ppm of sorbic acid after 5 h of incubation at room temperature. These results confirm that the detection of sublethal injury after PEF contributes to the identification of those treatment conditions under which PEF may act synergistically with other processes for food preservation.
In our opinion, the synergistic effect at pH 4.6 may be due either to the fact that sorbic acid acts on the damaged structures by increasing the degree of injury and, finally, inactivating the sublethally PEF-injured cells or to the fact that the altered membranes of sublethally injured cells may facilitate the entrance of a greater proportion of sorbic acid, even the dissociated forms, thereby increasing the inactivating effect. The classical weak-acid theory suggests that undissociated acid molecules are lipophilic and pass through the plasma membrane readily, affecting the cells in ways different from those of dissociated forms (20). According to the second hypothesis, membrane permeabilization caused by PEF may facilitate the entrance of both dissociated and undissociated forms, thus allowing their specific antimicrobial actions. Liu et al. (19) also described a synergistic killing effect of PEF and organic acids, such as sorbic and benzoic acid, on E. coli O157:H7 at pH 3.4, but not at pH 6.4.
The combination of PEF and 2,000 ppm of sorbic acid at pH 3.8 also allows us to describe a synergistic killing effect. As shown in Fig. 6, the combined treatment not only inactivated the sublethally PEF-injured cells, but it also injured and inactivated a new proportion of the surviving cell population. The combined treatment guaranteed the inactivation of more than log10 5 cycles of both yeast species. Under these treatment conditions, the synergistic inactivating effect seems to be due to the sum of diverse effects. PEF caused partial cell inactivation (fewer than log10 1 cycles) and the occurrence of a large proportion of sublethally injured cells (up to log10 2 extra cycles). As previously suggested, sorbic acid not only caused the inactivation of the sublethally PEF-injured cells, but it also injured a fraction of the surviving population, which would later be inactivated by the subsequent incubation at room temperature. The final inactivating effect was greater than the sum of the effects of all treatments acting separately.
We have described for the first time the occurrence of sublethal injury in yeast cells after exposure to PEF. The proportion of sublethally injured cells after PEF treatment depended on the type of yeast, the pH of the treatment medium, the treatment time, and the electric field strength used. Moreover, these studies confirm that membrane damage is an important event in the inactivation of yeast by PEF. The fact that sublethal injury after PEF treatments has been detected is a very relevant aspect to be taken into account, since, from a practical point of view, the occurrence of sublethally injured cells means that PEF may act synergistically with other treatments, thereby proving to be valuable in the development of appropriate combination processes. Our results have also demonstrated that sorbic acid, at a concentration of 2,000 ppm, may injure and inactivate yeast cells as a function of the treatment medium pH. We have described a new synergistic killing effect on yeast of the combination of PEF, sorbic acid at a concentration of 2,000 ppm, and subsequent incubation at room temperature for 120 h.
Acknowledgments
This work was supported by Framework Programme 6 (015710-2NOVELQ), CICYT (AGL2006-08856), and Ministerio Español de Educación, Cultura y Deporte, which provided M. Somolinos with a grant to carry out this investigation.
Our thanks to J. M. Soriano for his revision of the English manuscript.
Footnotes
Published ahead of print on 27 April 2007.
REFERENCES
- 1.Aronsson, K., and U. Rönner. 2001. Influence of pH, water activity and temperature on the inactivation of Escherichia coli and Saccharomyces cerevisiae by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2:105-112. [Google Scholar]
- 2.Aronsson, K., U. Rönner, and E. Borch. 2005. Inactivation of Escherichia coli, Listeria innocua and Saccharomyces cerevisiae in relation to membrane permeabilization and subsequent leakage of intracellular compounds due to pulsed electric field processing. Int. J. Food Microbiol. 99:19-32. [DOI] [PubMed] [Google Scholar]
- 3.Barbosa-Cánovas, G. V., M. M. Góngora, U. R. Pothakamury, and B. G. Swanson. 1999. Preservation of foods with pulsed electric fields. Academic Press, San Diego, CA.
- 4.Chatonnet, P., D. Dubourdieu, and J. N. Boidron. 1995. The influence of Brettanomyces/Dekkera sp. yeasts and lactic acid bacteria on the ethylphenol content of red wines. Am. J. Enol. Vitic. 46:463-468. [Google Scholar]
- 5.Condón, S., A. Palop, J. Raso, and F. J. Sala. 1996. Influence of the incubation temperature after heat treatment upon the estimated heat resistance values of spores of Bacillus subtilis. Lett. Appl. Microbiol. 22:149-152. [Google Scholar]
- 6.Dawson, R. M. C., D. C. Elliot, W. H. Elliot, and K. M. Jones. 1974. Data for biochemical research. Oxford at Clarendon Press, Oxford, United Kingdom.
- 7.Dutreux, N., S. Notermans, M. M. Góngora-Nieto, G. V. Barbosa-Cánovas, and B. G. Swanson. 2000. Effects of combined exposure of Micrococcus luteus to nisin and pulsed electric fields. Int. J. Food Microbiol. 60:147-152. [DOI] [PubMed] [Google Scholar]
- 8.Elez-Martínez, P., J. Escolá-Hernández, R. C. Soliva-Fortuny, and O. Martín-Belloso. 2004. Inactivation of Saccharomyces cerevisiae suspended in orange juice using high-intensity pulsed electric fields. J. Food Prot. 67:2596-2602. [DOI] [PubMed] [Google Scholar]
- 9.Fernández-Molina, J. J., B. Altunakar, D. Bermúdez-Aguirre, B. G. Swanson, and G. V. Barbosa-Cánovas. 2005. Inactivation of Pseudomonas fluorescens in skim milk by combinations of pulsed electric fields and organic acids. J. Food Prot. 60:1232-1235. [DOI] [PubMed] [Google Scholar]
- 10.García, D., N. Gómez, S. Condón, J. Raso, and R. Pagán. 2003. Pulsed electric fields cause sublethal injury in Escherichia coli. J. Appl. Microbiol. 36:140-144. [DOI] [PubMed] [Google Scholar]
- 11.García, D., N. Gómez, P. Mañas, S. Condón, J. Raso, and R. Pagán. 2005. Occurrence of sublethal injury after pulsed electric fields depending on the microorganism, the treatment medium pH and the intensity of the treatment investigated. J. Appl. Microbiol. 99:94-104. [DOI] [PubMed] [Google Scholar]
- 12.García, D., M. Hassani, P. Mañas, S. Condón, and R. Pagán. 2005. Inactivation of Escherichia coli O157:H7 during the storage under refrigeration of apple juice treated by pulsed electric fields. J. Food Safety 25:30-42. [Google Scholar]
- 13.García, D., N. Gómez, P. Mañas, J. Raso, and R. Pagán. 2007. Pulsed electric fields cause bacterial envelope permeabilization depending on the treatment intensity, the treatment medium pH and the microorganism investigated. Int. J. Food Microbiol. 113:219-227. [DOI] [PubMed] [Google Scholar]
- 14.Gásková, D., K. Sigler, B. Janderová, and J. Plásek. 1996. Effect of high-voltage electric pulses on yeast cells: factors influencing the killing efficiency. Bioelectrochem. Bioenerg. 39:195-202. [Google Scholar]
- 15.Hamilton, W. A., and A. J. H. Sale. 1967. Effects of high electric fields on microorganisms. II. Killing of bacteria and yeasts. Biochim. Biophys. Acta 148:789-800. [Google Scholar]
- 16.Heinz, V., I. Álvarez, A. Angersbach, and D. Knorr. 2002. Preservation of liquid foods by high intensity pulsed electric fields: basic concepts for process design. Trends Food Sci. Technol. 12:103-111. [Google Scholar]
- 17.Ho, S. Y., and G. S. Mittal. 1996. Electroporation of cell membranes: a review. Crit. Rev. Biotechnol. 16:349-362. [DOI] [PubMed] [Google Scholar]
- 18.Liang, Z., G. S. Mittal, and M. W. Griffiths. 2002. Inactivation of Salmonella typhimurium in orange juice containing antimicrobial agents by pulsed electric fields. J. Food Prot. 65:1081-1087. [DOI] [PubMed] [Google Scholar]
- 19.Liu, X., A. E. Yousef, and G. W. Chism. 1997. Inactivation of Escherichia coli O157:H7 by the combination of organic acids and pulsed electric fields. J. Food Safety 16:287-299. [Google Scholar]
- 20.Lund, B. M., and T. Eklund. 2000. Control of pH and use of organic acids, p. 175-200. In M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food. Aspen Publisher, Inc., Gaithersburg, MD.
- 21.Mackey, B. M. 2000. Injured bacteria, p. 315-341. In M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food. Aspen Publisher, Inc., Gaithersburg, MD.
- 22.Pagán, R., and P. Mañas. 2006. Fundamental aspects of microbial membrane electroporation, p. 73-94. In J. Raso and V. Heinz (ed.), Pulsed electric fields technology for the food industry. Springer Science & Business Media, LLC, New York, NY.
- 23.Pol, I. E., H. C. Mastwijk, P. V. Bartels, and E. J. Smid. 2000. Pulsed-electric field treatment enhances the bactericidal action of nisin against Bacillus cereus. Appl. Environ. Microbiol. 66:428-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin, B.-L., G. V. Barbosa-Cánovas, B. G. Swanson, P. D. Pedrow, and R. G. Olsen. 1998. Inactivating microorganisms using a pulsed electric field continuous treatment system. IEEE Trans. Ind. Appl. 34:43-50. [Google Scholar]
- 25.Raso, J., I. Álvarez, S. Condón, and F. J. Sala. 2000. Predicting inactivation of Salmonella senftenberg by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 1:21-30. [DOI] [PubMed] [Google Scholar]
- 26.Sale, A. J. H., and W. A. Hamilton. 1967. Effects of high electric fields on microorganisms. I. Killing of bacteria and yeast. Biochim. Biophys. Acta 148:781-788. [Google Scholar]
- 27.Stratford, M., P. D. Hofman, and M. B. Cole. 2000. Fruit juices, fruit drinks, and soft drinks, p. 836-869. In M. Lund, T. C. Baird-Parker, and G. W. Gould (ed.), The microbiological safety and quality of food. Aspen Publisher, Inc., Gaithersburg, MD.
- 28.Tsong, T. Y. 1991. Electroporation of cell membranes. Biophys. J. 24:271-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver, J. C., and Y. A. Chizmadzhev. 1996. Theory of electroporation: a review. Bioelectrochem. Bioenerg. 41:135-160. [Google Scholar]
- 30.Wouters, P. C., I. Alvarez, and J. Raso. 2001. Critical factors determining inactivation kinetics by pulsed electric field food processing. Trends Food Sci. Technol. 12:112-121. [Google Scholar]