Abstract
Very little is known about the biodiversity of freshwater autotrophic picoplankton (APP) in the Laurentian Great Lakes, a system comprising 20% of the world's lacustrine freshwater. In this study, the genetic diversity of Lake Superior APP was examined by analyzing 16S rRNA gene and cpcBA PCR amplicons from water samples. By neighbor joining, the majority of 16S rRNA gene sequences clustered within the “picocyanobacterial clade” consisting of freshwater and marine Synechococcus and Prochlorococcus. Two new groups of Synechococcus spp., the pelagic Lake Superior clusters I and II, do not group with any of the known freshwater picocyanobacterial clusters and were the most abundant species (50 to 90% of the sequences) in samples collected from offshore Lake Superior stations. Conversely, at station Portage Deep (PD), located in a nearshore urbanized area, only 4% of the sequences belonged to these clusters and the remaining clones reflected the freshwater Synechococcus diversity described previously at sites throughout the world. Supporting the 16S rRNA gene data, the cpcBA library from nearshore station PD revealed a cosmopolitan diversity, whereas the majority of the cpcBA sequences (97.6%) from pelagic station CD1 fell within a unique Lake Superior cluster. Thus far, these picocyanobacteria have not been cultured, although their phylogenetic assignment suggests that they are phycoerythrin (PE) rich, consistent with the observation that PE-rich APP dominate Lake Superior picoplankton. Lastly, flow cytometry revealed that the summertime APP can exceed 105 cells ml−1 and suggests that the APP shifts from a community of PE and phycocyanin-rich picocyanobacteria and picoeukaryotes in winter to a PE-rich community in summer.
The autotrophic picoplankton (APP) are the major primary producers in the vast oligotrophic segments of the ocean and large transparent lakes (2, 35). They can account for up to 80% of the total carbon fixation in certain oceanic areas (22). The APP of both marine and freshwater ecosystems are composed predominantly of cyanobacteria <3 μm in size. In the ocean, phycoerythrin (PE)-rich marine Synechococcus strains coexist with chlorophyll (Chl) b-containing Prochlorococcus, whereas the APP of oligotrophic lakes is dominated by the PE-rich freshwater Synechococcus (10, 29, 35). Different ecotypes of Synechococcus and Prochlorococcus are adapted to specific light and nutrient regimes that exist in the euphotic zone of the ocean (18, 35). Most likely, physical and chemical gradients yield differential vertical distribution and seasonal succession of ecotypes in marine (11, 18, 25, 32, 38) and freshwater (7, 8, 29) environments. Owing to the importance of the APP for global primary production and biomass, a great body of research was focused on the genetic and physiologic biodiversity and between different picocyanobacterial ecotypes in the ocean (18); however, the freshwater APP have received far less attention (2).
Traditional cultivation methods are biased towards larger phytoplankton and phycocyanin (PC)-rich cyanobacteria that represent only a minor fraction of the APP and thus tend to underestimate the true APP ubiquity (5). Furthermore, due to their small size, physiologically and genetically diverse Synechococcus strains are impossible to differentiate by microscopy (21, 30, 31). Molecular phylogeny of the ribosomal operon DNA sequences has proven to be useful for studying cyanobacterial biodiversity as it can resolve differences between morphologically similar strains (5-7, 15, 31). In a 16S rRNA gene tree, both marine and freshwater unicellular picocyanobacteria cluster within the “picocyanobacterial clade,” which separates from the rest of the cyanobacterial radiation with 100% bootstrap support (6, 7, 36). Phylogenetic analysis of the 16S rRNA gene has also been used to study the natural APP assemblages. PCR-based amplification of picoplankton 16S rRNA gene sequences from water samples allows direct analysis of the APP community, avoiding biased culturing techniques (12, 13, 15, 31, 33, 36, 38).
In this study, we characterized the phylogenetic diversity of Lake Superior cyanobacteria inferred by 16S rRNA gene and cpcBA phycocyanin operon intergenic spacer (IGS) sequences. cpcBA sequences have been useful in resolving closely related APP into defined clusters (6). Lake Superior is ultraoligotrophic and has the largest surface area of any freshwater lake in the world. PE-rich picocyanobacteria contribute significantly to total phytoplankton biomass and primary production in Laurentian Great Lakes, including Lake Superior (3, 9, 10). Indeed, recent surveys of pelagic Lake Superior revealed that APP contribute up to 50% of total Chl (9, 24). Despite their importance, the biodiversity of the Great Lakes APP is poorly understood. The results of the phylogenetic analyses of the 16S rRNA gene and cpcBA libraries suggest the presence of two novel clusters of 16S rRNA gene sequences in Lake Superior that lie within the picocyanobacterial clade sensu Urbach et al. (36). The novel clusters dominate pelagic waters, whereas picocyanobacterial sequences obtained nearshore represent the Synechococcus sp. diversity described previously.
MATERIALS AND METHODS
Sample collection.
Samples for phylogenetic analyses were collected during two research cruises in the western arm of Lake Superior in May and September 2004 (Fig. 1). Samples for flow cytometry and for photosynthetic rate measurements were obtained during cruises in March and August 2006. In May 2004, during isothermal mixing, water was collected from a depth of 5 m at stations Sterner B (SB; 46.81°N, 92.13°W), Castle Danger 1 (CD1; 47.30°N, 91.67°W), and Western Mid-lake (WM; 47.33°N, 89.90°W). In September 2004, when the water column was stratified, water was collected from the epilimnion and deep Chl maximum (DCM) from stations CD1 (5 m and 15 m respectively) and WM (5 m and 30 m, respectively) and from the surface mixed layer (5-m depth) from station SB and nearshore Keweenaw Waterway station Portage Deep (PD; 47.06°N, 88.50°W). Water from all stations except for SB and PD was collected using a trace metal clean pumping system (34). Water from stations SB and PD was sampled using an acid-clean Teflon-coated Go-Flo bottle (General Oceanics, Miami, FL). For phylogenetic analyses, water from all stations was passed through 0.45-μm polycarbonate filters (GE Osmonics, Trevose, PA), which were then placed into 1.6-ml microcentrifuge tubes containing TE buffer (10 mM Tris-HCl [pH.7.5], 1 mM EDTA-Na2) and stored at −20°C for DNA extraction.
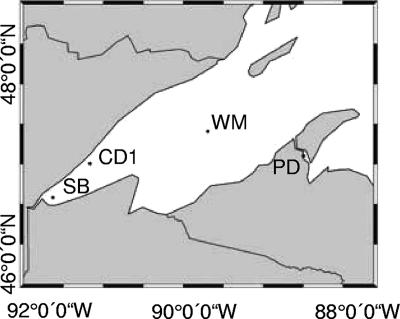
FIG. 1.
Map of Lake Superior, with the locations of hydrographic stations indicated. Stations CD1 and WM are pelagic stations. CD1 is close to shore by distance (∼10 km) but due to its depth (zmax = 249 m) and exclusion from the nearshore current exhibits the features of an offshore station. Station SB is located ∼12 km east of Duluth, MN, and station PD is positioned in the Keewenaw Waterway in the vicinity of Hancock and Houghton, MI.
Isolation of cyanobacterial strains from Lake Superior.
Whole water was collected as described above and drawn through a 0.2-μm polycarbonate filter, which was immediately placed onto a BG-11 agarose plate (1) containing 250 μg ml−1 cycloheximide, and the plates were stored in the dark until the end of the cruise. All BG-11 media were modified with reduced nitrate as described previously (16). In the laboratory, plates were incubated at 18°C in 10-μmol quanta m−2 s−1. Strains were also obtained by amendment of surface water collected from station CD1 in May 2004 with 30 μM NaNO3, 8 μM K2HPO3, 10 nM FeCl3, and 250 μg liter−1 cycloheximide. After 4 weeks of room temperature incubation under continuous illumination at 5 to 10 μmol quanta m−2 s−1, the culture was centrifuged at 3,000 × g for 15 min to collect picocyanobacteria and the sediment was spread onto BG-11 plates containing cycloheximide as described above. When single colonies of picocyanobacteria formed on plates, they were transferred into liquid BG-11 medium and grown under the same light conditions. After several rounds of retransfer into fresh BG-11, all cultures became unialgal but still were contaminated with heterotrophic bacteria. The strains included in this study were designated LS0417, LS0425, LS0427, LS0503, LC0530, LS0588, and LS0590.
DNA extraction.
For the extraction of DNA from environmental samples, each filter was aseptically cut into slices and placed into 50-ml Falcon tubes. Three millilters of STE buffer (10 mM Tris-HCl [pH 8.0], 0.1 M NaCl, 1 mM EDTA-Na2) was added to the tubes with lysozyme to a final concentration of 3 mg ml−1. The tubes were incubated with shaking for 2 h at 37°C. Furthermore, 300 μl of sodium dodecyl sulfate (10% [pH 7.0]) was added to the samples and incubated at 37°C for 1 h, 95°C for 15 min, −70°C for 15 min, and 37°C for 15 min. The tubes were then subjected to two rounds of phenol-chloroform extraction, and the DNA was precipitated with 96% ethanol and resuspended in 50 μl of Tris-EDTA buffer. DNA from the isolated cyanobacterial strains was extracted essentially as described by Crosbie et al. (6).
PCR amplification.
Oxygenic phototroph-specific primers 106F (5′ CGGACGGGTGAGTAACGCGTGA 3′) and 789R (5′ GACTACAT/AGGGGTATCTAATCCCA/TTT 3′) (27) were used to amplify cyanobacterial 16S rRNA genes from DNA preparations. PCR amplifications were performed in a 50-μl volume containing 5 μl of 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl [pH 9.0], 1% Triton X-100), 5 μl of the DNA preparation, 200 μM concentrations of deoxynucleoside triphosphates, 0.2 nM primers, and 0.25 μl of Taq DNA polymerase (Promega). The amplification steps included 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 58°C for 2 min, and 72°C for 3 min. In order to increase the efficiency of the following TOPO reaction, a final step of 30 min at 72°C was added to ensure the PCR products are 3′ polyadenylated. The same PCR and ligation conditions with the TOPO vector were used for the amplification of the 16S rRNA genes of isolated cyanobacterial strains. For amplification of cpcBA IGS sequences, the forward and reverse primers described previously were employed (6): forward, 5′ TAGTGTAAAACGACGGCCAGTTGYYTKCGCGACATGGA 3′; and reverse, 5′ TAGCAGGAAACAGCTATGACGTGGTGTARGGGAAYTT 3′. The PCR conditions were as described by Robertson et al. (31).
Construction of clone libraries.
The pCR 2.1-TOPO vector (Invitrogen) was used to clone the PCR amplicons as described in the user's manual. TOP10F′ chemically competent Escherichia coli cells (Invitrogen) were transformed with the TOPO cloning reaction with Luria-Bertani plates containing 100 μg liter−1 ampicillin used to select for transformants. The colonies were screened for positive clones by colony PCR with the primers used for the initial amplification. Positive colonies were stored in 25% glycerol at −70°C for subsequent sequencing.
DNA sequencing and phylogenetic analysis.
High-throughput sequencing of Escherichia coli cultures transformed with TOPO vector bearing inserts of environmental 16S rRNA gene and cpcBA IGS PCR amplicons was performed at the sequencing center of the Clemson University Genomic Institute. Four 96-well plates containing glycerol stocks were frozen at −70°C prior to shipping to Clemson. Sequencing was performed in an ABI 3730XL 96-capillary sequencer using 2 pM per reaction of M13 reverse sequencing primer (Invitrogen) for the pCR 2.1-TOPO vector. Sequences were aligned using the alignment program CLUSTALX 1.83 (14). The phylogenetic trees for each sampling site were constructed from 615 unambiguously aligned bases by the neighbor-joining method using MEGA 3.1 software (20).
Photosynthetic measurements.
To measure photosynthetic rates, NaH14CO3 (60 μCi; specific activity, 58 mCi mmol−1 [MP Biomedicals, Solon, OH]) was added to size-fractionated samples of 30 ml following a 30-min dark adaptation. The cell suspension was distributed as 1-ml aliquots into 7-ml glass scintillation vials that were incubated simultaneously under 24 different light intensities for 2 to 3 h using a temperature-controlled photosynthetron (CHPT Mfg. Inc., Georgetown, DE) as described by McKay et al. (23). The reaction was terminated by the addition of 50 μl of formaldehyde to each sample. Acid-stable 14C assimilation was measured by liquid scintillation counting following the addition of 4.5 ml of Ecolite (+) cocktail (MP Biomedicals) to each vial. Total activity of the added 14C was determined by adding 20 μl of the sample at time (t) = 0 to scintillation cocktail containing 200 μl of β-phenylethylamine (Sigma). Background activity was determined at t = 0 by dispensing a sample aliquot directly into formaldehyde prior to adding scintillation cocktail. Experiments were performed on unfiltered water and on a fraction that passed through a 5-μm (March) or 2-μm (August) polycarbonate membrane under gentle vacuum filtration (100 mm Hg). Rates of photosynthesis were determined by using a nonlinear regression curve-fitting function (SigmaPlot 9.0; Systat Software, Inc., San Jose, CA) based on the equation of Platt et al. (28). Chl a used to normalize rates of photosynthesis was extracted overnight at −20°C in 90% acetone and measured by fluorometry (37).
Flow cytometry.
Water samples were either analyzed by flow cytometry immediately following collection or preserved in 2% (wt/vol) paraformaldehyde. APP abundance was determined by flow cytometry using a FACSCalibur instrument (15-mW air-cooled argon-ion laser fixed at 488 nm [Becton Dickinson, Mountain View, CA]) with CellQuest software v5.1.1 (BD Biosciences). Double-distilled water was used as a sheath fluid, and the analyzed volume was calculated by measuring the sample flow rate as recommended in the Becton Dickinson manual and recording the time each sample was counted. Samples were run at the “Hi flow” setting (ca. 50 μl min−1) following methods based on Corzo et al. (4). Both eukaryotic APP and Synechococcus sp. cells were enumerated using FL2-versus-FL3 plots. To remove background electronic noise, an FL3 threshold of 52 was set.
Nucleotide sequence accession numbers.
The 16S rRNA gene and cpcBA IGS sequences obtained during this study were deposited in GenBank under accession no. DQ519592 to DQ520013, DQ525026 to DQ525059, DQ526396 to DQ526425, and DQ533710.
RESULTS
16S rRNA gene sequences obtained from pelagic station CD1.
Water samples were collected from a depth of 5 m at stations CD1, WM, and SB during spring isothermal mixing in May 2004 and from the epilimnion and DCM at stations CD1 and WM and mixed surface waters at stations SB and PD during September 2004 (Fig. 1). High-throughput sequencing of 480 plasmid clones resulted in 368 successful reactions. Out of the entire pool of sequences, 41 did not cluster within the oxygenic phototroph lineage in a neighbor-joining tree, and 29 clustered together with the plastids of the green alga Chlorella mirabilis (Chlorophyta) and diatoms (data not shown). All sequences that clustered within the plastid lineage and the majority (39 sequences) of those that did not cluster within the oxygenic phototroph lineage were from the May 2004 libraries.
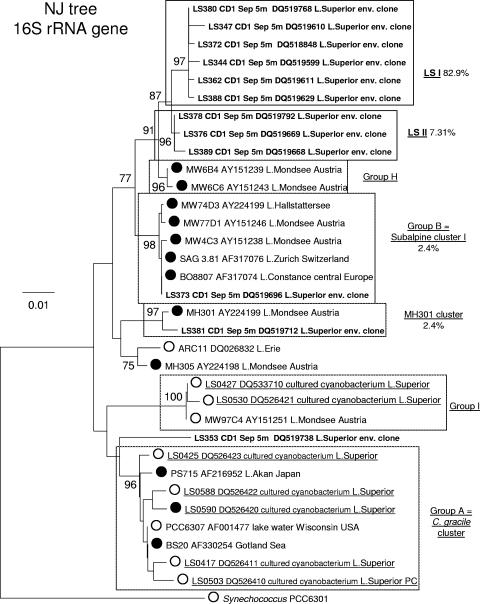
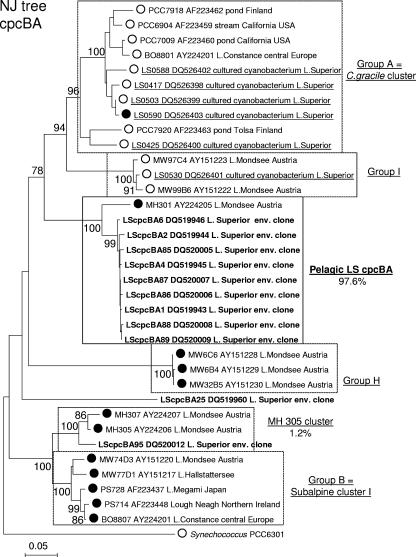
The remaining 298 sequences that did cluster within the cyanobacterial radiation were divided into nine libraries according to the sampling sites and dates. The phylogenetic analysis of the epilimnion (5-m depth) library from CD1 is presented in Fig. 2. Included in the analysis are strains LS0417, LS0425, LS0427, LS0503, LS0530, LS0588, and LS0590, which were isolated from pelagic (LS0417, LS0425, LS0427, and LS0588) and nearshore (LS0503, LS0530, and LS0590) stations in Lake Superior during the years 2004 and 2005. Other strains are Cyanobium gracile and Synechococcus freshwater isolates, as well as marine Synechococcus and Prochlorococcus, which together constitute the major clusters of the picocyanobacterial clade. The cluster designations are in accordance with previous studies on freshwater Synechococcus spp. (6, 7, 31). All major picocyanobacterial clusters described in previous studies (6, 7) were recovered by our analysis and retained high bootstrap values (Fig. 2). Thirty-one out of 32 unique sequences clustered within the picocyanobacterial clade sensu Urbach et al. (36) with 99% bootstrap support. The majority of the sequences clustered within two new picocyanobacterial groups, Lake Superior clusters I and II (LSI and LSII, respectively), with average pairwise sequence similarities of 99.4% and 99.7%, respectively. The two groups are closely related to each other and Crosbie group H (6), which consists of a number of PE-rich isolates from Lake Mondsee, Austria, and a PE-rich strain, LPB1, isolated from Lake Biwa, Japan. However, LSI and LSII sequences formed two independent clusters that did not include any of the strains isolated from any other lake previously sampled. Only three of the picocyanobacterial sequences did not cluster within groups LSI and LSII. LS373 was in group B (6), which consists of PE-rich isolates from several oligotrophic lakes in Europe; LS381 grouped with a PE-rich isolate, MH301, from Lake Mondsee, Austria, which remained unclustered in the 16S rRNA gene tree by Crosbie et al. (6). Another picocyanobacterial sequence (LS353) did not cluster with any of the known picocyanobacteria. One sequence in the library, LS365, was outside the picoplankton lineage and was most similar to the 16S rRNA gene of Synechocystis sp. strain PCC 6803 (data not shown). Similarly, the 16S rRNA sequences from the DCM at CD1 formed two new clusters, LSI and LSII (average pairwise sequence similarities of 99.5% and 99.7%, respectively), within the picoplankton clade that were closely related to but well separated from group H (data not shown).
FIG. 2.
Phylogeny of picocyanobacterial 16S rRNA gene sequences from pelagic station CD1. Shown is a 16S rRNA gene neighbor-joining (NJ) tree of the major clusters of picocyanobacteria described previously (6, 7), 12 representative environmental clones (in bold), seven Lake Superior isolates (underlined; LS0417, 0425, 0427, 0503, 0530, 0588, and 0590), and outgroup strain Synechococcus PCC 6301. Percentages of representation of each cluster within the 16S rRNA gene library are shown. Where known, the dominant pigment (PE rich [•] versus PC rich [○]) is indicated. Clusters that included LSI and LSII clones are highlighted in bold. The tree was inferred from 615 bases of the 16S rRNA gene. Numbers at nodes indicate the percent of bootstrap frequency (1,000 replicates) obtained by MEGA 3.1 using the Kimura two-parameter model of nucleotide substitution. Bootstrap values of <70% are not shown.
LSI and LSII clusters were also present on the trees obtained for the September epilimnion libraries from WM and SB and the DCM library from WM (data summarized in Fig. 4; see Fig. S1 to S3 in the supplemental material). The same trend was observed in the WM DCM library, although there were fewer LSI sequences compared to the sequences obtained from the epilimnion at that station.
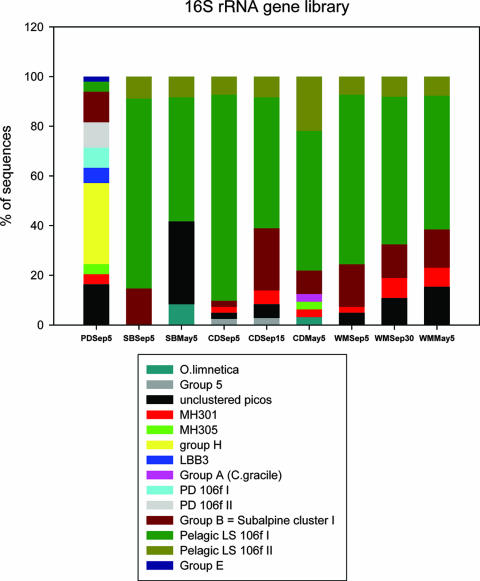
FIG. 4.
Comparison of the distributions of 16S rRNA sequences among picocyanobacterial (picos) clusters obtained from libraries constructed from samples taken from offshore stations WM, SB, and CD1 during May and September 2004 and nearshore Keweenaw Waterway station PD.
Libraries constructed from the spring (May 2004) samples contained fewer cyanobacterial sequences than did the September libraries. The distribution of sequences among picocyanobacterial clusters from station CD1 resembles that of the September libraries but with fewer sequences in LSI and more group B sequences compared to the epilimnion libraries (summarized in Fig. 4). Unlike the LSII cluster from the September trees, LSII had moderate bootstrap support. However, in a neighbor-joining tree of LSI and LSII sequences from libraries prepared from stations CD1 and PD, all LSII sequences clustered together with a 70% bootstrap (data not shown). The SB and WM libraries from May contained sequences from LSI and II, subalpine cluster I, the MH301 cluster (summarized in Fig. 4), as well as two more sequences clustering with Oscillatoria limnetica (19).
Overall, clusters LSI and LSII contain the most Lake Superior cyanobacterial 16S rRNA gene sequences in our data set from open lake stations. Both groups are closely related to Crosbie group H (6) but form independent clusters supported by high or moderate bootstrap values.
16S rRNA gene sequences obtained from station PD.
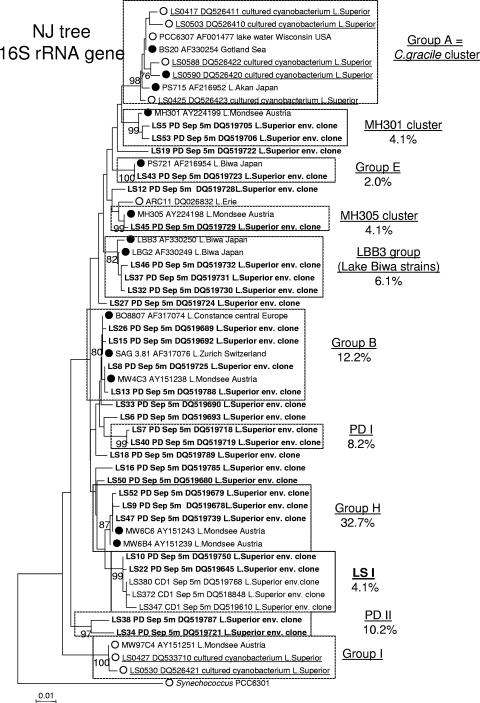
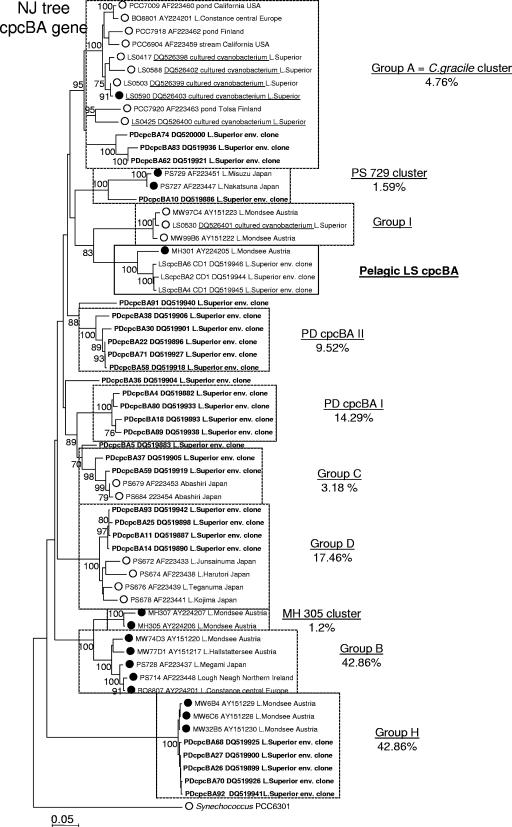
Unlike the cyanobacterial 16S rRNA gene sequences from SB and pelagic stations CD1 and WM, only 2 out of 30 unique sequences from surface waters at station PD clustered with the Lake Superior cluster LSI and cluster LSII was not represented in the library (Fig. 3). In contrast, members of each of the major freshwater picocyanobacterial clusters described in previous studies, except for the C. gracile cluster and group I sensu Crosbie (6), were represented in the PD library (Fig. 3). In addition, a new cluster, MH305, which previously consisted of a single PE-rich strain, MH305 (5, 6), contained two PD sequences (LS11 and LS45) and a PC-rich Lake Erie isolate, ARC11 (GenBank accession no. DQ026832; A. R. Cupp and G. S. Bullerjahn, unpublished data). Lastly, two minor PD-specific clusters named PDI and PDII, with average sequence similarities of 99.8 and 99.7%, respectively, were present and did not include any previously characterized cyanobacterial strains.
FIG. 3.
Phylogeny of picocyanobacterial 16S rRNA gene sequences from nearshore station PD. Shown is a 16S rRNA neighbor-joining tree of the major clusters of picocyanobacteria, 28 representative environmental clones (bold) with the addition of seven Lake Superior isolates (underlined), and outgroup strain Synechococcus PCC 6301. The percentages of representation of each cluster within the 16S rRNA library are shown. Where known, the dominant pigment (PE rich [•] versus PC rich [○]) is indicated. Clusters that included LSI and LSII clones are highlighted in boldface. Numbers at nodes indicate the percentage of bootstrap frequency from 1,000 replicates. Bootstrap values of <70% are not shown.
Figure 4 shows the overall distribution of 16S rRNA gene clones obtained in this study among different lineages within the cyanobacterial radiation. One striking feature of this data set is the apparent difference in the genetic diversities of cyanobacteria between the nearshore station PD and other sampling sites, including pelagic stations CD1 and WM and the western arm station SB near Duluth, MN. LSI is ubiquitous at CD1, WM, and SB (minimum of 50% of the clones), whereas at PD the LSI group accounted for only 4% of the sequences. Furthermore, cluster LSII is absent from the PD library but has representatives at all pelagic stations and SB. Conversely, picocyanobacterial clusters MH305, Crosbie group H, the Lake Biwa cluster, and PD clusters I and II are only present at PD. The major Lake Superior cluster, LSI, is more abundant in the mixed layer during the fall than in the deeper DCM layer or in the surface waters during the spring. The O. limnetica group (19) was present only in the spring libraries, as was the C. gracile cluster. Lake Superior strains LS0504 and LS0503, which cluster within these two groups, respectively, were also isolated in culture from the spring water samples.
cpcBA IGS sequences from CD1 and PD.
DNA sequencing and neighbor-joining analysis of 63 cpcBA IGS clones from nearshore station PD and 105 from pelagic station CD1 revealed the same overall patterns seen with the 16S rRNA gene sequences. Indeed, phylogenetic analysis revealed the presence of novel, low-diversity samples at station CD1 (Fig. 5), whereas the cpcBA IGS library from station PD both revealed high diversity and included the major picocyanobacterial clusters described previously (Fig. 6). The cpcBA analysis at station CD1 revealed that close to 98% of the sequences fell within a unique cluster, compared to 90% of 16S rRNA gene sequences comprising the novel LSI and LSII clusters. In contrast, none of the cpcBA sequences represented in the major CD1 cluster was represented in the PD cpcBA clone library. This disparity between the 16S and cpcBA data is likely due to biases introduced by the PCR. Nonetheless, these data support the conclusion from the 16S rRNA sequences indicating that the APP of pelagic Lake Superior are phylogenetically distinct from other Synechococcus sp. strains identified and characterized around the globe.
FIG. 5.
Phylogeny of picocyanobacterial cpcBA internal transcribed spacer sequences from offshore station CD1. Shown is a cpcBA neighbor-joining (NJ) tree of the major clusters of picocyanobacteria described previously (6, 7), containing 10 representative environmental clones (bold), six Lake Superior isolates (underlined), and outgroup strain Synechococcus PCC 6301. The percentages of representation of each cluster in the library are shown. Where known, the dominant pigment (PE rich versus PC rich) is indicated as in Fig. 1 and 2. Numbers at nodes indicate the percentages of bootstrap frequency from 1,000 replicates. Bootstrap values of <70% are not shown.
FIG. 6.
Phylogeny of picocyanobacterial cpcBA internal transcribed spacer sequences from nearshore station PD. Shown is a cpcBA neighbor-joining (NJ) tree of the major clusters of picocyanobacteria containing 27 representative environmental clones (bold), six Lake Superior isolates (underlined), and outgroup strain Synechococcus PCC 6301. The percentages of representation of each cluster in the library are shown. Where known, the dominant pigment (PE rich versus PC rich) is indicated as in Fig. 1 and 2. No sequences clustering with the cpcBA sequences from pelagic station CD1 were found among 63 clones sequenced. For comparison, station CD1 cpcBA clones LScpcBA2, LScpcBA4, and LScpcBA6 are included. Numbers at nodes indicate the percentages of bootstrap frequency from 1,000 replicates. Bootstrap values of <70% are not shown.
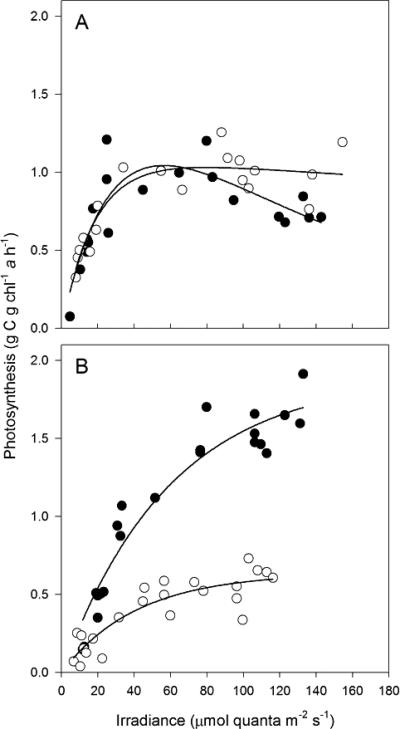
Photosynthetic performance of Lake Superior APP.
Photosynthesis versus irradiance (PE) curves were determined for the total phytoplankton community and for the APP assemblage at station CD1 during March and August 2006. At both times, the APP were an abundant component of the total plankton fraction at this site, comprising >50% of Chl biomass. For the total community, biomass-normalized rates of light-saturated photosynthesis (PmChl) were nearly 2× higher in August than in March (Fig. 7). Measurements of photosynthesis were conducted at in situ temperatures; thus, higher rates in August were consistent with the elevated temperature of midsummer surface waters (20°C) compared to March (4°C). Whereas PmChl did not vary between total phytoplankton and a <5-μm fraction in March, the picoplankton in August exhibited ca. 33% of the PmChl of the total community (Fig. 7). An assessment of photosynthetic efficiency (α) calculated from the initial slope of the PE curves showed no difference between total plankton and the <5-μm fraction during March but modest variation in August, with a picoplankton photosynthetic efficiency ca. 25% lower than the total. A temporal comparison clearly showed higher efficiency for those samples collected during March (α = 5.9 × 10−2) compared to August (α = 2.4 × 10−2 to 3.2 × 10−2), consistent with deep mixing and the resultant light-limiting conditions experienced by phytoplankton prior to the onset of thermal stratification at this site in late June to early July (17). Also consistent with the light-limiting conditions synonymous with vernal holomixis were depressed values of Ek, a parameter derived from PmChl/αChl to estimate the irradiance at which photosynthesis becomes light saturated. During March, Ek ranged between 17 and 18 μmol quanta m−2 s−1, whereas in August, Ek increased to 26 and 60 μmol quanta m−2 s−1 for the picoplankton and total phytoplankton, respectively.
FIG. 7.
Chl-normalized rates of photosynthesis for seston collected at station CD1 from 10-m depth during vernal holomixis in March 2006 (A) and from a 5-m depth during thermal stratification in August 2006 (B). Samples were assayed at a series of irradiances while maintaining near in situ temperature using a photosynthetron (March, 4°C; August, 20°C). Closed circles indicate the photosynthesis in whole water; replicate samples (open circles) were size fractionated prior to assay as follows: <5 μm for March and <2 μm for August.
Flow cytometry: seasonal progression.
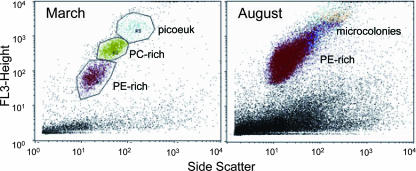
The phylogenetic studies in this paper, along with detailed characterization of cultured freshwater Synechococcus spp., suggest that PE-rich picocyanobacteria comprise the major fraction of Lake Superior APP. However, flow cytometry of water samples obtained during March and August 2006 indicates that the composition of the APP undergoes a large shift from late winter to summer. Indeed, winter to early spring APP included abundant picoeukaryotes and PC-rich picocyanobacteria, whereas summer APP were exclusively PE rich (Fig. 8). Typical abundance of the PE-rich population exceeded 104 cells ml−1 at the surface in summer, with 105 cells ml−1 in the DCM (Table 1). We propose that the PE-rich APP is dominated by picocyanobacteria of the LSI and LSII groups described herein.
FIG. 8.
Flow cytometry of water samples from station CD1 during late winter (March) and summer (August) 2006. The composition of the APP was determined by pigment fluorescence and is indicated on the plots. Red, PE-rich APP; green, PC-rich APP; blue, picoeukaryotes (picoeuk); and orange, PE-rich microcolonies.
TABLE 1.
Seasonal abundance of APP at station CD1 determined by flow cytometry
| Date and sampling depth | Group | No. of cells ml−1a |
|---|---|---|
| 24 March 2006 | ||
| 5 m | Microcolonies | 8.64 × 102 |
| Picoeukaryotes | 2.84 × 103 | |
| PC-rich APP | 1.18 × 104 | |
| PE-rich APP | 1.57 × 104 | |
| 8 August 2006 | ||
| 5 m | Microcolonies | 1.12 × 103 |
| Picoeukaryotes | 3.09 × 103 | |
| PC-rich APP | <103 | |
| PE-rich APP | 1.30 × 104 | |
| 25 m (DCM) | Microcolonies | 1.15 × 104 |
| Picoeukaryotes | 7.28 × 103 | |
| PC-rich APP | <103 | |
| PE-rich APP | 1.06 × 105 | |
| 80 m | Microcolonies | 1.25 × 103 |
| Picoeukaryotes | 1.41 × 103 | |
| PC-rich APP | <103 | |
| PE-rich APP | 1.97 × 103 |
The values reported are the average of three independent measurements.
DISCUSSION
The work presented here summarizes our efforts to determine the biodiversity of the Lake Superior APP that comprises up to 50% of total Chl in the open lake (9, 24). The vast majority of Lake Superior cyanobacterial 16S rRNA gene clones obtained in the present study belonged to the robust picoplankton clade sensu Urbach et al. (36), which consists of both marine and freshwater picocyanobacteria. Crosbie et al. (6) defined seven clusters within the nonmarine subdivision of the picoplankton clade. Certain freshwater clusters may possibly be restricted to a particular ecological niche, e.g., the cluster of Antarctic strains, or geographical location, e.g., Crosbie group E (6). In contrast, most clusters appear to be cosmopolitan, with members isolated from distant locations and lakes of different limnological characteristics. The examples of such clusters include the Cyanobium gracile cluster and Crosbie group H. Here, we present evidence for the existence of new freshwater picocyanobacterial clusters LSI and LSII within the freshwater group of picocyanobacteria. LSI and LSII were supported by relatively high bootstrap values and appeared to be closely related to group H, which consists of a number of PE-rich isolates from deep subalpine Lake Mondsee (Austria) as well as the PE-rich strain LBP1, isolated from the mesotrophic Lake Biwa (Japan). The LSI and LSII clusters were well represented at the open lake stations but were nearly absent from the nearshore station PD. Instead, the picocyanobacterial community at PD appeared more diverse than the pelagic community and consisted of members of the C. gracile cluster, group B (subalpine cluster I), the Lake Biwa cluster sensu Ernst et al. (7), group H, as well as two new minor PD-specific clusters, PDI and PDII.
Pelagic Lake Superior is an extremely oligotrophic system in which the combination of chemical and physical factors may create a unique environment in which phytoplankton are severely limited by low concentrations of phosphorus and iron, as well as constrained by light and temperature regimes unfavorable for photosynthesis (17, 24, 26, 34). The evident differences in the APP community between the pelagic western arm of Lake Superior and the nearshore station PD located in a region subject to high coastal influence allow us to suggest the existence of novel ecotypes of Synechococcus spp. adapted to the specific nutrient-poor conditions in the open lake. Accordingly, the abundance of PC-rich picocyanobacteria and picoeukaryotes among the APP in March likely reflects large differences in nutrient availability, temperature, and light during winter mixing compared to summer conditions, and the switch to PE-rich LSI- and LSII-dominated APP may result from selection of ecotypes specifically adapted to outcompete other APP under the ultraoligotrophic conditions following stratification. Whether the ecotype is unique to Lake Superior will remain uncertain until additional 16S rRNA gene/cpcBA sequence information from other lakes with similar characteristics becomes available.
We have isolated several PE-rich cyanobacterial strains from Lake Superior and analyzed their phylogenetic relatedness to known cyanobacteria (see Fig. 2, 3, 5, and 6). Despite the abundance of LSI and LSII sequences in our analysis, none of the isolates brought into culture lie within the LSI and LSII clusters. Nonetheless, the results of the phylogenetic analysis and flow cytometry indicate the predominance of PE-rich cyanobacteria in the lake. Despite the fact that grouping of picocyanobacteria based on pigment composition is not always consistent with phylogenetic clustering (7, 31), Crosbie groups B and H, which are the most closely related to LSI and LSII of all other groups of freshwater APP, consist entirely of PE-rich strains. Moreover, Fahnenstiel et al. (9) reported on the dominance of PE-rich Synechococcus-like cells in the epilimnion and hypolimnion of Lake Superior. All of the above findings strongly suggest that PE-rich Synechococcus spp. of clusters LSI and LSII are the dominant members of open lake APP. Future work will focus on culture and physiological characterization of these novel Synechococcus spp.
A parallel study in our lab is focused on the APP of pelagic Lake Erie, specifically the formation of a PE-rich DCM in the central basin during the onset of hypolimnetic hypoxia in late summer (39). Comparison of the 16S rRNA gene sequences from the Lake Erie picocyanobacterial assemblage reveals that they are predominantly related to MH301; thus, they are distinct from the Lake Superior Synechcococcus spp. identified at offshore stations (39; A. R. Cupp, S. W. Wilhelm, and G. S. Bullerjahn, unpublished data). These data suggest that the LSI and LSII clusters are restricted to Lake Superior and are not widespread throughout the Laurentian Great Lakes.
Supplementary Material
Acknowledgments
This material is based upon work supported by the National Science Foundation under award no. OCE-0352274 (R.M.L.M. and G.S.B.).
We thank Captain Mike King and the crew of the R/V Blue Heron, who assisted in the collection of samples. Robert Sterner, Sandra Brovold, Sanjeev Kumar, and Becky Stark (University of Minnesota) and Jason Agnich (Large Lakes Observatory) and Korinna Straube (BGSU) assisted in the collection of water samples. Vanessa Michelou (University of Delaware) provided valuable assistance with the flow cytometry studies. Jami Barnes and Ma'moon Al-Rshaidat provided assistance in preparing the figures for this paper. Anton Post provided helpful comments on the manuscript prior to publication.
Footnotes
Published ahead of print on 27 April 2007.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Allen, M. M. 1968. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 4:1-4. [DOI] [PubMed] [Google Scholar]
- 2.Callieri, C., and J. S. Stockner. 2002. Freshwater autotrophic picoplankton: a review. J. Limnol. 61:1-14. [Google Scholar]
- 3.Caron, D. A., F. R. Pick, and D. R. S. Lean. 1985. Chroococcoid cyanobacteria in Lake Ontario: vertical and seasonal distributions during 1982. J. Phycol. 21:171-175. [Google Scholar]
- 4.Corzo, A., F. Jiménez-Gómez, F. Gordillo, R. García-Ruíz, and F. Niell. 1999. Synechococcus and Prochlorococcus-like populations detected by flow cytometry in a eutrophic reservoir in summer. J. Plankton Res. 21:1575-1581. [Google Scholar]
- 5.Crosbie, N. D., M. Pöckl, and T. Weisse. 2003. Rapid establishment of clonal isolates of freshwater autotrophic picoplankton by single-cell and single-colony sorting. J. Microbiol. Methods 55:361-370. [DOI] [PubMed] [Google Scholar]
- 6.Crosbie, N. D., M. Pöckl, and T. Weisse. 2003. Dispersal and phylogenetic diversity of nonmarine picocyanobacteria, inferred from 16S rRNA gene and cpcBA-intergenic spacer sequence analyses. Appl. Environ. Microbiol. 69:5716-5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ernst, A., S. Becker, U. I. A. Wollenzien, and C. Postius. 2003. Ecosystem-dependent adaptive radiations of picocyanobacteria inferred from 16S rRNA and ITS-1 sequence analysis. Microbiology 149:217-227. [DOI] [PubMed] [Google Scholar]
- 8.Ernst, A., P. Marschall, and C. Postius. 1995. Genetic diversity among Synechococcus spp. (cyanobacteria) isolated from the pelagial of Lake Constance. FEMS Microbiol. Ecol. 17:197-204. [Google Scholar]
- 9.Fahnenstiel, G. L., L. Sicko-Goad, D. Scavia, and E. R. Stoermer. 1986. Importance of phytoplankton in Lake Superior. Can. J. Fish. Aquat. Sci. 43:235-240. [Google Scholar]
- 10.Fahnenstiel, G. L., and H. J. Carrick. 1992. Phototrophic picoplankton in Lakes Huron and Michigan: abundance, distribution, composition, and contribution to biomass and production. Can. J. Fish. Aquat. Sci. 49:379-388. [Google Scholar]
- 11.Ferris, M. J., and B. Palenik. 1998. Niche adaptation in ocean cyanobacteria. Nature 396:226-228. [Google Scholar]
- 12.Field, K. G., D. Gordon, T. Wright, M. Rappé, E. Urbach, K. Vergin, and S. J. Giovannoni. 1997. Diversity and depth-specific distribution of SAR11 cluster rRNA genes from marine planktonic bacteria. Appl. Environ. Microbiol. 63:63-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuller, N. J., D. Marie, F. Partensky, D. Vaulot, A. F. Post, and D. Scanlan. 2003. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Appl. Environ. Microbiol. 69:2430-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins, D. G., and P. M. Sharp. 1988. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene 73:237-244. [DOI] [PubMed] [Google Scholar]
- 15.Honda, D., A. Yokota, and J. Sugiyama. 1999. Detection of seven major evolutionary lineages in Cyanobacteria based on the 16S rRNA gene sequence analysis with new sequences of five marine Synechococcus strains. J. Mol. Evol. 48:723-739. [DOI] [PubMed] [Google Scholar]
- 16.Ivanikova, N. V., R. M. L. McKay, and G. S. Bullerjahn. 2005. Construction and characterization of a freshwater cyanobacterial bioreporter capable of assessing nitrate assimilatory capacity in freshwaters. Limnol. Oceanogr. Methods 3:86-93. [Google Scholar]
- 17.Ivanikova, N. V., R. M. L. McKay, G. S. Bullerjahn, and R. W. Sterner. Nitrate utilization in Lake Superior is impaired by low nutrient (P, Fe) availability and seasonal light limitation—a cyanobacterial bioreporter study. J. Phycol., in press.
- 18.Johnson, Z. I., E. R. Zinser, A. Coe, N. P. McNulty, E. M. Woodward, and S. W. Chisholm. 2006. Niche partitioning among Prochlorococcus ecotypes along ocean-scale environmental gradients. Science 311:1737-1740. [DOI] [PubMed] [Google Scholar]
- 19.Katano, T., M. Fukui, and Y. Watanabe. 2001. Identification of cultured and uncultured picocyanobacteria from a mesotrophic freshwater lake based on the partial sequences of 16S rDNA. Limnology 2:213-218. [Google Scholar]
- 20.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 21.Leppard, C. G., D. Urcioli, and F. R. Pick. 1987. Characterization of cyanobacterial picoplankton in Lake Ontario by transmission electron microscopy. Can. J. Fish. Aquat. Sci. 44:2173-2177. [Google Scholar]
- 22.Li, W. K. W., D. V. Subba Rao, W. G. Harrison, J. C. Smith, J. J. Cullen, B. Irwin, and T. Platt. 1983. Autotrophic picoplankton in the tropical ocean. Science 219:292-295. [DOI] [PubMed] [Google Scholar]
- 23.McKay, R. M. L., R. J. Geider, and J. La Roche. 1997. Physiological and biochemical response of the photosynthetic apparatus of two marine diatoms to Fe stress. Plant Physiol. 114:615-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay, R. M. L., D. Porta, G. S. Bullerjahn, M. M. D. Al-Rshaidat, J. A. Klimowicz, R. W. Sterner, T. M. Smutka, E. T. Brown, and R. M. Sherrell. 2005. Bioavailable iron in oligotrophic Lake Superior assessed using biological reporters. J. Plankton Res. 27:1033-1044. [Google Scholar]
- 25.Moore, L. R., G. Rocap, and S. W. Chisholm. 1998. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature 393:464-467. [DOI] [PubMed] [Google Scholar]
- 26.Nawelajko, C., and D. Voltolina. 1986. Effects of environmental variables on growth rates and physiological characteristics of Lake Superior phytoplankton. Can. J. Fish. Aquat. Sci. 43:1163-1170. [Google Scholar]
- 27.Nübel, U., F. Garcia-Pichel, and G. Muyzer. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl. Environ. Microbiol. 63:3327-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt, T., C. L. Gallegos, and W. G. Harrison. 1980. Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38:687-701. [Google Scholar]
- 29.Postius, C., and A. Ernst. 1999. Mechanism of dominance: coexistence of picocyanobacterial genotypes in a freshwater ecosystem. Arch. Microbiol. 172:69-75. [DOI] [PubMed] [Google Scholar]
- 30.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Genetic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 31.Robertson, B. R., N. R. Tezuka, and M. M. Watanabe. 2001. Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. Int. J. Syst. Evol. Microbiol. 51:861-871. [DOI] [PubMed] [Google Scholar]
- 32.Rocap, G., F. W. Larimer, J. Lamerdin, S. Malfatti, P. Chain, N. A. Ahlgren, A. Arellano, M. Coleman, L. Hauser, W. R. Hess, Z. I. Johnson, M. Land, D. Lindell, A. F. Post, W. Regala, M. Shah, S. L. Shaw, C. Steglich, M. B. Sullivan, C. S. Ting, A. Tolonen, E. A. Webb, E. R. Zinser, and S. W. Chisholm. 2003. Genome divergence in two Prochlorococcus ecotypes reflects oceanic niche differentiation. Nature 424:1042-1046. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt, T. M., E. F. DeLong, and N. R. Pace. 1991. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J. Bacteriol. 173:4371-4378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterner, R. W., T. M. Smutka, R. M. L. McKay, Q. Xiaoming, E. T. Brown, and R. M. Sherrell. 2004. Phosphorus and trace metal limitation of algae and bacteria in Lake Superior. Limnol. Oceanogr. 49:495-507. [Google Scholar]
- 35.Ting, C. S., G. Rocap, J. King, and S. W. Chisholm. 2002. Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends Microbiol. 10:134-142. [DOI] [PubMed] [Google Scholar]
- 36.Urbach, E., D. J. Scanlan, D. L. Distel, J. B. Waterbury, and S. W. Chisholm. 1998. Rapid diversification of marine picophytoplankton with dissimilar light-harvesting structures inferred from sequences of Prochlorococcus and Synechococcus (Cyanobacteria). J. Mol. Evol. 46:188-201. [DOI] [PubMed] [Google Scholar]
- 37.Welschmeyer, N. A. 1994. Fluorometric analysis of chlorophyll-a in the presence of chlorophyll-b and phaeopigments. Limnol. Oceanogr. 39:1985-1992. [Google Scholar]
- 38.West, N. J., and D. J. Scanlan. 1999. Niche-partitioning of Prochlorococcus populations in a stratified water column in the eastern North Atlantic Ocean. Appl. Environ. Microbiol. 65:2585-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilhelm, S. W., G. S. Bullerjahn, J. M. Rinta-Kanto, M. L. Eldridge, and R. A. Bourbonniere. 2006. Seasonal hypoxia and the genetic diversity of prokaryote populations in the central basin hypolimnion of Lake Erie: evidence for abundant picocyanobacteria and photosynthesis. J. Great Lakes Res. 32:657-671. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.