Abstract
The probiotic Lactobacillus rhamnosus GG is able to bind the potent hepatocarcinogen aflatoxin B1 (AFB1) and thus potentially restrict its rapid absorption from the intestine. In this study we investigated the potential of GG to reduce AFB1 availability in vitro in Caco-2 cells adapted to express cytochrome P-450 (CYP) 3A4, such that both transport and toxicity could be assessed. Caco-2 cells were grown as confluent monolayers on transmembrane filters for 21 days prior to all studies. AFB1 levels in culture medium were measured by high-performance liquid chromatography. In CYP 3A4-induced monolayers, AFB1 transport from the apical to the basolateral chamber was reduced from 11.1% ± 1.9% to 6.4% ± 2.5% (P = 0.019) and to 3.3% ± 1.8% (P = 0.002) within the first hour in monolayers coincubated with GG (1 × 1010 and 5 × 1010 CFU/ml, respectively). GG (1 × 1010 and 5 × 1010 CFU/ml) bound 40.1% ± 8.3% and 61.0% ± 6.0% of added AFB1 after 1 h, respectively. AFB1 caused significant reductions of 30.1% (P = 0.01), 49.4% (P = 0.004), and 64.4% (P < 0.001) in transepithelial resistance after 24, 48, and 72 h, respectively. Coincubation with 1 × 1010 CFU/ml GG after 24 h protected against AFB1-induced reductions in transepithelial resistance at both 24 h (P = 0.002) and 48 h (P = 0.04). DNA fragmentation was apparent in cells treated only with AFB1 cells but not in cells coincubated with either 1 × 1010 or 5 × 1010 CFU/ml GG. GG reduced AFB1 uptake and protected against both membrane and DNA damage in the Caco-2 model. These data are suggestive of a beneficial role of GG against dietary exposure to aflatoxin.
Mycotoxins are fungal metabolites contaminating 25% of cereals and grains destined for human consumption (3). One of the most toxic groups of mycotoxins are the aflatoxins (20, 47), and aflatoxin B1 (AFB1), the most toxic and carcinogenic of the naturally occurring aflatoxins, has been classified as a class 1A human carcinogen by the International Agency for Research on Cancer (20). Aflatoxins additionally cause growth impairment and immune suppression in a range of animal species (48). In West Africa, dietary aflatoxin exposure is frequent and occurs at high levels (12, 13, 38-42) and has been associated with growth faltering (12, 13, 39) and immune suppression (39) in exposed children. Aflatoxins are efficiently absorbed in the intestinal tract, probably by passive diffusion (19, 32). AFB1 is subsequently metabolized predominantly by cytochrome P-450 (CYP) enzyme systems (16) to produce a range of metabolites, including AFM1 (by CYP 1A2) and AFQ1 (by CYP 3A4). Aflatoxicol (AFL), regarded as a reservoir for AFB1, can also be generated by cytosolic reductases (5). However, the toxicity of AFB1 is generally regarded to occur via production of the highly reactive AFB1-8,9-epoxide (predominantly via CYP 3A4 but also by CYP 1A2), which forms covalent adducts with macromolecules such as proteins and DNA (5). This reactive epoxide is capable of causing damage to cells in the liver and at the intestinal interface. Given the observations of an association between aflatoxin exposure and growth faltering in West African infants (12, 13), it is possible that direct damage within the intestine may be occurring that alters nutrient uptake or leads to the “intestinal leakiness” that has been associated with growth faltering in Gambian infants by Lunn (25) at the time when weaning foods are introduced. Such weaning foods are commonly less hygienic than breast milk and additionally are likely to be contaminated with aflatoxins at high levels (12, 13). Considering the multiple adverse effects of aflatoxin exposure, the reduction of intestinal uptake and transport to the body are of major interest to reduce or prevent toxic events.
Intervention approaches at the individual level use either sorbent materials to prevent aflatoxin absorption in the gastrointestinal tract (enterosorption) or compounds that alter activation or detoxification of AFB1 (chemoprotection) (48). There is considerable interest in the protective role of probiotic bacteria in humans (33). Our previous work on >250 strains of lactic acid bacteria isolated from either dairy products or healthy human microbiota revealed that the efficacy of aflatoxin binding was highly variable depending on the genus and strain of bacteria. Two Lactobacillus rhamnosus strains, GG and LC-705, were the most efficient strains in binding a range of mycotoxins, including aflatoxins (6, 9, 10, 18, 28, 29). Carbohydrate and protein components of the bacterial surface are important for AFB1 binding (17, 18), and heat treatment does not reduce this binding (6, 23). Lactobacillus rhamnosus GG is currently used in various dairy products including yogurt and is therefore a good candidate for assessing protective effects with a view to its use in humans. Additionally, evidence from animal studies showed that AFB1 binding by probiotic bacteria successfully reduces tissue uptake of AFB1 in the duodenum of chicks (8, 14).
The aim of this study was to investigate the modulation of AFB1 transport by GG in the human intestinal cell line Caco-2. This cell line has previously been used as an in vitro model for studying the intestinal absorption of xenobiotics, including mycotoxins (1, 26). In this study, we used Caco-2 cells as a confluent monolayer and allowed differentiation to occur for 21 days to develop an apical brush border and to allow tight junction formation between cells (34). Further, we utilized the Caco-2 cell model to investigate the modulation of AFB1 metabolism and intestinal cell toxicity in the presence of GG. Since the expression of aflatoxin-metabolizing enzymes in the Caco-2 cells differs from that seen for normal human enterocytes, induction of one of the key activating enzymes (CYP 3A4) was established by preincubation with 1α,25-dihydroxy vitamin D3 [1,25(OH)2D3] (35).
MATERIALS AND METHODS
Bacterial strains.
Lactobacillus rhamnosus strain GG (ATCC 53013) was obtained from Valio Ltd. (Helsinki, Finland) as lyophilized powder and stored at −80°C. Bacteria were weighed, suspended in phosphate-buffered saline, boiled for 1 h in a water bath, and centrifuged (3,000 × g for 7 min, 4°C). After being washed once with culture medium (Dulbecco's modified Eagle's medium [DMEM]; Cambrex Bio Science, Verviers, Belgium) containing antibiotics/antimycotics (0.2% penicillin, streptomycin, amphotericin B; Invitrogen, Paisley, United Kingdom), bacteria were centrifuged again and then suspended in DMEM containing antibiotics, resulting in final concentrations of 1 × 1010 or 5 × 1010 CFU/ml.
Cell line and culture conditions.
The human colon cancer cell line Caco-2 (ATCC HTB-37) was cultivated according to standard procedures in DMEM with 20% fetal bovine serum (Invitrogen, Paisley, United Kingdom) and 0.2% antibiotic/antimycotic, and cultures were kept at 37°C at 5% CO2. Cells were grown in 12-well plates until confluent monolayers formed and cells differentiated into small intestinal cells containing microvilli in 21 days.
Cytochrome 3A4 induction with 1,25(OH)2D3.
The Caco-2 cell line has a low expression of the key enzyme CYP 3A4, which generates the aflatoxin epoxide. This enzyme was induced by adding 0.25 μM 1,25(OH)2D3 in DMEM (35) on day 21 of culture and incubating for 2 or 5 days prior to the addition of AFB1 and GG in culture medium. In all experiments, the bacterial suspension was applied to Caco-2 cells first and immediately followed by AFB1 solution in DMEM prior to further incubation.
Uptake of AFB1 and formation of free metabolites.
For transport experiments, cells were grown on Transwell filter inserts (12-mm diameter, 0.4-μm pore size; Corning Inc., Corning, NY) with medium volumes of 1 ml in the apical and 2 ml in the basolateral chamber. Medium was changed three times per week, and cells were differentiated for 21 days. Following induction with 1,25(OH)2D3 for 2 days, the transport of AFB1 from the apical to the basolateral side was investigated. Either AFB1 (150 μM) alone in culture medium or GG suspension (1 × 1010 or 5 × 1010 CFU/ml) and AFB1 (150 μM) in culture medium were added to the apical chamber. In order to avoid bacteria settling on the bottom of the filter inserts and physically blocking transport of AFB1 through the epithelial monolayer, cell culture dishes were placed on a plate shaker (shaking at 300 rpm) during incubations. Aliquots (15 μl) of culture medium were taken from both chambers at 0.5, 1, 24, 48, and 72 h after the addition of AFB1 and GG. To quantify free AFB1, AFM1, and AFL, aliquots were centrifuged, and the supernatant was diluted with Milli-Q water when necessary and analyzed using high-performance liquid chromatography (HPLC). For the analysis of AFB1 bound by GG in the apical chamber, the bacterial pellet was extracted twice with 0.5 ml methanol and analyzed by HPLC. The HPLC conditions used were as described previously (27). To assess the transport of AFB1 through the monolayer, permeability coefficients (Pe) were calculated according to the following equation: Pe = Vd/A × Δ%/Δt, where Vd is the volume of the donor compartment, A is the surface area of the monolayer, and Δ%/Δt is the percentage of mass transported per second (26). The identities of metabolites (AFM1 and AFL) were confirmed via spiking samples of cell culture medium with the respective aflatoxin standards. Amounts of metabolites were calculated by comparing peak areas of samples with peak areas of a standard.
TER.
The integrity of the epithelial monolayer was assessed by measuring the electrical potential difference (transepithelial resistance [TER]) between the apical and basolateral chambers of the two-chamber model. Once Caco-2 cells form a confluent monolayer, they start differentiating and producing tight junctions, which results in an increase in TER. TER was monitored using an EVOM TER-meter (World Precision Instruments, Sarasota, FL). In untreated cells, the TER is known to increase until tight junction formation between cells is complete, which takes 21 days; TER levels then plateau for at least 8 days (31). TER readings were recorded over the first 21-day differentiation process, after induction with 1,25(OH)2D3 and after the addition of either AFB1 (150 μM) alone or GG (1 × 1010 or 5 × 1010 CFU/ml) plus AFB1 (150 μM) as described above. TER measurements were always conducted in fresh culture medium and after bacteria were removed by washing the Caco-2 monolayer with phosphate-buffered saline (five washes with 0.5 ml). Following the final TER measurement, cells were trypsinized and cell viability was assessed using a trypan blue exclusion assay. Cell viability was always high (>90% viability).
AFB1-induced DNA damage.
Differentiated Caco-2 cells were cultured in the presence of 1,25(OH)2D3 for 5 days prior to AFB1 exposure. Following 72 h of incubation with AFB1 alone (150 μM) or with GG (1 × 1010 or 5 × 1010 CFU/ml) and AFB1 (150 μM), the cells were harvested using trypsin-EDTA and viability was assessed with trypan blue staining. Only cell cultures with viabilities of >90% were used for further assays. DNA damage was assessed using a DNA fragmentation assay. The DNA fragmentation assay includes lysis of the cells, extraction of DNA with phenol-chloroform, and gel electrophoresis as previously described in detail (24).
Statistics.
SPSS 11.5 for Windows was used for the statistical analysis of the data. Results were subjected to the Mann-Whitney U test. Differences in mean values are considered significant at P values of ≤0.05.
RESULTS
AFB1 binding.
In this study, the effect of nonviable probiotic bacteria on the transport, metabolism, and toxicity of AFB1 in Caco-2 cells grown on a two-chamber dish was investigated. Following apical incubation of Caco-2 cells with either AFB1 alone or GG plus AFB1, samples were taken from the apical chamber to assess AFB1 binding by GG and from the basolateral chamber to assess AFB1 transport across the epithelium. AFB1 bound by GG in the apical chamber was determined from the GG pellet after centrifugation (Fig. 1). GG binding of AFB1 in the apical chamber was apparent by the first time point (0.5 h; data not shown) and was similar in magnitude to that at 1 h. At 1 h, GG at 5 × 1010 CFU/ml had bound significantly (P = 0.002) more of the total AFB1 (61.0% ± 7.0%) than did GG at 1 × 1010 CFU/ml (40.1% ± 2.2%). After 24 h, there was a reduction in the total binding of AFB1 to GG for both the higher GG concentration (40.9% ± 2.2% of total AFB1) and the lower GG concentration (18.0% ± 8.0% of total AFB1). There was no significant change in the amount of AFB1 bound to GG at the later time points (data not shown). The overall mean recovery from each test well combining quantities within the media (apical and basolateral) and the pellet was 96.3%, though a number of samples at the earlier time point (1 h) had recoveries slightly greater than 100%. GG does not bind AFB1 covalently, and for this reason the GG pellet could not be exhaustively washed to remove free AFB1 within the pellet prior to the extraction of bound AFB1. Particularly at high concentrations of apical AFB1, as observed at the 1-h time point, this may have caused an overestimate of total AFB1 recovery.
FIG. 1.
Percentages of AFB1 (150 μM) recovered free from culture media of apical and basolateral chambers and bound by 0, 1 × 1010, or 5 × 1010 CFU/ml GG in the apical chamber of the Caco-2 culture dish. Apical and basolateral culture media were removed after 1 or 24 h of incubation and centrifuged, and supernatant was injected into HPLC. The bacterial pellet of the apical chamber was extracted with methanol to remove AFB1 bound by GG. A pellet was not obtained for the apical sample with no added GG. Values are presented as means of two replicates and two repeated experiments; error bars refer to standard deviation.
AFB1 transport.
The transport of AFB1 to the basolateral side increased with time such that after 1 h 11.1% ± 1.9% of total AFB1 had transferred; this transport increased to 38.7% ± 5.5% after 24 h (Fig. 1). After 48 and 72 h, there was no significant change in concentration. The amounts of AFB1 transported were significantly affected in the presence of GG, such that at 1 h the basolateral chamber contained less AFB1 with both 1 × 1010 CFU/ml GG (6.4% ± 2.5% of total AFB1) and 5 × 1010 CFU/ml GG (3.3% ± 1.8% of total AFB1) (P = 0.019 and P = 0.002, respectively) than what was seen for AFB1-only treatments. After 24 h, the reduction in AFB1 transport was less pronounced and significant only in the presence of 5 × 1010 CFU/ml GG (24.6% ± 5.6% [P = 0.01]). At later time points, AFB1 transport remained stable (data not shown). A permeability coefficient was calculated to estimate the transport of aflatoxin through the monolayer (Fig. 2). The permeability of AFB1 (24.6 × 10−6 ± 3.3 × 10−6 cm/s) was reduced slightly in the presence of 1 × 1010 CFU/ml (17.2 × 10−6 ± 6.1 × 10−6 cm/s [P = 0.06]) and significantly with 5 × 1010 CFU/ml (7.0 × 10−6 ± 4.8 × 10−6 cm/s [P = 0.005]) GG.
FIG. 2.
Pe for AFB1 (150 μM) transport through the Caco-2 monolayer calculated according to the following equation: Pe = Vd/A × Δ%/Δt, where Vd is the volume of the donor compartment, A is the surface area of the monolayer, and Δ%/Δt is the percentage of mass transported per second (27). Values are presented as means of two replicates and two repeated experiments; error bars refer to standard deviation.
Formation of free metabolites.
Following incubation with 150 μM AFB1, the release of free metabolites into the culture medium on both the apical and basolateral chambers was also measured. Without bacteria present, AFM1 and AFL were detectable in the culture media of both chambers, with basolateral concentrations of 17.4 ± 3.2 nM, 130.7 ± 5.9 nM, and 170.6 ± 16.5 nM for AFM1 and 46.0 ± 1.2 nM, 238.0 ± 7.6 nM, and 363.2 ± 35.2 nM for AFL after 24, 48, and 72 h of incubation, respectively (Fig. 3A and B). There was a GG dose-dependent reduction in levels of AFL in both chambers (basolateral only shown in Fig. 3A) at all time points. After 72 h of incubation, basolateral AFL concentration was reduced significantly to 259.3 ± 30.2 nM (P = 0.004) with 1 × 1010 CFU/ml GG and to 153.1 ± 15.6 nM (P = 0.002) with 5 × 1010 CFU/ml GG from what was seen for AFB1-only treatment (363.2 ± 35.2 nM). Surprisingly, the basolateral AFM1 concentration was increased after 72 h of incubation from 170.6 ± 16.5 nM with AFB1 only to 361.8 ± 36.0 nM (P = 0.002) in the presence of 1 × 1010 CFU/ml GG and to 248.2 ± 20.4 nM in the presence of 5 × 1010 CFU/ml GG (significantly [P = 0.01] lower than what was seen for 1 × 1010 CFU/ml GG) (Fig. 3B).
FIG. 3.
Effect of heat-treated Lactobacillus rhamnosus strain GG (1 × 1010 [1GG] and 5 × 1010 [5GG] CFU/ml) on the formation of AFL (A) and AFM1 (B) in differentiated Caco-2 cells measured in culture medium of a basolateral chamber at three time points (24, 48, and 72 h) after AFB1 addition (150 μM). Values are presented as means of three replicates and two repeated experiments; error bars refer to standard deviation.
Intestinal cell toxicity.
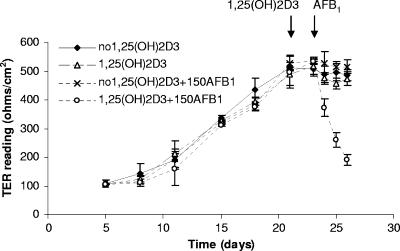
To assess the toxic effect of AFB1 on Caco-2 cells, we studied the integrity of the cell monolayer, by use of TER measurements, and AFB1-induced DNA damage. TER readings increased over 3 weeks of differentiation of the Caco-2 cells and reached a plateau towards day 21 (502 ± 40 Ω/cm2) (Fig. 4). Further incubation of cells with or without 1,25(OH)2D3 for 2 days had no significant effect on the TER (488 ± 28 and 496 ± 20 Ω/cm2), and further growth for 3 days in the absence of AFB1 or after adding AFB1 to cells without prior 1,25(OH)2D3 preactivation resulted in no significant change in TER. In Caco-2 cells induced with 1,25(OH)2D3 for 2 days and subsequently incubated with 150 μM AFB1, significant decreases in TER by 31.1% ± 3.3% (P = 0.01), 52.0% ± 2.9% (P = 0.004), and 64.4% ± 5.2% (P < 0.001) were observed at 24, 48, and 72 h after AFB1 addition, respectively. When bacteria were coincubated with AFB1, the TER reduction caused by AFB1 alone was significantly attenuated. With 1 × 1010 CFU/ml GG after 24 h of incubation, the TER was reduced by 18.4% ± 2.1%, compared to 31.1% ± 3.3% without GG (P = 0.002), and after 48 h the TER was reduced by 48.8% ± 1.6%, compared to 52.0% ± 2.9% without GG (P = 0.04) (Fig. 5). Higher bacterial numbers and longer incubation times were not suitable for this assay, since greater quantities of bacteria influenced TER readings, independent of the presence of AFB1 (data not shown).
FIG. 4.
Change in TER during 21-day differentiation of Caco-2 cells and induction with 1,25(OH)2D3 for 2 days and following incubation with AFB1 (150 μM) (150AFB1) for 3 days. TER was measured in fresh culture media of apical and basolateral chambers. Values are presented as means of three replicates and two repeated experiments; error bars refer to standard deviation.
FIG. 5.
Effect of heat-treated Lactobacillus rhamnosus strain GG (1 × 1010 CFU/ml) on AFB1 (150 μM)-induced reduction in TER in differentiated Caco-2 cells [following induction with 1,25(OH)2D3 for 48 h]. TER was measured in fresh culture medium. Values are presented as means of three replicates and two repeated experiments; error bars refer to standard deviation.
DNA damage.
As a marker of AFB1-induced DNA damage in the cells, DNA fragmentation of Caco-2 cells exposed to AFB1 following 1,25(OH)2D3 activation for 5 days was assessed using the DNA fragmentation assay. DNA fragmentation was observed for cells treated with 150 μM AFB1 for 72 h (Fig. 6, lane 2) but not for cells treated with AFB1 and GG (1 × 1010 or 5 × 1010 CFU/ml) (Fig. 6, lanes 3 and 4), or with GG alone (5 × 1010 CFU/ml) (Fig. 6, lane 5).
FIG. 6.
DNA fragmentation in 1,25(OH)2D3-induced Caco-2 cells caused by AFB1 (150 μM) and GG. Lanes: 1, untreated cells; 2, cells treated with AFB1 for 72 h; 3, cells treated with AFB1 and GG (1 × 1010 CFU/ml); 4, cells treated with AFB1 and GG (5 × 1010 CFU/ml); 5, cells treated with GG (5 × 1010 CFU/ml) only.
DISCUSSION
Following oral exposure, AFB1 is readily absorbed in the gut and reaches the liver and systemic circulatory system. In vitro cell culture models have been used to assess the bioavailability and transport of several mycotoxins (1, 26, 45). In our study, we used the Caco-2 cell culture model to study AFB1 transport and intestinal toxicity. We confirmed published results that AFB1 is transported rapidly through the intestinal cell monolayer and that permeability coefficients of AFB1 are in agreement with the literature (26, 45). The transport occurred in a time-dependent manner, though no further increase in the basolateral concentration of AFB1 occurred after 24 h. The amount of AFB1 transported to the basolateral chamber was significantly reduced when GG was added to the apical compartment. The reduction in transported AFB1 was also dependent on GG dose and at both doses of GG reached a plateau at 24 h. At the same time, additional AFB1 was recovered from the bacterial pellet, indicating that GG was able to bind AFB1 in this cell culture system and hence to reduce AFB1 transport through the intestinal cell monolayer. The binding of AFB1 by GG was initially high, with 40.1% ± 8.3% and 61.0% ± 7.0% of the total AFB1 being bound in the first hour for the lower and higher GG concentrations, respectively, but over the course of the study binding to GG was reduced to 18.0% ± 8.0% and to 40.9% ± 2.2%, respectively, after 24 h. This suggests that AFB1 is reversibly bound to the bacterial surface (24) and that as aflatoxin is transported to the basolateral chamber, the equilibrium between bound and free AFB1 in the apical chamber adjusts until the overall system is in equilibrium. The reduced transport of AFB1 in the presence of GG in this model is in agreement with our ex vivo findings showing that GG reduced AFB1 transport in duodenal loops (9). These data indicate that the Caco-2 model is useful to study the effect of bacterial binding on AFB1 absorption and could be further developed to study the role of food matrix in AFB1 uptake and bacterial binding (45).
The metabolism and toxicity of AFB1 have been studied with human cellular systems derived from liver (21, 43) or respiratory tract (44), but the impact of the intestinal metabolism and toxicity has not been fully investigated. We were able to detect AFM1 and AFL in the culture media of both the apical and basolateral chambers after incubating the apical chamber with AFB1, albeit at relatively low concentrations compared to the amount of AFB1 added. Total formation of AFM1 and that of AFL in both chambers after 72 h were only 0.4 and 0.8% of the AFB1 dose, respectively, and this likely reflects the low activity of the relevant enzymes. AFQ1, a metabolite generated by CYP 3A4, was not evident in the culture media. This was surprising, as the culture model included 1,25(OH)2D3 to upregulate CYP 3A4 expression (35). The lack of detection of this metabolite may reflect the detection limit for AFQ1, which is considerably (approximately 100-fold) higher than that for AFM1 (27). However, the fact that toxicity of AFB1 was observed only for 1,25(OH)2D3-pretreated cells is strongly supportive of an increased CYP 3A4 activity and generation of the highly reactive aflatoxin epoxide. In the presence of GG, the level of AFL in culture media was reduced, as would be predicted for a model that restricted AFB1 bioavailability. AFL can be readily oxidized back to AFB1 and has therefore been suggested as a “reservoir” for AFB1 in vivo rather than a detoxification product (5). The reduced level of this metabolite may therefore be relevant to the toxicity caused by AFB1. On the contrary, AFM1 levels in culture media increased in the presence of 1 × 1010 CFU/ml GG rather than decreased as would be expected after reduced cellular uptake of AFB1. Caloni and colleagues (1) recently reported that differentiated Caco-2 cells are particularly able to accumulate AFM1 from culture media such that the medium concentration can be reduced by up to 95% of the initial concentration. This property of AFM1 may in part explain the high frequency of its distribution into breast milk compared to that of other aflatoxin metabolites (30). It has also been shown that GG can effectively bind AFM1 (29). It is therefore possible that the equilibrium between AFM1 within the cell and that which is released into the medium, which usually favors cellular accumulation (1), is being disrupted due to the presence of GG in the medium. It is of note that AFM1 levels in the media subsequently decreased at the higher GG concentration from the level seen for the low GG concentration, further suggesting that GG is able to sequester the metabolite in this model. However, this study was not able to confirm if alterations in the levels of these metabolites reflect alterations in AFB1 availability due to GG binding or subsequent sequestering of the metabolites within the culture medium by GG or a combination of both processes. We previously observed increased fecal excretion of AFM1 in rats dosed with live GG (15); thus, the possible impact of probiotic bacteria on AFM1 formation or mobilization warrants further clarification.
The effects of GG on restricting transport of AFB1 would clearly have a positive effect on reducing the bioavailability and therefore the burden of aflatoxin-induced damage within the liver and associated hepatocarcinogenic risk. Chronic aflatoxin exposure has also been associated with growth faltering (12, 13), and in independent studies from the Gambia, intestinal enteropathy, characterized by “intestinal leakiness,” has been associated with early childhood growth faltering (2, 25). Given that (i) enterocytes have the metabolic enzymes to generate the reactive aflatoxin epoxide (4), (ii) aflatoxin exposure is frequent at high levels (38, 39) in regions reporting an association between growth faltering and intestinal leakiness (2, 25), and (iii) aflatoxin exposure has been associated with infant growth faltering (13, 14, 39), it is plausible that observations of aflatoxin-associated growth faltering may be explained at least in part by a direct action of the aflatoxin epoxide within the enterocytes. To assess this direct toxic effect of AFB1 on the Caco-2 monolayer, we studied its effect on monolayer integrity, using TER measurements. TER is a measure of epithelial barrier function, with higher TER values indicating intact tight junction complexes. The TER of the Caco-2 monolayer increased until a steady state was maintained after about 21 days (34). A decrease in TER is suggestive of damage to the tight junctions, which form part of the scaffolding between epithelial cells. Damage at this site makes the monolayer barrier more susceptible to the unregulated passage of solutes, or “leaky.” In this study, AFB1 caused a time-dependent decrease in TER if cells were induced with 1,25(OH)2D3. In the absence of 1,25(OH)2D3 pretreatment, there was no effect with AFB1 incubation, indicating the requirement for CYP 3A4 induction to generate sufficient quantities of the reactive aflatoxin epoxide. The reduction in TER was not associated with reduced cell viability, suggesting that AFB1 might act directly or indirectly on the protein scaffolding networks that maintain cell monolayer integrity. A recent study with rats suggests that the targeting of secretory proteins in hepatocytes, which are important structural and functional components of plasma membranes, was inhibited by AFB1 (36). It will be of interest to further assess aflatoxin-induced damage within the intestine in terms of junctional complex stability and barrier function, because damage to the integrity of the barrier may contribute to the intestinal enteropathy or “leakiness” observed by Lunn and colleagues (2, 25) in Gambian infants. In the presence of GG (1 × 1010 CFU/ml), the AFB1-induced decrease in TER was significantly restricted, suggesting that the bacteria were able to reduce AFB1 availability. Higher bacterial numbers (5 × 1010 CFU/ml) could not be used in this assay due to direct influence by the bacteria on TER readings.
The genotoxicity of AFB1 has been widely studied by use of hepatic cells with the Comet assay (39, 40, 43), but data from intestinal enterocytes are limited. AFB1-DNA adducts have been detected in animal and human intestinal samples (4, 22), but to our knowledge only one study in rat intestine investigated DNA damage (46). In the latter study, DNA damage was observed only in vitro, in isolated rat jejunal cells, but not in rats dosed with AFB1 in vivo. In our study, we used DNA fragmentation as a marker of AFB1-induced DNA damage in differentiated Caco-2 cells exposed to AFB1 following induction of CYP 3A4. DNA fragmentation was assessed by extracting DNA and separating intact and damaged DNA by use of gel electrophoresis. DNA damage was apparent following treatment with AFB1, while coincubation with GG reduced the AFB1-induced damage in this test system, further indicating the protective role of GG.
Our data indicate that the Caco-2 model is suitable for AFB1 transport studies evaluating the effect of probiotics. Nonviable bacteria were used because live bacteria multiply in the cell culture medium, affecting pH and nutrient availability. Nonviable GG bacteria have been shown to have an AFB1 binding capacity comparable to that of live GG bacteria (7), and thus this does not restrict their use in model systems. In fact, a major issue in the field of probiotic research is the survival of numbers of bacteria sufficient to cause colonization or to exert a beneficial physiological effect within the gut. Therefore, the possibility of using GG in a nonviable format to restrict aflatoxin uptake may be advantageous. One limitation in this study was that AFB1 was applied at a relatively high concentration and for a long time (up to 72 h). Based on the levels of CYP 3A4 induction by 1,25(OH)2D3 described in the literature (11, 35), it is likely that the levels of CYP 3A4 in our cell model are somewhat below those in intestinal cells in vivo. The use of a metabolically more active Caco-2/TC7 clone which expresses CYP 3A4 (34) might help overcome this problem for future studies. It is always difficult to directly translate the effects from a cell culture model to a role within humans. We would expect GG to colonize mainly the large intestine following repeated administration, but it might also adhere within the upper intestinal tract. The upper intestine is the major site for AFB1 absorption (34); thus, colonization of the intestinal track may impart a benefit in terms of restricting aflatoxin bioavailability. Approximately 50% of AFB1 metabolites from the liver will be excreted in the feces (mainly as AFB1-glutathione conjugate) via the bile (20). Release of the parent aflatoxin following colonic microflora activity can allow the reuptake and circulation of the parent compound. It is therefore possible that GG colonization of the large intestine will in part restrict this recirculation and shuttle GG-bound aflatoxin to be excreted via the fecal route. However, we anticipate that the maximum benefit will occur if GG is consumed immediately before or at the same time as aflatoxin-contaminated foods are consumed. This will allow the greatest opportunity for interaction within the gut lumen.
In summary, our results clearly show that AFB1 transport can be reduced by GG binding in the Caco-2 model. We found that 1,25(OH)2D3-induced Caco-2 cells can be used to estimate the intestinal metabolism and toxicity of AFB1 by use of TER measurements. The data suggest that bacterial binding of AFB1 will impact on bioavailability and therefore reduce the burden of toxicity. Amounts of GG are in line with quantities typically found in probiotic yogurts. It is important to note that the effects of GG were obtained with heat-inactivated bacteria; thus, there are no concerns of effective bacterial survival for efficacy. However, the possible role of live probiotics on intestinal AFB1 metabolism and toxicity still needs to be investigated.
Acknowledgments
This work was supported by grants to S. Gratz from the Juho Vainio Foundation and the Finnish Graduate School on Applied Bioscience: Bioengineering, Food and Nutrition, Environment.
Footnotes
Published ahead of print on 20 April 2007.
REFERENCES
- 1.Caloni, F., M. Spotti, G. Pompa, F. Zucco, A. Stammati, and I. De Angelis. 2002. Evaluation of Fumonisin B(1) and its metabolites absorption and toxicity on intestinal cells line Caco-2. Toxicon 40:1181-1188. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, D. I., M. Elia, and P. G. Lunn. 2003. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J. Nutr. 133:1332-1338. [DOI] [PubMed] [Google Scholar]
- 3.CAST. 2003. Mycotoxins: risks in plant, animal and human systems, task force report no. 139. CAST, Ames, IA.
- 4.Cupid, B. C., T. J. Lightfoot, D. Russell, S. J. Gant, P. C. Turner, K. H. Dingley, K. D. Curtis, S. H. Leveson, K. W. Turteltaub, and R. C. Garner. 2004. The formation of AFB1-macromolecular adducts in rats and humans at dietary levels of exposure. Food Chem. Toxicol. 42:559-569. [DOI] [PubMed] [Google Scholar]
- 5.Eaton, D. L., H. S. Ramsdell, and G. E. Neal. 1994. Biotransformation of aflatoxins, p. 45-71. In D. L. Eaton and J. Groopman (ed.), The toxicology of aflatoxins: human health, veterinary and agricultural significance. Academic Press, New York, NY.
- 6.El-Nezami, H., P. Kankaanpää, S. Salminen, and J. Ahokas. 1998. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food Chem. Toxicol. 36:321-326. [DOI] [PubMed] [Google Scholar]
- 7.El-Nezami, H., P. Kankaanpää, S. Salminen, and J. Ahokas. 1998. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 61:466-468. [DOI] [PubMed] [Google Scholar]
- 8.El-Nezami, H., H. Mykkänen, P. Kankaanpää, S. Salminen, and J. Ahokas. 2000. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B, from the chicken duodenum. J. Food Prot. 63:549-552. [DOI] [PubMed] [Google Scholar]
- 9.El-Nezami, H., N. Polychronaki, S. Salminen, and H. Mykkänen. 2002. Binding rather than metabolism may explain the interaction of two food-grade Lactobacillus strains with zearalenone and its derivative Ü-zearalenol. Appl. Environ. Microbiol. 68:3545-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Nezami, H. S., A. Chrevatidis, S. Auriola, S. Salminen, and H. Mykkänen. 2002. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 19:680-686. [DOI] [PubMed] [Google Scholar]
- 11.Engman, H. A., H. Lennernas, J. Taipalensuu, C. Otter, B. Leidvik, and P. Artursson. 2001. CYP3A4, CYP3A5, and MDR1 in human small and large intestinal cell lines suitable for drug transport studies. J. Pharm. Sci. 90:1736-1751. [DOI] [PubMed] [Google Scholar]
- 12.Gong, Y., A. Hounsa, S. Egal, P. C. Turner, A. E. Sutcliffe, A. J. Hall, K. Cardwell, and C. P. Wild. 2004. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environ. Health Perspect. 112:1334-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gong, Y. Y., K. Cardwell, A. Hounsa, S. Egal, P. C. Turner, A. J. Hall, and C. P. Wild. 2002. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: cross sectional study. Brit. Med. J. 325:20-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratz, S., H. Mykkänen, and H. El-Nezami. 2005. Aflatoxin B1 binding by a mixture of Lactobacillus and Propionibacterium: in vitro versus ex vivo. J. Food Prot. 68:2470-2474. [DOI] [PubMed] [Google Scholar]
- 15.Gratz, S., M. Täubel, R. O. Juvonen, M. Viluksela, P. C. Turner, H. Mykkänen, and H. El-Nezami. 2006. Lactobacillus rhamnosus strain GG modulates intestinal absorption, fecal excretion, and toxicity of aflatoxin B1 in rats. Appl. Environ. Microbiol. 72:7398-7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guengerich, F. P. 2001. Common and uncommon cytochrome P450 reactions related to metabolism and chemical toxicity. Chem. Res. Toxicol. 14:611-650. [DOI] [PubMed] [Google Scholar]
- 17.Haskard, C., C. Binnion, and J. Ahokas. 2000. Factors affecting the sequestration of aflatoxin by Lactobacillus rhamnosus strain GG. Chem. Biol. Interact. 128:39-49. [DOI] [PubMed] [Google Scholar]
- 18.Haskard, C. A., H. S. El-Nezami, P. E. Kankaanpää, S. Salminen, and J. T. Ahokas. 2001. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 67:3086-3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh, D., and J. J. Wong. 1994. Pharmacokinetics and excretion of aflatoxins, p. 73-88. In D. L. Eaton and J. Groopman (ed.), The toxicology of aflatoxins: human health, veterinary and agricultural significance. Academic Press, New York, NY.
- 20.IARC. 2002. IARC monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. IARC Monogr. 82:171. [PMC free article] [PubMed] [Google Scholar]
- 21.Knasmüller, S., V. Mersch-Sundermann, S. Kevekordes, F. Darroudi, W. W. Huber, C. Hoelzl, J. Bichler, and B. J. Majer. 2004. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology 198:315-328. [DOI] [PubMed] [Google Scholar]
- 22.Kolars, J. C., P. Benedict, P. Schmiedlin-Ren, and P. B. Watkins. 1994. Aflatoxin B1-adduct formation in rat and human small bowel enterocytes. Gastroenterology 106:433-439. [DOI] [PubMed] [Google Scholar]
- 23.Lee, Y. K., H. El-Nezami, C. A. Haskard, S. Gratz, K. Y. Puong, S. Salminen, and H. Mykkänen. 2003. Kinetics of adsorption and desorption of aflatoxin B1 by viable and nonviable bacteria. J. Food Prot. 66:426-430. [DOI] [PubMed] [Google Scholar]
- 24.Leist, M., F. Gantner, I. Bohlinger, G. Tiegs, P. G. Germann, and A. Wendel. 1995. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 146:1220-1234. [PMC free article] [PubMed] [Google Scholar]
- 25.Lunn, P. G. 2000. The impact of infection and nutrition on gut function and growth in childhood. Proc. Nutr. Soc. 59:147-154. [DOI] [PubMed] [Google Scholar]
- 26.Mata, J. E., Z. Yu, J. E. Gray, D. E. Williams, and R. Rodriguez-Proteau. 2004. Effects of chlorophyllin on transport of dibenzo(a, l)pyrene, 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine, and aflatoxin B1 across Caco-2 cell monolayers. Toxicology 196:117-125. [DOI] [PubMed] [Google Scholar]
- 27.Mykkänen, H., H. L. Zhu, E. Salminen, R. O. Juvonen, W. H. Ling, J. Ma, N. Polychronaki, H. Kemilainen, O. Mykkänen, S. Salminen, and H. El-Nezami. 2005. Fecal and urinary excretion of aflatoxin B1 metabolites (AFQ1, AFM1 and AFB-N-7-guanine) in young Chinese males. Int. J. Cancer 115:879-884. [DOI] [PubMed] [Google Scholar]
- 28.Peltonen, K., H. El-Nezami, C. Haskard, J. Ahokas, and S. Salminen. 2001. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 84:2152-2156. [DOI] [PubMed] [Google Scholar]
- 29.Pierides, M., H. El-Nezami, K. Peltonen, S. Salminen, and J. Ahokas. 2000. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Prot. 63:645-650. [DOI] [PubMed] [Google Scholar]
- 30.Polychronaki, N., R. M. West, P. C. Turner, H. Amra, M. Abdel-Wahhab, H. Mykkänen, and H. El-Nezami. A longitudinal assessment of aflatoxin M1 excretion in breast milk of selected Egyptian mothers. Food Chem. Toxicol., in press. [DOI] [PubMed]
- 31.Quaroni, A., and J. Hochman. 1996. Development of intestinal cell culture models for drug transport and metabolism studies. Adv. Drug Deliv. Rev. 22:3-52. [Google Scholar]
- 32.Ramos, A. J., and E. Hernandez. 1996. In situ absorption of aflatoxins in rat small intestine. Mycopathologia 134:27-30. [DOI] [PubMed] [Google Scholar]
- 33.Salminen, S., S. Gorbach, Y. K. Lee, and Y. Benno. 2004. Human studies on probiotics: what is scientifically proven today?, p. 515-530. In S. Salminen, A. von Wright, and A. Ouwehand (ed.), Lactic acid bacteria, microbiological and functional aspects, 3rd ed. Marcel Dekker, Inc., New York, NY.
- 34.Sambuy, Y., I. De Angelis, G. Ranaldi, M. L. Scarino, A. Stammati, and F. Zucco. 2005. The Caco-2 cell line as a model of the intestinal barrier: influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 21:1-26. [DOI] [PubMed] [Google Scholar]
- 35.Schmiedlin-Ren, P., K. E. Thummel, J. M. Fisher, M. F. Paine, K. S. Lown, and P. B. Watkins. 1997. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1alpha,25-dihydroxyvitamin D3. Mol. Pharmacol. 51:741-754. [DOI] [PubMed] [Google Scholar]
- 36.Singh, J., H. M. Dani, R. Sharma, and P. Steinberg. 2006. Inhibition of the biosynthesis of SRP polypeptides and secretory proteins by aflatoxin B1 can disrupt protein targeting. Cell Biochem. Funct. 24:507-510. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Turner, P. C., M. Mendy, H. Whittle, M. Fortuin, A. J. Hall, and C. P. Wild. 2000. Hepatitis B infection and aflatoxin biomarker levels in Gambian children. Trop. Med. Int. Health 5:837-841. [DOI] [PubMed] [Google Scholar]
- 39.Turner P. C., S. E. Moore, A. J. Hall, A. M. Prentice, and C. P. Wild. 2003. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environ. Health Perspect. 111:217-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turner, P. C., A. Sylla, M. S. Diallo, J. J. Castegnaro, A. J. Hall, and C. P. Wild. 2002. The role of aflatoxins and hepatitis viruses in the aetiopathogenesis of hepatocellular carcinoma: a basis for primary prevention in Guinea-Conakry, West Africa. J. Gastroenterol. Hepatol. 17:S441-S448. [DOI] [PubMed] [Google Scholar]
- 41.Turner, P. C., A. Sylla, Y. Y. Gong, M. S. Diallo, A. E. Sutcliffe, A. J. Hall, and C. P. Wild. 2005. Reduction in exposure to carcinogenic aflatoxins by simple post-harvest intervention measures in West Africa. Lancet 365:1950-1956. [DOI] [PubMed] [Google Scholar]
- 42.Turner, P. C., A. Sylla, S.-Y. Kuang, C. L. Marchant, M. S. Diallo, A. J. Hall, J. D. Groopman, and C. P. Wild. 2005. Absence of TP53 codon 249 mutations in Guinean infants with high aflatoxin exposure. Cancer Epidemiol. Biomarkers Prev. 14:2053-2055. [DOI] [PubMed] [Google Scholar]
- 43.Uhl, M., C. Helma, and S. Knasmüller. 2000. Evaluation of the single cell gel electrophoresis assay with human hepatoma (Hep G2) cells. Mutat. Res. 468:213-225. [DOI] [PubMed] [Google Scholar]
- 44.Van Vleet, T. R., P. J. Klein, and R. A. Coulombe. 2001. Metabolism of aflatoxin B1 by normal human bronchial epithelial cells. J. Toxicol. Environ. Health Part A 63:525-540. [DOI] [PubMed] [Google Scholar]
- 45.Versantvoort, C. H., A. G. Oomen, E. Van de Kamp, C. J. Rompelberg, and A. J. Sips. 2005. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 43:31-40. [DOI] [PubMed] [Google Scholar]
- 46.Watzl, B., C. Neudecker, G. M. Hansch, G. Rechkemmer, and B. L. Pool-Zobel. 1999. Short-term moderate aflatoxin B1 exposure has only minor effects on the gut-associated lymphoid tissue of Brown Norway rats. Toxicology 138:93-102. [DOI] [PubMed] [Google Scholar]
- 47.Wild, C. P., and P. C. Turner. 2002. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis 17:471-481. [DOI] [PubMed] [Google Scholar]
- 48.Williams, J. H., T. D. Phillips, P. E. Jolly, J. K. Stiles, C. M. Jolly, and D. Aggarwal. 2004. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 80:1106-1122. [DOI] [PubMed] [Google Scholar]