Abstract
Acinetobacter venetianus Rag1 produces an extracellular, polymeric lipoheteropolysaccharide termed apoemulsan. This polymer is putatively produced via a Wzy-dependent pathway. According to this model, the length of the polymer is regulated by polysaccharide-copolymerase (PCP) protein. A highly conserved proline and glycine motif was identified in all members of the PCP family of proteins and is involved in regulation of polymer chain length. In order to control the structure of apoemulsan, defined point mutations in the proline-glycine-rich region of the apoemulsan PCP protein (Wzc) were introduced. Modified wzc variants were introduced into the Rag1 genome via homologous recombination. Stable chromosomal mutants were confirmed by Southern blot analysis. The molecular weight of the polymer was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Five of the eight point mutants produced polymers having molecular weights higher than the molecular weight of the polymer produced by the wild type. Moreover, four of these five polymers had modified biological properties. Replacement of arginine by leucine (R418L) resulted in the most significant change in the molecular weight of the polymer. The R418L mutant was the most hydrophilic mutant, exhibiting decreased adherence to polystyrene, and inhibited biofilm formation. The results described in this report show the functional effect of Wzc modification on the molecular weight of a high-molecular-weight polysaccharide. Moreover, in the present study we developed a genetic system to control polymerization of apoemulsan. The use of selective exogenous fatty acid feeding strategies, as well as genetic manipulation of sugar backbone chain length, is a promising new approach for bioengineering emulsan analogs.
Apoemulsan is an anionic polysaccharide produced from a variety of hydrocarbon sources, including fatty acids and ethanol, by the gram-negative bacterium Acinetobacter venetianus strain Rag1 (ATCC 31012) (38). Apoemulsan forms an extracellular cell-associated capsule which is subsequently released into the medium as a protein-polysaccharide complex called emulsan (11). The polysaccharide main chain contains amino sugars (13) and has O-acyl- and N-acyl-bound side chain fatty acids with chain lengths ranging from C10 to C18. These fatty acid substituents constitute up to 23% (wt/wt) of the polymer (36). The combination of the hydrophilic anionic sugar main chain along with the hydrophobic side groups gives apoemulsan the amphipathic nature that allows it to function as a potent bioemulsifier by binding to oil-water interfaces and thus form emulsions (28). The utility of apoemulsan as a cleaner that effectively removes oil-type residues (14) and as a heavy metal-binding agent (37) has been reported previously. Recently, we demonstrated that apoemulsan can be employed as a biological adjuvant for vaccine delivery (26) or as a carrier for drug delivery (6).
We have previously demonstrated that apoemulsan properties can be modulated by modification of the fatty acid composition. Polymer variants have been obtained via different feeding strategies and through transposon mutagenesis (12, 16, 36). Since the sequence of the gene cluster responsible for apoemulsan biosynthesis was recently described (22), other possibilities for modulation of the structure of this important bioemulsifier can be envisioned. A combined strategy of modifying not only the fatty acid decorations but also the polysaccharide backbone provides the potential for generating a wide range of apoemulsan analogs.
The wee gene cluster (accession number AJ243431) consists of 20 open reading frames, most of which have putative functions based upon homologous sequences, as determined from database searches (22). Based on homology, it was proposed that emulsan is a member of the group 1 or 4 family of capsular polysaccharides. Bacteria belonging to these groups produce polysaccharides via a common mechanism called a Wzy-dependent pathway (34, 35). The monomeric units of the polymer are assembled on a lipid carrier on the cytoplasmic face of the inner membrane. Lipid-linked repeat units are then transferred by the Wzx protein to the periplasmic face of the membrane, where polymerization occurs by means of the Wzy polymerase. The last step in capsule assembly is release of polymer from the lipid intermediate and transport of the polysaccharide through the periplasm and across the outer membrane. Wza is an outer membrane protein involved in transport of the polymer, playing a role as an export channel (23).
Although polymerization of the polysaccharides is Wzy dependent, a number of studies have reported that the length of the polymer is regulated by polysaccharide-copolymerase (PCP) proteins (2, 7, 8, 10, 17, 21). Three groups of PCP proteins have been described: (i) PCP1 proteins, which are associated with lipopolysaccharide (LPS)-O-antigen (like Wzz); (ii) PCP2 proteins, involved in the synthesis of high-molecular-weight polysaccharides like capsular polysaccharide and exopolysaccharide (like Wzc and ExoP); and (iii) PCP3 proteins, which function in capsular polysaccharide production using a different ABC-2 type transporter system (20). Members of the PCP protein family are located in the inner membrane and have two transmembrane regions (TM1 and TM2). Between TM1 and TM2 PCPs have a large, hydrophilic, periplasmic region with coiled-coil potential. A highly conserved proline and glycine motif was identified in all PCP proteins, which is located just before and within TM2. A number of studies have indicated that amino acid substitution in this region modifies the polymer length (4, 7).
The aim of the present study was to develop a genetic system to control polymerization of apoemulsan. In order to accomplish this objective, first a wzc deletion mutant and then wzc replacement mutants carrying point mutations had to be constructed. According to analysis by gel electrophoresis, five of the eight substitutions resulted in the production of a polymer with a molecular weight higher than that of the wild-type polymer. The R418L substitution caused the most dramatic change in the molecular weight of the polymer, influencing the biological functions of apoemulsan. The results described in this report demonstrate the functional effect of Wzc modification on the molecular weight of high-molecular-weight polysaccharides. Application of these genetic approaches could lead to generation of new bioemulsifiers with improved control of polymer structure and function.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
A. venetianus Rag1 (ATCC 31012) and its derivatives were grown at 30°C in Luria-Bertani (LB) broth or ethanol-mineral medium (EtOH-MM) containing (per liter): K2HPO4, 17 g; KH2PO4, 7.26 g; MgSO4·7H2O, 0.5 g; (NH4)2SO4, 4 g; ethanol, 10 ml; and a trace metal solution, 0.3 ml. Escherichia coli strains were cultivated in LB medium at 37°C. Antibiotics were added as required at the following concentrations: 50 μg/ml of kanamycin, 15 μg/ml of gentamicin sulfate, 20 μg/ml of chloramphenicol, and 100 μg/ml of streptomycin sulfate. In order to assess colony morphology, cells were plated by spotting 2 μl of cells diluted to an optical density at 600 nm (OD600) of 0.13 on EtOH-MM-0.75% agar plates supplemented with Congo red (40 μg/ml) and Coomassie brilliant blue (15 μg/ml). The plates were incubated for 6 days at 30°C. The morphology of cells growing on LB medium plates was assessed after 2 days of incubation at 30°C. Cells were streaked on LB medium plates using an inoculating loop. The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant features | Source |
|---|---|---|
| E. coli strains | ||
| GeneHogs | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara-leu)7697 galU galK λrpsL(Strr) nupG | Invitrogen |
| SM10 | Integrated RP4-2 plasmid provides transfer functions | S. Stibitz |
| A. venetianus strains | ||
| Rag1 | Wild type, emulsan producer, Cmr | ATCC 31012 |
| Rag1-Sm | Smr derivative of Rag1, emulsan producer, Cmr | W. Blank |
| Δwzc | Rag1-Sm, deletion in wzc gene, Gms Smr | This study |
| R418L | Rag1-Sm, R418L substitution in Wzc, Gms Smr | This study |
| A423P | Rag1-Sm, A423P substitution in Wzc, Gms Smr | This study |
| P432S | Rag1-Sm, P432S substitution in Wzc, Gms Smr | This study |
| L451G | Rag1-Sm, L541G substitution in Wzc, Gms Smr | This study |
| G445A | Rag1-Sm, G445A substitution in Wzc, Gms Smr | This study |
| G449A | Rag1-Sm, G449A substitution in Wzc, Gms Smr | This study |
| G445-9A | Rag1-Sm, G445A and G449A substitutions in Wzc, Gms Smr | This study |
| G445-6-9A | Rag1-Sm, G445A, G446A, and G449A substitutions in Wzc, Gms Smr | This study |
| Δ2 | Rag1-Sm, 5,630-bp deletion in fragment 2, Gms Smr | This study |
| Plasmids | ||
| pSS2141 | Replacement vector, Amr GmrrpsL oriT | S. Stibitz |
| pSS2141/Δwzc | pSS2141, Δwzc cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/R418L | pSS2141, NK/R418L cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/A423P | pSS2141, NK/A423P cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/P432S | pSS2141, NK/P432S cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/L451G | pSS2141, NK/L451G cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/G445A | pSS2141, NK/G445A cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/G449A | pSS2141, NK/G449A cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/G445-9A | pSS2141, NK/G445-9A cloned into ScaI site, Ams Gmr | This study |
| pSS2141/NK/G445-6-9A | pSS2141, NK/G445-6-9A cloned into ScaI site, Ams,Gmr | This study |
| pSS2141/Δ2 | pSS2141, Δ2 cloned into ScaI site, Ams Gmr | This study |
DNA methods.
Genomic DNA was isolated using an EasyDNA kit (Invitrogen) according to the manufacturer's recommendations. PCR amplifications were carried out by using either AccuPrime Pfx DNA polymerase (Invitrogen) or Platinum High Fidelity PCR SuperMix (Invitrogen) with the following conditions: denaturation at 95°C for 2 min and then 35 cycles of annealing at 55°C for 15 s, extension at 68°C for 1 min per 1 kb DNA, and denaturation at 95°C for 15 s. PCR and plasmid DNA fragments were purified using a QIAquick gel extraction kit (QIAGEN) according to the manufacturer's protocol. A purified PCR product was cloned into the pCR Blunt II TOPO vector using a Zero Blunt TOPO PCR cloning kit (Invitrogen) as recommended by the supplier. E. coli cells were transformed by electroporation using a Bio-Rad Gene-Pulser II (Bio-Rad) at a voltage of 1.7 kV, a resistance of 200 Ω, and a capacitance of 25 μF. Plasmid DNA was isolated using a QIAprep Spin miniprep kit (QIAGEN) as recommended by the manufacturer. All digestions, dephosphorylations, fill-in reactions, and ligations were performed using standard techniques (32). Restriction and modifying enzymes were supplied by New England BioLabs (Beverly, MA). DNA was sequenced by automated cycle sequencing at the Tufts University Core Facility with standard M13 forward and reverse primers.
Construction of mutants. (i) Construction of wzc deletion mutant.
The wild-type wzc gene was amplified using proofreading AccuPrime Pfx DNA polymerase and primers F1 and WzcR. These primers correspond to the following nucleotide numbers in the wee gene cluster sequence available in the GenBank database under accession number AJ243431: F1, nucleotides (nt) 535 to 550; and WzcR, nt 2982 to 3000. The PCR product was purified and cloned into the pCR Blunt II TOPO vector. Positive constructs were sequenced. Defined in-frame deletion mutations were constructed by digestion of pCR Blunt II TOPO/wzc with AhdI, filling in with Klenow polymerase, and then digestion with SspI. The religated plasmid pCR Blunt II TOPO/Δwzc was sequenced. The Δwzc insert was excised with EcoRI, and the 5′ overhangs were filled in using Klenow polymerase and ligated into the ScaI restriction site of the pSS2141 replacement vector. The pSS2141 vector contains Gmr and Amr cassettes, the rpsL gene conferring Sms as a dominant trait, and the RP4 plasmid transfer origin (S. Stibitz, personal communication). In order to use this vector, wild-type A. venetianus Rag1 cells were cultivated on LB plates containing streptomycin sulfate, and spontaneous Smr mutants were selected. Rag1-Sm cells are Rag1 derivatives that can tolerate streptomycin sulfate at a concentration of >400 μg/ml, compared to the <25 μg/ml that the wild-type Rag1 cells can tolerate. The resulting plasmid, pSS2141/Δwzc, was electroporated into E. coli SM10 cells, which possessed the integrated RP4-2 plasmid providing transfer functions. SM10(pSS2141/Δwzc) cells were mated with Rag1-Sm cells by mixing a loopful of the donor cells with a loopful of the recipient cells in 500 μl of LB medium and incubating the preparation at 30°C for 8 h. After cells were spread on LB medium plates and incubated overnight at 30°C, they were plated on LB medium plates containing chloramphenicol and gentamicin sulfate. Exconjugant cells containing the pSS2142/Δwzc plasmid integrated into the wzc region were incubated for 2 days at 30°C in LB medium without an antibiotic and then spread on LB plates containing streptomycin sulfate. Streptomycin-resistant colonies were recovered by eliminating the plasmid through a second recombination event, which could be processed in two ways: (i) reconstitution of the wild-type wzc gene or (ii) substitution with the deleted gene. In order to analyze the cells, colony PCR and Southern blot hybridization were performed. For colony PCR primers WzcF (position according to the accession no. AJ243431 sequence, nt 710 to 725) and WzcR were used.
(ii) Construction of Wzc amino acid substitution derivatives.
Site-directed mutagenesis was performed by overlapping extension PCR methods in a two-step process. First, two PCR products were amplified using a correct combination of primers. WzcF and mutagenic reverse primers were used to amplify one product, and mutagenic forward and MutR primers were used for amplification of the second fragment. The mutagenic primers are indicated in Table 2. After gel purification the two PCR products containing complementary ends were mixed and amplified with the MutF and MutR primers. The resultant DNA fragments were purified and after cloning into the pCR Blunt II TOPO vector were sequenced for mutation verification. Fragments of the amplified product were excised using naturally occurring restriction sites in the wzc gene (BglII and BsmBI) and were cloned into complementary ends of wild-type wzc in the pCR Blunt II TOPO/NKwzc plasmid. The pCR Blunt II TOPO/NKwzc plasmid was previously cloned by insertion of a 2,990-bp PCR product containing the wzc gene and flanking regions of the wee cluster into pCR Blunt II TOPO. The NKwzc fragment was generated using the NKwzc1F and NKwzc2R primers. Next, mutated wzc was digested with SpeI and XhoI, filled in with Klenow polymerase, and cloned into the ScaI site of the replacement vector pSS2141. The resulting plasmid was used to integrate the mutated wzc gene through homologous recombination as described above. Rag1 Δwzc cells were used as recipients for gene replacement. Mutants were screened by the colony PCR method using the WzcF and WzcR primers and were confirmed by Southern blot hybridization. Mutated residues are shown in Fig. 2.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| General primers | |
| NKwzc1F | GCG CAT GTG CAT CGC GGA AAC CAG TAA |
| NKwzc2R | TGC CGT AGT CCA ATG GCC GAA |
| F1 | CGG GTA CCG AGA TGT CCC GAT ATG |
| Wzc F | GCT CGA GTT AGT CTT CTT TAT TGG C |
| WzcR | CGG ATC CTG ACC CAT ACC AAC ACG |
| MUT F | GCC ACG ACG CAT ATC TGC ATC GAT CAG AAG |
| MUT R | CCC TCC ATC TCG ATC TAG AGA TTG CAA GCG |
| F2 | CAT GCC TTG ATC TCG CAT TGG |
| R2 | GGT GAA ATA GGC CTA CTA GG |
| Mutagenic primers | |
| L451G R | TTG GTG GCT TCT TAG GGA CTG GGC TTG CA |
| L451G F | GCA TAT TTC GTA ATA ATG CAA GCC CAG TCC C |
| G449A R | CCT TGG TGG CTT CTT AGC GAC TCT GCT TG |
| G449A F | CGT AAT AAT GCA AGC AGA GTC GCT AAG AAG CC |
| G445A R | CTT TCT ATT TTC CTT GCT GGC TTC TTA GGG |
| G445A F | CAA GCA GAG TCC CTA AGA AGC CAG CAA GG |
| P432S R | GCT GTA GAA CCT GTA GAA CCA ATC AAA TCT AAG |
| P432S F | GAA AGA ATT AAA ATT TGT AAT TTC TTA GAT TTG ATT GG |
| A423P R | ATG TTC GGA TTG TGG ATA CTC CTG TAG AAC C |
| A423P F | TGG TTC TAC AGG TTC TAC AGG AGT ATC CAC |
| R418L R | AGC AGG TGA AAT TGG TAA TGT TCT GAT TGT GG |
| R418L F | AGG TTC TAC AGC AGT ATC CAC AAT CAG AAC AT |
| G445-9A F | CAA GCA GAG TCG CTA AGA AGC CAG CAA GG |
| G445-9A R | CCT TGC TGG CTT CTT AGC GAC TCT GCT TG |
| G445-6-9A F | CAA GCA GAG TCG CTA AGA AGG CAG CAA GG |
| G445-6-9A R | CCT TGC TGC CTT CTT AGC GAC TCT GCT TG |
FIG. 2.
Comparison of proline and glycine motifs of wild-type (WT) and modified Wzc proteins. The bold type indicates changed amino acids.
(iii) Construction of apoemulsan-defective mutant.
Fragment 2 (Frag2) of the wee gene cluster containing the promoter region and six adjacent genes was amplified using the proofreading AccuPrime Pfx DNA polymerase and primers 2F and 2R. These primers correspond to the following nucleotide numbers in the wee gene cluster sequence: 2F, nt 3627 to 3647; and 2R, nt 10207 to 10226. The blunt-end PCR product generated was purified and cloned into the pCR Blunt II TOPO vector. The deletion mutation was constructed by digestion of pCR Blunt II TOPO/Frag2 with ScaI and SwaI. After purification and religation the resulting plasmid, pCR Blunt II TOPO/Δ2, was sequenced. Next, insert Δ2 was excised with EcoRI, and the 5′ overhangs were filled in using Klenow polymerase and ligated into the ScaI restriction site of the pSS2141 replacement vector. The pSS2141/Δ2 plasmid was used for construction of a apoemulsan-defective mutant via homologous recombination as described above. In order to analyze the cells, colony PCR and Southern blot hybridization were performed. For colony PCR primers 2F and 2R were used.
Southern blot hybridization.
For hybridization, 20 μg of genomic DNA was digested and separated overnight in a 1% agarose gel. The MfeI restriction enzyme was used for DNA digestion of wzc derivative mutants, and StuI was used for analysis of apoemulsan-defective mutants. After depurination in 0.25 M HCl and denaturation in 0.5 M NaOH-1.5 M NaCl, DNA was transferred by overnight capillary blotting to a Hybond-N+ positively charged nylon membrane (Amersham Bioscience) in denaturation solution and cross-linked to the membrane with UV. The fragment of Δwzc excised with MfeI was used as a probe for analysis of wzc derivatives, and the StuI Δ2 fragment was used as a probe for detection of gene replacement in apoemulsan-negative mutants. Probe labeling and detection were performed by using the North2South direct system (Pierce) according the manufacturer's recommendations.
Polymer production and purification.
For polymer production, A. venetianus wild-type strain Rag1 and its derivatives were grown in 2-liter baffled flasks containing 1 liter EtOH-MM and incubated at 30°C in an orbital shaker (250 rpm) for 6 days. Cell cultures were centrifuged for 30 min at 10,000 rpm, the supernatants were collected, and the polymer was precipitated with ammonium sulfate (274 g/liter) at 4°C for 24 h. The precipitated product was resuspended in water, and the associated proteins were removed by hot phenol extraction. After phenol residues were removed by dialysis against several changes of distilled water, the polymer was lyophilized.
Separation of polysaccharides on an SDS-PAGE gel.
Ten micrograms of purified polysaccharide was separated on a Novex 8 to 16% Tris-glycine gel (Invitrogen) according to the manufacturer's protocol. Following electrophoresis, the gel was washed in water for 1 h, and then polysaccharides were stained with Alcian blue (Sigma) by incubation for 1 h in 0.1% Alcian blue-40% ethanol-5% acetic acid and then destained overnight in a 40% ethanol-5% acetic acid solution.
Solid-surface-associated biofilm formation assay.
Overnight cultures grown in LB medium at 30°C were diluted to a final OD600 of 0.013 in EtOH-MM, and 150 μl was transferred to 96-well polyvinylchloride (PVC) microtiter plates and allowed to stand at 30°C for the required amount of time. A solid-surface-associated biofilm was demonstrated by washing the wells under tap water and then staining them by addition of 200 μl of 0.3% crystal violet (25). After incubation for 15 min at room temperature, samples were washed with tap water and allowed to dry. For quantitative analysis, 200 μl of dimethyl sulfoxide was added to each well, and after 10 min of incubation 125 μl was transferred to a polystyrene microtiter dish to determine the OD580.
Bacterial adherence to polystyrene.
A replica method for studying bacterial adherence to polystyrene has been described previously (29). After growing at 30°C for 48 h on EtOH-MM plates, the cells were replicated on flat polystyrene plates. The replica formed was washed with tap water, fixed by dipping it in methanol, and stained with 0.3% crystal violet.
RESULTS
Generation of A. venetianus Rag1 mutants.
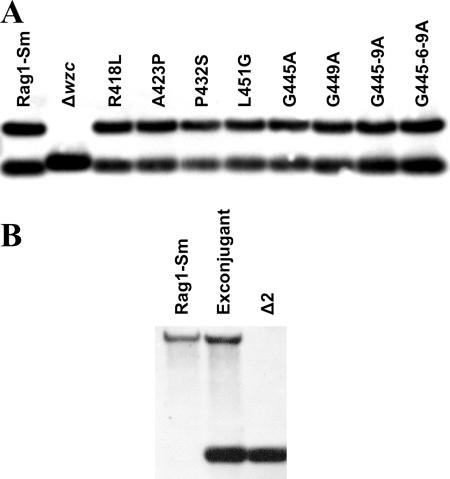
One of the characteristics of PCP protein family members is the appearance the highly conserved proline and glycine motif which is located just before and within TM2 (Fig. 1) (20). Several studies indicated that amino acid substitutions in this region resulted in modification of the molecular weight of the polysaccharide (4, 7). Based on previously published data, we selected specific amino acid substitutions within the Wzc protein that would likely result in the production of the polymer with a modified molecular weight (Fig. 2). Moreover, one additional amino acid substitution, A423P, was analyzed. This substitution was investigated since all analyzed PCP proteins involved in LPS polymerization had proline at the corresponding position, whereas the PCP of high-molecular-weight polymers contained alanine. Defined point mutations were introduced into the genome of Rag1-Sm cells via homologous recombination. In order to be able to distinguish the wild-type wzc gene and derivatives carrying point mutations, we first generated wzc deletion mutants as described in Materials and Methods. In order to confirm the deletions obtained, Southern blot hybridization was performed using an MfeI-excised fragment of Δwzc as a probe (Fig. 3A). MfeI-digested genomic DNA isolated from Rag1-Sm formed two bands at around 1,500 and 680 bp. After counterselection, the mutated Rag1 Δwzc colony lost both fragments, indicating that the wild-type wzc gene was replaced with the deleted form, which resulted in a single DNA fragment at around 740 bp (Fig. 3A). The wzc truncated mutant was used as a recipient strain to generate defined point mutation variants.
FIG. 1.
Alignment of the proline and glycine motif of PCP proteins involved in production of polysaccharides, including LPS, capsular polysaccharides (CPS), and exopolysaccharides (EPS). Abbreviations: K12 Wzz, E. coli K-12 Wzz (GenBank accession number Y07559); SF Wzz, Shigella flexneri Wzz (CAA50783); SE Wzz, Shigella dysenteriae Wzz (CAA78946); K30 Wzc, E. coli K30 Wzc (AF104912); RM ExoP, Rhizobium meliloti ExoP (AAQ87031); K12Co Wzc, E. coli K-12 colanic acid Wzc (U38473); EA AmsA, Erwinia amylovora AmsA (X77921); AL Wzc, A. venetianus Rag1 Wzc (AJ243431). The putative transmembrane region is underlined.
FIG. 3.
Southern blot analysis of point mutation derivatives (A) and a capsule-negative mutant (B). (A) Genomic DNA digested with MfeI was hybridized with the excised fragment of Δwzc. The Rag1-Sm derivative was used for generation of the wzc deletion mutant (Δwzc). Next, the Δwzc mutant served as the recipient strain for generation of point mutation derivatives. (B) Genomic DNA digested with StuI was hybridized with the excised fragment of Δ2.
The point mutations were introduced into the wzc gene by overlapping extension PCR with the primers indicated in Table 2. The replacement vectors carrying correct point mutations were prepared as described in Materials and Methods. The wzc point mutation was introduced into the Δwzc mutant genome via another round of homologous recombination. Colonies were identified by colony PCR and were confirmed by Southern blotting as described above (Fig. 3A). The truncated form of wzc was replaced with variants with point mutations that were the wild-type length; thus, the new mutant generated two bands at around 1,500 and 680 bp. Each amino acid substitution mutant was formed separately, and Southern blot analysis confirmed that there was correct incorporation of the mutated fragments (Fig. 3A).
In order to better understand the biological function of apoemulsan, a mutant unable to produce apoemulsan was generated. The wee gene operon is composed of two clusters transcribed in opposite directions. The 607-bp region between the two clusters contains putative σ promoter sequences in both upstream and downstream directions (22). Putative promoter regions together with six adjacent genes were deleted from the A. venetianus Rag1 genome as described in Materials and Methods. The obtained deletion was confirmed by Southern blot hybridization (Fig. 3B). StuI-digested genomic DNA isolated from Rag1-Sm cells formed single bands at approximately 6000 bp. In the exconjugant containing pSS2142/Δ2 the 6,000-bp fragment was joined by an additional 900-bp band, which came from the integrated plasmid. After counterselection, the mutated Δ2 colony lost the 6,000-bp band, indicating that fragment 2 of the wee gene cluster was replaced by the deleted form, which formed single a 900-bp band.
Amino acid substitutions in Wzc modulated the molecular weight of apoemulsan.
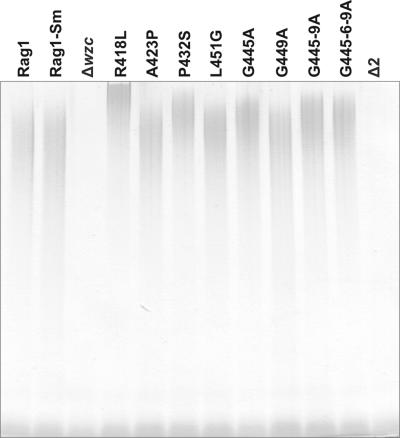
Wild-type and mutated derivative cells were grown in batch culture for 6 days in EtOH-MM. Both deletion mutants (Δwzc and Δ2) were characterized by a high tendency to aggregate, easily observed as the cells settled after the shaking stopped. After separation of the cells and supernatant, the polymer was recovered from the supernatant via ammonium sulfate precipitation and purified as described previously (12). The yield of apoemulsan obtained from amino acid substitution variants was similar to the yield of the wild-type polymer (data not shown). From deletion mutants we were able to recover very small amounts of the polymer; the yield was about 14% of the yield from the wild type. In order to analyze the effect of amino acid substitutions in Wzc on the molecular weight of apoemulsan, 10-μg portions of purified polymers were separated on SDS-PAGE gels. Five of eight polymer analogs had higher molecular weights than the wild-type polysaccharide (Fig. 4). The most dramatic change was the replacement of arginine by leucine at position 418 (R418L) and then the replacement of proline by alanine at position 432 (P432A). The polymers produced by the mutants, G445-9A and G445-6-9A, had higher but similar molecular weights, and the G445A mutant polymer had the lowest molecular weight of all five modified polymers. Moreover, except for the high-molecular-weight polymer, all of the variants analyzed showed the presence of low-molecular-weight polysaccharide (Fig. 4). Neither of the deletion mutants (ΔWzc and Δ2) produced a high-molecular-weight polymer; only low-molecular-weight polysaccharide was produced.
FIG. 4.
SDS-PAGE analysis of purified polymers from the wild type and its derivatives. Ten-microgram portions of purified polysaccharides were separated in an 8 to 16% polyacrylamide gel and stained with Alcian blue.
Apoemulsan analog-modified properties of Rag1 cells. (i) Morphology.
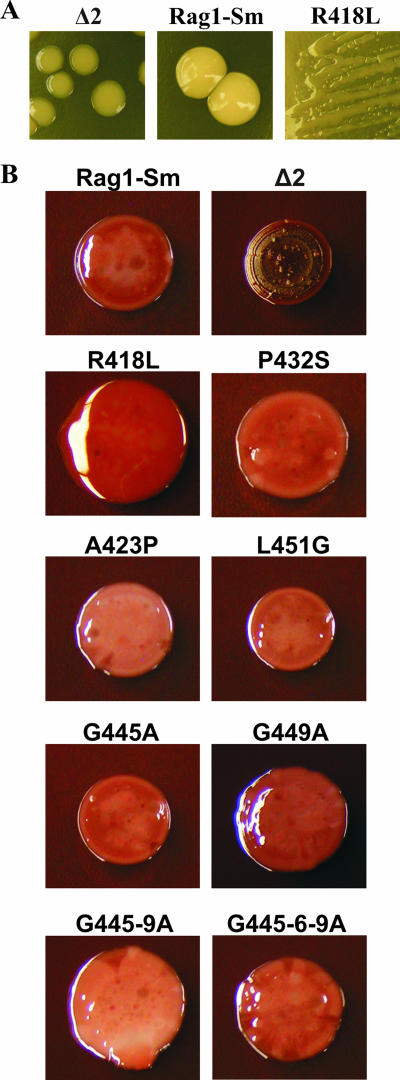
When apoemulsan-defective mutants were grown on LB agar plates at 30°C, they formed colonies that were more translucent than the opaque colonies formed by Rag1-Sm cells (Fig. 5A). This observation was consistent with previous observations (3) indicating that Δ2 mutants are not able to produce an apoemulsan-like polymer. Moreover, the morphology of one of the amino acid substitution mutants, R418L, was highly mucoid (Fig. 5A), indicating that there was a significant change in cell properties. The R418L mutant was the only mutant which displayed a modified morphology when it was grown on an LB agar plate. Since EtOH-MM is routinely used for apoemulsan production, we investigated the colony morphology of cells growing on EtOH-MM plates. As indicated in Fig. 5A, the Rag1-Sm derivative and emulsan-defective mutant colonies grown on LB agar plates were smooth. When cells were grown on EtOH-MM plates containing 0.75% agar supplemented with Congo red and Coomassie brilliant blue, the colony morphology changed dramatically (Fig. 5B). The apoemulsan-defective strain produced brown, rough, dry colonies. The Rag1-Sm cells formed brighter colonies with glossy, smooth surfaces; however, some wrinkled structures were visible. The morphology of the R418L mutant was the most modified compared to the morphology of the Rag1-Sm strain. The colonies were much bigger, and their surfaces were smooth and glossy. Mutants P432S, G449A, G445-9A, and G445-6-9A also formed bigger colonies; however, they were much flatter than the colonies of R418L cells. Moreover, on their surfaces some wrinkled structures were visible, in contrast to colonies of R418L. Mutants A423P, L451G, and G445A produced colonies whose morphology was the most similar to the morphology of the colonies produced by Rag1-Sm cells.
FIG. 5.
Colony morphology of Rag1-Sm and its derivatives grown on LB plates containing 0.75% agar (A) and EtOH-MM plates containing 40 mg of Congo red/liter and 15 mg of Coomassie brilliant blue/liter (B). (A) The magnification for the R418L cells is not the same as the magnification for the Δ2 and Rag1-Sm cells.
(ii) Hydrophobicity and bacterial attachment.
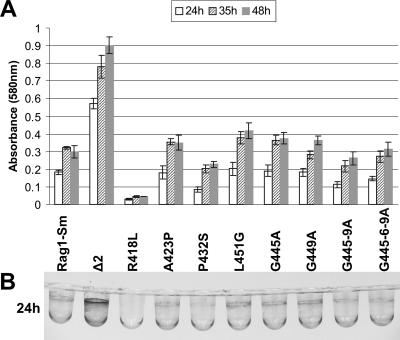
It has been shown previously that the presence of apoemulsan on the cell surface makes Rag1 cells more hydrophilic (33). Since bacterial adherence to polystyrene reflects bacterial hydrophobicity (29), we evaluated how the modified polymers affected bacterial adherence to a hydrophobic surface. As shown in Fig. 6, an apoemulsan-negative mutant was strongly attached to polystyrene plates, while the attachment of Rag1-Sm cells was less than that of the Δ2 variant. The R418L mutant exhibited the most significant reduction in attachment to polystyrene. Moreover, the P432S mutant and also mutants G445-9A and G445-6-9A exhibited reduced attachment, manifested as slightly decreased dye intensity, which represented a lower number of adhered cells. Since emulsan has been described as a biosurfactant and secreted low-molecular-weight biosurfactants were demonstrated to inhibit cell attachment to abiotic surfaces (5, 18), we determined whether the observed inhibitory effect was the result of accumulation of released emulsan after incubation for 48 h or whether emulsan could influence attachment of the cells during growth of the culture. In order to investigate this, an assay of biofilm formation on microtiter PVC surfaces was performed. After 24 h of incubation the most robust biofilm was formed by the apoemulsan-negative mutant (Fig. 7B). The biofilm of Rag1-Sm cells was less extensive, and the biofilm of R418L cells was hardly visible. Mutant P432S and the derivatives G445-9A and G445-6-9A showed lower biofilm accumulation than the Rag1-Sm cells. Up to 48 h, the apoemulsan-negative mutant continued to accumulate on the PVC surface, while Rag1-Sm cells, after forming a mature biofilm, did not (Fig. 7A). The biofilm of the R418L derivative was still hardly visible. In contrast, the P432S biofilm continued to form after 24 h but at a lesser extent than the biofilm formed by the wild-type cells. After 48 h the biofilms of the G445-9A and G445-6-9A derivatives reached the level of the biofilm of Rag1-Sm cells.
FIG. 6.
Adherence of Rag1-Sm and its derivatives to polystyrene. After 48 h of growth on an EtOH-MM plate, cells were replicated on polystyrene plates, washed, and stained with crystal violet. Greater dye intensity indicates a higher number of adherent cells.
FIG. 7.
Biofilm formation on the surfaces of microtiter dish wells under standing culture conditions. (A) Crystal violet-stained, surface-attached cells were quantified by solubilization in 100% dimethyl sulfoxide, and absorbance was measured at 580 nm. Each bar represents the average of six independent samples. The error bars indicate standard deviations. (B) Rag1-Sm and its derivatives were grown for 24 h at 30°C in EtOH-MM. The biofilms were visualized using crystal violet dye.
DISCUSSION
We have previously described how to modulate the structure of apoemulsan by modifying the fatty acid composition, mainly by culture manipulation (12, 36). However, the recently described wee gene cluster responsible for apoemulsan formation and secretion (22) offered new options for generating emulsan-like analogs. With knowledge of the genes involved, genetic engineering could be used to modify the sugar backbone and thus the polymer properties. This approach, together with control of the fatty acid composition, can be used for the generation of new types of the polymer with the potential to impact a broader range of applications.
The open reading frames of the wee cluster encode enzymes that have putative functions in catalyzing the production of nucleotide amino sugar precursors, transglycosylation, transacetylation, and polymerization, as well as proteins involved in polymer transport (22). These open reading frames are typical of those found in gene clusters involved in the synthesis capsular polysaccharides, which are polymerized via the Wzy-dependent pathway (34). Although the mechanism of assembly and transport of capsular polymer is still under investigation, some data are useful for the approach used in the present work. Consideration was given to the proteins that control the process of polysaccharide polymerization. According to this model, the polymer chain length distribution is dependent on PCP proteins (2, 7, 8, 10, 17, 21). There are some differences between members of the PCP family. However, one characteristic is highly conserved in all PCP proteins: the proline-glycine-rich region located just before and within TM2 (Fig. 1) (20). Bastin et al. (2) proposed that this region may be involved in protein-protein interactions with integral membrane proteins connected with polymerization and transport of the polymer. Daniels and Morona (7) examined this region in order to localize essential amino acids. Site-directed mutagenesis was used to show that single residue changes in the glycine motif had no effect on the regulation of polymer length. Also, some multiple changes in glycine residues, like G305A/G309A and G305A/G306A/G309A, had no effect on chain length. However, some other multiple changes (G305A/G311A) resulted in reduction in the chain length. Moreover, a single proline substitution caused a loss of function of the Wzz protein (P292A) or a reduced level of formation of the O-antigen chain (P286A) (7). There are some data that indicate the involvement of a proline- and glycine-rich motif of PCP proteins in modulation of polymerization of high-molecular-weight polymers. In Sinorhizobium meliloti two of seven mutations (R443L and P457S) in this region of ExoP resulted in enhanced production of low-molecular-weight succinoglycan at the expense of a high-molecular-weight polymer (4). Our results showed that five of eight single amino acid substitutions or combinations of amino acid substitutions caused changes in the molecular weight of the polymer (Fig. 4). The most significant substitution was the R418L substitution, which corresponded to the R443L substitution in the S. meliloti study. In our case, the point mutation at position 418 increased the molecular weight of the polymer. The same effect was observed for the P432S substitution, which corresponds to the P457S modification in S. meliloti; however, the molecular weight of apoemulsan was slightly decreased compared to that of the R418L mutant, but it was still higher than that of the wild type. Moreover, the P432S substitution corresponds to the P292A modification in the study of Daniels and Morona (7). In the model of these authors this substitution caused a loss of function of the Wzz protein. This indicates that the last proline in the proline-glycine-rich region not only is the most conservative but also impacts the function of PCP proteins. Another important residue seems to be the glycine at position 445. In our study replacement of this amino acid caused the production of a polymer with a higher molecular weight. In the Wzz model the single amino acid modification in the glycine region did not affect the pattern of polymerization (7). Only the G305A/G311A combination resulted in a reduction in chain length. Wzz glycine residues 305 and 311 correspond to glycine 445 and leucine 451, respectively, in our model. Since glycine does not occur at position 451, the mutant with the single G445A substitution in apoemulsan could correspond to the mutant with the G305A/G311A combination in the Wzz model. It is interesting that the G445A/G449A and G445A/G446A/G449A substitutions were involved in production of a modified polymer. This is in contrast to the corresponding substitutions in the Wzz model (G305A/G309A and G305A/G306A/G309A, respectively). Again, in our PCP protein glycine does not occur at position 451, which could explain the differences in the results observed. However, position 451 does not seem to be critical, since the L451G single substitution did not influence the polymerization pattern.
Most of the available data about relationships between functional effects of mutated PCP proteins on polymer distribution concerns the proteins involved in LPS-O-antigen synthesis, since they can be easily analyzed on silver-stained SDS-PAGE gels. The functional effect of PCP2 modification is usually expressed as the presence or absence of the capsule or as discrimination between high and low molecular weights (4, 9). Becker and Puhler (4) reported changing the ratio of high-molecular-weight polymers to low-molecular-weight polymers, but they did not investigate the change in the molecular weight of the exopolysaccharide. Since high-molecular-weight polysaccharides tend to aggregate, it is difficult to analyze chain length. In this study we tried to use size exclusion chromatography; however, the results were not sufficiently consistent. In addition, we explored matrix-assisted laser desorption ionization mass spectroscopy; however, this technique also suffers from limitations when it is used to characterize high-molecular-weight polymers. Therefore, as an alternative, we analyzed the molecular weights of the polymers via separation of the polysaccharides on denaturing PAGE gels. This approach did not allow determination of exact molecular weights or provide detailed information about the distribution of the polymers; however, it did highlight the difference between the wild-type and modified polysaccharides. The results obtained from the gel analysis supported the functional effect of Wzc modification on the molecular weight of high-molecular-weight polysaccharide. However, since we are aware of the low resolution of gel analysis, we further supported our results by analyzing the functional effects of modified polymers on Rag1 biology. In terms of morphology the most significant change was observed with the mutant that produced the polymer with the highest molecular weight (R418L). The differences were observed on both LB and EtOH-MM agar plates. The observed change in morphology indicated the different cell surface properties. Moreover, we investigated whether higher-molecular-weight apoemulsan influenced Rag1 hydrophobicity. A. venetianus Rag1 cells adhere to various abiotic and biotic hydrophobic surfaces, like hexadecane, polystyrene, or epithelial cells (29, 31). Previous results demonstrated that accumulation of emulsan on the cell surface did not have a beneficial effect on cell adherence (24). Furthermore, adverse effects on cell hydrophobicity were reported (33). As a consequence of the solubilization of the emulsan capsule, Rag1 cells became more hydrophobic (33). Our results support the correlation between the presence of apoemulsan and cell hydrophobicity. An apoemulsan-negative mutant (Δ2) had the most hydrophobic features; it bound strongly to polystyrene plates and formed the most robust biofilm (Fig. 6 and 7). Rag1-Sm cells exhibited hydrophobicity, since they adhered to polystyrene and were able to form a biofilm but to a lesser extent than the apoemulsan-negative mutant. The hydrophobicity of mutant R418L was significantly reduced. Attachment to polystyrene and the formation of a biofilm were decreased or nearly abolished. Moreover, mutants producing a higher-molecular-weight polymer, like P432S, G445-9A, and G445-6-9A, adhered to the polystyrene plates to a lesser extent than Rag1-Sm cells. However, the differences were not as evident as they were for the R418L mutant. The same three mutants exhibited inhibition of biofilm formation after 24 h of incubation. However, after 48 h, mutants G445-9A and G445-6-9A were able to form biofilms comparable to the biofilms formed by Rag1-Sm cells. According to the gel electrophoresis analysis, five mutants produced polymers with higher molecular weights. Four of them had modified biological properties. Only one mutant (G445A) did not exhibit changes in morphology and attachment to hydrophobic surfaces. However, this mutant produced the polymer with the smallest difference in molecular weight.
One possible explanation for the observed reduction in hydrophobicity is an enhanced effect of masking hydrophobic sites on the cell surface by the modified polymer. Direct contact with the hydrocarbon was shown to be mediated by fimbriae (30). The analysis of Henrichsen and Blom (15) of various strains of Acinetobacter calcoaceticus demonstrated the presence of two kinds of fimbriae: thin fimbriae about 3 nm in diameter and thick fimbriae about 5 nm in diameter. The presence of thick fimbriae was correlated with twitching motility (15), while the thin fimbriae were shown to be directly involved in binding to hydrophobic surfaces (30). Moreover, Pines and Gutnick (27) postulated that other hydrophobic sites exist on the Rag1 surface. A mutant that did not bind to hydrocarbon did not assemble thin fimbriae; however, it still expressed emulsan. A derivative of this mutant, which was not able to produce emulsan, adhered to hexadecane (27); however, the nature of the sites was not described. Mutants that produce higher-molecular-weight polymers may mask not only alternate hydrophobic sites but also thin fimbriae, which could explain the significantly decreased cell attachment to hydrophobic surfaces. R418L cells produced the polymer with the highest molecular weight, which may extend above the thin fimbria level. The polymers of mutants P432S, G445-9A, and G445-6-9A may be at the level of thin fimbriae, which influence but do not abolish the attachment to hydrophobic surfaces. Although mutant G445A produces a polymer whose molecular weight is higher than the wild-type molecular weight, this polymer may not cover the thin fimbriae and thus not modify the hydrophobicity of the cells. However, the observed loss of hydrophobicity of modified cells needs to be investigated further, which was beyond the scope of the present study. The modified polymer whose molecular weight is higher than the molecular weight of the wild-type polymer influences cell properties. The analysis of mutants indicated that cell surface properties are modified, which supports our conclusion based on polymer separation in polyacrylamide gels.
Conclusions.
The goal of this study was to develop a genetic system to control polymerization of apoemulsan. We showed that amino acid substitutions in the PCP (Wzc) successfully altered the molecular weight of the polymer. Moreover, mutants produced the highest-molecular-weight polymer and displayed modified biological properties. Changed morphology and attachment to hydrophobic surfaces indicated that modification of the polymer molecular weight had a direct impact on the function of the cells and also supported the analytical results based on SDS-PAGE. The strategy presented here can be applied to explore more apoemulsan variants. Moreover, the use of selective exogenous fatty acid feeding strategies, as well as the types of genetic manipulations of the sugar backbone chain length described here, is a promising approach for bioengineering emulsan analogs to match future specific applications.
Acknowledgments
We thank Scott Stibitz for the generous gifts of pSS2141 and E. coli strain SM10. We also thank Walter Blank for his early contributions to this study and Bruce Paniliatis for general discussions relevant to the overall project.
The USDA provided support for this study (grant 35504-12878).
Footnotes
Published ahead of print on 20 April 2007.
REFERENCES
- 1.Reference deleted.
- 2.Bastin, D. A., G. Stevenson, P. K. Brown, A. Haase, and P. R. Reeves. 1993. Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol. Microbiol. 7:725-734. [DOI] [PubMed] [Google Scholar]
- 3.Bayer, E. A., E. Skutelsky, S. Goldman, E. Rosenberg, and D. L. Gutnick. 1983. Immunochemical identification of the major cell surface agglutinogen of Acinetobacter calcoaceticus RAG-92. J. Gen. Microbiol. 129:1109-1119. [DOI] [PubMed] [Google Scholar]
- 4.Becker, A., and A. Puhler. 1998. Specific amino acid substitutions in the proline-rich motif of the Rhizobium meliloti ExoP protein result in enhanced production of low-molecular-weight succinoglycan at the expense of high-molecular-weight succinoglycan. J. Bacteriol. 180:395-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boles, B. R., M. Thoendel, and P. K. Singh. 2005. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 57:1210-1223. [DOI] [PubMed] [Google Scholar]
- 6.Castro, G. R., R. R. Kamdar, B. Panilaitis, and D. L. Kaplan. 2005. Triggered release of proteins from emulsan-alginate beads. J. Control. Release 109:149-157. [DOI] [PubMed] [Google Scholar]
- 7.Daniels, C., and R. Morona. 1999. Analysis of Shigella flexneri wzz (Rol) function by mutagenesis and cross-linking: wzz is able to oligomerize. Mol. Microbiol. 34:181-194. [DOI] [PubMed] [Google Scholar]
- 8.Dodgson, C., P. Amor, and C. Whitfield. 1996. Distribution of the rol gene encoding the regulator of lipopolysaccharide O-chain length in Escherichia coli and its influence on the expression of group I capsular K antigens. J. Bacteriol. 178:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drummelsmith, J., and C. Whitfield. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol. Microbiol. 31:1321-1332. [DOI] [PubMed] [Google Scholar]
- 10.Franco, A. V., D. Liu, and P. R. Reeves. 1998. The Wzz (cld) protein in Escherichia coli: amino acid sequence variation determines O-antigen chain length specificity. J. Bacteriol. 180:2670-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman, S., Y. Shabtai, C. Rubinovitz, E. Rosenberg, and D. L. Gutnick. 1982. Emulsan in Acinetobacter calcoaceticus RAG-1: distribution of cell-free and cell-associated cross-reacting material. Appl. Environ. Microbiol. 44:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorkovenko, A., J. Zhang, R. A. Gross, A. L. Allen, and D. L. Kaplan. 1997. Bioengineering of emulsifier structure: emulsan analogs. Can. J. Microbiol. 43:384-390. [DOI] [PubMed] [Google Scholar]
- 13.Gutnick, D. L. 1987. The emulsan polymer: prospectives on a microbial capsule as an industrial product. Biopolymers 26:S223-S240. [Google Scholar]
- 14.Gutnick, D. L., and E. Rosenberg. 1977. Oil tankers and pollution: a microbiological approach. Annu. Rev. Microbiol. 31:379-396. [DOI] [PubMed] [Google Scholar]
- 15.Henrichsen, J., and J. Blom. 1975. Correlation between twitching motility and possession of polar fimbriae in Acinetobacter calcoaceticus. Acta Pathol. Microbiol. Scand. Sect. B 83:103-115. [DOI] [PubMed] [Google Scholar]
- 16.Johri, A. K., W. Blank, and D. L. Kaplan. 2002. Bioengineered emulsans from Acinetobacter calcoaceticus RAG-1 transposon mutants. Appl. Microbiol. Biotechnol. 59:217-223. [DOI] [PubMed] [Google Scholar]
- 17.Klee, S. R., B. D. Tzschaschel, K. N. Timmis, and C. A. Guzman. 1997. Influence of different rol gene products on the chain length of Shigella dysenteriae type 1 lipopolysaccharide O antigen expressed by Shigella flexneri carrier strains. J. Bacteriol. 179:2421-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuiper, I., E. L. Lagendijk, R. Pickford, J. P. Derrick, G. E. Lamers, J. E. Thomas-Oates, B. J. Lugtenberg, and G. V. Bloemberg. 2004. Characterization of two Pseudomonas putida lipopeptide biosurfactants, putisolvin I and II, which inhibit biofilm formation and break down existing biofilms. Mol. Microbiol. 51:97-113. [DOI] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Morona, R., L. Van Den Bosch, and C. Daniels. 2000. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled-coil regions. Microbiology 146:1-4. [DOI] [PubMed] [Google Scholar]
- 21.Morona, R., L. van den Bosch, and P. A. Manning. 1995. Molecular, genetic, and topological characterization of O-antigen chain length regulation in Shigella flexneri. J. Bacteriol. 177:1059-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakar, D., and D. L. Gutnick. 2001. Analysis of the wee gene cluster responsible for the biosynthesis of the polymeric bioemulsifier from the oil-degrading strain Acinetobacter lwoffii RAG-1. Microbiology 147:1937-1946. [DOI] [PubMed] [Google Scholar]
- 23.Nesper, J., C. M. Hill, A. Paiment, G. Harauz, K. Beis, J. H. Naismith, and C. Whitfield. 2003. Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J. Biol. Chem. 278:49763-49772. [DOI] [PubMed] [Google Scholar]
- 24.Ng, T. K., and W. S. Hu. 1989. Adherence of emulsan-producing Acinetobacter calcoaceticus to hydrophobic liquids. Appl. Microbiol. Biotechnol. 31:480-485. [Google Scholar]
- 25.O'Toole, G. A., L. A. Pratt, P. I. Watnick, D. K. Newman, V. B. Weaver, and R. Kolter. 1999. Genetic approaches to study of biofilms. Methods Enzymol. 310:91-109. [DOI] [PubMed] [Google Scholar]
- 26.Panilaitis, B., A. Johri, W. Blank, D. Kaplan, and J. Fuhrman. 2002. Adjuvant activity of emulsan, a secreted lipopolysaccharide from Acinetobacter calcoaceticus. Clin. Diagn. Lab. Immunol. 9:1240-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pines, O., and D. Gutnick. 1984. Alternate hydrophobic sites on the cell surface of Acinetobacter calcoaceticus RAG-1. FEMS Microbiol. Lett. 22:307-311. [Google Scholar]
- 28.Rosenberg, E., A. Zuckerberg, C. Rubinovitz, and D. L. Gutnick. 1979. Emulsifier of Arthrobacter RAG-1: isolation and emulsifying properties. Appl. Environ. Microbiol. 37:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg, M. 1981. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl. Environ. Microbiol. 42:375-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg, M., E. A. Bayer, J. Delarea, and E. Rosenberg. 1982. Role of thin fimbriae in adherence and growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. Appl. Environ. Microbiol. 44:929-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenberg, M., A. Perry, E. A. Bayer, D. L. Gutnick, E. Rosenberg, and I. Ofek. 1981. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect. Immun. 33:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 33.Shoham, Y., M. Rosenberg, and E. Rosenberg. 1983. Bacterial degradation of emulsan. Appl. Environ. Microbiol. 46:573-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitfield, C., and A. Paiment. 2003. Biosynthesis and assembly of group 1 capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. Carbohydr. Res. 338:2491-2502. [DOI] [PubMed] [Google Scholar]
- 35.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 36.Zhang, J., A. Gorkovenko, R. A. Gross, A. L. Allen, and D. Kaplan. 1997. Incorporation of 2-hydroxyl fatty acids by Acinetobacter calcoaceticus RAG-1 to tailor emulsan structure. Int. J. Biol. Macromol. 20:9-21. [DOI] [PubMed] [Google Scholar]
- 37.Zosim, Z., D. L. Gutnick, and E. Rosenberg. 1983. Uranium binding by emulsan and emulsanosols. Biotechnol. Bioeng. 25:1725-1735. [DOI] [PubMed] [Google Scholar]
- 38.Zuckerberg, A., A. Diver, Z. Peeri, D. L. Gutnick, and E. Rosenberg. 1979. Emulsifier of Arthrobacter RAG-1: chemical and physical properties. Appl. Environ. Microbiol. 37:414-420. [DOI] [PMC free article] [PubMed] [Google Scholar]