Abstract
Ventilator-associated pneumonia (VAP) is the second most common hospital-acquired infection among pediatric intensive care unit (ICU) patients. Empiric therapy for VAP accounts for approximately 50% of antibiotic use in pediatric ICUs. VAP is associated with an excess of 3 days of mechanical ventilation among pediatric cardiothoracic surgery patients. The attributable mortality and excess length of ICU stay for patients with VAP have not been defined in matched case control studies. VAP is associated with an estimated $30,000 in attributable cost. Surveillance for VAP is complex and usually performed using clinical definitions established by the CDC. Invasive testing via bronchoalveolar lavage increases the sensitivity and specificity of the diagnosis. The pathogenesis in children is poorly understood, but several prospective cohort studies suggest that aspiration and immunodeficiency are risk factors. Educational interventions and efforts to improve adherence to hand hygiene for children have been associated with decreased VAP rates. Studies of antibiotic cycling in pediatric patients have not consistently shown this measure to prevent colonization with multidrug-resistant gram-negative rods. More consistent and precise approaches to the diagnosis of pediatric VAP are needed to better define the attributable morbidity and mortality, pathophysiology, and appropriate interventions to prevent this disease.
INTRODUCTION
Ventilator-associated pneumonia (VAP) is pneumonia in mechanically ventilated patients that develops later than or at 48 h after the patient has been placed on mechanical ventilation. VAP is the second most common hospital-acquired infection among pediatric and neonatal intensive care unit (ICU) (NICU) patients (41, 43). Overall, VAP occurs in 3 to 10% of ventilated pediatric ICU (PICU) patients (1, 28). Surveillance studies of nosocomial infections in NICU patients indicate that pneumonia comprises 6.8 to 32.3% of nosocomial infections in this setting (26, 39, 48). The incidence of VAP is higher in adult ICU patients, ranging from 15 to 30% (8, 31, 50, 70, 90).
NICU VAP rates vary by birth weight category as well as by institution. Two large studies are summarized in Table 1. The most recent National Nosocomial Infection Surveillance (NNIS) data from 2002 to 2004 show NICU VAP rates ranging from 1.4 to 3.5 per 1,000 ventilator days (68). In 1998, a cross-sectional study of hospital-acquired infections in 50 children's hospitals was performed by the Pediatric Prevention Network (88). Of 43 children's hospitals that returned questionnaires reporting NICU and PICU surveillance data, the VAP rate by device days was reported by 19 hospitals, and 12 hospitals provided VAP rates stratified by birth weight groups (Table 1). In this cross-sectional survey, VAP rates were highest for the 1,001- to 1,500-g and <1,000-g birth weight categories.
TABLE 1.
VAP rates stratified by birth weighta
Differences in study methodology and case mix can influence the reported incidence of VAP (6). Surveillance methods differ across study institutions, introducing variability into the reported incidence of VAP. The influence of surveillance intensity on the reported prevalence of nosocomial infections is illustrated by a 41-month surveillance study in a children's hospital. Infection control surveillance was conducted twice a week for the first 2 years of the study and then daily for the second 2 years of the study through a nursing sentinel sheet. Those investigators found a 50% increase in the incidence of reported nosocomial infections following the introduction of daily surveillance (39).
NNIS definitions for VAP were revised in 2002, resulting in a more stringent definition of VAP. Studies of VAP incorporating these revised definitions reported lower rates of VAP, making it difficult to know if VAP was previously overdiagnosed or is now underdiagnosed. The revised definitions must also be considered when VAP rates are compared over time. Applying the Centers for Disease Control and Prevention (CDC) definitions for VAP in low-birth-weight infants introduces additional complexity in defining the incidence of VAP. CDC definitions for VAP exist for infants <1 year of age, but there are no specific definitions for low- or very-low-birth-weight infants. These patients often have comorbidities such as bronchopulmonary dysplasia, hyaline membrane disease, bloodstream infections (BSIs), and necrotizing enterocolitis that obscure clinical, laboratory, and radiographic evidence of VAP.
OUTCOMES
VAP is associated with increased morbidity in PICU patients, specifically, a longer duration of mechanical ventilation. Fischer et al. (34) performed a prospective cohort study to determine the delay of extubation attributable to VAP among neonates and children undergoing repair of congenital heart disease. Twenty-six of the 272 patients enrolled over a 22-month period developed VAP (9.6%). VAP diagnosis was made when the following criteria were met: fever exceeding 38.5°C, tachypnea and/or otherwise unexplained increased oxygen requirement, elevated white blood cell count (>15 × 109 cells/liter), a cultured pathogen from tracheal aspirate together with a positive gram stain, and increased leukocyte contents, plus an infiltrate on chest radiographs persisting for 48 h or more (29). Using a Cox proportional hazards model to control for complexity of surgery, other respiratory complications, and secondary surgeries, those investigators found that the median delay of extubation attributable to VAP was 3.7 days (average of 5.2 versus 1.5 for patients with and without VAP, respectively). VAP rates increased dramatically for patients intubated for long periods of time. Among patients extubated within the first 3 days of surgery, only 4% developed VAP, compared to 40% of postoperative cardiothoracic surgery patients intubated longer than 30 days (34). Of the 26 VAP cases, 19 occurred within the first 3 to 6 days after surgery.
Presumed VAP is also associated with additional resource utilization with respect to antibiotic administration. VAP is the most common reason for the initiation of empirical antibiotics among PICU patients. A prospective cohort study at an academic tertiary care center performed in a PICU (n = 456) found that over half (56.6%) of all patients (n = 258) received antibiotics (33). Treatment for suspected VAP comprised 616 of 1,303 (47%) of the antibiotic treatment days. Those authors reviewed medical records to determine whether patients had evidence of an alternative explanation for the symptoms attributed to VAP, such as a viral infection. For 40% of the antibiotic days (552/1,303 treatment days), patients were classified as having no infection (i.e., did not meet clinical criteria as defined by the CDC) or as having a viral infection. Those authors concluded that an intervention targeted at decreasing antibiotic use for VAP would have the greatest impact on antibiotic use.
In pediatric populations, the published data are unmatched for severity of illness and univariate but suggest that pediatric patients with VAP may have excess mortality and length of PICU and NICU stay. The European Multicenter Trial examined the epidemiology of hospital-acquired infections in 20 units (5 PICUs, 7 neonatal units, 2 hematology-oncology units, and 8 general pediatric units) in eight countries, with a total of 14,675 admissions (710 admission in PICUs) (77). Those investigators found the infected patients had a longer mean length of stay in the PICU (26.1 ± 17.3 versus 10.6 ± 6 days; P < 0.001) than uninfected patients. The mortality rate was 10% for PICU patients with nosocomial infections. The mortality and length of stay associated specifically with VAP were not reported, although VAP accounted for 53% of the nosocomial infections in PICU patients. Mortality among uninfected PICU patients was not reported. Similarly, PICU length of stay in a 9-month prospective cohort study in an academic tertiary care center revealed that patients with VAP (n = 30) had a mean PICU length of stay of 27 days versus 6 days for uninfected patients (n = 595) (P = 0.001) (28). In that same study, the mortality rates with and without VAP were 20% and 7%, respectively (P = 0.065). Outcomes between patients on mechanical ventilation for more than 8 days with VAP (n = 30) and those without VAP (n = 62) were also compared. PICU length of stay was longer for VAP patients (27.53 ± 20.09 versus 18.72 ± 35 days), as was hospital length of stay (52.63 ± 37.43 versus 33.77 ± 49.51 days), but no differences in mortality rates for VAP (20%) or uninfected patients (21%) were found. Almuneef et al. (1) determined in a prospective cohort study (n = 361) that PICU lengths of stay with (n = 37) and without (n = 324) VAP were longer for patients with VAP (33.70 ± 30.28 versus 14.66 ± 17.34 days; P = 0.001). Statistically significant differences in mortality rates between patients with VAP and those without VAP were not found (P = 0.362). Both of those studies performed only univariate analyses to compare mortality rates among patients with and without VAP. Multivariate analysis of predictors of mortality among PICU patients with sufficient numbers of VAP controlling for severity of illness both at admission and at the time of VAP as well as other potential predictors of death is necessary to determine the true attributable mortality of VAP in pediatric patients.
VAP has also been shown to increase hospital costs. The cost of VAP was analyzed in a 2-year study of PICU patients (n = 1919) with a single admission (38). The direct cost for patients with VAP (n = 56) was $38,614, and that for patients without VAP was $7,682. In a multivariate analysis controlling for other predictors of cost including age, severity of illness, underlying disease, and ventilator days, VAP was independently associated with a direct cost of $30,931 (95% confidence interval [CI], $18,349 to $82,638) (38). This is a single study from an academic tertiary care center; further studies are needed to determine whether the results from this single center are generalizable.
Recommendations for Current Practice and Future Research
Differences in the incidence of VAP occur as a result of the definitions used, persons doing surveillance, and frequency of surveillance. Standardization of surveillance methodology and validation of current definitions against a histopathologic or microbiologic “gold standard” would make interinstitutional comparisons meaningful, particularly in light of mandatory reporting of health care-associated infections. Recent literature suggests that pediatric VAP is associated with increased morbidity, antibiotic use, PICU cost, and PICU and hospital length of stay. Prospective studies using consistent definitions of VAP and controlling for severity of illness both at admission and at the time of VAP as well as other possible predictors of death and length of stay are necessary to define the true attributable mortality and cost associated with VAP in pediatric patients.
DIAGNOSIS
Clinical Criteria
The lack of a gold standard for the diagnosis of VAP in both adults and children makes an interpretation of the literature complex. The clinical criteria for the diagnosis of VAP have been established by the NNIS and the CDC (22). Patients who are mechanically ventilated for more than or equal to 48 h must have two or more abnormal chest radiographs with at least one of the following symptoms: new or progressive and persistent infiltrate, consolidation, cavitation, and/or pneumatoceles (in infants ≤1 year of age). However, in patients without underlying pulmonary or cardiac disease (respiratory distress syndrome, bronchopulmonary dysplasia, pulmonary edema, or chronic obstructive pulmonary disease), one definitive chest radiograph is acceptable. In addition to abnormal chest radiographs, a patient must have at least one of the following symptoms: fever (>38°C) with no other recognized cause, leukopenia (<4,000 white blood cells [WBC]/mm3) or leukocytosis (≥12,000 WBC/mm3), and at least two of the following criteria: new onset of purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements; new onset of or worsening cough, dyspnea, or tachypnea; rales or bronchial breath sounds; and worsening gas exchange (e.g., O2 desaturations [e.g., PaO2/FiO2 levels of ≤240], increased oxygen requirements, or increased ventilation demand). The criteria described above may be used to diagnose VAP in children; however, specific diagnostic criteria for VAP have been developed for infants ≤1 year of age and children >1 and ≤12 years of age. Infants that are ≤1 year old must have worsening gas exchange (oxygen desaturations, increased oxygen requirements, or increased ventilator demand) and at least three of the following criteria: temperature instability with no other recognized cause; new onset of purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements; apnea, tachypnea, nasal flaring with retraction of chest wall, or grunting; wheezing, rales, or rhonchi; cough; and bradycardia (<100 beats/min) or tachycardia (>170 beats/min). Children >1 and ≤12 years of age must meet at least three of the following criteria: fever (>38.4°C or >101.1°F) or hypothermia (<37°C or 97.7°F) with no other recognized cause; leukopenia (<4,000 WBC/mm3) or leukocytosis (≥15,000 WBC/mm3); new onset of purulent sputum, change in character of sputum, increased respiratory secretions, or increased suctioning requirements; rales or bronchial breath sounds; and worsening gas exchange (O2 desaturations [pulse oximetry of <94%], increased oxygen requirements, or increased ventilation demand). NNIS/CDC criteria do not require microbiologic confirmation to diagnose pneumonia.
In summary, many of the diagnostic criteria are similar for the ≤1-year-old and >1- or ≤12-year-old age groups. Temperature instability is a diagnostic criterion for the ≤1-year-old age group; either temperature elevation or hypothermia is a criterion for the >1- and ≤12-year-old age group. For the <1-year-old group, cough, bradycardia, tachycardia, nasal flaring, grunting, and wheezing are diagnostic criteria not listed for the >1- or ≤12-year-old age groups, although for the older age group, dyspnea without further specific definition is a diagnostic criterion. Worsening gas exchange, change in character or amount of sputum, cough, rales, or bronchial breath sounds are criteria for diagnosis in all three age groups. We suggest that a consistent use of the age-specific definitions are preferred, although we were unable to find any published studies directly comparing the sensitivity and specificity of the age-specific definitions to those for any age group.
Clinical definitions for VAP may be applied inconsistently, and the lack of specific definitions of components of the clinical definition such as worsening gas exchange, oxygen desaturations, increased oxygen requirements, and increased ventilator demand may exacerbate this. Cordero et al. (19) determined differences in the application of the CDC definitions using NICU patients (n = 37) diagnosed with VAP by interpretation of CDC definitions by infection control practitioners (ICPs) and a positive tracheal aspirate culture. A panel of neonatologists reviewed the clinical and laboratory evidence as well as the radiographs. The neonatologists diagnosed VAP in only seven patients. The neonatologists categorized the other patients as having asymptomatic airway colonization (n = 12), BSI (n = 7), and airway colonization with equivocal signs of infection (n = 11). Among 8 of the 11 patients with equivocal signs of infection, the general radiologist report stated that the radiographic changes were suggestive of VAP; the neonatologist panel, reviewing the same radiographs, concluded that VAP was unlikely in these patients. Those authors concluded that an isolated positive tracheal aspirate does not distinguish between airway colonization and VAP and that routine radiology reports without definitive clinical and laboratory evidence may be misleading.
Invasive Testing in Adults
NNIS/CDC criteria for VAP do not require microbiologic confirmation. A brief review of invasive testing to confirm the diagnosis of VAP in adults will be performed, given the paucity of literature regarding invasive testing in children. It is unclear whether the adult experience can be extrapolated to children. Several studies have examined the accuracy of invasive testing for the diagnosis of VAP in critically ill adults (Table 2). Microbiologic examination of specimens obtained from bronchoalveolar lavage (BAL) or protected specimen brush (PSB) have an estimated 70% sensitivity and 77% specificity compared to histopathology and/or lung tissue culture (13, 30, 80).
TABLE 2.
Accuracy of invasive diagnostic techniques for the diagnosis of VAP in adults and childrena
| Age group and source (reference) | No. of patients | Diagnostic technique | Gold standard(s) | SE (%) | SP (%) |
|---|---|---|---|---|---|
| Adults | |||||
| Rouby et al. (80) | 26 | Protected mini-BAL | Histopathology, lung tissue culture | 70 | 69 |
| Chastre et al. (13) | 26 | PSB | Histopathology, lung tissue culture | 100 | 60 |
| Fabregas et al. (30) | 25 | TBA | Histopathology, lung tissue culture | 69 | 92 |
| Protected BAL | 39 | 100 | |||
| BAL | 77 | 58 | |||
| PSB | 62 | 75 | |||
| Any invasive diagnostic technique | 85 | 50 | |||
| Children | |||||
| Gauvin et al. (42) | 10 | BAL (104 CFU/ml) | Expert opinion; 2/3 blinded to BAL and PSB results | 50 | 80 |
| Bacterial index >5 | 78 | 86 | |||
| ICB | 30 | 95 | |||
| Endotracheal cultures | 90 | 40 | |||
| Labenne et al. (60) | 29 | BAL (104 CFU/ml) | (i) Positive pleural fluid culture, (ii) computed tomography scan with abscess, (iii) histopathology, (iv) lung tissue culture, (v) blood and tracheal aspirate positive for same organism without other source, (vi) expert opinion; 2/3 blinded to BAL/PSB | 72 | 88 |
| PSB | 69 | 95 | |||
| ICB on gram stain and + BAL | 79 | 88 | |||
| ICB on gram stain and + BAL and + PSB | 90 | 88 |
SE, sensitivity; SP, specificity; TBA, tracheobronchial aspirates; ICB, intracellular bacteria.
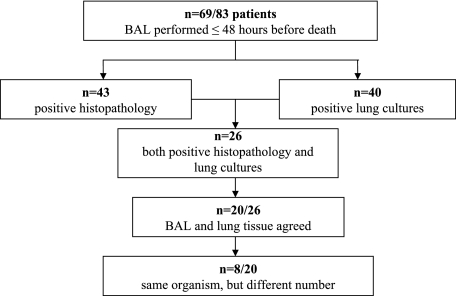
In one of the most comprehensive studies of VAP in mechanically ventilated adults, Rouby et al. (80) sought to describe the histologic and bacteriologic characteristics of human nosocomial pneumonia and to evaluate the accuracy of protected mini-BAL for the diagnosis of VAP compared to lung tissue cultures and lung histology in patients who died while on mechanical ventilation. Twenty-six patients had both positive pathology and lung tissue culture; in 20 of these patients, the BAL and lung tissue culture results were concordant (Fig. 1).
FIG. 1.
Comparison of protected minibronchoalveolar lavage with histopathology and bacteriology for diagnosing VAP.
Chastre (13) compared the accuracy of the bronchoscopic PSB to that of histologic and bacteriologic examinations of pulmonary specimens in adults (n = 26). PSB and lung cultures were highly correlated (r = 0.65; n = 28; P < 0.001) and higher in patients not on antibiotics within 1 week before death than in patients on antibiotics before death (r = 0.55; n = 33; P < 0.001). Pneumonia was not found by histology or lung tissue culture when PSB culture organisms were <103 CFU per ml. PSB cultures with ≥103 CFU/ml identified all patients with histologically proven pneumonia. In patients treated with antibiotics, four patients had microorganisms isolated by PSB with concentrations of >103 CFU/ml not found in the lung tissue cultures.
Fabregas and colleagues (30) sought to determine the accuracy of clinical criteria and microbiologic testing for the diagnosis of VAP. The clinical pulmonary infection score (CPIS) was used to compare microbiological criteria, clinical criteria, and sampling techniques. Lung biopsies were performed for 25 mechanically ventilated patients immediately after death. The reference standard was the presence of positive histology for pneumonia or positive lung cultures. Chest X-ray infiltrates and at least two of three clinical criteria achieved sensitivity and specificity of 69% and 75%, respectively. The CPIS sensitivity and specificity were 77% and 42%, respectively. Noninvasive and invasive techniques achieved similar results. All diagnostic techniques combined (PSB, BAL fluid, and protected BAL fluid) achieved sensitivity and specificity of 85% and 50%, respectively.
A meta-analysis done in 2005 (n = 628) determined whether invasive testing altered the management and mortality of VAP in critically ill adults (84). VAP was confirmed bronchoscopically in 44 to 69% of the patients. Overall, antibiotics were almost three times as likely to be changed if a bronchoscopy was performed. In a separate pooled analysis of prospective uncontrolled trials, alteration in antibiotic prescription occurred 50% (36 to 65%) of the time. To our knowledge, no similar meta-analyses exist for pediatric populations.
Invasive Testing in Children
A few studies have examined the sensitivity and specificity of lower airway sampling in PICU patients and found the sensitivity and specificity of BAL (104 CFU/ml) to be 50 to 72% and 80 to 88%, respectively (42, 60) (Table 2). The nonbronchosopic BAL (NB-BAL) has been used in children as an alternative to fiberoptic BAL to diagnose pneumonia. This procedure is performed by placing a suction catheter into the endotracheal tube until resistance is met and then placing and suctioning back a small amount of sterile normal saline from the lower airway. The presence of bubbles in the returned fluid is suggestive that a lower airway sample containing surfactant has been obtained. Quantitative cultures of the fluid are then performed by the microbiology laboratory. This procedure may be advantageous compared to fiberoptic bronchoscopic techniques because bronchoscopic equipment is not required, a trained bronchoscopist is not necessary, and the diagnostic accuracy is comparable to that of fiberoptic techniques (36, 37, 71).
Gauvin et al. (42) performed a 27-month prospective cohort study of PICU patients suspected of having VAP in a tertiary academic care center. Of 30 patients, 10 were diagnosed with VAP and 9 were diagnosed with ventilator-associated tracheitis by an expert panel. The expert panel was used as the reference standard; they were given clinical, radiographic, and microbiologic data but were blinded to the BAL results. A bacterial index (sum of the log of all species obtained from BAL) of >5 had the highest correlation with the reference standard (concordance, 83%; kappa = 0.61), a sensitivity of 78%, a specificity of 86%, a positive predictive value of 70%, and a negative predictive value of 90% (42). Intracellular bacteria and gram stain from BAL were specific (95% and 81%, respectively) but not sensitive (30% and 50%, respectively) for the diagnosis of pediatric VAP, whereas clinical criteria and endotracheal cultures were sensitive (100% and 90%, respectively) but not specific (15% and 40%, respectively). That study concluded that blind BALs with a bacterial index of >5 are the most reliable method for diagnosing VAP in mechanically ventilated children. The study did not describe what proportion of patients were on antibiotics or the duration of antibiotic exposure prior to BAL.
Labenne et al. (60) also investigated the sensitivity and specificity of PSB and BAL in PICU patients with suspected VAP. The gold standards used by those investigators were a positive pleural fluid culture, computed tomography scan with pulmonary abscesses, histopathological evidence, positive lung biopsy (>104 CFU/gram), the same bacteria isolated in blood and endotracheal aspirate without another source, or clinical diagnosis using CDC guidelines established independently by two investigators blinded to PSB/BAL culture results. Of 103 patients, 29 were diagnosed with VAP, 10 were labeled as “uncertain,” and 64 were classified as not having VAP. Thirteen of 64 patients with negative PSB and BAL cultures had antibiotics stopped after 48 h, 25 of 64 had negative cultures, and antibiotics were not used at all, and 28 of 38 had a positive tracheal aspirate culture but negative PSB and BAL, so antibiotics were discontinued prior to the standard 7-day treatment in that center. The sensitivity and specificity for BAL fluid culture were 72% and 88%, respectively. The intracellular bacteria and the BAL combined had sensitivity and specificity of 79% and 88%, respectively. Use of PSB culture results in combination with intracellular bacteria and BAL further increased the sensitivity and specificity to 90% and 88%, respectively. The PSB and BAL are effective methods of collecting distal samples and were helpful in diagnosing VAP. However, a combined diagnostic approach was superior to either one alone.
The safety of the NB-BAL in children has also been determined by several studies; few adverse experiences (n = 18) have been reported (12, 42, 60, 66, 79). The types of adverse events were sustained oxygen desaturation requiring increased ventilatory support (n = 11), pneumothorax (n = 4), hypotension (n = 2), and significant increase in intracranial pressure (n = 1). Of the 11 patients who experienced sustained oxygen desaturations, 7 patients were diagnosed with acute respiratory distress syndrome and saturations in the low 80s before the procedure was performed. Pneumothorax occurred in patients less than 1 month of age (n = 4). Hypotension occurred in patients requiring dopamine before the NB-BAL procedure began.
The safety of NB-BAL and BAL have been examined in a study evaluating the diagnosis of infectious and interstitial lung disease in children (n = 32) (79). That study found that both NB-BAL and the BAL were safe, as respiratory rate, heart rate, and oxygen saturation were monitored during the procedure and a minimum of 6 h afterwards. Patients did not require increased supplemental oxygen after the procedure, and no major airway bleeding occurred.
Computerized Surveillance
Recently, a retrospective study was performed to determine the accuracy of computerized surveillance to detect nosocomial pneumonia in two NICUs over a 2-year period (n = 2,932 patients) (46). The automated monitoring system was a natural language processor, referred to as the Medical Language Extraction and Encoding system, that converted the electronic narrative reports to coded descriptions to identify patients with pneumonia. The automated monitoring system was compared to diagnosis of VAP by an ICP who performed prospective surveillance for pneumonia using NNIS definitions. A total of 1,277 patients had chest radiographs. In NICU 1, seven cases of VAP were identified by the ICP prospective surveillance; five of the seven cases found by the ICP were also identified by automated computer surveillance, which flagged an additional 61 patients with possible nosocomial pneumonia. Nine were considered to be inappropriately flagged by a second independent ICP review. The sensitivity and specificity of the computerized surveillance were 71% and 99.8%, respectively. The positive predictive value was 7.9%, and the negative predictive value was >99%. In NICU 2, 10 cases of VAP were identified by the ICP; only 2 of these were flagged by the computer. Further investigation revealed that 7 of the original 10 cases were not flagged by the computer because the original chest radiograph reports could not be found. Eight hundred thirty-six patients had chest radiographs performed; 84 were flagged by the computer as VAP. The sensitivity and specificity for NICU 2 could not be calculated. The findings of that study indicate that computerized surveillance may be useful in streamlining the identification of patients with possible VAP who require a more time-consuming chart review by an ICP. This system was not linked with microbiology reports. An unstudied area is computerized surveillance linking both radiology and microbiology reports.
Recommendations for Current Practice and Future Research
The lack of a gold standard plagues all literature regarding VAP in both adults and children. The current literature suggests that NB-BAL, BAL, and PSB are safe in older children who do not have severe hypoxemia, increased intracranial pressure, hemodynamic instability, or bleeding problems and that invasive testing is sensitive and specific compared to a reference of expert opinion. Comparison of the clinical definitions, including the age-specific definitions, with and without invasive testing against histopathology and/or lung tissue culture would be a valuable addition to the literature. The feasibility of using NB-BAL in a general patient population and the effect on antibiotic use remain to be determined.
Computerized surveillance has the potential for considerable time savings, particularly if electronic surveillance of the radiographic reports could be combined with that of microbiology and vital signs and validated against the current practical gold standard of application of CDC/NNIS definitions by an experienced clinician who has reviewed the complete medical record. Additional computerized surveillance studies are necessary to help further understand the impact that computerized surveillance may have on diagnosing pneumonia.
MICROBIOLOGY
Understanding the microbiology of VAP is critical for guiding decisions regarding empirical antibiotic therapy. A retrospective cohort study of the microbiologic etiology of VAP in the ICU setting was performed in three hospital settings: a large teaching hospital, a community hospital, and a children's hospital (5). The most commonly isolated organisms were similar across adult and pediatric hospitals: Staphylococcus aureus (28.4%), Pseudomonas aeruginosa (25.2%), and other gram-negative bacilli (26.6%). The microbiologies of early-onset and late-onset infections differed in the adult populations, but this was not the case in the children's hospital. Pseudomonas aeruginosa and Staphylococcus aureus were the most commonly isolated organisms in the children's hospital. Pseudomonas aeruginosa was more common in the PICU than in the NICU (33.3% versus 17%; P = 0.01), while Staphylococcus aureus was more common in the NICU than in the PICU (38% versus 17.6%; P < 0.001). A prospective cohort study of VAP in the same NICU was performed. Most of the tracheal isolates from patients with VAP grew polymicrobial cultures; the organisms most commonly isolated included Staphylococcus aureus (23%), Pseudomonas aeruginosa (38.4%), Enterobacter spp. (38.4%), and Klebsiella spp. (23%) (3). A limitation of these studies is that the vast majority of isolates were from endotracheal aspirates rather than from invasive sampling of the lower airway, and thus, the results may represent oropharyngeal flora.
Two studies reported differences in the microbiologies of early-onset and late-onset nosocomial pneumonia among children. Group B streptococci were most commonly isolated from infants with maternally acquired pneumonia (31.8%), while these organisms were rarely isolated in cases of late-onset pneumonia (1.3%). The frequency of Staphylococcus aureus increased from 2.4% of maternally acquired cases to 18.7% of non-maternally-acquired cases of pneumonia, and Pseudomonas aeruginosa frequency increased from 2.9% of maternally acquired cases to 12.9% of non-maternally-acquired cases of pneumonia (43).
A 41-month prospective surveillance study of nosocomial infections in a NICU divided pneumonia into early-onset (onset of symptoms within first 48 h of life) and late-onset (onset of symptoms more than 48 h after birth) infections (98). There were 35 cases of definite or probable early-onset pneumonia. In 26 of these cases, potential pathogens were identified: 18 (76.9%) group B streptococci, 1 (3.8%) group F streptococcus, 3 (11.5%) Streptococcus pneumoniae isolates, and 2 (7.7%) nontypeable Haemophilus influenzae infections. Late-onset pneumonia occurred in 36 of 358 (10%) neonates who were ventilated for over 24 h. Cultures were taken from endotracheal tubes or nasopharyngeal secretions for 41 episodes of late-onset nosocomial pneumonia. The most commonly isolated organisms were coliform spp. (n = 18; 43.9%), Pseudomonas aeruginosa (n = 14; 34.1%), and Staphylococcus aureus (n = 6; 14.6%).
RISK FACTORS FOR VAP IN NICU PATIENTS
In pediatric populations, the pathogenesis of VAP is not well studied. In adult patients, aspiration of oropharyngeal secretions, inhalation of aerosols containing bacteria, hematogenous spread, and bacterial translocation from the gastrointestinal tract are all considered to be mechanisms of the development of VAP (89).
Neonates have unique characteristics predisposing them to nosocomial infections. These patients’ immature immune systems place them at increased risk for infection (24). Skin and mucous membranes are more permeable and are less effective barriers to infection (47). Abnormal granulocyte migration and bacterial digestion in these patients have been demonstrated. Additionally, decreased activity of complement, particularly complement opsonization, occurs in newborns (40). Lastly, hypogammaglobulinemia occurs in premature newborns. Maternal immunoglobulin G (IgG) is transported to the fetus in the second and last trimesters of pregnancy, and fetal IgG levels reach maternal levels by term (58). Levels of IgG are lower in premature newborns, as maternal levels have not yet been attained. In the initial months following birth, maternal IgG levels drop, and it takes the infant months to produce ample levels of IgG and other immunoglobulins.
Low birth weight has been shown to be a risk factor for the development of nosocomial pneumonia. A 41-month surveillance study demonstrated a significant association between a birth weight of <1,500 g and a higher rate of nosocomial pneumonia (48). However, low birth weight may be a marker for an increased duration of mechanical ventilation. That study was limited by the lack of a specific control for the duration of mechanical ventilation. Apisarnthanarak et al. (3) focused on estimated gestational age (EGA) rather than birth weight in their 10-month-long case control study of 211 intubated NICU patients. VAP rates were much higher in babies with an EGA of <28 weeks (19 VAP cases) than in babies with an EGA of ≥28 weeks (5 VAP cases) (P < 0.001) (3). The VAP rate per 1,000 ventilator days was also higher in babies with an EGA of <28 weeks (6.5/1,000 ventilator days) than in babies with an EGA of ≥28 weeks (4.0/1,000 ventilator days) but was not statistically significant (P = 0.34) (3). Not all investigators found an inverse relationship between birth weight and frequency of nosocomial pneumonia. A prospective surveillance study of nosocomial infections in seven Brazilian NICUs found that the rate of nosocomial pneumonia was actually higher in neonates with birth weights of >1,500 g than in babies with birth weights of ≤1,500 g (4.4/1,000 patient days versus 2.8/1,000 patient days) (72).
Prior BSIs have been identified as a being a risk factor for VAP in NICU patients. In babies with an EGA of <28 weeks, history of a prior BSI was the only significant risk factor for the development of VAP in multivariate analyses after controlling for the duration of mechanical ventilation (P = 0.03). Although none of the cases of VAP were caused by the same organism as that which caused the BSI, those authors suggested that prior BSI may serve as a surrogate for severity of illness rather than actually contributing to VAP (3).
The design of the NICU may also have an effect on the incidence of nosocomial infections and specifically VAP. A 5-year prospective study of nosocomial infections in a NICU was performed (44). Midway through that study, the NICU location was moved from cramped quarters adjacent to a busy medical ward to a new facility. The new nursery had a 50% increase in staffing and improved infection control features. In the old nursery, 16 of 492 patients had pneumonia, whereas in the new nursery, only 1 patient of 419 had pneumonia. While the new nursery had improved structural infection control measures such as more space per patient, a large number of sinks, and a separate isolation room, it is not clear if other practices of care, such as head-of-bed elevation or suctioning, changed after the move to the new unit. Those authors did not report any changes in infection control surveillance or diagnosis in the new nursery.
RISK FACTORS FOR VAP IN PICU PATIENTS
Several prospective cohort studies described risk factors for pediatric VAP. In a prospective cohort study at a tertiary care center, genetic syndrome (odds ratio [OR], 2.37; 95% CI, 1.01 to 5.46), transport out of the PICU (OR, 8.90; 95% CI, 3.82 to 20.74), and reintubation (OR, 2.71; 95% CI, 1.18 to 6.21) were all independent predictors of pediatric VAP (28). That study also found that primary BSIs were associated with the development of VAP, as five of the nine patients with primary BSIs and VAP had the BSI first. Another prospective cohort study identified prior antibiotic use (OR, 2.45; 95% CI, 1.112 to 5.405), continuous enteral feeding (OR, 2.29; 95% CI, 1.093 to 4.798), and bronchoscopy (OR, 5.04; 95% CI, 1.665 to 15.266) as being independent predictors of pediatric VAP (1). Immunosuppressant drugs (OR, 4.8; P = 0.04), immunodeficiency (OR, 6.9; P = 0.06), and neuromuscular blockade (OR, 11.4; P = 0.002) were also found to be independent predictors in another prospective cohort study (32). Torres et al. (94) identified several factors associated with increased risk of developing VAP: reintubation (OR, 4.95; 95% CI, 3.48 to 7.04; P = 0.000012), gastric aspiration (OR, 5.05; 95% CI, 3.28 to 7.77; P = 0.00018), mechanical ventilation for >3 days (OR, 1.17; 95% CI, 1.15 to 1.19; P = 0.015), chronic obstructive pulmonary disease (OR, 1.89; 95% CI, 1.38 to 2.59; P = 0.048), and positive end-expiratory pressure (OR, 1.85; 95% CI, 1.30 to 2.64; P = 0.092). In nosocomial pneumonia patients, factors associated with increased mortality risk were a rapidly fatal underlying condition (OR, 8.84; 95% CI, 3.52 to 22.22; P = 0.0018), worsening acute respiratory failure from developing pneumonia (OR, 11.94; 95% CI, 4.75 to 30; P = 0.0096), septic shock (OR, 2.83; 95% CI, 1.41 to 5.78; P = 0.016), inappropriate antibiotic treatment (OR, 5.81; 95% CI, 2.70 to 12.48; P = 0.02), and non-cardiac-surgery ICU patients (OR, 3.38; 95% CI, 1.70 to 6.71; P = 0.08) (78). Medications associated with the development of VAP are NADP, steroids, and histamine type 2 receptor blockers (28).
Recommendations for Current Practice and Future Research
Several factors have been identified as being risk factors for VAP in NICU and PICU patients. Many of these factors reflect a risk for aspiration such as that which may occur during reintubation, physical movement out of the ICU, and bronchoscopy. In addition, neuromuscular weakness and immunodeficiency may predispose a patient to VAP, as does prolonged mechanical ventilation. A risk stratification system incorporating preventable and nonpreventable risk factors for pediatric VAP might assist intensivists in the development of pediatric VAP prevention bundles and methods for identifying meaningful indicators as a measure of an institution's success at VAP prevention. Larger, multicenter, randomized controlled trials using a standard reference definition of VAP to test interventions to prevent aspiration in children would be useful. Testing the efficacy of a standardized assessment of readiness to wean mechanical ventilatory support would also be useful in this patient population, as would a standardized assessment of pain and the need for sedation and neuromuscular blockade.
PREVENTION
Several recommendations have been given to decrease VAP. The CDC and Healthcare Infection Control Practices Advisory Committee suggest using orotracheal tubes (instead of nasotracheal tubes) when patients require mechanical ventilation, changing breathing circuits of ventilators only if they malfunction or if they are visibly contaminated, and using endotracheal tubes with dorsal lumens to allow respiratory secretions to drain (89). There are no recommendations for the preferential use of sucralfate, histamine 2 receptor antagonists, or antacids for stress bleeding prophylaxis (89).
Head-of-Bed Elevation
Supine position has been associated with VAP in adult patients, which is thought to be related to an increase in gastroesophageal reflux and aspiration. Semirecumbent positioning has been demonstrated to decrease surrogate outcomes such as aspiration and gastroesophageal reflux in adults (16), and one clinical trial demonstrated a dramatic decrease in the incidence of confirmed VAP in patients with head-of-bed elevation (5% versus 23%; OR, 6.8; 95% CI, 1.7 to 26.7) (25). The efficacy of semirecumbent positioning in preventing VAP in children has not been established. One age- and sex-matched case control study of nosocomial pneumonia in children found that head-of-bed elevation did not differ between cases and controls. That study was limited by small numbers of cases and controls (n = 9 for each group) (10). Additionally, size-related factors must be considered in the utility of semirecumbent positioning in children. For instance, elevating the head >30° is logistically challenging for small pediatric patients such as infants and toddlers.
In-Line Suctioning
Endotracheal suctioning is used for eliminating bronchopulmonary secretions from the airway. Traditional open endotracheal suction requires disconnection from the ventilator. This process has been shown to result in increased intracranial pressure, increased mean blood pressure, and hypoxia in mechanically ventilated children (27, 57). The introduction of a closed multiuse suction catheter in the 1980s allowed endotracheal suctioning without disconnection from the ventilator. In critically ill adult populations, closed suction systems have been shown to result in fewer physiologic disruptions such as arterial and venous desaturations and arrhythmias (54).
Closed endotracheal suction systems present the potential for bacterially contaminated secretions to pool in the lumen of the tube, with reinoculation of the respiratory tract with each repeated suctioning. On the other hand, a closed system could potentially decrease environmental contamination of the respiratory device. Many studies of critically ill adults have compared the incidence of airway colonization and nosocomial pneumonia in patients on a closed multiuse system to that in patients on a single-use open suction system. The frequency of airway colonization has been shown to be significantly more frequent in patients on the closed suction system (23). However, studies have not demonstrated an increased frequency of nosocomial pneumonia in patients on the closed suction system (23, 54). Indeed, a more recent prospective randomized study of 102 ventilated adults demonstrated an increased risk of VAP for patients with an open suction system compared to a closed suction system (adjusted risk, 3.5; 95% CI, 1.0 to 12.33) (17). There are currently no CDC recommendations regarding the preferential use of closed or open suction systems, nor are there recommendations regarding the frequency of change for multiuse closed suctioning systems in a single patient (89).
A single study has compared open and closed suction systems in critically ill children. Cordero et al. (20) monitored 133 ventilated NICU patients who were alternately assigned to a closed or open suction system for bacterial airway colonization, nosocomial pneumonia, BSI, and bronchopulmonary dysplasia. A definition of nosocomial pneumonia required radiographic evidence of “probable” pneumonia (new airspace disease or a parenchymal process) and positive blood cultures and tracheal culture for a respiratory pathogen. Colonization patterns from tracheal cultures were comparable between groups, with gram-positive colonization occurring by the second week of intubation and gram-negative colonization occurring after the third week of ventilation. There were no significant differences in the incidences of VAP or BSIs or mortality between patient groups. Additionally, the numbers of endotracheal suctions per day, the numbers of reintubations, the incidences and severities of bronchopulmonary dysplasia, and the numbers of infants discharged on supplemental oxygen were similar between groups. Finally, 40 of 44 (91%) NICU nurses judged the closed suction system to be easier to use, less time-consuming, and better tolerated by NICU patients.
H2 Blockers/Sucralfate
The acidification of gastric contents is thought to decrease colonization with potentially pathogenic bacteria. Stress ulcer prophylactic medications that increase gastric pH, like H2-antagonists and antacids, may increase colonization with pathogenic organisms and increase the risk of VAP (18). Sucralfate is an alternative stress ulcer prophylactic agent that does not alter gastric pH, and this medication may lower the risk of VAP while maintaining stress ulcer prophylaxis. Over 20 clinical trials with adults have investigated the risk of VAP associated with these medications. Of seven meta-analyses of these clinical trials, four found a significant reduction in the incidence of VAP in patients treated with sucralfate compared to patients receiving H2 antagonists. The same effect occurred in the other three analyses but did not reach statistical significance. Three of these meta-analyses demonstrated a significant reduction in mortality associated with sucralfate therapy (16).
Two clinical trials compared the risk of VAP with various methods of stress ulcer prophylaxis in pediatric patients. A retrospective study included 155 PICU patients who had a nasogastric tube in place and were mechanically ventilated for >48 h: 54 were given ranitidine, 53 were give sucralfate, and 48 were not on stress ulcer prophylaxis (62). There was no significant difference in the incidences of VAP between patients treated with ranitidine and patients treated with sucralfate (11.1% versus 7.5%; χ2 = 0.40; P = 0.52). That study had several limitations. Patients were not randomized into study groups, and patient characteristics differed between patients given stress ulcer prophylaxis and those who were not given prophylaxis. The retrospective nature of the study may have resulted in errors in diagnosing VAP.
A prospective study was performed to study the incidence of VAP and associated mortality among patients randomized to one of four groups for stress ulcer prophylaxis in Turkey (101). That study included 160 PICU patients: 38 received sucralfate, 42 received ranitidine, 38 received omeprazole, and 42 did not receive prophylaxis. VAP occurred in 70 of 160 (44%) patients, ranging from 41 to 48% in individual treatment groups. There was no difference in the incidence of VAP across treatment groups. The overall mortality rate was 35 of 160 (22%) and did not differ significantly among treatment groups, ranging from 21 to 23% across groups. The overall incidence of VAP (44%) in this study was much higher than that reported in other pediatric studies from referral hospitals (5.1% to 10.2%) (1, 28). It is possible that VAP was overdiagnosed in that study, although diagnostic criteria used in that study were similar to criteria used in this country. If VAP was overdiagnosed, this effect would likely be distributed throughout all study groups. That study may also have been underpowered to detect differences in the incidences of VAP among these patient groups.
Both of those studies failed to demonstrate a difference in the incidence of VAP in patients treated with sucralfate compared to those treated with agents that alter gastric pH. Additionally, neither study demonstrated an increased risk of VAP in patients treated with agents that alter gastric pH compared to that in patients with no treatment. The microbiologies of infections were similar across treatment groups, and many infections were caused by organisms that are not likely to be affected by stress ulcer prophylaxis. It is possible that the study sizes presented were simply too small to appreciate a significant difference in the incidence of VAP, or it is possible that stress ulcer prophylaxis is not associated with VAP in the pediatric population. Larger prospective randomized studies of children are needed to asses the impact of stress ulcer prophylaxis on VAP and whether sucralfate has a protective effect compared to medications that decrease gastric acidity.
Hand Hygiene
Efforts at reducing person-to-person transmission of bacteria are crucial for preventing nosocomial infections. Significant bacterial contamination of hospital employees’ hands during routine patient care has been demonstrated (75). The concept that routine hand washing by health care workers reduces nosocomial infections is not new, but the first study investigating the impact of hand hygiene on the rate of hospital-acquired infections in NICU patients was recently performed (99). A 2-year-long multimodal intervention was instigated, which consisted of formal lectures, written and posted instruction regarding proper hand hygiene technique, covert observation, financial incentives, and regular feedback of observed hand hygiene rates. Surveillance of hand washing compliance and nosocomial infections from the pre- and postintervention periods were compared. The rate of hand hygiene compliance increased from 43% at baseline to 80% during the intervention, and the rate of respiratory infections decreased from 3.35 to 1.06 per 1,000 patient days (P = 0.002) in the pre- and postintervention periods. The two parameters were statistically correlated (r = −0.385; P = 0.014). That study is helpful in demonstrating an association of hand hygiene and prevention of nosocomial pneumonia, but it has limitations. The before-after design of the study makes it difficult to assess if a reduction in the rate of pneumonia is attributable exclusively to the increase in hand hygiene. An intervention of this magnitude may have altered other clinical practices related to the spread of bacterial contamination, as employees’ awareness of preventing nosocomial infections was increased. Those authors did point out that no changes in the use of surfactant or suction procedures occurred during the study period but did not comment on other procedural changes that may have occurred such as head-of-bed elevation, stress ulcer prophylaxis, or oral hygiene changes. Additionally, while rates of hand hygiene compliance remained at 81% during the 16-month postintervention period, it is unclear how sustainable this effect was after observations were discontinued.
A prospective study of a 3-month-long implementation of an intervention to decrease rates of nosocomial infection in NICU patients was undertaken (69). The intervention consisted of three parts: (i) grouping of all blood-taking tasks to reduce the number of daily blood draws, (ii) reducing the frequency of blood investigations after stabilization of acute illness, and (iii) using an aseptic delivery system of drugs though a central venous catheter to reduce peripheral intravenous access. The incidences of nosocomial infection in the NICU between the 1-year preintervention period and the 1-year postintervention period were compared. VAP rates declined from 3.3/1,000 ventilator days to 1.0/1,000 ventilator days after the intervention. Again, that study was limited by the before-after nature of the design. Those authors acknowledged that practices regarding mechanical ventilation also changed during the study period, as patients were weaned from the ventilator more aggressively and as soon as possible. Earlier weaning may have contributed to lowering the VAP rates, as prolonged intubation is a risk factor for VAP in children (62).
The importance of hand hygiene in preventing horizontal transmission of pathogens among mechanically ventilated patients was highlighted by a study performed by Sole et al. (86) to evaluate the proportion of suctioning devices colonized with pathogenic bacteria and to correlate the bacteria found on respiratory equipment with those found in patients’ mouths and sputum. Those investigators found that within 24 h of changing to new suctioning equipment, 94% of tonsil suction tubing, 83% of in-line suction tubing, and 61% of distal suction connectors were colonized with pathogenic bacteria similar to those found in the patients’ oropharynx and sputum (86).
Selective Decontamination
The impact of using topical antibiotics on tracheostomy sites on exogenous colonization or infection of the lower airways has been studied. A 2-year-long prospective observational cohort study was performed with 23 children who were treated with 2% paste of polymyxin E and tobramycin on the tracheostoma four times a day for the first two postoperative weeks (65). Only 1 of 23 (4%) patients developed exogenous colonization or infection of the lower respiratory tract, which was lower than that in historical controls (6/22 [27%]). While topical antibiotics may be useful in preventing exogenous colonization or infection of the lower airways in children with tracheostoma, the risk of endogenous colonization remains high. Endogenous colonization or infection occurred in 15 episodes in 14 of 23 (61%) patients during the 2-week postoperative period.
Many investigators have studied the efficacy of selective digestive tract decontamination (SDD) in preventing VAP. SDD traditionally consists of a regimen of topical antimicrobials applied to the oropharynx and through a nasogastric tube, with the aim of reducing the burden of pathogenic bacteria in aspirated secretions. While the majority of trials have focused exclusively on the use of topical antimicrobials, many have also used a short course of intravenous antimicrobial therapy. Seven meta-analyses of randomized, controlled trials of SDD in adults all showed a significant reduction in the risk of VAP, and four of those analyses also demonstrated a significant reduction in mortality in patients treated with SDD (16). One recent meta-analysis divided trials into those that used topical antibiotics alone and those that used a combination of topical and systemic antimicrobials for the prevention of nosocomial respiratory infections (61). That analysis included 32 randomized, controlled trials including a total of 5,185 adult patients. A protective effect was demonstrated in trials comparing patients on a combination of systemic and topical antibiotics with controls (OR, 0.35; 95% CI, 0.29 to 0.41) and in trials comparing patients on topical antibiotics alone with controls (OR, 0.52; 95% CI, 0.43 to 0.63). A significant reduction in mortality was seen only in trials that used a combination of topical and systemic therapy (OR, 0.78; 95% CI, 0.68 to 0.89). Mortality from VAP was not reduced when topical therapy alone was used (OR, 0.97; 95% CI, 0.81 to 1.16).
Recent evidence suggests that results from some of those trials may be overly optimistic. A meta-analysis of 32 primary trials of SDD was performed to assess the impact of study methodology on results (97). Study methodology was evaluated based on allocation and concealment, patient selection, patient characteristics, blinding, and definition of nosocomial pneumonia. That analysis found an inverse relationship between the methodologic quality and benefit of SDD on the incidence of pneumonia, suggesting that the benefit of SDD for the prevention of VAP may be overestimated by many clinical trials (97).
Studies focusing on the use of SDD to prevent VAP in children have conflicting results. A prospective study of SDD in 226 PICU patients randomized study subjects into a treatment (n = 116) or control (n = 110) group (81). The treatment group received colistin, tobramycin, and nystatin orally or through a nasogastric tube every 6 h, and patients were monitored for the development of nosocomial infection in any body site. There were 87 episodes of any nosocomial infection in 65 of 226 (28.8%) patients. The most common nosocomial infections were catheter-related bacteremia, sepsis, pneumonia, and urinary tract infection. The overall incidence of nosocomial infection across all sites did not differ between treatment and control groups. However, when infections were studied by body site, patients in the treatment group had a significantly lower frequency of pneumonia (2.6% versus 7.2%). In multivariate analyses, SDD retained a protective effect against pneumonia (OR, 0.21; 95% CI, 0.06 to 0.8). There was no significant difference in overall mortality between the treatment and control groups (six versus five patients). Patients in that study were randomized and were well matched for most variables with the exception of severity of illness; the treatment group had more severely ill patients. This difference in severity of illness would be expected to skew the results toward the null hypothesis, but there were actually fewer cases of pneumonia in the more severely ill group who were treated with SDD.
A prospective, randomized, double-blinded study was performed to determine the efficacy of SDD in preventing nosocomial infections in severely burned (>30% total body surface area) PICU patients (7). Patients were randomized to the treatment group (n = 11) or control group (n = 12). The treatment group was given a mixture of polymyxin E, tobramycin, and amphotericin B four times daily by nasogastric tube. No significant differences regarding demographics, underlying conditions, inhalation, injury, or percent of surface area burned between patient and control groups existed. There was no difference in the proportion of patients with colonization of wounds, feces, nasogastric aspirates, or sputum between groups at the start of the study or throughout the study. No significant differences between groups were noted with regard to the serious complications measured: sepsis, pneumonia, gastrointestinal bleeding, respiratory distress syndrome, and mortality. The group treated with SDD had a higher incidence of diarrhea than the control group (82% versus 17%; P = 0.003). Results from that study suggest that SDD may not prevent nosocomial infections in pediatric patients. However, that study was limited by a small sample size (n = 23). Additionally, results of that study may not be generalizable to all PICU patients, as that study was restricted to burn patients.
A prospective nonrandomized cohort study was performed to determine the impact of SDD on nosocomial infections in NICU patients (49). The decision to administer decolonization was left to attending physicians. Investigators later determined if patients had received well-performed decolonization (decolonization within the first 5 days with oral polymyxin E, tobramycin, and nystatin), incorrect decolonization (started after 5 days or less than three drugs used), or no decolonization. The incidence of nosocomial respiratory infection was lower in patients given well-performed (2.5%) or incorrect (7%) decolonization (P value not given). Interestingly, the incidence of nosocomial respiratory infection was lowest in patients who were not decolonized (1%). However, because patients were not randomized into treatment groups, significant underlying differences between groups, including gestational age, birth weight, NICU length of stay, exposure to central catheters, and respiratory support, existed. To control for these differences, investigators performed logistic regression and found that well-performed selective intestinal decolonization exerted a protective effect toward nosocomial infections of intestinal origin (OR, 0.17; 95% CI, 0.03 to 0.83). This group of infections included respiratory tract infections, sepsis, surgical wound infections, and urinary tract infections. The investigators did not supply a separate analysis of the impact of SDD on respiratory infections alone.
Oral Hygiene
The CDC suggests that health care facilities develop and implement a comprehensive oral hygiene program for patients in acute-care settings or residents in long-term care facilities who are at high risk for health care-associated pneumonia (89).
Fitch et al. (35) demonstrated that an oral care protocol and scores developed by a dental hygienist could be used by ICU nurses to improve oral health in critically ill adult patients. Mean oral inflammation scores were significantly lower after the implementation of a standard oral care protocol using toothpaste, antibacterial mouthwash, and oral gel (3.9 [standard error of the mean, 3.0] versus 12.4 [standard error of the mean, 2.2]; P = 0.03). Those investigators also noted lower mean scores for oral candidiasis, purulence, bleeding, and plaque, but the differences were not statistically significant. The dental hygienist and nurses’ assessments had a high degree of interrater reliability (kappa = 0.64). The scores used in that study were developed by one of the investigators and reviewed by other dental faculty members but were not validated in other patient populations. In addition, those investigators did not examine the effect of the standard oral care protocol on the incidence of VAP or bacterial oropharyngeal colonization.
Bergmans et al. (9) performed a prospective, randomized, placebo-controlled, double-blind study in adult ICU patients to determine if VAP was preventable by the modulation of bacterial flora in the oropharynx. Those investigators compared topical prophylaxis to the buccal cavities with 2% each gentamicin, colistin, and vancomycin (n = 87) to an Orabase placebo group (n = 78) (group A) and a second control group of patients admitted to an ICU where no topical preparation was used (n = 61) (group B). Topical prophylaxis eradicated a significantly higher proportion of organisms present on admission in the oropharynx in the treatment group than in either control group (75% of the treatment group versus 0% in the placebo group and 9% in the no-preparation ICU group; P < 0.00001). Topical prophylaxis was also effective in eradicating organisms from the trachea (treatment group, 52%; group A, 22%; group B, 7% [P ≤ 0.03]). The incidence of VAP was also lower in the treatment group (10%) than in the controls (group A, 31% [P = 0.001]; group B, 23% [P = 0.04]). That study concluded that preventing oropharyngeal colonization is protective against VAP, with an absolute risk reduction of 0.21 (95% CI, 0.09 to 0.33); treating five patients with topical antibiotics would prevent one case of VAP. VAP was defined prospectively using CDC definitions and confirmed with BAL or PSB. However, it is unclear whether the person who determined whether the patients had VAP was blinded to the treatment group. In addition, the treatment group received enteral feeds more frequently than controls, which could alter oropharyngeal flora. The placebo group (group A) was significantly more likely than the treatment group to receive sucralfate, another potential confounder of oropharyngeal colonization.
Pineda et al. (73) performed a meta-analysis to determine if oral chlorhexidine treatment reduced the incidence of VAP. Four randomized controlled trials including 1,202 patients met inclusion criteria for the meta-analysis. Patients in the chlorhexidine treatment group were less likely to develop VAP than those in the control group (4% [24 of 587] versus 7% [41 of 615]), although the difference did not reach statistical significance (OR, 0.42; 95% CI, 0.16 to 1.06; P = 0.07). ICU length of stay and duration of mechanical ventilation did not differ between the groups. Mortality was not significantly different between the two groups (OR, 0.77; 95% CI, 0.28 to 2.11; P = 0.6). The magnitude of the protective OR is striking, as is the proximity of the CIs to statistical significance, suggesting that additional studies with larger sample sizes might demonstrate a significant protective effect from oral chlorhexidine rinses. Of note, patients in those studies received either a 0.12% chlorhexidine rinse twice a day (n = 914) or 0.2% chlorhexidine gel three times a day (n = 288).
A meta-analysis of seven randomized controlled trials (n = 1,650 patients) performed by Chlebicki and Safdar (15) revealed a similar protective effect with a relative risk (RR) of 0.74 (95% CI, 0.56 to 0.96; P = 0.02) using a fixed-effects model and a RR of 0.70 (95% CI, 0.47 to 1.04; P = 0.07) using a random-effects model for patients treated orally with chlorhexidine. The risk reduction was even higher in cardiac surgery patients (RR, 0.41; 95% CI, 0.17 to 0.98; P = 0.04) (15).
The Bundle Approach
In December 2004, the Institute for Healthcare Improvement (IHI) challenged hospitals to save 100,000 lives by June 2006 (21). One of the six evidence-based guidelines to be implemented was the prevention of VAP. The VAP bundle for adults is to (i) avoid/decrease endotracheal intubation and duration of mechanical ventilation whenever possible, (ii) use orotracheal and orogastric tubes to decrease the risk of hospital-acquired sinusitis, (iii) avoid heavy sedation and neuromuscular blockade with depression of cough reflexes, (iv) maintain endotracheal cuff pressures to greater than 20 cm water, (v) prevent condensate in tubing from entering the lower respiratory tract, (vi) maintain head-of-bed elevation at 30° to 45°, (vii) maintain oral care, and (viii) maintain hand hygiene (21, 67).
The team approach using the IHI bundle has been shown to be successful in reducing VAP (21). The bundle approach has been used at the Children's Hospital in Boston and at Vanderbilt Children's Hospital. In the latter, an education and intervention termed “ZAP VAP” was put into practice, with their efforts emphasizing the IHI bundle (21). Prevention included hand washing, elevating the head of the bed 30° to 45°, monitoring gastric residuals every 4 h to prevent aspiration, providing aggressive oral care (and documentation) every 2 h, managing hypopharyngeal secretions, providing in-line endotracheal suction, and providing equipment care. During the first 6 months of implementation, the time between VAP occurrences has nearly tripled.
Educational Interventions
Identifying effective measures for preventing VAP is only as useful as the proper implementation of these measures in the clinical setting. Many studies have shown a reduction in rates of VAP following initiatives to educate health care workers about the epidemiology of VAP and infection control measures used to prevent VAP (89). Most of those studies were performed in the adult ICU setting. A recent educational intervention was performed in an integrated health system, with results compared across a large adult teaching hospital, two community hospitals, and a pediatric teaching hospital (4). The targeted health care workers were respiratory care practitioners and nursing staff working in the ICU setting. This intervention centered on a 10-page self-study module that focused on multiple aspects of VAP and also included posters, fact sheets, and in-services for nursing staff and respiratory therapists. VAP rates between the 12-month preintervention period and the 18-month postintervention period were compared. Nursing compliance rates were highest among nurses at the pediatric hospital (100%) and one of the community hospitals (98.9%). The adult teaching hospital and the other community hospital had significantly lower compliance rates among nurses (64.9% and 44.2%; P < 0.001). Three hospitals had a significant drop in the VAP rates from the preintervention period to the postintervention period. The VAP rate at the pediatric hospital fell 38%, from 7.9 episodes to 4.9 episodes per 1,000 ventilator days (P < 0.001). The community hospital with no change in the rate of VAP had the lowest compliance of respiratory therapists compared to the other three hospital combined (56.3% versus 95.2%; P < 0.001).
Not all lapses in infection control measures result from a lack of knowledge. A survey of NICU health care workers was performed to investigate the knowledge, beliefs, and practices regarding nosocomial infections and infection control measures (56). The survey revealed some areas in which health care workers’ actions arose from unawareness of data related to infection control. For instance, few participants believed that nosocomial infections were related to health care workers’ rings (40%), artificial fingernails (61%), or long fingernails (48%). However, that study also revealed some disconnects between knowledge and practice. Although 96% of respondents believed that using sterile techniques for catheter insertion and care reduces a patient's risk for BSI, only 67% reported using full sterile barriers at least 76% of the time when participating in inserting a line. Likewise, 91% of participants believed gloves are important for preventing the spread of nosocomial infections, but only 53% reported changing their gloves in all indicated situations. That study demonstrated the need for increased educational efforts to bridge the gaps in knowledge of infection control recommendations. Additionally, the study demonstrated that a lack of knowledge alone does not account for the lapses in infection control practices in the NICU studied. The most common barriers to infection control perceived by respondents included logistics (54%), time (48%), and lack of supplies (47%).
Interventions that lower rates of VAP may have temporary effects, with VAP rates eventually rising following the conclusion of the intervention, indicating the need for continuous reinforcement of interventional measures (55). Factors associated with noncompliance with hand hygiene exist at the individual, group, and institutional levels (74). A proposed framework for the promotion of hand hygiene includes 12 factors: (i) education, (ii) routine observation and feedback, (iii) engineering controls, (iv) patient education, (v) reminders in the workplace, (vi) administrative sanctions and rewards, (vii) change in hand hygiene agents, (viii) promotion of workers’ skin care, (ix) active participation at the individual and institutional level, (x) maintenance of an institutional safety climate, (xi) enhancement of individual and institutional self-efficacy, and (xii) avoidance of overcrowding, understaffing, and excessive workload (74). The diversity of these factors emphasizes the need for a multipronged and continuous approach necessary to maintain high levels of compliance with infection control measures.
A summary of interventional measures to decrease the incidence of VAP in children is provided in Table 3.
TABLE 3.
Summary of interventions to prevent VAPa
| Intervention and source (reference) | Design | Patient description | Outcome |
|---|---|---|---|
| Enteral feeds | |||
| Almuneef et al. (1) | Prospective active surveillance | 361 PICU patients(37 with VAP, 324 without VAP) | Cases had higher frequency of enteral feeds (48.6% vs 26.8%; OR, 2.58; P = 0.006) |
| Lopriore et al. (62) | Retrospective case control | 155 PICU patients (13 with VAP, 142 without VAP) | No significant difference between cases and controls (53.8% vs 43.6%) |
| Motility agents | |||
| Lopriore et al (62) | Retrospective case control | 155 PICU patients (13 with VAP, 142 without VAP) | Cases had higher frequency of motility agent use (P < 0.05) (association not significant in logistic regression) |
| Head-of-bed elevation | |||
| Black et al. (10) | Matched case control study | 18 PICU patients (9 with VAP, 9 without VAP) | No significant difference between cases and controls in head-of-bed elevation |
| Closed vs open suctioning | |||
| Cordero et al. (20) | Prospective, randomized | 133 NICU patients (67 closed, 66 open) | No difference in diagnosis of nosocomial pneumonia between groups (n = 5 patients each) |
| SDD | |||
| Barret et al. (7) | Prospective, randomized, double-blinded | 23 burn patients (11 SDD, 12 placebo) | No difference in rates of pneumonia (1 case in SDD group, 0 in placebo group) |
| Ruza et al. (81) | Prospective, randomized, nonblinded | 226 PICU patients (116 with SDD, 110 without SDD) | SDD protective toward respiratory infection in logistic regression (OR, 0.21; 95% CI, 0.06-0.8) |
| Herruzo-Cabrera et al. (49) | Prospective cohort, nonrandomized | 536 neonates (58 WP SID, 88 IP SID, 392 no SID) | WP SID protective toward NI of intestinal origin in logistic regression (OR, 0.17; 95% CI, 0.03-0.83). |
| Stress ulcer prophylaxis | |||
| Lopriore et al. (62) | Retrospective case control | 155 PICU patients (54 ranitidine, 53 sucralfate, 48 no prophylaxis) | No significant difference in upper airway colonization with GNB, no significant difference in incidence of VAP |
| Yildizdas et al. (101) | Prospective, randomized, nonblinded | 160 PICU patients (38 sucralfate, 42 ranitidine, 38 omeprazole, 42 no prophylaxis) | No significant difference in VAP between patient groups |
| Infection control interventions | |||
| Won et al. (99) | Prospective study of hand hygiene campaign | NICU patients admitted during hand hygiene campaign | Rate of respiratory infections dropped from 3.35 to 1.06 per 1,000 patient days (P = 0.002) |
| Babcock et al. (4) | Prospective study of educational intervention | PICU patients at pediatric teaching hospital | 38% reduction in VAP rate from 7.9 to 4.9 episodes per 1,000 ventilator days (P < 0.001) |
| Nursing practice (decreasing peripheral intravenous access) | |||
| Ng et al. (69) | Prospective surveillance | 493 NICU patients (227 before period, 266 after period) | Non-statistically-significant decrease in VAP rate from 3.3 to 1.0 per 1,000 ventilator days (P = 0.22) |
| Oral hygiene | |||
| Chlebicki and Safdar (15) | Meta-analysis, seven randomized controlled trials | 1,650 patients total (n = 812 [topical chlorhexidine]; n = 838 [comparator]) | Topical chlorhexidine reduced VAP incidence (RR, 0.74; P = 0.02); risk reduction was even higher in cardiac surgery patients (RR, 0.41; P = 0.04). |
| Pineda et al. (73) | Meta-analysis, four randomized controlled trials | 1202 patients total (n = 587 [chlorhexidine group]; n = 615 [control group]) | Chlorhexidine group less likely to develop VAP (4%) compared to control group (7%)(P = 0.07); ICU length of stay, duration of mechanical ventilation, and mortality not significantly different |
| Bergmans et al. (9) | Prospective, randomized, double-blinded, placebo controlled | 87 patients (treatment group), 78 patients, (control group A), and 61 patients (control group B) | VAP incidences were less in treatment group (10%) than in groups A (31%; P = 0.001) and B (23%; P = 0.04) |
| Fitch et al. (35) | Longitudinal design, repeated measures | ICU nurses and dental hygienist | Nurses following oral care protocols can help improve ICU patient oral health |
WP, well performed; IP, incorrectly performed; GNB, gram-negative bacilli; NI, nosocomial infection.
Recommendations for Current Practice and Future Research
There is scant literature regarding testing the efficacy of head-of-bed elevation, in-line suctioning, and preferential use of sucralfate over histamine type 2 receptor antagonists in pediatric VAP prevention. However, head-of-bed elevation and other measures to prevent aspiration, a consistent approach to oral hygiene, meticulous hand hygiene, and regular assessment of readiness to wean are biologically plausible as effective VAP prevention measures in children. Further studies documenting that head-of-bed elevation in children decreases aspiration and risk of pneumonia as well as determining the natural history of aerodigestive tract colonization and its relationship to gastric acidity in children may shed light on the risk/benefit ratio of sucralfate and/or H2 blockers and the number needed to treat to prevent pediatric VAP.
VAP TREATMENT
Treatment of suspected VAP is centered on an approach of initial empirical therapy with broad-spectrum antibiotics followed by de-escalation to specific antimicrobial therapy once culture results are known or discontinuation of antibiotics if VAP is no longer suspected. The American Thoracic Society and Infectious Disease Society of America have recently published an updated version of their evidence-based guidelines for the management of VAP in adults (2). Key recommendations in the new document include the use of early, appropriate, and broad-spectrum antibiotics for empirical therapy; utilization of empirical antibiotics from a different class than antibiotics that the patient has recently received; judicious use of combination therapy in hospital-acquired pneumonia; the potential use of linezolid as an alternative to vancomycin for VAP caused by methicillin-resistant Staphylococcus aureus (MRSA); the use of colistin for patients with VAP caused by carbapenem-resistant Acinetobacter species; the potential use of aerosolized antibiotics as adjunctive therapy for patients with VAP caused by certain antibiotic-resistant organisms; de-escalation of antibiotics based on patients’ culture results and clinical improvement; and a shorter duration of antibiotics for patients with uncomplicated health care-associated pneumonia from bacteria other than nonfermenting gram-negative bacilli. These guidelines are based on data from clinical trials of hospital-acquired pneumonia in adult patients. There have been few clinical studies regarding the optimal treatment for VAP in children.
Empirical Therapy
The importance of prompt initiation of appropriate empirical therapy for suspected VAP has been demonstrated in adults, with many studies describing higher mortality in patients who received delayed appropriate treatment for VAP (51, 59, 63). However, inappropriate use and overuse of antibiotics can lead to increased hospital expenditures and could potentially promote antibiotic resistance (33, 83). Empirical antibiotic therapy for suspected VAP accounts for a major proportion of inappropriate antibiotic use in pediatric patients, with up to 33% of patients receiving unwarranted antimicrobial therapy for suspected VAP (33). Prescribing patterns have also shifted toward more expensive and broader-spectrum antibiotics in hospitalized children in recent years, with the proportion of total antibiotic expenditure used for vancomycin increasing from 0.2% in 1984 to 17.2% in 1994. Additionally, broad-spectrum cephalosporins accounted for 17.7% of antibiotic expenditures in 1984 and 49.6% in 1994 (96). Thus, prescribing patterns for empirical therapy for suspected VAP should maintain a balance between adequately covering patients who are potentially infected and minimizing unnecessary and prolonged exposure to antimicrobials.
Infection with potentially antibiotic-resistant organisms accounts for a large proportion of VAP in adults (95). When selecting empirical therapy, physicians should be aware of the patient's risk factors for infection with multidrug-resistant (MDR) bacteria, the antibiotics that the patient has recently received, and the local antibiotic resistance patterns. Risk factors for VAP with MDR pathogens in adults include mechanical ventilation for at least 7 days, prior antibiotic use, and prior exposure to broad-spectrum antibiotics (imipenem, broad-spectrum cephalosporins, or fluoroquinolones) (95). In children, it has been postulated that patients may be colonized with organisms from their own preexisting endogenous flora in response to antibiotic pressure (92). Risk factors for colonization with antibiotic-resistant gram-negative organisms in PICU patients include younger age, increasing PRISM (pediatric risk of mortality) score, previous PICU admissions, intravenous antibiotic use in the past 12 months, and exposure to chronic care facilities (91, 93). Additional special considerations for the pediatric population include premature infants with an increased risk of Staphylococcus epidermidis infections and immunocompromised patients with an additional need for empirical antifungal therapy (52).
Monotherapy for empirical coverage is recommended for adult patients with early-onset VAP without risk factors for infection with MDR pathogens, while combination therapy should be used for coverage of potential infection with MDR organisms or late-onset VAP based on a local antibiogram. Additionally, patients should be treated with antibiotics differing in class from those that they have recently received in case colonizing bacteria have developed antibiotic resistance from previous exposures (2). No consensus guidelines for empirical coverage of suspected VAP in children exist.
Empirical therapy should be discontinued or altered based on culture results and clinical status. The fear that negative culture results may have missed an infection in critically ill children often leads to prolonged empirical antimicrobial therapy in neonates (87). A study of late-onset sepsis evaluations in neonates was undertaken to determine a sufficient time point for the discontinuation of empirical therapy. Those investigators found that 99% of blood cultures were positive within 48 h, and investigators used this finding as a basis for decreasing empirical coverage from 72 h to 48 h for suspected late-onset sepsis in neonates. That study examined cultures from sterile body sites only. Cultures from the respiratory tract and the vagaries of diagnosing VAP may not lend themselves to an easily defined time point for the discontinuation of empirical therapy in suspected VAP.
Singh et al. (85) used the CPIS to guide the duration of empirical therapy in adults. Intensive care patients with new-onset pulmonary infiltrate who were suspected of having pneumonia were evaluated at baseline with five of the seven CPIS items on a scale of 0 to 2 each (temperature, blood leukocytes, tracheal secretions, oxygenation, and pulmonary radiography). Patients with a total CPIS of ≤6 were considered to have a low likelihood of pneumonia and were randomized to receive standard care as determined by their attending physician or experimental therapy with ciprofloxacin monotherapy. All patients were reevaluated at 3 days, and of those with CPIS remaining at ≤6, 100% of patients in the experimental arm had a cessation of antimicrobial therapy, compared to 4% of patients in the standard therapy arm. There was no difference in mortality or length of stay between treatment groups; antibiotic resistance, superinfections, or both occurred more frequently in patients receiving standard therapy than patients receiving experimental therapy (35% versus 15%; P = 0.017). Replication of these results in children could offer clinical guidelines for the rapid cessation of empirical antimicrobial therapy for suspected VAP.
Specific Treatment
Combination therapy exposes patients to multiple antibiotics and is more expensive; a prompt de-escalation of empirical therapy to more specific antimicrobial therapy should occur once culture results and susceptibilities are known. Monotherapy is recommended for patients who are not at risk for infection with MDR pathogens and for patients infected with gram-positive pathogens including MRSA. Additionally, patients with severe VAP should initially be treated with combination therapy but may be changed to monotherapy if lower respiratory tract cultures do not identify an antibiotic-resistant pathogen (2). Agents that have demonstrated efficacy for monotherapy in adult patients infected with susceptible organisms include fluoroquinolones, carbapenems, cefepime, and piperacillin-tazobactam (2).
Recent interest surrounding the substitution of linezolid for vancomycin in the treatment of VAP caused by MRSA has emerged. A meta-analysis of two prospective, randomized, double-blind, multicenter, multinational trials in adults with nosocomial pneumonia comparing clinical cure from linezolid to vancomycin was performed (100). Patients with MRSA pneumonia who were treated with linezolid had survival that was significantly higher than that of patients treated with vancomycin (80.0% versus 63.5%; P = 0.03). Clinical cure rates were also higher for patients treated with linezolid than those treated with vancomycin (59.0% versus 35.5%; P < 0.01). Both of these effects remained significant in logistic regression models.
A prospective, randomized, open-label, multicenter, multinational trial was performed to compare the efficacy and safety of linezolid and vancomycin for antibiotic-resistant gram-positive bacteremia and nosocomial pneumonia in hospitalized children (53). Among patients with nosocomial pneumonia, patients randomized to the linezolid group were more often mechanically ventilated (63.6% versus 10.0%; P = 0.011) and had more multiple-lobe involvement (90.9% versus 50.0%; P = 0.038). No significant difference in clinical cure was seen between patients treated with linezolid and those treated with vancomycin. However, patients treated with linezolid appeared to experience a faster resolution of dyspnea/tachypnea/grunting than patients with vancomycin, with fewer than 40% of patients receiving linezolid and more than 80% of patients receiving vancomycin demonstrating these symptoms of VAP on day 3 of treatment (P value not given). Additionally, patients treated with linezolid experienced a shorter mean duration of total therapy (10.5 days versus 12.8 days; P = 0.03) and intravenous therapy (8.5 days versus 11.3 days; P = 0.004). The frequency of drug-related adverse events was similar between groups. That analysis was a subset of a larger study, and it analysis was not powered for nosocomial pneumonia or bacteremia. Other subanalyses of this same patient population have demonstrated the safety of linezolid compared to vancomycin in children (64, 82).
Aerosolized antibiotics have been studied most extensively in children in the setting of cystic fibrosis. The FDA approval of tobramycin solution for inhalation is only for maintenance therapy in patients with cystic fibrosis known to be colonized with Pseudomonas aeruginosa (76). In two randomized controlled trials, a modest improvement in the forced expiratory volume in 1 min occurred after 24 weeks of therapy with tobramycin solution for inhalation; there were significant decreases in CFU/ml of Pseudomonas aeruginosa in sputum, decreases in hospital admission days, and decreases in numbers of parenteral antibiotic days for treatment of Pseudomonas aeruginosa infections. To our knowledge, there are no data showing that treatment of acute pulmonary infections with aerosolized antibiotics is beneficial in children. Inhaled aminoglycosides do produce low but measurable serum concentrations (1 to 4 μg/ml). Sputum concentrations vary. No ototoxicity or nephrotoxicity has been associated with the use of inhaled aminoglycosides, although many of those studies excluded children with serum creatinine levels greater than 2. The potential disadvantages of use include bronchospasm, increased MICs of the targeted organism, increased isolation of Candida and Aspergillus species from sputum, nebulization of microorganisms, and antibiotic contamination of the environment (76).
Finally, subpopulations of pediatric patients deserve special consideration. Neurologically impaired children are at increased risk for aspiration or tracheostomy-associated pneumonia. The mixed microbiology of these infections, often including anaerobic organisms, warrants specific antimicrobial therapy against likely pathogens. A retrospective study of 57 neurologically impaired children with aspiration or tracheostomy-related pneumonia was performed to evaluate the efficacy of various antimicrobial therapies (11). Children with either type of pneumonia had better clinical improvement and microbiological response when treated with agents effective against penicillin-resistant anaerobic bacteria (ticarcillin-clavulanate or clindamycin) than patients treated with ceftriaxone (P < 0.05 for both tracheostomy-related pneumonia and aspiration pneumonia groups). Although this was a small study within a focused population, it underscores the need to account for unique underlying conditions in children that may predispose them to infections with specific pathogens.
Duration of Therapy
No consensus exists regarding the appropriate duration of antimicrobial therapy for VAP in adults, and the appropriate duration for proven infections in critically ill children has not been established (45). A multicenter, randomized, controlled trial was performed using adults with VAP to compare patients treated with appropriate empirical therapy for 8 days to patients treated for 15 days (14). There was no difference in mortality or recurrent infections between groups. Among patients infected with nonfermenting gram-negative bacilli, patients treated for 8 days did have a higher relapse rate (32.8% versus 19.0%; 13.8% risk difference; 90% CI, 7.8% to 19.7%). However, among patients who experienced recurrent infections, patients treated for 8 days were less likely to become infected with MDR pathogens (42.1% versus 62.0%; P = 0.04). These data suggest that limiting treatment of VAP in patients infected with pathogens other than nonfermenting gram-negative bacilli is safe and may decrease the incidence of reinfection with MDR pathogens.
Recommendations for Current Practice and Future Research
No consensus guidelines exist for empirical coverage of suspected VAP in children.
Empirical therapy should be discontinued or altered based on clinical status and culture results, preferably from lower airway samples. Patients who are severely ill and/or with previous exposure to the health care system should be treated with combination therapy. Areas for future research in children include the efficacy of linezolid compared to vancomycin for VAP treatment, the duration of optimal antibiotic therapy for VAP, and validation of the CPIS in children as well as its use in treatment decisions.
CONCLUSION
VAP is the second most common hospital-acquired infection among PICU patients. Empirical therapy for VAP accounts for approximately 50% of antibiotic use in PICUs. VAP is associated with an excess of 3 days of mechanical ventilation among pediatric cardiothoracic surgery patients. The attributable mortality and excess length of ICU stay of VAP have not been defined in matched case control studies. VAP is associated with an estimated $30,000 in attributable cost. Surveillance for VAP is complex and usually performed using clinical definitions established by the CDC. Invasive testing via BAL increases the sensitivity and specificity of the diagnosis. The pathogenesis is poorly understood in children, but several prospective cohort studies suggest that aspiration and immunodeficiency are risk factors. In children, educational interventions and efforts to improve adherence to hand hygiene have been associated with decreased VAP rates. More consistent and precise approaches to the diagnosis of pediatric VAP are needed to better define the attributable morbidity and mortality, pathophysiology, and appropriate interventions to prevent this disease.
REFERENCES
- 1.Almuneef, M., Z. A. Memish, H. H. Balkhy, H. Alalem, and A. Abutaleb. 2004. Ventilator-associated pneumonia in a pediatric intensive care unit in Saudi Arabia: a 30-month prospective surveillance. Infect. Control Hosp. Epidemiol. 25:753-758. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society. 2005. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 171:388-416. [DOI] [PubMed] [Google Scholar]
- 3.Apisarnthanarak, A., G. Holzmann-Pazgal, A. Hamvas, M. A. Olsen, and V. J. Fraser. 2003. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. Pediatrics 112:1283-1289. [DOI] [PubMed] [Google Scholar]
- 4.Babcock, H. M., J. E. Zack, T. Garrison, E. Trovillion, M. Jones, V. J. Fraser, and M. H. Kollef. 2004. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Chest 125:2224-2231. [DOI] [PubMed] [Google Scholar]
- 5.Babcock, H. M., J. E. Zack, T. Garrison, E. Trovollion, M. H. Kollef, and V. J. Fraser. 2003. Ventilator-associated pneumonia in a multi-hospital system: differences in microbiology by location. Infect. Control Hosp. Epidemiol. 24:853-858. [DOI] [PubMed] [Google Scholar]
- 6.Baltimore, R. S. 1998. Neonatal nosocomial infections. Semin. Perinatol. 22:25-32. [DOI] [PubMed] [Google Scholar]
- 7.Barret, J. P., M. G. Jeschke, and D. N. Herndon. 2001. Selective decontamination of the digestive tract in severely burned pediatric patients. Burns 27:439-445. [DOI] [PubMed] [Google Scholar]
- 8.Bercault, N., and T. Boulain. 2001. Mortality rate attributable to ventilator-associated nosocomial pneumonia in an adult intensive care unit: a prospective case-control study. Crit. Care Med. 29:2303-2309. [DOI] [PubMed] [Google Scholar]
- 9.Bergmans, D. C., M. J. Bonten, C. A. Gaillard, J. C. Paling, S. van der Geest, F. H. van Tiel, A. J. Beysens, P. W. de Leeuw, and E. E. Stobberingh. 2001. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am. J. Respir. Crit. Care Med. 164:382-388. [DOI] [PubMed] [Google Scholar]
- 10.Black, S. R., E. Lo, M. Madriaga, M. Zimmerman, and J. Segreti. 2002. Nosocomial pneumonia in the PICU. Abstr. 40th Intersci. Conf. Antimicrob. Agents Chermother., abstr. K-452.
- 11.Brook, I. 1996. Treatment of aspiration or tracheostomy-associated pneumonia in neurologically impaired children: effect of antimicrobials effective against anaerobic bacteria. Int. J. Pediatr. Otorhinolaryngol. 35:171-177. [DOI] [PubMed] [Google Scholar]
- 12.Burmester, M., and Q. Mok. 2001. How safe is non-bronchoscopic bronchoalveolar lavage in critically ill mechanically ventilated children? Intensive Care Med. 27:716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chastre, J., F. Viau, P. Brun, J. Pierre, M. C. Dauge, A. Bouchama, A. Akesbi, and C. Gibert. 1984. Prospective evaluation of the protected specimen brush for the diagnosis of pulmonary infections in ventilated patients. Am. Rev. Respir. Dis. 130:924-929. [DOI] [PubMed] [Google Scholar]
- 14.Chastre, J., M. Wolff, J. Y. Fagon, S. Chevret, F. Thomas, D. Wermert, E. Clementi, J. Gonzalez, D. Jusserand, P. Asfar, D. Perrin, F. Fieux, S. Aubas, and the PneumA Trial Group. 2003. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA 290:2588-2598. [DOI] [PubMed] [Google Scholar]
- 15.Chlebicki, M. P., and N. Safdar. 2007. Topical chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. Crit. Care Med. 35:595-602. [DOI] [PubMed] [Google Scholar]
- 16.Collard, H. R., S. Saint, and M. A. Matthay. 2003. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann. Intern. Med. 138:494-501. [DOI] [PubMed] [Google Scholar]
- 17.Combes, P., B. Fauvage, and C. Oleyer. 2000. Nosocomial pneumonia in mechanically ventilated patients, a prospective randomized evaluation of the Stericath closed suctioning system. Intensive Care Med. 26:878-882. [DOI] [PubMed] [Google Scholar]
- 18.Cook, D. J., B. K. Reeve, G. H. Guyatt, D. K. Heyland, L. E. Griffith, L. Buckingham, and M. Tryba. 1996. Stress ulcer prophylaxis in critically ill patients. Resolving discordant meta-analyses. JAMA 275:308-314. [PubMed] [Google Scholar]
- 19.Cordero, L., L. W. Ayers, R. R. Miller, J. H. Seguin, and B. D. Coley. 2002. Surveillance of ventilator-associated pneumonia in very-low-birth-weight infants. Am. J. Infect. Control 30:32-39. [DOI] [PubMed] [Google Scholar]
- 20.Cordero, L., M. Sananes, and L. W. Ayers. 2000. Comparison of a closed (Trach Care MAC) with an open endotracheal suction system in small premature infants. J. Perinatol. 3:151-156. [DOI] [PubMed] [Google Scholar]
- 21.Curley, M. A., E. Schwalenstocker, J. K. Deshpande, C. C. Ganser, D. Bertoch, J. Brandon, and P. Kurtin. 2006. Tailoring the Institute for Health Care Improvement 100,000 Lives Campaign to pediatric settings: the example of ventilator-associated pneumonia. Pediatr. Clin. N. Am. 53:1231-1251. [DOI] [PubMed] [Google Scholar]
- 22.Department of Health and Human Services. 23 August 2006, accession date. Criteria for defining nosocomial pneumonia. http://www.cdc.gov/ncidod/hip/NNIS/members/pneumonia/Final/PneumoCriteriaV1.pdf.
- 23.Deppe, S. A., J. W. Kelly, L. L. Thoi, J. H. Chudy, R. N. Longfield, J. P. Ducey, C. L. Truwit, and M. R. Antopol. 1990. Incidence of colonization, nosocomial pneumonia, and mortality in critically ill patients using a Trach Care closed-suction system versus an open-suction system: prospective, randomized study. Crit. Care Med. 18:1389-1393. [DOI] [PubMed] [Google Scholar]
- 24.Donowitz, L. G. 1989. Nosocomial infection in neonatal intensive care units. Am. J. Infect. Control 17:250-257. [DOI] [PubMed] [Google Scholar]
- 25.Drakulovic, M. B., A. Torres, T. T. Bauer, J. M. Nicolas, S. Nogué, and M. Ferrer. 1999. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet 354:1851-1858. [DOI] [PubMed] [Google Scholar]
- 26.Drews, M. B., A. C. Ludwig, J. U. Leititis, and F. D. Daschner. 1995. Low birth weight and nosocomial infection of neonates in a neonatal intensive care unit. J. Hosp. Infect. 30:65-72. [DOI] [PubMed] [Google Scholar]
- 27.Durand, M., B. Sangha, L. A. Cabal, T. Hoppenbrouwers, and J. E. Hodgman. 1989. Cardiopulmonary and intracranial pressure changes related to endotracheal suctioning in preterm infants. Crit. Care Med. 17:506-510. [DOI] [PubMed] [Google Scholar]
- 28.Elward, A. M., D. K. Warren, and V. J. Fraser. 2002. Ventilator-associated pneumonia in pediatric intensive care unit patients: risk factors and outcomes. Pediatrics 109:758-764. [DOI] [PubMed] [Google Scholar]
- 29.Ely, E. W., A. M. Baker, D. P. Dunagan, H. L. Burke, A. C. Smith, P. T. Kelly, M. M. Johnson, R. W. Browder, D. L. Bowton, and E. F. Haponik. 1996. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N. Engl. J. Med. 335:1864-1869. [DOI] [PubMed] [Google Scholar]
- 30.Fabregas, N., S. Ewig, A. Torres, M. El-Ebiary, J. Ramirez, J. P. de La Bellacasa, T. Bauer, and H. Cabello. 1999. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax 54:867-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fagon, J. Y., J. Chastre, A. J. Hance, P. Montravers, A. Novara, and C. Gibert. 1993. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am. J. Med. 94:281-288. [DOI] [PubMed] [Google Scholar]
- 32.Fayon, M. J., M. Tucci, J. Lacroix, C. A. Farrell, M. Gauthier, L. Lafleur, and D. Nadeau. 1997. Nosocomial pneumonia and tracheitis in a pediatric intensive care unit: a prospective study. Am. J. Respir. Crit. Care Med. 155:162-169. [DOI] [PubMed] [Google Scholar]
- 33.Fischer, J. E., M. Ramser, and S. Fanconi. 2000. Use of antibiotics in pediatric intensive care and potential savings. Intensive Care Med. 26:959-966. [DOI] [PubMed] [Google Scholar]
- 34.Fischer, J. E., P. Allen, and S. Fanconi. 2000. Delay of extubation in neonates and children after cardiac surgery: impact of ventilator-associated pneumonia. Intensive Care Med. 26:942-949. [DOI] [PubMed] [Google Scholar]
- 35.Fitch, J. A., C. L. Munro, C. A. Glass, and J. M. Pellegrini. 1999. Oral care in the adult intensive care unit. Am. J. Crit. Care 8:314-318. [PubMed] [Google Scholar]
- 36.Flanagan, P. G. 1999. Diagnosis of ventilator-associated pneumonia. J. Hosp. Infect. 41:87-99. [DOI] [PubMed] [Google Scholar]
- 37.Flanagan, P. G., G. P. Findlay, J. T. Magee, A. Ionescu, R. A. Barnes, and M. Smithies. 2000. The diagnosis of ventilator-associated pneumonia using non-bronchoscopic, non-directed lung lavages. Intensive Care Med. 26:20-30. [DOI] [PubMed] [Google Scholar]
- 38.Foglia, E., C. Hollenbeak, V. Fraser, and A. Elward. 2006. Costs associated with nosocomial bloodstream infections and ventilator-associated pneumonia in pediatric intensive care unit patients. Abstr. 16th Annu. Meet. Soc. Healthcare Epidemiol. America, abstr. 109.
- 39.Ford-Jones, E. L., C. M. Mindorff, J. M. Langley, U. Allen, L. Navas, M. L. Patrick, R. Milner, and R. Gold. 1989. Epidemiologic study of 4684 hospital-acquired infections in pediatric patients. Pediatr. Infect. Dis. J. 8:668-675. [DOI] [PubMed] [Google Scholar]
- 40.Forman, M. L., and E. R. Stiehm. 1969. Impaired opsonic activity but normal phagocytosis in low-birth-weight infants. N. Engl. J. Med. 281:926-931. [DOI] [PubMed] [Google Scholar]
- 41.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1996. CDC definitions for nosocomial infections, p. A1-A19. In R. N. Olmsted (ed.), APIC infection control and applied epidemiology: principles and practice. Mosby, St. Louis, MO.
- 42.Gauvin, F., C. Dassa, M. Chaibou, F. Proulx, C. A. Farrell, and J. Lacroix. 2003. Ventilator-associated pneumonia in intubated children: comparison of different diagnostic methods. Pediatr. Crit. Care Med. 4:437-443. [DOI] [PubMed] [Google Scholar]
- 43.Gaynes, R. P., J. R. Edwards, W. R. Jarvis, D. H. Culver, J. S. Tolson, W. J. Martone, and the National Nosocomial Infection Surveillance System. 1996. Nosocomial infections among neonates in high-risk nurseries in the United States. Pediatrics 98:357-361. [PubMed] [Google Scholar]
- 44.Goldmann, D. A., J. Freeman, and W. A. Durbin, Jr. 1983. Nosocomial infection and death in a neonatal intensive care unit. J. Infect. Dis. 147:635-641. [DOI] [PubMed] [Google Scholar]
- 45.Gordon, A., and D. Isaacs. 2004. Late-onset infection and the role of antibiotic prescribing policies. Curr. Opin. Infect. Dis. 17:231-236. [DOI] [PubMed] [Google Scholar]
- 46.Haas, J. P., E. A. Mendonca, B. Ross, C. Friedman, and E. Larson. 2005. Use of computerized surveillance to detect nosocomial pneumonia in neonatal intensive care unit patents. Am. J. Infect. Control 33:439-443. [DOI] [PubMed] [Google Scholar]
- 47.Harpin, V. A., and N. Rutter. 1983. Barrier properties of the newborn infant's skin. J. Pediatr. 102:419-425. [DOI] [PubMed] [Google Scholar]
- 48.Hemming, V. G., J. C. Overall, Jr., and M. R. Britt. 1976. Nosocomial infections in a newborn intensive-care unit. Results of forty-one months of surveillance. N. Engl. J. Med. 294:1310-1316. [DOI] [PubMed] [Google Scholar]
- 49.Herruzo-Cabrera, R., J. I. Garcia Gonzalez, P. Garcia-Magan, and J. del Rey-Calero. 1994. Nosocomial infection in a neonatal intensive care unit and its prevention with selective intestinal decolonization: a multivariant evaluation of infection reduction. Eur. J. Epidemiol. 10:573-580. [DOI] [PubMed] [Google Scholar]
- 50.Heyland, D. K., D. J. Cook, L. Griffith, S. P. Keenan, C. Brun-Buisson, et al. 1999. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am. J. Respir. Crit. Care Med. 159:1249-1256. [DOI] [PubMed] [Google Scholar]
- 51.Iregui, M., S. Ward, G. Sherman, V. J. Fraser, and M. H. Kollef. 2002. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest 122:262-268. [DOI] [PubMed] [Google Scholar]
- 52.Jacobs, R. F. 1991. Nosocomial pneumonia in children. Infection 19:64-72. [DOI] [PubMed] [Google Scholar]
- 53.Jantausch, B. A., J. Deville, S. Adler, M. R. Morfin, P. Lopez, B. Edge-Padbury, S. Naberhuis-Stehouwer, and J. B. Bruss. 2003. Linezolid for the treatment of children with bacteremia or nosocomial pneumonia caused by resistant gram-positive bacterial pathogens. Pediatr. Infect. Dis. J. 22:S164-S171. [DOI] [PubMed] [Google Scholar]
- 54.Johnson, K. L., P. A. Kearney, S. B. Johnson, J. B. Niblett, N. L. MacMillan, and R. E. McClain. 1994. Closed versus open endotracheal suctioning: costs and physiologic consequences. Crit. Care Med. 22:658-666. [DOI] [PubMed] [Google Scholar]
- 55.Kelleghan, S. I., C. Salemi, S. Padilla, M. McCord, G. Mermilliod, T. Canola, and L. Becker. 1993. An effective continuous quality improvement approach to the prevention of ventilator-associated pneumonia. Am. J. Infect. Control 21:322-330. [DOI] [PubMed] [Google Scholar]
- 56.Kennedy, A. M., A. M. Elward, and V. J. Fraser. 2004. Survey of knowledge, beliefs, and practices of neonatal intensive care unit healthcare workers regarding nosocomial infections, central venous catheter care, and hand hygiene. Infect. Control Hosp. Epidemiol. 25:747-752. [DOI] [PubMed] [Google Scholar]
- 57.Kerem, E., I. Yatsiv, and K. J. Goitein. 1990. Effect of endotracheal suctioning on arterial blood gases in children. Intensive Care Med. 16:95-99. [DOI] [PubMed] [Google Scholar]
- 58.Kohler, P. F., and R. S. Farr. 1966. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature 210:1070-1071. [DOI] [PubMed] [Google Scholar]
- 59.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 60.Labenne, M., C. Poyart, C. Rambaud, B. Goldfarb, B. Pron, P. Jouvet, C. Delamare, G. Sebag, and P. Hubert. 1999. Blind protected specimen brush and bronchoalveolar lavage in ventilated children. Crit. Care Med. 27:2537-2543. [DOI] [PubMed] [Google Scholar]
- 61.Liberati, A., R. D'Amico, Pifferi, V. Torri, and L. Brazzi. 2004. Antibiotic prophylaxis to reduce respiratory infections and mortality in adults receiving intensive care. Cochrane Database Syst. Rev. 2004(1):CD000022. [DOI] [PubMed] [Google Scholar]
- 62.Lopriore, E., D. G. Markhorst, and R. J. Gemke. 2002. Ventilator-associated pneumonia and upper airway colonisation with gram negative bacilli: the role of stress ulcer prophylaxis in children. Intensive Care Med. 28:763-767. [DOI] [PubMed] [Google Scholar]
- 63.Luna, C. M., P. Vujacich, M. S. Niederman, C. Vay, C. Gherardi, J. Matera, and E. C. Jolley. 1997. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676-685. [DOI] [PubMed] [Google Scholar]
- 64.Meissner, H. C., T. Tonwsend, W. Wenman, S. L. Kaplan, M. R. Morfin, B. Edge-Padbury, S. Naberhuis-Stehouwer, and J. B. Bruss. 2003. Hematologic effects of linezolid in young children. Pediatr. Infect. Dis. J. 22:S186-S192. [DOI] [PubMed] [Google Scholar]
- 65.Morar, P., Z. Makura, A. Jones, P. Baines, A. Selby, J. Hughes, and R. van Saene. 2000. Topical antibiotics on tracheostoma prevents exogenous colonization and infection of lower airways in children. Chest 117:513-518. [DOI] [PubMed] [Google Scholar]
- 66.Morrow, B., and A. Argent. 2001. Risks and complications of nonbronchoscopic bronchoalveolar lavage in a pediatric intensive care unit. Pediatr. Pulmonol. 32:378-384. [DOI] [PubMed] [Google Scholar]
- 67.Munro, C. L., and M. J. Grap. 2004. Oral health and care in the intensive care unit: state of the science. Am. J. Crit. Care 13:25-34. [PubMed] [Google Scholar]
- 68.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 69.Ng, S. P., J. M. Gomez, S. H. Lim, and N. K. Ho. 1998. Reduction of nosocomial infection in a neonatal intensive care unit (NICU). Singapore Med. J. 39:319-323. [PubMed] [Google Scholar]
- 70.Papazian, L., F. Bregeon, X. Thirion, R. Gregoire, P. Saux, J. P. Denis, G. Perin, J. Charrel, J. F. Dumon, J. P. Affray, and F. Gouin. 1996. Effect of ventilator-associated pneumonia on mortality and morbidity. Am. J. Respir. Crit. Care Med. 154:91-97. [DOI] [PubMed] [Google Scholar]
- 71.Perkins, G. D., S. Chatterjee, S. Giles, D. F. McAuley, S. Quinton, D. R. Thickett, and F. Gao. 2005. Safety and tolerability of nonbronchoscopic lavage in ARDS. Chest 127:1358-1363. [DOI] [PubMed] [Google Scholar]
- 72.Pessoa-Silva, C. L., R. Richtmann, R. Calil, R. M. Santos, M. L. Costa, A. C. Frota, and S. B. Wey. 2004. Healthcare-associated infections among neonates in Brazil. Infect. Control Hosp. Epidemiol. 25:772-777. [DOI] [PubMed] [Google Scholar]
- 73.Pineda, L. A., R. G. Saliba, and A. A. El Solh. 2006. Effect of oral decontamination with chlorhexidine on the incidence of nosocomial pneumonia: a meta-analysis. Crit. Care 10:R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pittet, D. 2000. Improving compliance with hand hygiene in hospitals. Infect. Control Hosp. Epidemiol. 21:381-386. [DOI] [PubMed] [Google Scholar]
- 75.Pittet, D., S. Dharan, S. Touveneau, V. Sauvan, and T. V. Perneger. 1999. Bacterial contamination of the hands of hospital staff during routine patient care. Arch. Intern. Med. 159:821-826. [DOI] [PubMed] [Google Scholar]
- 76.Prober, C. G., P. D. Walson, J. Jones, and the Committee on Infectious Diseases and Committee on Drugs. 2000. Technical report: precautions regarding the use of aerosolized antibiotics. Pediatrics 106:e89. [DOI] [PubMed] [Google Scholar]
- 77.Raymond, J., Y. Aujard, and the European Study Group. 2000. Nosocomial infections in pediatric patients: a European, multicenter prospective study. Infect. Control Hosp. Epidemiol. 21:260-263. [DOI] [PubMed] [Google Scholar]
- 78.Richards, M. J., J. R. Edwards, D. H. Culver, R. P. Gaynes, and the National Nosocomial Infections Surveillance System. 1999. Nosocomial infections in pediatric intensive care units in the United States. Pediatrics 103:e39. [DOI] [PubMed] [Google Scholar]
- 79.Riedler, J., J. Grigg, and C. F. Robertson. 1995. Role of bronchoalveolar lavage in children with lung disease. Eur. Respir. J. 8:1725-1730. [DOI] [PubMed] [Google Scholar]
- 80.Rouby, J. J., E. M. De Lassale, P. Poete, M. H. Nicolas, L. Bodin, V. Jarlier, Y. Le Charpentier, J. Grosset, and P. Viars. 1992. Nosocomial bronchopneumonia in the critically ill. Histologic and bacteriologic aspects. Am. Rev. Respir. Dis. 146:1059-1066. [DOI] [PubMed] [Google Scholar]
- 81.Ruza, F., F. Alvarado, R. Herruzo, M. A. Delgado, S. Garcia, P. Dorao, and F. Goded. 1998. Prevention of nosocomial infection in a pediatric intensive care unit (PICU) through the use of selective digestive decontamination. Eur. J. Epidemiol. 14:719-727. [DOI] [PubMed] [Google Scholar]
- 82.Saiman, L., J. Goldfarb, S. A. Kaplan, K. Wible, B. Edge-Padbury, S. Naberhuis-Stehouwer, and J. B. Bruss. 2003. Safety and tolerability of linezolid in children. Pediatr. Infect. Dis. J. 22:S193-S200. [DOI] [PubMed] [Google Scholar]
- 83.Shlaes, D. M., D. N. Gerding, J. F. John, Jr., W. A. Craig, D. L. Bornstein, R. A. Duncan, M. R. Eckman, W. E. Farrer, W. H. Greene, V. Lorian, S. Levy, J. E. McGowan, Jr., S. M. Paul, J. Ruskin, F. C. Tenover, and C. Watanakunakorn. 1997. Society for Healthcare Epidemiology of America and Infectious Diseases Society of America Joint Committee on the Prevention of Antimicrobial Resistance: guidelines for the prevention of antimicrobial resistance in hospitals. Clin. Infect. Dis. 25:584-599. [DOI] [PubMed] [Google Scholar]
- 84.Shorr, A. F., J. H. Sherner, W. L. Jackson, and M. H. Kollef. 2005. Invasive approaches to the diagnosis of ventilator-associated pneumonia: a meta-analysis. Crit. Care Med. 33:46-53. [DOI] [PubMed] [Google Scholar]
- 85.Singh, N., P. Rogers, C. W. Atwood, M. M. Wagener, and V. L. Yu. 2000. Short-course empirical antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am. J. Respir. Crit. Care Med. 162:505-511. [DOI] [PubMed] [Google Scholar]
- 86.Sole, M. L., F. E. Poalillo, J. F. Byers, and J. E. Ludy. 2002. Bacterial growth in secretions and on suctioning equipment of orally intubated patients: a pilot study. Am. J. Crit. Care 11:141-149. [PubMed] [Google Scholar]
- 87.Squire, E. N., Jr., H. M. Reich, G. B. Merenstein, B. E. Favara, and J. K. Todd. 1982. Criteria for the discontinuation of antibiotic therapy during presumptive treatment of suspected neonatal infection. Pediatr. Infect. Dis. 1:85-90. [DOI] [PubMed] [Google Scholar]
- 88.Stover, B. H., S. T. Shulman, D. F. Bratcher, M. T. Brady, G. L. Levine, W. R. Jarvis, and the Pediatric Prevention Network. 2001. Nosocomial infection rates in US children's hospitals’ neonatal and pediatric intensive care units. Am. J. Infect. Control 29:152-157. [DOI] [PubMed] [Google Scholar]
- 89.Tablan, O. C., L. J. Anderson, R. Besser, C. Bridges, R. Hajjeh, CDC, and Healthcare Infection Control Practices Advisory Committee. 2004. Guidelines for preventing health-care-associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. Morb. Mortal. Wkly. Rep. Recomm. Rep. 53:1-36. [PubMed] [Google Scholar]
- 90.Tejerina, E., F. Frutos-Vivar, M. I. Restrepo, A. Anzueto, F. Abroug, F. Palizas, M. Gonzalez, G. D'Empaire, C. Apezteguia, A. Esteban, et al. 2006. Incidence, risk factors, and outcome of ventilator-associated pneumonia. J. Crit. Care 21:56-65. [DOI] [PubMed] [Google Scholar]
- 91.Toltzis, P., C. Hoyen, S. Spinner-Block, A. E. Salvator, and L. B. Rice. 1999. Factors that predict preexisting colonization with antibiotic-resistant gram-negative bacilli in patients admitted to a pediatric intensive care unit. Pediatrics 103:719-723. [DOI] [PubMed] [Google Scholar]
- 92.Toltzis, P., and J. L. Blumer. 2001. Nosocomial acquisition and transmission of antibiotic-resistant gram-negative organisms in the pediatric intensive care unit. Pediatr. Infect. Dis. J. 20:612-618. [DOI] [PubMed] [Google Scholar]
- 93.Toltzis, P., T. Yamashita, L. Vilt, and J. L. Blumer. 1997. Colonization with antibiotic-resistant gram-negative organisms in a pediatric intensive care unit. Crit. Care Med. 25:538-544. [DOI] [PubMed] [Google Scholar]
- 94.Torres, A., R. Aznar, J. M. Gatell, P. Jimenez, J. Gonzalez, A. Ferrer, R. Celis, and R. Rodriguez-Roisin. 1990. Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am. Rev. Respir. Dis. 142:523-528. [DOI] [PubMed] [Google Scholar]
- 95.Trouillet, J. L., J. Chastre, A. Vuagnat, M. L. Joly-Guillou, D. Combaux, M. C. Dombret, and C. Gibert. 1998. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 157:531-539. [DOI] [PubMed] [Google Scholar]
- 96.van Houten, M. A., M. Laseur, and J. L. Kimpen. 1998. Shift in antibiotic prescribing patterns in relation to antibiotic expenditure in paediatrics. Eur. J. Pediatr. 157:479-481. [DOI] [PubMed] [Google Scholar]
- 97.van Nieuwenhoven, C. A., E. Buskens, F. H. van Tiel, and M. J. Bonten. 2001. Relationship between methodological trial quality and the effects of selective digestive decontamination on pneumonia and mortality in critically ill patients. JAMA 286:335-340. [DOI] [PubMed] [Google Scholar]
- 98.Webber, S., A. R. Wilkinson, D. Lindsell, P. L. Hope, S. R. Dobson, and D. Isaacs. 1990. Neonatal pneumonia. Arch. Dis. Child. 65:207-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Won, S. P., H. C. Chou, W. S. Hsieh, C. Y. Chen, S. M. Huang, K. I. Tsou, and P. N. Tsao. 2004. Handwashing program for the prevention of nosocomial infections in a neonatal intensive care unit. Infect. Control Hosp. Epidemiol. 25:742-746. [DOI] [PubMed] [Google Scholar]
- 100.Wunderink, R. G., J. Rello, S. K. Cammarata, R. V. Croos-Dabrera, and M. H. Kollef. 2003. Linezolid vs vancomycin: analysis of two double-blind studies of patients with methicillin-resistant Staphylococcus aureus nosocomial pneumonia. Chest 124:1789-1797. [PubMed] [Google Scholar]
- 101.Yildizdas, D., H. Yapicioglu, and H. L. Yilmaz. 2002. Occurrence of ventilator-associated pneumonia in mechanically ventilated pediatric intensive care patients during stress ulcer prophylaxis with sucralfate, ranitidine, and omeprazole. J. Crit. Care 17:240-245. [DOI] [PubMed] [Google Scholar]