Abstract
The major goals of veterinary vaccines are to improve the health and welfare of companion animals, increase production of livestock in a cost-effective manner, and prevent animal-to-human transmission from both domestic animals and wildlife. These diverse aims have led to different approaches to the development of veterinary vaccines from crude but effective whole-pathogen preparations to molecularly defined subunit vaccines, genetically engineered organisms or chimeras, vectored antigen formulations, and naked DNA injections. The final successful outcome of vaccine research and development is the generation of a product that will be available in the marketplace or that will be used in the field to achieve desired outcomes. As detailed in this review, successful veterinary vaccines have been produced against viral, bacterial, protozoal, and multicellular pathogens, which in many ways have led the field in the application and adaptation of novel technologies. These veterinary vaccines have had, and continue to have, a major impact not only on animal health and production but also on human health through increasing safe food supplies and preventing animal-to-human transmission of infectious diseases. The continued interaction between animals and human researchers and health professionals will be of major importance for adapting new technologies, providing animal models of disease, and confronting new and emerging infectious diseases.
INTRODUCTION
In its original concept, vaccination aims to mimic the development of naturally acquired immunity by inoculation of nonpathogenic but still immunogenic components of the pathogen in question, or closely related organisms. The term “vaccine” (from the Latin term “vacca,” meaning cow) was first coined by Edward Jenner to describe the inoculation of humans with the cowpox virus to confer protection against the related human smallpox virus and illustrates the close relationship between human and animal infectious disease sciences. The criteria for successful animal or veterinary vaccines can be very different from those for human vaccines depending on the animal groups under consideration. For example, criteria for companion animal vaccines are similar to those for human vaccines in that the health and welfare of the individual animal are primary concerns. The main objective of livestock vaccines, on the other hand, is to improve overall production for the primary producers, and the cost-benefit resulting from vaccination is the bottom line for this industry. Vaccination against zoonotic or food-borne infections is aimed at reducing or eliminating the risk for the consumer and in some cases to improve the productivity of the individual animal. Vaccination of wildlife is generally considered only with respect to infections that are transmittable to humans (zoonotic diseases), although welfare concerns are of increasing importance.
While veterinary vaccines comprise only approximately 23% of the global market for animal health products, the sector has grown consistently due mainly to new technological advances in vaccine development, the continuous development of drug resistance by pathogens, and the emergence of new diseases. Apart from improving animal health and productivity, veterinary vaccines have a significant impact on public health through reductions in the use of veterinary pharmaceuticals and hormones and their residues in the human food chain. This will be an increasing impetus for activity with the more stringent requirements of regulatory agencies and consumer groups, particularly in the major markets of Europe and the United States (166). For example, the use of antibiotics in animal production has already been severely restricted, and the European Union has recently banned the use of coccidiostats for poultry. In addition, vaccines contribute to the well-being of livestock and companion animals, and their use is favored by the growing animal welfare lobby.
The process of developing veterinary vaccines has both advantages and disadvantages over human vaccine development. On the one hand, the potential returns for animal vaccine producers are much less than those for human vaccines, with lower sales prices and smaller market sizes, resulting in a much lower investment in research and development in the animal vaccine area than in the human vaccine area, although the complexity and range of hosts and pathogens are greater. For example, the market size for the recently launched human vaccine (Gardasil) against papillomavirus and cervical cancer is estimated to be greater than 1 billion U.S. dollars, while the most successful animal health vaccines (e.g., against foot-and-mouth disease [FMD] virus in cattle and Mycoplasma hyopneumoniae in pigs) enjoy a combined market size that is 10 to 20% of this figure. On the other hand, veterinary vaccine development generally has less stringent regulatory and preclinical trial requirements, which can make up the largest cost in human vaccine development, and a shorter time to market launch and return on investment in research and development. In contrast to human vaccine development, veterinary scientists are also able to immediately perform research in the relevant target species. This is an obvious advantage over human vaccine development, as experimental infections, dose-response studies, and challenge inoculations need not be carried out in less relevant rodent models.
Immunity acquired through natural infection can take on several forms depending on the type and life cycle of the pathogen, as schematically represented in Fig. 1. Vaccines may be used to prevent clinical signs of disease after infection or to help control, eliminate, or even eradicate an infection at the population level (e.g., the expected global eradication of rinderpest virus through vaccination). Both vaccine effectiveness and mechanism of action may vary depending on the required outcome. New technologies to achieve the selective induction of effective immune responses in the development of new vaccines are becoming available to vaccine researchers and have been extensively reviewed in recent papers (33, 136, 163, 166). Notwithstanding the scientific advances, the single factor that determines the success of an experimental vaccine is its successful commercialization and/or use in the field. This outcome requires a combination of basic research, commercial imperatives, local requirements, and global perspectives depending on the particular disease under investigation. These four aspects are represented by the authors of this review, which will concentrate on recent advances in veterinary vaccines that have reached the marketplace or are actively produced by veterinary institutes for use by local farming communities.
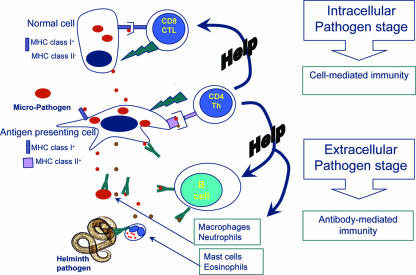
FIG. 1.
Simplified schematic representation of immune mechanisms that can act to protect animals against invading viral, bacterial, and protozoal pathogens or against multicellular helminth parasites. Viral, bacterial, or protozoal pathogens (red ovals) that infect non-antigen-presenting cells can be killed by cytotoxic T cells (CTL) that recognize pathogen-derived epitopes presented in conjunction with major histocompatibility complex (MHC) class I on infected cells or by antibody-dependent lysis or opsonization of infected cells expressing pathogen molecules. Extracellular pathogens, or intracellular pathogens on their way to infect other cells, can be attacked by specific circulating antibodies and either killed by lysis or agglutination or phagocytosed by macrophages and neutrophils. Both antibody and CTL induction requires help from pathogen-specific CD4 helper T cells that are activated after interaction with pathogen-derived epitopes presented in conjunction with MHC class II molecules on the surface of MHC class II+ antigen-presenting cells. If pathogens infect antigen-presenting cells, they can be killed directly by CD4 T cells as well as CD8 CTL through the induction of mediators such as gamma interferon (IFN-γ), reactive oxygen and nitrogen species, and indoleamine 2,3-dioxygenase (IDO). Toxins released by pathogens (red circles) can be neutralized by circulating antibodies, thereby decreasing clinical signs of infection. Multicellular helminth parasites generally do not reside within host cells and are too large to be phagocytosed; therefore, they usually require alternative immune killer mechanisms mediated by antibody-directed actions of mast cells and eosinophils. Essential secreted proteins and toxins derived from the worms (brown circles) may also be neutralized by antibodies and thereby interfere with parasite growth.
VETERINARY VIRAL VACCINES
As there are no broad-spectrum antiviral pharmaceuticals available, hygienic measures to limit exposure and vaccination are the only means to prevent or control viral infections. Viruses (especially RNA viruses) are highly variable, and many viral infections are due to viruses with multiple serotypes (e.g., FMD virus, bluetongue virus, and influenza viruses). As a consequence, many of the existing viral vaccines are often unable to cope with the prevailing strains in the field, and new ones have to be generated from field strains with new outbreaks. Numerous conventional live and inactivated viral vaccines have been produced by animal health companies and have been used for many decades in routine vaccination protocols for both companion and production animals. Increasingly, a number of rationally designed and subunit vaccines are reaching the market, and this section will concentrate mainly on these “second-generation” viral vaccines (summarized in Table 1).
TABLE 1.
Second-generation licensed/commercialized veterinary viral vaccines
| Target pathogen | Target animal | Brand name(s)a | Distributor | Characteristic(s) | Reference(s) |
|---|---|---|---|---|---|
| PCV2 | Pigs | Porcilis-PCV2 | Intervet | Inactivated baculovirus expressed PCV2 ORF2 protein; adjuvanted | 20 |
| PCV2 | Pigs | Suvaxyn PCV2 | Fort Dodge | Inactivated PCV1-2 chimera; adjuvanted | 55 |
| Pseudorabies virus | Pigs | Suvaxyn Aujeszky | Fort Dodge | gE- and thymidine kinase-deleted marker vaccine | 56 |
| Classical swine fever virus | Pigs | Porcilis Pesti | Intervet | Baculovirus recombinant E2 protein without emulsion | 181 |
| Classical swine fever virus | Pigs | Bayovac CSF E2 | Bayer Leverkusen | Baculovirus recombinant E2 protein without emulsion | 116 |
| BHV-1 | Cattle | Bovilis IBR Marker | Intervet | Live or inactivated gE-deleted marker vaccine | 185 |
| Equine influenza virus | Horses | PROTEQ-FLU (European Union), Recombitek (United States) | Merial | Canarypox virus-vectored vaccine | 113 |
| WNV | Horses | PreveNile | Intervet | Live flavivirus chimera vaccine | 114 |
| WNV | Horses | West Nile-Innovator DNA | Fort Dodge | DNA vaccine | 45 |
| WNV | Horses | RECOMBITEKEquine WNV | Merial | Canarypox virus-vectored vaccine | 113 |
| MDV (HTV) and IBDV | Poultry | Vaxxitek HVT+IBD | Merial | Live recombinant chimera virus expressing VP2 gene of IBD on HTV virus | 43 |
| Newcastle disease virus | Poultry | NA | Dow AgroSciences | HN recombinant produced in plant cell lines (registered but not on market) | |
| Newcastle disease virus | Poultry | Vectormune FP-ND | Biomune | Fowlpox virus vectored | |
| Avian influenza virus (H5N1) and NDV | Poultry | Intervet | Chimera virus on NDV backbone; field trials in 2007 | 133, 187 | |
| Avian influenza virus | Poultry | Poulvac FluFend I AI H5N3 RG | Fort Dodge | Chimera H5N3 virus, inactivated in oil-based adjuvant | |
| Avian influenza virus | Poultry | Trovac AI H5 | Merial | Fowlpox virus-vectored H5 | 29 |
| Rabies virus | Wildlife, canines | Raboral | Merial | Vaccinia virus recombinant | 26, 100, 152, 170 |
| Rabies virus | Cats | Purevax Feline Rabies | Merial | Canarypox virus-vectored vaccine | |
| Rabies virus | Cats | PUREVAX Feline Rabies | Merial | Canarypox virus-vectored vaccine | |
| Feline leukemia virus | Cats | EURIFEL FeLV | Merial | Canarypox virus-vectored vaccine | |
| Canine parvovirusl | Dogs | RECOMBITEK Canine Parvo | Merial | Modified live virus | |
| Canine coronavirus | Dogs | RECOMBITEK Corona MLV | Merial | Modified live virus | 132 |
| Canine distemper virus | Dogs | RECOMBITEK rDistemper | Merial | Canarypox virus-vectored vaccine (HA and F antigens) | |
| Canine distemper virus | Fur animals | PUREVAXFerret Distemper | Merial | Canarypox virus-vectored vaccine | |
| IHN virus | Salmon | Apex-IHN | Novartis (Aqua Health) | DNA vaccine |
Brand names may differ between countries. NA, not applicable.
Conventional Live and Inactivated Viral Vaccines
As with the first vaccine for human smallpox, most live veterinary viral vaccines induce mild infections with live organisms derived from nontarget hosts or attenuated through passage in different cell line cultures or chicken embryos (eggs). Attenuated viral strains are also obtained by inducing random mutations and selecting for reduced virulence. As the live organism can still infect target cells, these vaccines can replicate and induce both cellular and humoral immunity and generally do not require an adjuvant to be effective. Live products also offer the advantage of ease of administration, potentially in drinking water, intranasally, intraocularly, etc. However, they can pose a risk of residual virulence and reversion to pathogenic wild types as well as provide a potential source of environmental contamination. Although modern regulatory processes require data to provide assurance on these issues, problems in the field can arise. This was highlighted during a program to control porcine respiratory and reproductive syndrome (PRRS) in Denmark. This disease first emerged in North America in the late 1980s and spread quickly in Europe in the early 1990s. The two main types of PRRS virus, European and North American, are only 55 to 80% identical at the nucleotide level (122) and cause distinguishable serological responses. Following vaccination with the live, attenuated North American PRRS vaccine against the European PRRS virus type present in Denmark in 1996, the vaccine virus reverted and spread within vaccinated herds as well as from vaccinated to nonvaccinated herds, leaving both virus types in the Danish pig population (120).
Despite such drawbacks of live viral vaccines, they have played a major role in successful disease control and eradication. For example, the virtual eradication of rinderpest virus from the globe is widely believed to have been critically dependent on the use of the “Plowright” vaccine (12, 150). This is an attenuated vaccine produced from the Kabete O strain passaged 90 times in tissue culture (141). The vaccine virus was recently found to have attenuating mutations in most of its genes, none of which are sufficiently debilitating to induce strong pressure for reversion (11). Although there are examples of stable attenuations from a single point mutation in the polymerase gene, the high rate of spontaneous mutations of RNA viruses increases the risk for reversion to virulence. Safe live viral vaccines are therefore likely to require a number of attenuating mutations distributed throughout the genome.
Whole inactivated or killed viral vaccines are generally more stable and do not pose the risk of reversion to virulence compared to live vaccines, but their inability to infect cells and activate cytotoxic T cells makes them much less protective. Consequently, they generally require strong adjuvants and several injections to induce the required level of immunity and are usually effective in controlling only clinical signs rather than infection (113). Inactivated adjuvanted vaccines also pose a greater risk of causing autoimmune diseases, allergic disorders, and vaccine injection site sarcomas (46). Viral inactivation is commonly achieved through heat or chemicals (e.g., formaldehyde, thiomersal, ethylene oxide, and β-propriolactone). The higher production cost and need for adjuvants make these vaccines more expensive to manufacture. Inactivated viral vaccines for a wide range of viral diseases have been available for several decades (reviewed in references 113 and 121) and are still being developed for some recently emergent diseases. For example, a one-dose inactivated porcine circovirus type 2 (PCV2) vaccine has recently been licensed in the United States for the prevention of postweaning multisystemic wasting syndrome in pigs (Table 1). Much of the recent research in this area has concentrated on the development of improved adjuvanted formulations to overcome the effects of maternal antibodies on young animals (see, for example, reference 17).
Inactivated vaccines for several viral diseases need to be continuously adapted to contain the appropriate serotypes, as exemplified by equine influenza virus vaccines. Vaccines for equine influenza virus, mostly inactivated, have been available since the 1960s (28). The most important equine subtypes are H7N7 and H3N8, although H7N7 has not been detected for several decades and is no longer included in vaccines, at least in Europe and the United States (130). Conversely, vaccination against H3N8 has been less effective, possibly due to antigenic drift, and there are now considered to be two distinct lineages, European and United States, and vaccines therefore tend to contain both. Over the years, improvements have been attempted, and more potent adjuvants have been used. Several European vaccines now produce high antibody responses that last for up to 1 year (113). Until recently, equine influenza virus vaccines produced in the United States have been considered to be of limited efficacy and sometimes lacking the relevant H3N8 strains (M. Mellencamp and A. Schultze, presented at the Proceedings Quality Control of Equine Influenza Vaccines, Budapest, Hungary, 2001).
DIVA Vaccines
For several viral infections of livestock, effective conventional vaccines are available but cannot be used, as they would interfere with disease surveillance based on serological testing and may result in the loss of a country's disease-free status. A classic example is FMD in cattle. Although inactivated FMD vaccines have been available for many years and are quite effective in controlling clinical disease (49), they are not used in FMD-free countries, as this would compromise this status and hence international trade. Nevertheless, conventional vaccines have reduced the prevalence of disease in enzootic areas, and in a recent outbreak in The Netherlands, vaccination was used to reduce the spread of the disease (142), although the vaccinates were subsequently slaughtered to enable the rapid reestablishment of the FMD-free status of the country.
The ability to identify and selectively delete genes from a pathogen has allowed the development of “marker vaccines” that, combined with suitable diagnostic assays, allow differentiating infected from vaccinated animals (DIVA) by differentiation of antibody responses induced by the vaccine (no antibodies generated to deleted genes) from those induced during infection with the wild-type virus (e.g., see Fig. 2). Such DIVA vaccines and their companion diagnostic tests are now available or in development for several diseases including infectious bovine rhinotracheitis (IBR), pseudorabies, classical swine fever (CSF), and FMD, as detailed below.
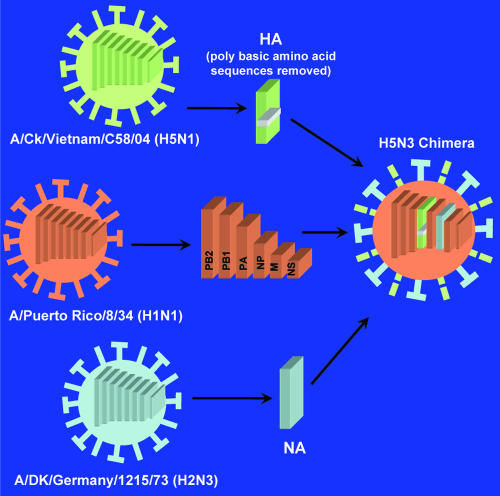
FIG. 2.
Simplified representation of the reverse genetic approach used to construct the chimera vaccine Poulvac FluFend i AI H5N3 RG to protect poultry against the pathogenic H5N1 virus. The HA gene was removed from an H5N1 virus (from a recent Asian outbreak), inactivated by removing the polybasic amino acid sequences, and combined with the NA gene from an H2N3 virus onto an H1N1 “backbone” virus. An immunoassay able to specifically detect antibodies against N3 and N1 proteins could be used for DIVA (i.e., N3+ N1− indicates vaccinated, and N3− N1+ indicates infected). (Modified from Fort Dodge Poulvac FluFend i AI H5N3 RG promotional flyer with permission.)
IBR, caused by bovine herpesvirus type 1 (BHV-1) infection of cattle, and pseudorabies (Aujeszky's disease) in pigs have been identified internationally as being candidates for eradication from national herds, and so there has been an impetus for the development of DIVA vaccines and diagnostics. The demand for a marker (DIVA) vaccine for IBR in Europe was met by the development of a glycoprotein E (gE)-deleted vaccine using conventional methodology (reviewed in reference 185). The gE protein is not essential for viral replication, but it plays a major role in intercellular spread, particularly along nerves. Specific diagnostic tests based on gE deletion have been developed using both gE-blocking enzyme-linked immunosorbent assay (ELISA) techniques and PCR amplification (138, 159).
Deletion of the gE gene has also been used to enable a DIVA approach for an Aujeszky's disease vaccine (137). The gene for thymidine kinase is also deleted in some formulations (e.g., Suvaxyn Aujesky), adding to the degree of attenuation (e.g., see reference 56). These deletion vaccines have been available since the 1980s, and their use has contributed to disease control and eradication in the United States and several European countries (e.g., see reference 24).
CSF is on the World Organization for Animal Health list of notifiable diseases and is one of the most important contagious diseases of pigs worldwide. In its classical clinical form, it is an acute hemorrhagic disease accompanied by high fever, depression, anorexia, and conjunctivitis. Morbidity and mortality are both very high and may reach 100%. However, it can also present as a subacute, chronic, or even subclinical condition. Countries in which the disease is enzootic tend to vaccinate with a very effective live, attenuated vaccine, while those that are free of disease do not (reviewed in reference 13). Two subunit vaccines based on the viral envelope glycoprotein E2 produced in a baculovirus/insect cell system, formulated in a water-in-oil adjuvant, and accompanied by discriminatory ELISA tests are available (116, 181). These vaccines will allow a DIVA approach to emergency vaccination and disease control in the case of new outbreaks, although these have not yet been used widely in the field and appear to be less protective than conventional live, attenuated CSF vaccines (13).
As there are now major doubts about the sustainability of “stamping-out” policies in areas of high animal population density, there is considerable investment in DIVA vaccine approaches for FMD (reviewed in references 8 and 66). Subunit antigen approaches to vaccination have been largely ineffective, as they present only a limited number of epitopes to the animal's immune system, and multiple antigens are generally required for protection. Current research is focused largely on combinations of capsid proteins, including empty capsid delivered by various expression systems, and the development of sensitive tests (ELISA) for antibodies against nonstructural proteins (66). Other diseases for which a DIVA approach is highly desirable but currently unavailable include bluetongue virus in cattle, Newcastle disease virus and avian influenza virus in poultry, bovine viral diarrhea, and equine viral arteritis.
Molecularly Defined Subunit Vaccines
Identification of the protective viral antigens potentially allows their isolation and/or recombinant production so that they can be administered as safe, nonreplicating vaccines. However, as isolated antigens generally induce poor protective immunity, subunit vaccines usually require repeated administration with strong adjuvants, making them less competitive. Notwithstanding these limitations, there are some examples of effective subunit vaccines.
PCV2 is considered to be the major pathogen in the etiology of postweaning multisystemic wasting syndrome (2). A recombinant baculovirus producing the protective ORF2 protein of PCV2 has recently become available as a vaccine for pigs (20).
Dow AgroSciences successfully registered the first plant-based vaccine for Newcastle disease virus in poultry in the United States in 2005. Recombinant viral HN protein was generated in plant cell lines via Agrobacterium transformation and could successfully protect chickens from viral challenge (G. A. Cardineau, H. S. Mason, J. Van Eck, D. D. Kirk, and A. M. Walmsley, 2004, PCT patent application 60/467,998, WO 2004/098533; C. A. Mihaliak, S. Webb, T. Miller, M. Fanton, D. Kirk, G. Cardineau, H. Mason, A. Walmsley, C. Arntzen, and J. Van Eck, presented at the 108th Annual Meeting of the United States Animal Health Association, Greensboro, NC, 2005). This process was a proof-of-concept exercise designed to test regulatory feasibility, and the product is not on the market.
Genetically Engineered Viral Vaccines
The availability of complete DNA sequences and a better understanding of gene function have allowed specific modifications or deletions to be introduced into the viral genome, with the aim of producing well-defined and stably attenuated live or inactivated viral vaccines.
Gene-deleted vaccines (glycoprotein I and/or glycoprotein X) against pseudorabies allowed a DIVA approach and control of Aujeszky's disease in swine (96); however, the potential for recombination between pseudorabies virus strains has raised concern (101). Similarly, thymidine kinase deletion in BHV-1 vaccines has been associated with latency and reactivation after treatment with dexamethasone (194), and deletion of multiple genes has been proposed in order to improve safety (14).
An interesting development in genetically engineered viral vaccines is the production of chimera viruses that combine aspects of two infective viral genomes. A chimera PCV1-2 vaccine has the immunogenic capsid gene of PCV2 cloned into the backbone of the nonpathogenic PCV1 and induces protective immunity to wild-type PCV2 challenge in pigs (55). A further sophistication of this approach is a recently developed vaccine against avian influenza virus (Poulvac FluFend), where the hemagglutinin (HA) gene has been removed from an H5N1 virus, inactivated by removing the polybasic amino acid sequences, and combined with the NA gene from an H2N3 virus onto an H1N1 “backbone” virus (Fig. 2). A vaccine containing the resultant inactivated H5N3-expressing virus administered in a water-in-oil emulsion protects chickens and ducks against the highly pathogenic H5N1 strain.
Similarly, a live Flavivirus chimera vaccine against West Nile virus (WNV) in horses (PreveNile) was registered in the United States in 2006. In this chimera vaccine, the structural genes of the attenuated yellow fever YF-17D backbone virus have been replaced with structural genes of the related WNV. The resulting chimera vaccine express the PreM and E proteins of WNV, while the nucleocapsid (C) protein, nonstructural proteins, and nontranslated termini responsible for virus replication remain those of the original yellow fever 17D virus (114). After a single shot, the vaccine stimulates both cell-mediated and humoral responses without causing any clinical illness or spreading to sentinel horses and provides protection against WNV challenge for up to 12 months (PreveNile package insert). A similar vaccine could be a candidate for a human WNV vaccine (115).
Live Viral Vector Vaccines
Poxviruses including vaccinia virus, fowlpox virus, and canarypox virus have been used as vectors for exogenous genes, as first proposed in 1982 (131), both for the delivery of vaccine antigens and for human gene therapy. Poxviruses can accommodate large amounts of foreign genes and can infect mammalian cells, resulting in the expression of large quantities of encoded protein. For example, modified vaccinia virus Ankara is a highly attenuated strain produced by several hundred passages of the virus in chicken cells. Modified vaccinia virus Ankara lacks about 10% of the vaccinia virus genome, including the ability to replicate in mammalian cells (reviewed in reference 139).
A particular success story has been the development of an oral recombinant vaccinia-rabies vaccine in bait for wild carnivores such as foxes in Europe (26) and foxes, raccoons, and coyotes in the United States (152, 170). Rabies is caused by a negative-stranded Rhabdoviridae RNA virus transmitted mainly via saliva following a bite from an infected animal. The main source of infection for humans is domestic reservoir species including dogs and cats. There are seven rabies virus genotypes, all of which, excluding type 2, produce similar effects in humans. Rabies can infect most if not all mammals. The virus enters the central nervous system, causing an encephalomyelitis that is always fatal once symptoms develop. Worldwide, the disease causes many thousands of human deaths each year. One type of oral vaccine is in the form of a bait containing a recombinant vaccinia virus vector expressing the protective glycoprotein G of rabies virus (100, 135). After several years of vaccination campaigns against fox rabies virus in several Western European countries, rabies could be eliminated from its wildlife terrestrial reservoir, as exemplified by the successful elimination of terrestrial rabies virus from Belgium and France (26, 135, 136).
The canarypox virus vector system ALVAC has been used as a platform for a range of veterinary vaccines including WNV, canine distemper virus, feline leukemia virus, rabies virus, and equine influenza virus (176) (Table 1). Canarypox virus was originally isolated from a single pox lesion in a canary and serially passaged 200 times in chicken embryo fibroblasts and serially plaque purified under agarose (Merial bulletin TSB-4-0019-FTB). Canarypox viruses and fowlpox viruses have the advantage of being more host restricted than vaccinia virus. While they produce an abortive infection in mammalian cells, canarypox virus recombinants still effectively express inserted foreign genes. Several veterinary viral vaccines have been produced using the ALVAC vector system (Table 1). Most notably, a novel equine influenza virus vaccine using the canarypox vector to express the hemagglutinin genes of the H3N8 Newmarket and Kentucky strains has recently been registered in the European Union (Proteq-Flu) (113) and the United States (Recombitek). It contains a polymer adjuvant (Carbopol; Merial Ltd.), and through the induction of both cell-mediated and humoral immunity, it is claimed to produce sterile immunity 2 weeks after the second of two doses. The new vaccine is also designed to protect horses against the highly virulent N/5/03 American strain of equine influenza virus and to prevent the virus from spreading through the elimination of viral shedding.
Trovac AI H5 is a recombinant fowlpox virus expressing the H5 antigen of avian influenza virus. This product has had a conditional license for emergency use in the United States since 1998 and has been widely used in Central America, with over 2 billion doses administered (29). As the vaccinated birds will not develop antibodies against matrix protein/nucleoprotein, this vaccine can also be used with a DIVA approach.
Several vaccines are available based on inactivated adjuvanted formulations for equine herpesvirus type 1 and equine herpesvirus type 4, equine herpesviruses that are major causes of abortion and respiratory disease. None of these vaccines are considered to provide complete clinical or virological protection (113). A canarypox virus-vectored vaccine containing the genes for gB, gC, and gD has been developed, but the latest reports suggested that it did not completely protect against challenge (113).
A further application of vectored vaccines is the use of an attenuated viral pathogen as the vector, with the aim of inducing protection against two diseases, as with the live recombinant vaccine against both Marek's disease virus (MDV) and infectious bursal disease virus (IBDV) in chickens (Vaxxitek HVT + IBD). MDV is a highly contagious neoplastic disease of poultry caused by gallid herpesvirus type 2, while IBDV replicates in the bursa of Fabricius, the primary lymphoid organ in birds, and causes a serious immunosuppressive condition in poultry flocks worldwide. Turkey herpesvirus (HVT) is nonpathogenic in chickens but confers cross-protection against MDV and has traditionally been used in live vaccines against MDV. The new vaccine is based on a recombinant parent HVT virus expressing the VP2 gene of IBDV (44) and can be given to embryonated eggs or 1-day-old chicks without interference from maternally derived antibodies. Data from large-scale field trials for this vaccine have not yet been reported, but those studies may encounter difficulties in maintaining high efficacy. This is because these tightly regulated recombinant vaccines cannot easily adapt to meet the emergence of very virulent strains of both IBDV and MDV, apparently induced by the comprehensive numbers of vaccinations performed against these diseases (81, 146).
Chimera avian influenza virus vaccines have also recently been produced on a backbone of an existing, attenuated Newcastle disease virus vaccine strain. Both Asian H5N1 and the pathogenic H7N7 strain, responsible for the chicken influenza virus outbreak in The Netherlands in 2003, were produced as chimeras with the Newcastle disease virus strain. This chimera vaccine induced strong protection against the respective wild-type influenza virus as well as against Newcastle disease virus (133, 187).
DNA Vaccines
Immunization of animals with naked DNA encoding protective viral antigens would in many ways be an ideal procedure for viral vaccines, as it not only overcomes the safety concerns of live vaccines and vector immunity but also promotes the induction of cytotoxic T cells after intracellular expression of the antigens. Furthermore, DNA vaccines are very stable and do not require a cold chain. While DNA vaccination of large animals has not been as effective as initially demonstrated in mice, several groups have obtained significant improvements in immune responses using innovative technologies such as specific targeting of the vaccine antigen to antigen-presenting cells (85), priming-boosting with stimulating CpG oligodeoxynucleotides (85, 97), and in vivo electroporation of DNA (155).
Considerable research into DNA vaccines for fish viruses, where this approach seems to be particularly effective (73, 99), has been ongoing. Notably, the first DNA vaccine for an edible species (Apex-IHN) was registered in 2005 in Canada to protect Atlantic salmon from infectious hematopoietic necrosis (IHN) (167). IHN disease is enzootic in wild salmon populations and can cause devastating outbreaks in farm-raised salmon that have had no prior exposure. The DNA vaccine encodes a surface glycoprotein of IHN virus and is administered intramuscularly (99).
A DNA vaccine to protect horses against viremia caused by WNV (West Nile-Innovator DNA) received a license from the USDA at approximately the same time as the fish DNA vaccine. WNV infection is caused by a flavivirus belonging to the Japanese encephalitis virus complex. It is enzootic in parts of Africa and Asia but was first detected in the United States in 1999 in an outbreak involving birds, horses, and humans in New York, and it subsequently spread rapidly to many states (62). The DNA plasmid codes for the WNV outer coat proteins and is administered with a proprietary adjuvant (143). The vaccine was, however, produced as part of a building-platform technology rather than as a commercial product, as the manufacturer already has a WNV vaccine on the market.
The success of these two DNA vaccines may be due more to good fortune than to any specific technological advances, as DNA uptake into fish muscle seems to be unusually efficient (99), and the WNV viral protein may be particularly effective because it naturally produces highly immunogenic virus-like particles (161). It is likely that a wider application of DNA vaccines will require further improvements and optimization for each host-pathogen combination.
VETERINARY BACTERIAL VACCINES
Many attenuated live or inactivated (killed) bacterial vaccines have been available for decades as prophylaxis against bacterial diseases in veterinary medicine. For most of the attenuated bacterial strains, the nature of the attenuation is not known, and since they have a proven track record, little is done to characterize underlying genetics. In some cases, however, the old and well-recognized live strains are not highly protective, and continued research is being performed to improve and develop new vaccines or vaccination strategies against, e.g., bovine tuberculosis, paratuberculosis, and brucellosis, as described below. Inactivated vaccines generally consist of bacterins of one or more bacterial species or serotypes (i.e., crude formalin-killed whole bacterial cultures and supernatants) or more well-defined subunit antigens formulated most often in an oil or aluminum hydroxide adjuvant.
Many of the established bacterial vaccines are highly efficacious, but since the technology has been available for many years, these conventional vaccines will not be dealt with in this review, and the reader is referred to company websites for information on specific diseases and vaccines. Both live and inactivated autogenous bacterial vaccines are also produced by local veterinary institutions or specialized companies for on-farm specific demands where no commercial vaccines are available.
This section will review some of the more recent additions to bacterial veterinary vaccines (summarized in Table 2), with particular focus on the more molecularly defined vaccines.
TABLE 2.
Recently commercialized veterinary bacterial vaccines
| Target pathogen(s) | Target animal | Brand name | Distributor | Characteristic(s) | Reference(s) |
|---|---|---|---|---|---|
| Lawsonia intracellularis | Pigs | Enterisol Ileitis | Boehringer-Ingelheim Vetmedica | Live oral vaccine | 67, 90 |
| Porphyromonas gulae, P. denticanis, and P. salivosa | Dogs | Periovac | Pfizer Animal Health | Killed vaccine against periodontitis | |
| Yersinia ruckeri | Fish | AquaVac ERM | Schering-Plough Animal Health | Killed oral vaccine | 64 |
| Aeromonas salmonicida | Fish | AquaVac Furuvac | Schering-Plough Animal Health | Killed oral vaccine | |
| Vibrio anguillarum | Fish | AquaVac Vibrio | Schering-Plough Animal Health | Killed oral vaccine | |
| Streptococcus equi | Horses | Equilis StrepE | Intervet | Live submucosal vaccine; deletions in aroA gene | 80 |
| Chlamydophila abortus | Sheep | Ovilis Enzovax | Intervet | Live temperature-sensitive mutant strain for subcutaneous or intramuscular injection | 34 |
| Mycoplasma synoviae | Chickens | Vaxsafe MS | Bioproperties | Live temperature-sensitive mutant strain; eye drop administration | 119 |
| Mycoplasma gallisepticum | Chickens | Vaxsafe MG | Bioproperties | Live temperature-sensitive mutant strain; eye drop administration | 10 |
| Bordetella avium | Turkeys | Art Vax | Schering-Plough Animal Health | Live temperature-sensitive mutant strain; spray inhalation or drinking water | 79 |
| Actinobacillus pleuropneumoniae | Pigs | PleuroStar APP | Novartis Animal Health | Recombinant ApxII, TbpB, CysL, OmlA(1), and OmlA(2) proteins | 186 |
| Actinobacillus pleuropneumoniae | Pigs | Porcilis APP | Intervet | Extracted ApxI, ApxII, ApxIII, and outer membrane proteins | 35 |
| Salmonella | Chickens and hens | Megan Vac1 MeganEgg | Lohman Animal Health International | Double gene-deleted S. enterica serovar Typhimurium strain | 5 |
| Brucella abortus | Cattle | RB-51 | Colorado Serum Company CZ Veterinaria | Spontaneous rifampin-resistant rough mutant | 118 |
Conventional Live Vaccines
In spite of modern technological advances, new live vaccines based on strains without identification of the attenuating characteristics continue to reach the market. One such example is a new live vaccine (Enterisol Ileitis) against porcine proliferative enteropathy caused by the obligate intracellular bacterium Lawsonia intracellularis. Identification of L. intracellularis as the cause of this disease was established in 1993 (107), and many features of the causal bacteria as well as the immunopathogenesis remain to be elucidated. The vaccine strain was cultivated from a clinical isolate, and there are no phenotypic or genotypic characteristics to separate this strain from wild-type strains. Following oral administration of the vaccine, there appear to be no or delayed fecal shedding of bacteria and a low or absent induction of systemic humoral or cell-mediated immunity, but fecal shedding upon challenge is reduced and weight gain is increased compared to unvaccinated pigs (67, 90). The vaccine has been licensed to improve weight gain and to reduce growth variability associated with ileitis in pigs and is administered through drinking water.
Conventional Inactivated Vaccines
An interesting new addition to the repertoire is a vaccine consisting of inactivated bacterins of Porphyromonas gulae, P. denticanis, and P. salivosa for vaccination against periodontal disease in dogs (Periovac). The vaccine is based on research identifying these three bacteria as being the most common black-pigmenting anaerobic bacteria in periodontal pockets of dogs and were all pathogenic in a mouse model (70). A vaccine prepared from P. gulae and administered subcutaneously to mice was able to significantly reduce alveolar bone loss in this model (71). There are no published details on the performance of the trivalent vaccine in dogs, but efficacy and potency trials are ongoing with, e.g., a clinical trial running at the University of Minnesota Veterinary Medical Center. Despite the lack of published efficacy data, the vaccine is currently fully licensed in New Zealand and conditionally licensed in the United States.
A killed oral vaccine (AquaVac ERM) against enteric redmouth disease caused by Yersinia ruckeri in rainbow trout has been available in United Kingdom since 2001 and is now further approved for a number of European countries. Enteric redmouth disease is a serious infectious disease of farmed rainbow trout in many countries characterized by congestive or hemorrhagic zones in various tissues and organs, particularly around the mouth and in the intestines. The disease has a very high mortality rate, and Y. ruckeri is able to form biofilms on fish tank surfaces and thus persist and remain infective in the aquatic environment, with the possibility of recurrent infections (39). Immersion of fry for 30 s into a vaccine soup at the hatchery provides initial protection for fingerlings but rarely lasts throughout the production cycle. Follow-up booster vaccination by injection is effective, but this is time-consuming and labor-intensive and may be stressful for the handled fish. The oral vaccine protocol recommends a primary immersion vaccination followed 4 to 6 months later by an oral booster of the vaccine, which is mixed and absorbed into the feed pellets. Both the primary and booster vaccine formulations are inactivated bacterial cultures, but for oral vaccination, the bacteria are incorporated into an “antigen protection vehicle,” bypassing the acidic environment of the gut and delivering the antigens to the area of the hindgut (64). There are no data available on the nature of the antigen protection vehicle, but the product résumé claims the presence of lecithin and fish oil, indicating that killed bacteria are likely incorporated into liposome structures (61). Similar vaccines against furunculosis caused by Aeromonas salmonicida and vibriosis caused by Vibrio anguillarum have also been developed, and an oral vaccine against infectious pancreatic necrosis virus is registered in Chile for use in salmon. To our knowledge, these are the only licensed inactivated mucosal vaccines against bacterial diseases in veterinary medicine.
Gene-Deleted Vaccines
Traditionally, attenuation of bacteria for the preparation of live vaccines has been performed by multiple passages in various media in the hope that some random mutation would deliver a nonvirulent, but replicable, type of the agent. With currently used molecular methods, the obtained deletions/mutations can be identified, but this technology also allows a more targeted design of live vaccines with specific deletions of predetermined known genes. Good targets for these deletions are genes responsible for key metabolic processes that inhibit the spread of the infection but allow the development of immune responses against virulence factors. Alternatively, deletions of virulence-associated genes are targets, but this may be more problematic when a protective immune response is desired.
Gene-deleted vaccines have been produced against strangles, a highly contagious disease in horses caused by infection with Streptococcus equi subsp. equi. The disease is characterized by fever, profuse nasal discharge, and abscess formation in the lymph nodes of the head and the neck. The pus discharged from bursting abscesses is highly infectious, and the swelling of involved lymph nodes may, in severe cases, cause airway restriction, hence the name. Commercial bacterin or protein extract vaccines for parenteral administration can induce high levels of serum bactericidal antibodies, but the protective effects of these antibodies are questionable (177), and the protective efficacy of inactivated vaccines in the field has been disappointing (174). A live intranasal vaccine based on a nonencapsulated attenuated strain (Pinnacle IN) has been widely used in North America since it was launched in 1998. However, the attenuating mutations of this strain have not been defined, and the vaccine strain sometimes reverts to an aggressive mucoid phenotype indistinguishable from that of wild-type strains. The Pinnacle strain has since been refined into a more stable hyaluronate synthase-defective mutant (191). It is, however, not clear if this new strain has replaced the original vaccine strain in the commercial product. Recently, the Equilis StrepE vaccine, a live recombinant bacterial vaccine prepared from the S. equi TW928 deletion mutant lacking bp 46 to 978 of the aroA gene (80, 84), was licensed in Europe. This mutant was constructed by the electroporation of gene knockout and gene deletion constructs. No foreign DNA such as antibiotic resistance markers was introduced, but the vaccine strain can allegedly be identified by an aroA PCR identifying the partial gene deletion (84). The live gene-deleted attenuated vaccine strain was originally developed for intranasal application, but protection was accomplished only by intramuscular injections, which in turn resulted in the local swelling of muscle tissue and the eventual formation of abscesses at the vaccination site (80). However, submucosal administration of the vaccine in the upper lip was shown to confer protection comparable to that of intramuscular administration but with only minimal local reactions (80), and it is with this unusual route of administration that the vaccine is now licensed.
Chlamydiae are obligate intracellular bacteria with a wide host range and with a wide spectrum of diseases, several of which are zoonotic. The most important veterinary species are Chlamydophila psittaci, causing respiratory infections in poultry (psittacosis/ornithosis), and Chlamydophila abortus (formerly Chlamydia psittaci serotype 1), causing ovine enzootic abortion, one of the most important causes of ovine and caprine abortion worldwide. Both infections are zoonotic. While no vaccines are available for birds and poultry, inactivated vaccines against ovine enzootic abortion have been available for many years (reviewed in reference 145). More recently, a temperature-sensitive mutant strain, TS1B, of the C. abortus reference strain AB7 obtained by nitroguanidine mutagenesis (148) is used to prevent abortion in sheep (Ovilis Enzovax). The temperature-sensitive mutant strain has an optimal growth temperature at 38°C, but at the restrictive temperature, 39.5°C, growth is impaired. The normal body temperature of adult sheep is 38.5 to 40.0°C. The vaccine induces good and long-lasting protection in sheep (34), goats (149), and mice (even though the body temperature of mice is within the permissive growth range). However, the vaccine is licensed only for sheep, not goats, and there is continued research into the development of an effective subunit-based vaccine (59, 190). Temperature-sensitive-mutant vaccines have also been developed and marketed as eye drop, spray, or inhalation vaccines. These include vaccines against Mycoplasma synoviae and M. gallisepticum in chickens (Vaxsafe MS and Vaxsafe MG, respectively) (10, 119) and Bordetella avium rhinotracheitis (coryza) in turkeys (Art Vax) (79).
Gene-deleted live bacteria with well-defined targeted attenuations also offer an attractive option as mucosal vectors for passenger antigens, with many potential advantages over traditionally injectable vaccines. Unlike viral vaccines, there are at present no commercialized vector vaccines based on a bacterial backbone carrier delivering antigens from other pathogens, although several bacterial vectors have shown very promising results (reviewed in references 61 and 108).
Subunit Vaccines
Porcine contagious pleuropneumonia is a widespread and severe disease of pigs with hemorrhagic necrotizing pneumonia and high mortality in the acute form. The disease is caused by Actinobacillus pleuropneumoniae, and prevention by vaccination with whole-cell bacterin vaccines has been severely restricted by the prevalence of 15 different serotypes. A second generation of acellular A. pleuropneumoniae subunit vaccines has been developed with four extracted (Porcilis APP) or five recombinant (PleuroStar APP) proteins, which confer some degree of cross-protection against all serotypes. Most of the pathological consequences of A. pleuropneumoniae infection are caused by pore-forming RTX (repeats in the structural toxin) exotoxins ApxI, ApxII, ApxIII, and APxIV, of which at least a combination of two are expressed in all serotypes. The serotypes expressing both ApxI and ApxII are particularly virulent (58). Vaccination with RTX toxins alone protects against mortality but does not reduce the typical lung lesions. The pentavalent recombinant vaccine containing only the ApxII toxin but supplemented with other common antigens such as transferring-binding proteins appears to provide protection that is at least as good as or better than that of the vaccine with three extracted Apx toxins supplemented with a single outer membrane protein (35, 69, 186). However, the precautions needed when such subunit vaccines are to be designed is evidenced by a study of vaccination against PalA of the peptidoglycan-associated protein family and the most “immunopredominant” outer membrane protein of A. pleuropneumoniae (and related to, e.g., the P6 protein of Haemophilus influenza). Antibodies induced against PalA alone aggravated the consequences of a challenge infection, and PalA vaccination in combination with RTX toxins even counteracted the protective effect of anti-ApxI and anti-ApxII antibodies (182).
Vaccines against Zoonotic Bacteria
Clinical salmonellosis in animals is often due to host-restricted serotypes such as Salmonella enterica serovar Choleraesuis in pigs, Salmonella enterica serovar Gallinarum in poultry, and Salmonella enterica serovar Dublin in young cattle with severe systemic infections, which may result in the death of the animal. In contrast, non-host-specific Salmonella serotypes usually induce a self-limiting gastrointestinal infection but with the capability of causing systemic infections in a wide range of host animals, including humans. The desired immunity of vaccines against zoonotic infections not only requires the induction of a local mucosal immunity, preventing colonization of the gut of the individual animal, but should ideally also prevent or eliminate the presence of the bacteria in the flock as a whole to prevent cross-contamination of meat products at the slaughterhouse. This is a very difficult task, and available vaccines have so far yielded variable success rates (reviewed for poultry in reference 184).
It is generally recognized that cell-mediated immunity is more important than humoral responses in protection against Salmonella, and together with the need for local mucosal immunity, this calls for live, attenuated vaccines as the most effective type. This is supported by a comparison of a live double-gene-deleted Salmonella enterica serovar Typhimurium vaccine (MeganVac 1) with an inactivated Salmonella enterica serovar Enteritidis vaccine (Poulvac SE), which showed reduced fecal shedding following live vaccination, while chickens receiving a killed vaccine experienced inhibited cell-mediated immune responses, enhanced antibody responses, and an increased bacterial load (5). The MeganVac 1 organism has recently been reformulated for immunization of laying hens (MeganEgg). The Megan vaccines for broilers and hens were licensed by the USDA in 1998 and 2003, respectively, but worldwide, there are at least 10 other live Salmonella vaccines available for Salmonella enterica serovar Enteritidis, Salmonella enterica serovar Typhimurium, or Salmonella enterica serovar Gallinarum infection in poultry.
Campylobacter jejuni is one of the most important causes of food-borne human bacterial gastroenteritis. Although several vaccines aimed at preventing human disease are in the pipeline, an effective vaccination intervention strategy for infected poultry flocks would be the most effective means of preventing human disease. Similar to the requirements of a vaccine against non-host-specific Salmonella serotypes, such a vaccine must, however, be able to provide a very high degree of protection in the flock to eliminate the subsequent contamination of meat products. Experimental vaccines, mainly killed whole-cell cultures or flagellum preparations, have been tested in poultry but provide only partial protection against a challenge with Campylobacter, and the development of an attenuated live strain may be more promising although not yet commercially available.
Brucellosis continues to be a major zoonotic threat to humans and a common cause of animal disease, especially in developing countries. In many industrialized countries, a test-and-slaughter policy has been effective for the eradication of the disease, while vaccines, although providing a fairly high level of protection, also induce antibodies that interfere in subsequent surveillance programs. Numerous attempts to produce a protective killed vaccine have so far been disappointing, and the most successful vaccines against brucellosis have been those employing live, attenuated Brucella spp. (158). Of these, Brucella abortus strain 19 (first described in 1930) and Brucella melitensis Rev.1 (first described in 1957) vaccines have been widely used in cattle and in small ruminants, respectively. The S19 and Rev.1 vaccines are, however, far from perfect, as absolute protection is not achieved, allowing for subclinical carrier animals, and both strains have retained some virulence and may induce abortions with variable frequency. Furthermore, both of these vaccines are infectious for humans (Rev.1 is also resistant to streptomycin), and they will induce antibodies against smooth lipopolysaccharide, making them incompatible with test-and-slaughter procedures in countries with an ongoing eradication program. More recently, a vaccine based on a stable spontaneous rifampin-resistant rough mutant of B. abortus, named RB-51 (157), has replaced S19 in many countries including the United States. RB-51 carries IS711 inserted into the wboA glycosyl transferase gene (188), but experimental data with other wboA mutants indicate that additional unknown defects are carried in this strain (118). Rough strains do not carry smooth lipopolysaccharide, and therefore, vaccination with RB-51 does not induce antibodies that are detectable in routine serological tests. This is an obvious advantage in many cases but may also result in the late diagnosis of accidental human infections, although RB-51 appears to be much less virulent for humans than S19 and Rev.1 vaccines (3). At present, several million animals have been vaccinated with the RB-51 mutant strain, but the protective efficacy in cattle compared to S19 remains controversial (118, 124, 158), and the protection against Brucella suis in pigs (172) and against B. abortus in elk (89) is very limited, if present at all.
Rickettsia Vaccines
The rickettsiae Ehrlichia, Anaplasma, and Coxiella are all small obligate intracellular pathogens that cause significant animal diseases. With the exception of Coxiella, all are transmitted by arthropod vectors (e.g., ticks, mites, lice, or fleas).
Heartwater is the most important tick-borne disease of domestic and wild ruminants in sub-Saharan Africa and the West Indies, which is caused by Ehrlichia ruminantium. The only commercially available vaccination procedure is based on the controlled infection of animals with cryopreserved infected sheep blood, followed by antibiotic treatments with tetracyclines when fever develops. A nonvirulent strain has recently been generated through in vitro cultivation and shown to confer good protection (198). Progress in developing cost-effective in vitro cultivation processes may lead to the development of inactivated vaccines (103).
Bovine anaplasmosis is another tick-borne disease caused by Anaplasma marginale infection of red blood cells. Transmission can occur by mechanical means via blood contamination or through blood-sucking arthropods and transplacentally from cow to calf. Cattle that survive acute infection are resistant to the disease but develop persistent, cyclic, low-level infections and therefore remain as “carriers.” Calves are less susceptible to infection and clinical disease than adult cattle. Infected blood containing a less pathogenic isolate or subspecies of A. marginale, generally referred to as Anaplasma centrale, remains the most widely used live vaccine in Africa, Australia, Israel, and Latin America. Infection with A. marginale followed by treatment of the patent infections with low doses of tetracycline drugs has also been used but requires close supervision for timely treatment. Following large-scale production of A. marginale antigen from infected bovine blood, a killed vaccine was effectively marketed and used in the United States until withdrawal in 1999 (88). Apart from its higher cost and need for yearly boosters, the killed vaccine was generally less effective in inducing protective immunity than live vaccines.
VETERINARY PARASITE VACCINES
Protozoal Vaccines
Protozoal infections in animals cause significant production losses and are a major impediment to the introduction of high-productivity breeds in poorer, mainly tropical areas around the world. Many also cause zoonotic diseases in humans or have close relationships to human parasites, increasing their significance as infection reservoirs or animal models for human diseases. While no vaccines for human protozoa are available as yet, several veterinary vaccines have been on the market or have been produced by agriculture/veterinary departments for local use for many decades. Most of these vaccines are based on live organisms; however, an increasing number of killed subunit vaccines have been developed and commercialized in recent years. The following overview will exemplify currently used protozoal vaccines according to increasing sophistication of vaccine production. A list of currently used protozoal vaccines is provided in Tables 3 and 4.
TABLE 3.
Available veterinary live protozoal vaccines
| Pathogen(s) | Host | Brand name(s) | Distributor(s) | Characteristic(s) | Referencea |
|---|---|---|---|---|---|
| Eimeria spp. | Poultry | Coccivac, Immucox, Paracox, Advent, Nobilis Cox ATM | Shering-Plough, Vetech Labs, Novus International, Intervet | Sporulated oocysts of several or all of the avian species | 164 |
| Eimeria spp. | Poultry | Inovocox | Embrex | In ovo delivery using proprietary platform injection system | 164 |
| E. tenella | Poultry | Livacox | BIOPHARM | Precocious and egg-passaged lines | 196 |
| Theileria parva | Cattle | Centre for Ticks and Tick-borne Disease, Malawi | Infection followed by drug treatment | 23 | |
| Theileria annulata and T. hirci | Cattle | Local veterinary institutes | Culture-derived schizonts | 165 | |
| Toxoplasma gondii | Sheep | Ovilis Toxovax | Intervet | S48 strain with a lost ability to form cysts after passages in mice | 30 |
| Babesia bovis and B. bigemina | Cattle | Local veterinary institutes | Infected blood from splenectomized calves | 48 |
See also company websites.
TABLE 4.
Available veterinary killed/subunit protozoal vaccines
| Pathogen(s) | Host | Brand name(s) | Distributor(s) | Characteristic(s) | Reference or sourcea |
|---|---|---|---|---|---|
| Neospora caninum | Cattle | Bovilis, Neoguard | Intervet | Killed tachyzoites, reduces abortion | Heuer et al.,b151 |
| Giardia duodenalis | Dogs | Giardiavax | Fort Dodge | Cultured trophozoites, reduces disease and cyst shedding | 128 |
| Sarcocystis neurona | Horses | Epm vaccine | Fort Dodge | In vitro-cultured merozoites, chemically inactivated | 104 |
| Babesia canis | Dogs | Pirodog and Nobivac Piro | Merial and Intervet (respectively) | In vitro-cultured supernatant antigens, reduce clinical disease | 117, 156 |
| Leishmania donovani | Dogs | Leishmune | Fort Dodge | Native fucose-mannose-ligand antigen complex | 21 |
| Eimeria maxima | Poultry | CoxAbic | Novartis AH | Gametocyte antigen(s); transmission blocking through maternal antibody transfer | 192 |
See also company websites.
C. Heuer, C. Nicholson, D. Russell, and J. Weston, presented at the 19th International Conference of the World Association for the Advancement of Veterinary Parasitology, New Orleans, LA, 2003.
Live protozoal parasite vaccines.
Protozoal parasites have a high degree of genetic complexity. The difficulty of vaccine development for these organisms is further exacerbated by the antigenic diversity displayed by their different life cycle stages within the host as well as between different species and strains and, in the case of hemoprotozoal parasites, even within the same life cycle stage. While most protozoal infections induce various degrees of immunity after previous infections, the immunological mechanisms involved in protection and the stages involved have mostly not been defined. It is therefore not surprising that most vaccines make use of the live organism itself to elicit the required protective immune response. Depending on the characteristics of infection, these vaccines can take several formats, as discussed below.
(i) Vaccines based on complete life cycle infections.
Vaccination with low doses of infective organisms has been used extensively in the poultry industry to combat coccidiosis, the major economic parasitic disease of poultry worldwide. Coccidiosis in poultry is caused by the obligate intracellular protozoal parasite Eimeria species, which undergoes a defined number of asexual cycles of merozoite production in gut epithelial cells (three to four merogenic cycles) before the final sexual stages develop and produce the infective oocysts. The infection is therefore self-limiting, and vaccination with small doses of oocysts, while producing minimal pathology, induces solid protection against homologous challenge. More recently developed live vaccines contain oocysts selected from naturally occurring “precocious” Eimeria strains that produce less merogenic cycles and are therefore safer to use. Although there are many problems with this kind of vaccine, including the need for simultaneous administration to prevent infection of susceptible birds by vaccine-produced oocysts and species- and strain-specific immunity, live coccidian vaccines have been used successfully for over 50 years and are produced as a commercial product by many animal health companies (Table 3) (reviewed in reference 164). The commercial success lies primarily in breeder and layer flocks where anticoccidial drugs have to be withdrawn to prevent the carryover of drugs into eggs.
(ii) Vaccines based on drug-abbreviated infections.
Hemoprotozoal parasite infections are not self-limiting, and parasites can proliferate continuously in the blood stages if not checked by the immune response or drug treatment. In contrast to most hemoprotozoal pathogens, including the closely related Theileria annulata, Theileria parva causes a highly fatal disease in cattle by transforming infected lymphocytes, while the erythrocyte stage of this parasite is much less pathogenic. Solid, sterile immunity develops after primary infection, and vaccination of cattle by infection with pathogenic wild-type T. parva followed by drug treatment (long-acting tetracyclines) has been used for many years to control East Coast fever. This vaccination regimen confers solid protection against homologous challenge and limited protection against heterologous challenge but is expensive to administer. Resistance is thought to be conferred mainly by cell-mediated immunity, more specifically, CD8+ cytotoxic T cells, against the intracellular schizont (106), and the targets of the protective cytotoxic-T-lymphocyte (CTL) response are currently being defined (65).
(iii) Vaccines based on infections with parasites with a truncated life cycle.
Several protozoal parasites produce cysts within the host that are a persistent source of infection when eaten by carnivores. These cysts can also cause reinfection when the immune system is compromised or can be reactivated during pregnancy, causing congenital disease and abortion. Toxoplasma gondii infects a wide variety of hosts, including humans, and is the major cause of abortion in sheep and goats. As immunity to primary infection develops, the intracellularly replicating tachyzoites become encysted in a dormant stage (zoitocysts), which can persist for several years, containing hundreds of infective bradyzoites. T. gondii parasites that were continuously passaged in mice to produce diagnostic antigens were later found to have lost their ability to form cysts. The “incomplete” S48 strain of T. gondii now forms the basis of a commercial vaccine conferring long-lasting immunity (∼18 months) of susceptible ewes against Toxoplasma-induced abortion when administered prior to mating (30).
(iv) Vaccines based on infection with virulence-attenuated strains.
Continuous passage of the tick-borne piroplasms Babesia bovis and Babesia bigemina in splenectomized calves was shown to result in attenuated infections while still inducing immunity in young calves. Live vaccines using infected blood collected from acute infections of splenectomized calves were developed in Australia several decades ago (31, 41) and are still used in most countries to protect against babesiosis, usually produced by local departments of agriculture or veterinary institutions (reviewed in reference 48). In some cases, this is supplemented with A. centrale-infected blood where A. marginale is enzootic. To increase shelf life and allow more rigorous safety testing, several veterinary institutes now produce frozen-blood vaccines stored in liquid nitrogen using either dimethyl sulfoxide or glycerol as the cryoprotectant. For reasons that are still unknown, young cattle up to 9 months of age are more resistant to Babesia infections, but vaccination of susceptible adult cattle often requires additional drug treatment even using the attenuated strains. Continuous exposure to natural tick infections is generally required to ensure continuous and long-lasting immunity.
A live, attenuated Theileria annulata vaccine has been produced by continuous in vitro passaging of the intracellular macroschizont stage and is used in many tropical and subtropical countries for the control of tropical theileriosis in cattle. In contrast to T. parva, immunity to the erythrocytic pathogen T. annulata is short-lived and wanes after 6 months in the absence of natural challenge infections (reviewed in references 140 and 165).
Killed or subunit protozoal parasite vaccines.
Several inactivated vaccines consisting of crude whole organisms or, more recently, defined antigenic structures have been registered and target mostly the companion animal market (Table 4). In general, these vaccines are not as effective as live organisms but can ameliorate disease or transmission to various degrees. They may also form the basis for the development of recombinant vaccines.
The final host of the coccidial parasite Neospora caninum is the dog, but its economic impact is felt mostly in the intermediate cattle host, where it is a major cause of abortion (51, 78). A crude N. caninum vaccine has been licensed in the United States to aid in the reduction of N. caninum-induced abortion in healthy pregnant cattle and prevent the transmission of the parasite to calves in utero. The vaccine consists of inactivated N. caninum tachyzoites with an adjuvant administered subcutaneously. A large field study in Costa Rica (151), where infection is highly prevalent in diary herds, demonstrated an overall twofold (46%) reduction in abortion rates through vaccination (49/438 versus 91/438 in saline-injected controls). As also reported in a multiherd vaccination trial in New Zealand (C. Heuer, C. Nicholson, D. Russell, and J. Weston, presented at the 19th International Conference of the World Association for the Advancement of Veterinary Parasitology, New Orleans, LA, 2003), there was a high variability in efficacy between farms, which is likely due to abortions being caused by other infections or by noninfectious causes (87). Timing of vaccination is also likely to play a role in preventing abortion and transmission (78).
A vaccine to alleviate a neurological disease in horses caused by infection with Sarcocystis neurona, equine protozoal myeloencephalitis, has recently been released and is being tested under conditional USDA license by Fort Dodge Animal Health. It consists of in vitro-cultured merozoites, originally obtained from the spinal cord of a horse, which are chemically inactivated and mixed with a proprietary adjuvant for intramuscular injection (104).
Giardia lamblia (synonyms, Giardia duodenalis and Giardia intestinalis) is an enteric parasite of many animal species. Infection is generally self-limiting, but severe gastrointestinal disease can develop in young and immunocompromised individuals. Its importance is mainly in animal-to-human transmission, and Giardia is a major cause of outbreaks of waterborne infections. Only one commercial vaccine has been licensed for use in dogs and cats in the United States (GiardiaVax). It is licensed to prevent clinical disease in dogs and significantly reduce the incidence, severity, and duration of cyst shedding. The vaccine consists of a crude preparation of disrupted, axenically cultured G. duodenalis trophozoites (sheep isolate) and has been shown to eliminate most clinical signs of infection and significantly reduce the total number of cysts shed in the feces in puppies and, to a lesser extent, in kittens. Some efficacy in the clearing of chronic infections resistant to chemotherapeutic agents may also be achieved through vaccination, but this requires more extensive testing (128, 129). It is thought that the vaccine acts mainly through the neutralization of parasite toxins by antibodies.
Two subunit vaccines have been developed to protect dogs against canine babesiosis caused by Babesia canis (Table 4). Both vaccines consist of soluble parasite antigens (SPA) released into the culture supernatant by in vitro-cultured parasites, combined with adjuvant. The first vaccine released, Pirodog, contains SPA from B. canis cultures only (117), while the recently released NobivacPiro contains SPA from B. canis and Babesia rossi in an attempt to broaden the strain-specific immunity. The protective effect of this vaccine seems to be based on the antibody-dependent neutralization of a soluble parasite substance that causes hypotension and clinical disease, rather than acting through reducing parasitemia per se (156). This vaccine approach was also evaluated in cattle but did not confer sufficient protection (165).
A killed subunit vaccine has been developed against coccidiosis in poultry by ABIC Veterinary Products, Israel, particularly for use in the broiler industry (192, 193). Interestingly, this vaccine does not target the merozoite stages, as most live vaccines are thought to do, but rather targets the final sexual, macrogametocyte stages that develop to form the disease-transmitting oocysts. The principle behind this vaccine strategy is that it will still allow immunity against the asexual stages to be generated by natural infections while reducing oocyst shedding and parasite transmission. Added advantages of this approach are that the laying hens, rather than the chicks, are immunized, transferring protective immunoglobulins into the egg yolk and subsequently the hatchlings. Considering that each hen lays more than 100 eggs in her lifetime, this considerably reduces the number of vaccinations and animal handling. In contrast to the species and strain specificity of live vaccines, this gametocyte vaccine was shown to confer partial protection across the three major Eimeria species. A major disadvantage of the vaccine is that it is expensive to produce, consisting of affinity-purified native gametocyte antigens derived from infected chickens, and is still a fairly complex preparation (15). Three major components of affinity-purified native gametocyte antigens have recently been cloned and characterized with a view to identifying the protective components and develop a recombinant vaccine (16). It remains to be seen if the translation of native vaccine to recombinant vaccine will be successful, as this has been a major stumbling block for much of the parasite development area (123).
Human visceral leishmaniasis, or kala-azar, is a devastating human disease caused by the intracellular parasite Leishmania chagasi or Leishmania infantum and transmitted through sand flies. Dogs are the principal carriers of the disease and are also clinically affected. Recently, a subunit vaccine against canine visceral leishmaniasis, based on a strongly antigenic surface glycoprotein complex, fucose mannose ligand, or FML antigen, from Leishmania donovani and saponin adjuvant has been developed in Brazil. Vaccine efficacy was reported to be 76 to 80% against both homologous and heterologous challenge with L. chagasi and to last for at least 3.5 years (21, 44). A concomitant reduction in human incidence of the disease was also reported, which is likely due to the transmission-blocking properties of the vaccine (42, 125). The vaccine may also have a therapeutic effect on infected dogs (22).
Helminth and Ectoparasite Vaccines
Multicellular parasites are the most complex pathogens, with genome sizes that approach those of their hosts. Apart from their genetic complexity, they are also the only pathogens that, due to their physical size, cannot be internalized by phagocytic cells of the immune system or killed by classical cytotoxic T cells (Fig. 1). In fact, the immune system had to develop a whole new mechanism to deal with these parasites, which is generally referred to as the type 2 or allergic-type immune response, typified by the recruitment and activation of potent effector leukocytes, mast cells, and eosinophils (9, 102).
There are three different families of helminths or worms, nematodes (roundworms), trematodes (flatworms), and cestodes (tapeworms), that infect both animals and humans. At present, only one worm vaccine is on the market in Europe for the cattle lung nematode Dictyocaulus viviparous (Bovilis Lungworm), consisting of irradiated infective L3 larvae that cannot develop into the adult stage (7). Vaccination with irradiated L3 larvae of the economically important gastrointestinal nematodes has been attempted but was not successful due mainly to their lack of efficacy in inducing immunity in young animals (reviewed in reference 6). The increasing drug resistance of gastrointestinal nematodes has renewed intense interest in developing vaccines for these important veterinary pathogens (reviewed in references 19, 123, and 189).
Tapeworms have a larval stage in intermediate hosts that is uniquely susceptible to immune killing after a single infection. Antigens from this early larval stage (oncospheres) were the first to confer protection against a multicellular pathogen as recombinant proteins (82). Commercial or field application of anticestode vaccines is, however, still in progress (reviewed in reference 98).
The most important veterinary trematode species are liver flukes (Fasciola hepatica and Fasciola gigantica). Vaccine development against these parasites is hindered by the fact that they do not seem to induce immunity in their natural ruminant hosts, even after repeated infections. Recently, a unique breed of sheep (Indonesian thin tail) was shown to develop immunity against F. gigantica, and further dissection of this protective mechanism may offer new approaches to vaccine development (109).
Ectoparasitic arthropods would seem to be the ultimate challenge in vaccine development, as they not only are large and complex but also spend most of their life outside or on the surface of the host. Interestingly, the only recombinant parasite antigen vaccine commercially available is against a tick parasite, Boophilus microplus, and was first introduced commercially in Australia in 1994 (TickGUARD; Fort Dodge Australia) and later in Cuba and a few South American countries (Gavac; Heber Biotec SA, Cuba). This vaccine is unique in that it is not based on natural antigens recognized by the immune system during infection but takes advantage of the ferocious blood-feeding habits of the tick. High antibody levels are generated by vaccinating cattle against a tick gut membrane-bound protein, Bm86, using a recombinant protein in a potent adjuvant. These antibodies bind to the tick's gut surface when taking a blood meal, causing the rupture of gut wall and tick death. The vaccine induces significant levels of protection against tick infestation and, in some cases, against tick-borne diseases (195). However, as the molecule is not seen during natural infection (“hidden” or “concealed” antigen), antibody levels are not boosted by infection and need to be maintained at high levels by repeated immunization. The vaccine is best used in conjunction with drug administration, which limits its practical and commercial appeal. The presence of a tick immunoglobulin excretion system seems to hamper the effectiveness of this vaccine approach in other ticks (126).
VETERINARY VACCINES FOR NONINFECTIOUS DISEASES
Allergy Vaccines
As is the case in humans, there is a genetic predisposition in some animals, especially cats, dogs, and horses, to develop allergic skin disease or atopic dermatitis in response to environmental allergens such as grass pollen, weeds, mold spores, and house dust mites. This can be exacerbated by secondary bacterial or yeast infections resulting in urticaria. The most common treatment against atopic dermatitis is vaccination with an allergen extract to which the animal has been shown to react, as determined by intradermal injection or allergen-specific serum immunoglobulin E assays. This “allergen-specific immunotherapy” (ASIT) consists of administering gradually increasing amounts of the allergen extract, either aqueous or precipitated with alum, over a period of several months, followed by yearly boosters. The reported effectiveness of this treatment varies widely, from 20% to close to 100% in dogs, depending on such factors as study design, parameters used, source of vaccine, and concurrent treatment for secondary infections (37). It is clear that a more rigorous evaluation of ASIT vaccine effectiveness is required, which would be aided greatly by a better understanding of the mechanisms by which ASIT works to reduce allergies. In parallel to human studies, it is likely that this involves the induction of specific regulatory T cells and/or immunoglobulin G antibodies that compete with and mask the allergens (94, 180). The identification of the exact mechanisms and mediators associated with successful ASIT may provide more reliable correlates of vaccine effectiveness and more rational and standardized vaccination protocols.
Cancer Vaccines
With longer life spans of domestic pets and higher value placed on the animals by their owners, treatment of spontaneous cancers has become of increasing interest. Canine malignant melanoma (CMM) is the most common oral tumor in dogs. CMM is similar to some malignant melanomas in humans, and despite treatment, most dogs die within a year of diagnosis. Several groups have anticancer vaccines against CMM in phase III clinical trials, and Merial launched a CMM DNA vaccine under conditional license from the USDA in 2006. These experimental vaccines are based primarily on studies for human cancer vaccines and include immunizations with canine tumor cell lines transfected with human granulocyte-macrophage colony-stimulating factor (75) or human gp100 (1) or DNA vaccination with human tyrosinase (18). The latter two immunizations with human melanocyte-specific proteins are based on the demonstration that immune tolerance against self-antigens can be broken through cross-reaction between a xenogeneic antigen and a self-antigen. The overall response rate in these studies was estimated to be around 17%, with occasional complete remission in individual dogs and prolongation of survival times. The experimental designs of the studies are, however, limited by small sample sizes, differences in breeds and clinical status, and comparisons to historical, stage-matched controls. No clear correlations between immune parameters and the likelihood of tumor control could be established.
Local vaccination with bacillus Calmette-Guérin (BCG) has long been used as a therapeutic treatment for superficial cancer of the urinary tract in humans and has been shown to be effective in the treatment of equine sarcoids and, to a lesser extent, bovine ocular squamous cell carcinoma (105, 153). The mode of action of this vaccination regimen is unknown but may involve the upregulation of tumor-specific antigens through local inflammation and activation of the innate immune system (162).
VETERINARY VACCINES FOR FERTILITY AND PRODUCTION CONTROL
Immunocontraceptive vaccination is a fast-moving area of vaccine research and development in the human and animal health areas, with a number of products for livestock and companion animals recently brought to the market.
Since before written history, humans have practiced neutering of animals used for food and transport and to be kept as pets. This has been termed “man's first attempt at bioengineering” (175). Following the discovery of the reproductive hormone system, attempts were made to control reproduction by immunization against key hormones in both humans and animals. Many early attempts gave encouraging results but were variable due to a lack of knowledge of how to consistently elicit an effective neutralizing immune response against self-proteins.
There are two goals of reproductive control vaccines that can be simply categorized as (i) immunocontraception and (ii) immunoneutering. Immunocontraceptive vaccines aim to prevent either fertilization of the oocyte by sperm or implantation of the fertilized egg yet retain sexual behavior patterns and competition in mating; this approach is most suited to the control of feral animal pests and native wildlife. Immunoneutering vaccines aim to prevent all sexual behaviors in both male and female animals as well as controlling fertility; these outcomes are suitable for companion animals, livestock, and, in some instances, feral animal pest control.
Design of Reproduction Control Vaccines
The hormone cascade involved in reproduction is shown in Fig. 3. T-cell help has been incorporated into vaccine formulations by using whole proteins or defined T-cell helper epitopes as peptides (36, 63, 92); however, all commercialized vaccines to date have been based on carrier protein-peptide conjugates. Commercialized peptide-carrier protein vaccines have used bacterial toxoids, including tetanus and diphtheria toxoids or ovalbumin as carriers, to which peptides of the self-antigens being targeted are conjugated. The main criterion for the selection of a carrier has been to provide strong antibody responses and potency, and the relative effectivenesses of different carriers have been identified (57). Other important factors for the selection of carrier proteins are abundance for large-scale manufacture, cost, and compliance with regulatory requirements. In some instances, the target sequence and carrier have been produced as a recombinant fusion protein and have shown good efficacy in species as diverse as cats and cattle (38, 147).
FIG. 3.
The key hormones of the hypothalmic-pituitary-gonadal axis. Tissues are in orange, and hormones are in green. *, hormones and gametes that have been targeted in constructing experimental and commercial vaccines.
Variable results from studies using anti-luteinizing hormone-releasing hormone (LHRH) vaccines under late-stage commercial development in pigs (52) and horses (173) have shown efficacies as low as 66 to 75%, leading to the view that it remains difficult to consistently stimulate a strong immune response to self-antigens. While this may still be the case for the efficacious induction of anti-self-T-cell responses due to tolerance and apoptosis of autoreactive T cells, recently commercialized vaccines have shown that strong anti-self-antibody responses can be induced with high efficacy and potency, provided that the elements of T-cell help against a foreign antigen, in conjunction with self-epitopes, are presented in combination with an appropriate and strong adjuvant.
Vaccines against Reproductive Hormones
The targeting of specific hormones involved in sexual development and function has resulted in the most scientifically and commercially successful approach for the control of reproduction by vaccination. The key hormone targets have been those of the hypothalamic-pituitary-gonad axis (Fig. 3).
The best-studied and best-characterized hormone, used as a vaccine target, has been LHRH, also known as gonadotropin-releasing hormone and gonadotropin-releasing factor. LHRH is the key hormone controlling reproductive function and development and is released from the hypothalamus. It is a simple 10-amino-acid peptide that is conserved across all species of mammals, with variants identified in other organisms from lampreys to birds and fish. Immunoneutralization of this pivotal hormone of the pituitary-gonad axis prevents reproductive function, provides contraception in all mammals, and controls estrus behavior in females and sexual and aggressive behaviors in males. Because of its simple structure and central controlling role, LHRH was the target of vaccine research soon after its discovery (36). Since then, there have been many research programs for the development of anti-LHRH vaccines both in academia and commercially; however, the commercial successes have been relatively few, as summarized in Table 5, and will be discussed further here.
TABLE 5.
Commercialized reproduction control vaccines
| Target antigen | Target species | Brand name | Distributor | Characteristic(s) | Reference or source |
|---|---|---|---|---|---|
| LHRH | Female cattle | Vaxstrate | Websters Animal Health (withdrawn in 1996) | LHRH peptide conjugated to ovalbumin; oil emulsion adjuvant | 76 |
| LHRH | Male pigs | Improvac | CSL Ltd. (now Pfizer Animal Health) | Peptide-protein conjugate and water-miscible adjuvant; control of boar taint | 53 |
| LHRH | Female horses | Equity | CSL Ltd. (now Pfizer Animal Health) | Peptide-protein conjugate and proprietary adjuvant (immunostimulating complex); control of estrus and estrus-related behavior | 54 |
| LHRH | Male dogs | Canine gonadotropin-releasing factor immunotherapeutic | Pfizer Animal Health | Peptide-protein conjugate and water-miscible adjuvant; treatment of benign prostatic hyperplasia | USDA |
| LHRH | Deer, bison, horses | GonaCon | USDA (registration reported to be in progress) | Peptide conjugated to keyhole limpet hemocyanin in mycobacterium-oil adjuvant (AdjuVac); contraceptive vaccine to control wildlife | 112 |
| ZP (ZPC/ZP3) antigen | Several species | SpayVac | ImmunoVaccine Technologies, Canada (no longer available) | Crude ZPA preparation; supplied to research groups only | 27 |
| Androstenedione | Ewes | Fecundin | Coopers Animal Health (withdrawn) | Linked to human serum albumin; increased ovulation/twinning | |
| Androstenedione | Ewes | Androvax | Agvax Pty. Ltd. (now Intervet) | Increased ovulation/twinning | |
| Androstenedione | Ewes | Ovastim | Virbac | Increased ovulation/twinning |
The first commercial vaccine developed against LHRH was Vaxstrate, comprising a conjugate of ovalbumin and LHRH peptide, presented in an oil emulsion adjuvant (76). It was sold for use as an immunospaying vaccine for extensively grazed female cattle in northern Australia. It was launched in the late 1980s and withdrawn from the market in 1996 due to poor sales, resulting mainly from being highly reactogenic (about 40% of animals with abscesses) and poor efficacy in the field. The two doses required for the administration of Vaxstrate prevented its wider use, as this did not fit well with the single annual mustering of cattle in northern Australia.
A more advanced anti-LHRH vaccine, Improvac, was developed for use in entire male pigs to control boar taint (Table 5). The formulation comprises a carrier protein conjugated to a modified form of LHRH peptide with a water-soluble adjuvant. This vaccine was launched in 1998 and has been sold since then in Australia and New Zealand and was recently launched in the Philippines, Mexico, Brazil, and South Africa. To date, it is the most successful of all the reproduction control vaccines. Improvac is given as two doses, with the first dose at least 8 weeks prior to slaughter and the second dose 4 weeks before slaughter, which is sufficient to induce an anamnestic anti-LHRH antibody response that in turn suppresses LHRH production, levels of gonadotrophins, and testicular function and allows the washout of the taint steroid androstenone and other taint compounds such as skatole, which are fat soluble. The main feature that distinguishes Improvac from the many noncommercialized vaccines is that it achieves a very high level of efficacy in the field (53). Another key feature that has ensured commercial success is that there is no impact on growth rates and more efficient use of feed over the last 4 weeks after the second dose. This is despite highly suppressed levels of the anabolic hormone testosterone for this period (53). The production advantages are probably a result of behavior modification resulting from the suppression of testosterone and have been shown to be significant compared to raising boars and are more pronounced than those of castrated boars, such as those that are raised in most pig-producing countries.
An anti-LHRH vaccine, Equity, has also been developed for use in female horses (Table 5). This product is used for the control of estrus and estrus-related behavior during the breeding season and was commercialized in 2001 in Australia. It comprises a peptide-protein conjugate and the Iscom-related immunostimulating complex adjuvant. This formulation is an advance over other commercial formulations trialed in mares and stallions that were reactogenic (50, 178).
An anti-LHRH vaccine was also conditionally licensed in the United States in 2004 for the treatment of benign prostatic hyperplasia in entire male dogs and together with Improvac are the only anti-LHRH vaccines commercialized outside of Australia and New Zealand; the dog vaccine is labeled canine gonadotropin-releasing factor immunotherapeutic under a USDA conditional license. Benign prostatic hyperplasia is very common in entire male dogs over 4 to 5 years of age, and the condition is dependent on the conversion of testosterone to dihydrotestosterone by cells in the prostate gland. Suppression of testosterone via an anti-LHRH response leads to a reduction in dihydrotestosterone and indirectly controls prostatic hyperplasia. A related application for anti-LHRH vaccines in men is for treatment of prostatic cancer, and a number of phase I/II studies have shown some degree of efficacy in men with this tumor (168).
Control of wildlife through an anti-LHRH formulation has been pursued by researchers at the National Wildlife Research Center of the USDA (86, 112). This vaccine, termed GonaCon, is based on a peptide-keyhole limpet hemocyanin carrier protein conjugate antigen formulated in a commercially available vaccine for Johne's disease in an oil-based adjuvant (AdjuVac). This formulation has the effect of making the skin of vaccinated animals test positive for Mycobacterium avium. Its advantage is that it is effective with a single vaccination, and efficacy has been shown in valued wildlife such as deer, bison, and horses (112) and in controlling feral pigs (86). Registration of this formulation is reportedly being undertaken by the Wildlife Research Group of the USDA through the EPA, as this agency is able to register products that would be restricted to use in wildlife.
Vaccines against Gamete Antigens: Wildlife Control
For the control of wildlife, the widely held view is that the maintenance of libido and sexual behavior would be optimal to achieve this goal, and hence, the hormone system that drives those behaviors has not been generally targeted. The exception to this is the control of native animal species by an anti-LHRH vaccine (see above). Alternate strategies to control wildlife have been to develop vaccines that prevent the fertilization of the oocyte by sperm or to prevent the implantation of the embryo and allow immunized animals to continue to compete in mating rituals. With this approach, antigens of the gametes (sperm and oocytes) have been widely targeted to prevent fertilization.
Sperm antigens.
Over 20 sperm antigens have been identified and characterized, and many have been tested as vaccine candidates in animals. Most of these are surface proteins and include sperm antigen 10 (SP10), SP17, FA-1, LDH-C4, and PH-20 (47). While some effect could be expected in vaccinated male animals, the large number of sperm in the male reproductive tract and observed autoimmune-mediated orchitis have focused efforts instead on vaccinating the female. Fertility levels in vaccinated females are generally reduced from levels around 75 to 80% to 25 to 30% in a range of species including mice (95), baboons (127, 171), and guinea pigs (179). The potency of the range of antigens is similar, and no one sperm antigen gives an exceptional contraceptive effect.
Oocyte antigens.
A family of surface antigens from the zona pellucida (ZP) has been identified as providing effective immunocontraception. These surface antigens have been referred to by a variety of terms that may vary between species. The major antigens are ZPA (also termed ZP2), ZPB (ZP1), and ZPC (ZP3). Most work has focused on ZPC, with a range of approaches. Vaccine studies have used formulations containing porcine ZPC, as it cross-reacts with the ZPC of many other species.
For a period between approximately 2002 and 2005, a vaccine called SpayVac was commercially available (Table 5), which was based on a crude porcine ZP antigen preparation, probably purified from pig ovaries. This was shown to have efficacy in a number of species (27). SpayVac was supplied to researchers for experimental wildlife population control and should not be considered to be a major commercialized vaccine product.
Many of the experimental ZP-based vaccines have induced reasonably high levels of efficacy sufficient to engender strong interest in further development and commercialization, with ZP antigens alone or in combination with sperm antigens to increase efficacy. Such combination vaccines as recombinant fusion antigens have been tested in a range of species to good effect (95).
Despite considerable scientific advances, there has been only very limited commercial success of gamete antigen-based vaccines. This approach has some major difficulties for wild or feral animal populations, including developing a delivery system to mass vaccinate wild animal populations without capture or restraint and being capable of delivering a booster dose, i.e., allow revaccination; ensuring the specificity of the delivery system to ensure vaccination of the target species only and to prevent unintentional vaccination and downregulation of fertility in bystander and native animal species; ensuring reasonable duration of efficacy (the duration required would be related to the frequency and effectiveness of boost vaccinations); and being able to induce and maintain high levels of antibody in the female reproductive tract.
Currently, these issues remain unresolved, and it is unlikely that fertility control vaccines will be used in wildlife management programs or commercialized until such technical hurdles are overcome. For application of gamete antigen vaccines in humans, the safety of the formulation would need to be demonstrated. Many constructs of ZP-based vaccines resulted in inflammation and immunopathology of the ovaries. The use of sperm antigens in the female would be less likely to induce safety problems.
Vaccines To Increase Fertility
There have been three fecundity vaccines for sheep that have been commercialized, all based on stimulating an immune response to the steroid androstenedione. Vaccination of ewes against this intermediate steroid leads to a reduction in estrogen levels, and estrogen (β-estradiol) has a negative-feedback effect on the production of follicle-stimulating hormone. Thus, immunoneutralization of androstenedione leads to the increased production of follicle-stimulating hormone, and this has the effect of increasing the frequency of multiple ovulations. The immunogen in the first available vaccine, Fecundin, was polyandroalbumin that contained androstenedione linked to human serum albumin. Similar vaccines, Androvax and Ovastim, are now marketed in New Zealand and Australia, respectively. Vaccination is carried out 5 and 2 weeks before mating for 6 to 8 weeks; in subsequent years, a booster dose is given to the flock 2 weeks before mating. The claimed increase in twinning is about 20 to 25% across a flock of ewes. The actual increased yield of lambs achieved with Fecundin was variable, and the vaccine was withdrawn from market. For these vaccines that increase fecundity, the correct nutritional maintenance of ewes with twins is not always easily managed, and vaccination will not correct underlying fertility problems.
CONCLUSION AND FUTURE DIRECTIONS
Vaccinology has become a recognized science that combines disciplines of immunology, microbiology, protein chemistry, and molecular biology with practical considerations of production costs, regulatory affairs, and commercial returns. The ultimate aim of any new vaccine is to provide a product that will be used to protect animals and humans against disease. More recently, vaccines have also found applications in animal production and reproduction processes. Veterinary vaccines have already made enormous impacts not only on animal health, welfare, and production but also on human health. A continuous interchange between animal and human disease control agencies and scientists will be essential to be prepared for the ever-present threat of new, emerging diseases (83). This is exemplified most recently with the advent of avian influenza virus, where poultry and wildfowl are identified as the major carriers of the disease, but recent data have shown that both wild and domestic cats can also become infected and may present a source of disease for humans (91). Pigs are susceptible to both avian and human influenza viruses, and it is speculated that coinfection of pigs with highly pathogenic avian influenza virus and human influenza virus may create viral reassortant strains with the ability for human-to-human transmission (40). Increasing animal travel and wildlife-human interactions promoted by global climate changes will also require sustained surveillance for the spread of diseases in different parts of the world, with both domestic, production, and wild animals forming important reservoirs of many vector-borne human diseases; e.g., the emergence of WNV in the United States and Europe requires continuous surveillance and control programs for the presence of the virus in birds and horses as well as humans (72). A novel addition to veterinary vaccines for human disease is a cattle vaccine against Escherichia coli O157:H7 that recently received a conditional license for distribution from the Canadian Food Inspection Agency. E. coli O157:H7 is a leading cause of food-borne disease in humans worldwide, and ruminant livestock are considered to be its major reservoir.
As highlighted in this review, much progress has been made in expanding the range of veterinary vaccines available as well as increasing efficacy and reducing side effects of existing vaccines. Many problems remain to be resolved, and there is ample scope to incorporate new knowledge and technologies into vaccine design. In particular, most vaccines are still based on live, attenuated pathogen strains. Apart from the obvious dangers involved with this type of immunization, this approach is not generally desirable for commercial companies, as it exposes them to risks of mitigation, and the short shelf life and strain/region specificity of many vaccines make them uneconomical to produce. While several variably defined subunit vaccines are available on the veterinary market, they are generally much less protective than live organisms. A better understanding of the molecular and immunological disease processes is likely to be required to improve the effectiveness of killed or subunit vaccines. In particular, while it is well established that the immune system has several effector mechanisms to deal with different pathogens depending on their individual life cycles and microenvironments (Fig. 1), most killed and subunit vaccines still rely predominantly on the induction of neutralizing antibodies (93). An increased ability to target pathogens at different stages of their life cycle is likely to open up new avenues for antigen discovery and increase the effectiveness of killed or subunit vaccines. One way that this may be achieved is through novel delivery systems such as plasmid DNA, liposomes, nano- or microparticles, and live vectors that introduce the vaccine antigens into the intracellular compartment (reviewed in references 4, 25, 61, and 154). Another major advance in immunology that will have an impact on an often neglected part of vaccine development is the increased awareness of the central role that innate immunity plays in the action of vaccine adjuvants (144). The recently discovered innate immune receptors are currently being screened for active novel adjuvant compounds (134), and their corresponding ligands (pathogen-associated molecular patterns) are being used to increase or modulate vaccine responses (77, 85, 169). The use of adjuvants in veterinary vaccinology is much less restricted than that in human vaccines, and a large number of different types and formulations of adjuvants are currently used in licensed veterinary vaccines, compared to only three adjuvants licensed for human vaccine use (134). In many cases, the details of the veterinary adjuvants are unfortunately withheld as proprietary information, but hopefully, this area will be reviewed in the near future.
Apart from the scientific challenges that are being addressed, the development of a commercially successful veterinary vaccine also needs to meet the regulatory hurdles that pave the route to the marketplace. For example, under current U.S. law, vaccines that target noninfectious disease (e.g., production gains and reproduction) come under the more stringent jurisdiction of the FDA and are treated as pharmaceuticals, whereas most animal vaccines come under the USDA, with more rapid and lower-cost routes to registration. In the European Union, regulatory matters are based on European Union legislation, and company dossiers are assessed and legalized by the European Medicines Evaluation Agency. In principle, three different procedures can be used to register a vaccine. During the centralized procedure, new and innovative vaccines are assessed and legalized in all member states in one procedure. During the mutual recognition procedure, the company selects a single reference country to evaluate the vaccine dossier, which is followed by an application in the relevant countries to have the vaccine registered afterwards. The third possibility is to apply for the recently introduced decentralized procedure, which can be chosen when a more expedient registration is desired. In this case, the vaccine dossier is reviewed by all selected countries at the same time to save the first step in the mutual recognition procedure. The Veterinary International Committee for Harmonization brings together the regulatory authorities of the European Union, Japan, and the United States and representatives from the animal health industry in the three regions to harmonize technical requirements for the registration of veterinary products. The Veterinary International Committee for Harmonization harmonizes guidelines that represent scientific consensus regarding regulatory requirements for the three regions. Expert working groups, under the supervision of the Steering Committee, are created to draft and recommend the harmonized guidelines.
Research and development form the basis for the generation of new and improved veterinary vaccines. Animal scientists can borrow heavily from medical research, particularly in the areas of welfare and geriatric medicine for companion animals, which are becoming increasingly lucrative markets for animal health companies (166). On the other hand, animal research scientists can also significantly contribute to human vaccine development, as they are able to bridge the gap between results obtained in small-rodent models, which are often not directly translatable to humans. Due to their similar sizes and anatomies, large-animal models are particularly useful for the testing of different delivery systems (160, 197) and have been extensively used to optimize the uptake of plasmid DNA for effective DNA vaccination (85, 155, 183). New animal health vaccines are also likely to be therapeutic rather than prophylactic, with cancer and osteoarthritis in longer-lived companion animals being obvious targets. Expected reductions in the cost of recombinant antibodies should make the passive immunotherapy of dogs and cats feasible. Due to their less stringent regulatory requirements and quicker route to the market, veterinary vaccines are also at the forefront of the testing and commercialization of innovative technologies, as exemplified by the recent successful licensing of two DNA vaccines for horses and fish and the conditional license of a DNA vaccine against CMM.
Acknowledgments
We thank all the companies that provided up-to-date information for their products and the scientific reviewers of the manuscript for their useful comments and corrections.
REFERENCES
- 1.Alexander, A. N., M. K. Huelsmeyer, A. Mitzey, R. R. Dubielzig, I. D. Kurzman, E. G. Macewen, and D. M. Vail. 2006. Development of an allogeneic whole-cell tumor vaccine expressing xenogeneic gp100 and its implementation in a phase II clinical trial in canine patients with malignant melanoma. Cancer Immunol. Immunother. 55:433-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, J. Ellis, S. Krakowka, A. Botner, K. McCullough, H. Nauwynck, S. Kennedy, B. Meehan, and C. Charreyre. 2004. PMWS: experimental model and co-infections. Vet. Microbiol. 98:165-168. [DOI] [PubMed] [Google Scholar]
- 3.Ashford, D. A., J. di Pietra, J. Lingappa, C. Woods, H. Noll, B. Neville, R. Weyant, S. L. Bragg, R. A. Spiegel, J. Tappero, and B. A. Perkins. 2004. Adverse events in humans associated with accidental exposure to the livestock brucellosis vaccine RB51. Vaccine 22:3435-3439. [DOI] [PubMed] [Google Scholar]
- 4.Azad, N., and Y. Rojanasakul. 2006. Vaccine delivery—current trends and future. Curr. Drug Deliv. 3:137-146. [DOI] [PubMed] [Google Scholar]
- 5.Babu, U., R. A. Dalloul, M. Okamura, H. S. Lillehoj, H. Xie, R. B. Raybourne, D. Gaines, and R. A. Heckert. 2004. Salmonella enteritidis clearance and immune responses in chickens following Salmonella vaccination and challenge. Vet. Immunol. Immunopathol. 101:251-257. [DOI] [PubMed] [Google Scholar]
- 6.Bain, R. K. 1999. Irradiated vaccines for helminth control in livestock. Int. J. Parasitol. 29:185-191. [DOI] [PubMed] [Google Scholar]
- 7.Bain, R. K., and G. M. Urquhart. 1988. Parenteral vaccination of calves against the cattle lungworm Dictyocaulus viviparus. Res. Vet. Sci. 45:270-271. [PubMed] [Google Scholar]
- 8.Balamurugan, V., R. M. Kumar, and V. V. Suryanarayana. 2004. Past and present vaccine development strategies for the control of foot-and-mouth disease. Acta Virol. 48:201-214. [PubMed] [Google Scholar]
- 9.Balic, A., V. M. Bowles, and E. N. Meeusen. 2000. The immunobiology of gastrointestinal nematode infections in ruminants. Adv. Parasitol. 45:181-241. [DOI] [PubMed] [Google Scholar]
- 10.Barbour, E. K., S. K. Hamadeh, and A. Eidt. 2000. Infection and immunity in broiler chicken breeders vaccinated with a temperature-sensitive mutant of Mycoplasma gallisepticum and impact on performance of offspring. Poult. Sci. 79:1730-1735. [DOI] [PubMed] [Google Scholar]
- 11.Baron, M. D., A. C. Banyard, S. Parida, and T. Barrett. 2005. The Plowright vaccine strain of rinderpest virus has attenuating mutations in most genes. J. Gen. Virol. 86:1093-1101. [DOI] [PubMed] [Google Scholar]
- 12.Barrett, T., P.-P. Pastoret, and W. Taylor. 2006. Rinderpest and peste des petits ruminants, virus plagues of large and small ruminants. Elsevier, London, United Kingdom.
- 13.Beer, M., I. Reimann, B. Hoffmann, and K. Depner. 4 January 2007, posting date. Novel marker vaccines against classical swine fever. Vaccine doi: 10.1016/j.vaccine.2006.12.036. [DOI] [PubMed]
- 14.Belknap, E. B., L. M. Walters, C. Kelling, V. K. Ayers, J. Norris, J. McMillen, C. Hayhow, M. Cochran, D. N. Reddy, J. Wright, and J. K. Collins. 1999. Immunogenicity and protective efficacy of a gE, gG and US2 gene-deleted bovine herpesvirus-1 (BHV-1) vaccine. Vaccine 17:2297-2305. [DOI] [PubMed] [Google Scholar]
- 15.Belli, S. I., M. Lee, P. Thebo, M. G. Wallach, B. Schwartsburd, and N. C. Smith. 2002. Biochemical characterisation of the 56 and 82 kDa immunodominant gametocyte antigens from Eimeria maxima. Int. J. Parasitol. 32:805-816. [DOI] [PubMed] [Google Scholar]
- 16.Belli, S. I., K. Mai, C. D. Skene, M. T. Gleeson, D. M. Witcombe, M. Katrib, A. Finger, M. G. Wallach, and N. C. Smith. 2004. Characterisation of the antigenic and immunogenic properties of bacterially expressed, sexual stage antigens of the coccidian parasite, Eimeria maxima. Vaccine 22:4316-4325. [DOI] [PubMed] [Google Scholar]
- 17.Bergman, J. G., M. Muniz, D. Sutton, R. Fensome, F. Ling, and G. Paul. 2006. Comparative trial of the canine parvovirus, canine distemper virus and canine adenovirus type 2 fractions of two commercially available modified live vaccines. Vet. Rec. 159:733-736. [DOI] [PubMed] [Google Scholar]
- 18.Bergman, P. J., J. McKnight, A. Novosad, S. Charney, J. Farrelly, D. Craft, M. Wulderk, Y. Jeffers, M. Sadelain, A. E. Hohenhaus, N. Segal, P. Gregor, M. Engelhorn, I. Riviere, A. N. Houghton, and J. D. Wolchok. 2003. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin. Cancer Res. 9:1284-1290. [PubMed] [Google Scholar]
- 19.Bethony, J. M., A. Loukas, P. J. Hotez, and D. P. Knox. 2006. Vaccines against blood-feeding nematodes of humans and livestock. Parasitology 133:S63-S79. [DOI] [PubMed] [Google Scholar]
- 20.Blanchard, P., D. Mahe, R. Cariolet, A. Keranflec'h, M. A. Baudouard, P. Cordioli, E. Albina, and A. Jestin. 2003. Protection of swine against post-weaning multisystemic wasting syndrome (PMWS) by porcine circovirus type 2 (PCV2) proteins. Vaccine 21:4565-4575. [DOI] [PubMed] [Google Scholar]
- 21.Borja-Cabrera, G. P., N. N. Correia Pontes, V. O. da Silva, E. Paraguai de Souza, W. R. Santos, E. M. Gomes, K. G. Luz, M. Palatnik, and C. B. Palatnik de Sousa. 2002. Long lasting protection against canine kala-azar using the FML-QuilA saponin vaccine in an endemic area of Brazil (Sao Goncalo do Amarante, RN). Vaccine 20:3277-3284. [DOI] [PubMed] [Google Scholar]
- 22.Borja-Cabrera, G. P., A. Cruz Mendes, E. Paraguai de Souza, L. Y. H. Okada, F. A. de A Trivellato, J. K. Kawasaki, A. C. Costa, A. B. Reis, O. Genaro, L. M. Batista, M. Palatnik, and C. B. Palatnik-de-Sousa. 2004. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine 22:2234-2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulter, N., and R. Hall. 1999. Immunity and vaccine development in the bovine theilerioses. Adv. Parasitol. 44:41-97. [DOI] [PubMed] [Google Scholar]
- 24.Bouma, A. 2005. Determination of the effectiveness of Pseudorabies marker vaccines in experiments and field trials. Biologicals 33:241-245. [DOI] [PubMed] [Google Scholar]
- 25.Bramwell, V. W., and Y. Perrie. 2006. Particulate delivery systems for vaccines: what can we expect? J. Pharm. Pharmacol. 58:717-728. [DOI] [PubMed] [Google Scholar]
- 26.Brochier, B., M. P. Kieny, F. Costy, P. Coppens, B. Bauduin, J. P. Lecocq, B. Languet, G. Chappuis, P. Desmettre, K. Afiademanyo, et al. 1991. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature 354:520-522. [DOI] [PubMed] [Google Scholar]
- 27.Brown, R. G., W. D. Bowen, J. D. Eddington, W. C. Kimmins, M. Mezei, J. L. Parsons, and B. Pohajdak. 1997. Temporal trends in antibody production in captive grey, harp and hooded seals to a single administration immunocontraceptive vaccine. J. Reprod. Immunol. 35:53-64. [DOI] [PubMed] [Google Scholar]
- 28.Bryans, J. T., E. R. Doll, J. C. Wilson, and W. H. McCollum. 1966. Immunization for equine influenza. J. Am. Vet. Med. Assoc. 148:413-417. [PubMed] [Google Scholar]
- 29.Bublot, M., N. Pritchard, D. E. Swayne, P. Selleck, K. Karaca, D. L. Suarez, J. C. Audonnet, and T. R. Mickle. 2006. Development and use of fowlpox vectored vaccines for avian influenza. Ann. N. Y. Acad. Sci. 1081:193-201. [DOI] [PubMed] [Google Scholar]
- 30.Buxton, D., and E. A. Innes. 1995. A commercial vaccine for ovine toxoplasmosis. Parasitology 110:S11-S16. [DOI] [PubMed] [Google Scholar]
- 31.Callow, L. L., R. J. Dalgliesh, and A. J. de Vos. 1997. Development of effective living vaccines against bovine babesiosis—the longest field trial? Int. J. Parasitol. 27:747-767. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Carter, P. B. 2003. The current state of veterinary vaccines: is there hope for the future? J. Vet. Med. Ed. 30:152-154. [DOI] [PubMed] [Google Scholar]
- 34.Chalmers, W. S., J. Simpson, S. J. Lee, and W. Baxendale. 1997. Use of a live chlamydial vaccine to prevent ovine enzootic abortion. Vet. Rec. 141:63-67. [DOI] [PubMed] [Google Scholar]
- 35.Chiers, K., I. van Overbeke, P. De Laender, R. Ducatelle, S. Carel, and F. Haesebrouck. 1998. Effects of endobronchial challenge with Actinobacillus pleuropneumoniae serotype 9 of pigs vaccinated with inactivated vaccines containing the Apx toxins. Vet. Q. 20:65-69. [DOI] [PubMed] [Google Scholar]
- 36.Clarke, I. J., H. M. Fraser, and A. S. McNeilly. 1978. Active immunization of ewes against luteinizing hormone releasing hormone, and its effects on ovulation and gonadotrophin, prolactin and ovarian steroid secretion. J. Endocrinol. 78:39-47. [DOI] [PubMed] [Google Scholar]
- 37.Colombo, S., P. B. Hill, D. J. Shaw, and K. L. Thoday. 2005. Effectiveness of low dose immunotherapy in the treatment of canine atopic dermatitis: a prospective, double-blinded, clinical study. Vet. Dermatol. 16:162-170. [DOI] [PubMed] [Google Scholar]
- 38.Cook, R. B., J. D. Popp, J. P. Kastelic, S. Robbins, and R. Harland. 2000. The effects of active immunization against gnRH on testicular development, feedlot performance, and carcass characteristics of beef bulls. J. Anim. Sci. 78:2778-2783. [DOI] [PubMed] [Google Scholar]
- 39.Coquet, L., P. Cosette, L. Quillet, F. Petit, G. A. Junter, and T. Jouenne. 2002. Occurrence and phenotypic characterization of Yersinia ruckeri strains with biofilm-forming capacity in a rainbow trout farm. Appl. Environ. Microbiol. 68:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cyranoski, D. 2005. Bird flu spreads among Java's pigs. Nature 435:390-391. [DOI] [PubMed] [Google Scholar]
- 41.Dalgliesh, R. J., L. L. Callow, L. T. Mellors, and W. McGregor. 1981. Development of a highly infective Babesia bigemina vaccine of reduced virulence. Aust. Vet. J. 57:8-11. [DOI] [PubMed] [Google Scholar]
- 42.Dantas-Torres, F. 2006. Leishmune vaccine: the newest tool for prevention and control of canine visceral leishmaniosis and its potential as a transmission-blocking vaccine. Vet. Parasitol. 141:1-8. [DOI] [PubMed] [Google Scholar]
- 43.Darteil, R., M. Bublot, E. Laplace, J. F. Bouquet, J. C. Audonnet, and M. Riviere. 1995. Herpesvirus of turkey recombinant viruses expressing infectious bursal disease virus (IBDV) VP2 immunogen induce protection against an IBDV virulent challenge in chickens. Virology 211:481-490. [DOI] [PubMed] [Google Scholar]
- 44.da Silva, V. O., G. P. Borja-Cabrera, N. N. Correia Pontes, E. P. de Souza, K. G. Luz, M. Palatnik, and C. B. Palatnik de Sousa. 2000. A phase III trial of efficacy of the FML-vaccine against canine kala-azar in an endemic area of Brazil (Sao Goncalo do Amaranto, RN). Vaccine 19:1082-1092. [DOI] [PubMed] [Google Scholar]
- 45.Davis, B. S., G. J. Chang, B. Cropp, J. T. Roehrig, D. A. Martin, C. J. Mitchell, R. Bowen, and M. L. Bunning. 2001. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J. Virol. 75:4040-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Day, M. J. 2006. Vaccine side effects: fact and fiction. Vet. Microbiol. 117:51-58. [DOI] [PubMed] [Google Scholar]
- 47.Delves, P. J., T. Lund, and I. M. Roitt. 2002. Antifertility vaccines. Trends Immunol. 23:213-219. [DOI] [PubMed] [Google Scholar]
- 48.de Waal, D. T., and M. P. Combrink. 2006. Live vaccines against bovine babesiosis. Vet. Parasitol. 138:88-96. [DOI] [PubMed] [Google Scholar]
- 49.Doel, T. R. 2003. FMD vaccines. Virus Res. 91:81-99. [DOI] [PubMed] [Google Scholar]
- 50.Dowsett, K. F., W. A. Pattie, L. M. Knott, A. E. Jackson, R. M. Hoskinson, R. P. Rigby, and B. A. Moss. 1991. A preliminary study of immunological castration in colts. J. Reprod. Fertil. Suppl. 44:183-190. [PubMed] [Google Scholar]
- 51.Dubey, J. P., and D. S. Lindsay. 1996. A review of Neospora caninum and neosporosis. Vet. Parasitol. 67:1-59. [DOI] [PubMed] [Google Scholar]
- 52.Dufour, R., C. Roulet, C. Chouvet, and M. B. Bonneau. December 1996. Method for improving the organoleptic qualities of the meat from uncastrated male domestic animals, vaccines which are usable in this method, new peptide, in particular, for producing these vaccines and vaccination kit relating thereto. U.S. patent 5573767.
- 53.Dunshea, F. R., C. Colantoni, K. Howard, I. McCauley, P. Jackson, K. A. Long, S. Lopaticki, E. A. Nugent, J. A. Simons, J. Walker, and D. P. Hennessy. 2001. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 79:2524-2535. [DOI] [PubMed] [Google Scholar]
- 54.Elhay, M., A. Newbold, A. Britton, P. Turley, K. Dowsett, and J. Walker. 2007. Suppression of behavioural and physiological oestrus in the mare by vaccination against GnRH. Aust. Vet. J. 85:35-45. [DOI] [PubMed] [Google Scholar]
- 55.Fenaux, M., T. Opriessnig, P. G. Halbur, F. Elvinger, and X. J. Meng. 2004. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 78:6297-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrari, M., A. Brack, M. G. Romanelli, T. C. Mettenleiter, A. Corradi, N. Dal Mas, M. N. Losio, R. Silini, C. Pinoni, and A. Pratelli. 2000. A study of the ability of a TK-negative and gI/gE-negative pseudorabies virus (PRV) mutant inoculated by different routes to protect pigs against PRV infection. J. Vet. Med. B Infect. Dis. Vet. Public Health 47:753-762. [DOI] [PubMed] [Google Scholar]
- 57.Ferro, V. A., and W. H. Stimson. 1998. Investigation into suitable carrier molecules for use in an anti-gonadotrophin releasing hormone vaccine. Vaccine 16:1095-1102. [DOI] [PubMed] [Google Scholar]
- 58.Frey, J. 1995. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 3:257-261. [DOI] [PubMed] [Google Scholar]
- 59.Garcia de la Fuente, J. N., C. B. Gutierrez-Martin, N. Ortega, E. F. Rodriguez-Ferri, M. L. del Rio, O. R. Gonzalez, and J. Salinas. 2004. Efficacy of different commercial and new inactivated vaccines against ovine enzootic abortion. Vet. Microbiol. 100:65-76. [DOI] [PubMed] [Google Scholar]
- 60.Reference deleted.
- 61.Gerdts, V., G. K. Mutwiri, S. K. Tikoo, and L. A. Babiuk. 2006. Mucosal delivery of vaccines in domestic animals. Vet. Res. 37:487-510. [DOI] [PubMed] [Google Scholar]
- 62.Gerhardt, R. 2006. West Nile virus in the United States (1999-2005). J Am. Anim. Hosp. Assoc. 42:170-177. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh, S., J. Walker, and D. C. Jackson. 2001. Identification of canine helper T-cell epitopes from the fusion protein of canine distemper virus. Immunology 104:58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gould, C. 2003. Successful oral vaccination. Skretting Outlook 20:10-11. [Google Scholar]
- 65.Graham, S. P., R. Pelle, Y. Honda, D. M. Mwangi, N. J. Tonukari, M. Yamage, E. J. Glew, E. P. de Villiers, T. Shah, R. Bishop, E. Abuya, E. Awino, J. Gachanja, A. E. Luyai, F. Mbwika, A. M. Muthiani, D. M. Ndegwa, M. Njahira, J. K. Nyanjui, F. O. Onono, J. Osaso, R. M. Saya, C. Wildmann, C. M. Fraser, I. Maudlin, M. J. Gardner, S. P. Morzaria, S. Loosmore, S. C. Gilbert, J. C. Audonnet, P. van der Bruggen, V. Nene, and E. L. Taracha. 2006. Theileria parva candidate vaccine antigens recognized by immune bovine cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 103:3286-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grubman, M. J. 2005. Development of novel strategies to control foot-and-mouth disease: marker vaccines and antivirals. Biologicals 33:227-234. [DOI] [PubMed] [Google Scholar]
- 67.Guedes, R. M., and C. J. Gebhart. 2003. Onset and duration of fecal shedding, cell-mediated and humoral immune responses in pigs after challenge with a pathogenic isolate or attenuated vaccine strain of Lawsonia intracellularis. Vet. Microbiol. 91:135-145. [DOI] [PubMed] [Google Scholar]
- 68.Reference deleted.
- 69.Haesebrouck, F., F. Pasmans, K. Chiers, D. Maes, R. Ducatelle, and A. Decostere. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100:255-268. [DOI] [PubMed] [Google Scholar]
- 70.Hardham, J., K. Dreier, J. Wong, C. Sfintescu, and R. T. Evans. 2005. Pigmented-anaerobic bacteria associated with canine periodontitis. Vet. Microbiol. 106:119-128. [DOI] [PubMed] [Google Scholar]
- 71.Hardham, J., M. Reed, J. Wong, K. King, B. Laurinat, C. Sfintescu, and R. T. Evans. 2005. Evaluation of a monovalent companion animal periodontal disease vaccine in an experimental mouse periodontitis model. Vaccine 23:3148-3156. [DOI] [PubMed] [Google Scholar]
- 72.Hayes, E. B., and D. J. Gubler. 2006. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu. Rev. Med. 57:181-194. [DOI] [PubMed] [Google Scholar]
- 73.Heppell, J., and H. L. Davis. 2000. Application of DNA vaccine technology to aquaculture. Adv. Drug Deliv. Rev. 43:29-43. [DOI] [PubMed] [Google Scholar]
- 74.Reference deleted.
- 75.Hogge, G. S., J. K. Burkholder, J. Culp, M. R. Albertini, R. R. Dubielzig, N. S. Yang, and E. G. MacEwen. 1999. Preclinical development of human granulocyte-macrophage colony-stimulating factor-transfected melanoma cell vaccine using established canine cell lines and normal dogs. Cancer Gene Ther. 6:26-36. [DOI] [PubMed] [Google Scholar]
- 76.Hoskinson, R. M., R. D. Rigby, P. E. Mattner, V. L. Huynh, M. D'Occhio, A. Neish, T. E. Trigg, B. A. Moss, M. J. Lindsey, G. D. Coleman, et al. 1990. Vaxstrate: an anti-reproductive vaccine for cattle. Aust. J. Biotechnol. 4:166-170, 176. [PubMed] [Google Scholar]
- 77.Huleatt, J. W., A. R. Jacobs, J. Tang, P. Desai, E. B. Kopp, Y. Huang, L. Song, V. Nakaar, and T. J. Powell. 2007. Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine 25:763-775. [DOI] [PubMed] [Google Scholar]
- 78.Innes, E. A., S. Wright, P. Bartley, S. Maley, C. Macaldowie, I. Esteban-Redondo, and D. Buxton. 2005. The host-parasite relationship in bovine neosporosis. Vet. Immunol. Immunopathol. 108:29-36. [DOI] [PubMed] [Google Scholar]
- 79.Jackwood, M. W., and Y. M. Saif. 1985. Efficacy of a commercial turkey coryza vaccine (Art-Vax) in turkey poults. Avian Dis. 29:1130-1139. [PubMed] [Google Scholar]
- 80.Jacobs, A. A., D. Goovaerts, P. J. Nuijten, R. P. Theelen, O. M. Hartford, and T. J. Foster. 2000. Investigations towards an efficacious and safe strangles vaccine: submucosal vaccination with a live attenuated Streptococcus equi. Vet. Rec. 147:563-567. [DOI] [PubMed] [Google Scholar]
- 81.Jarosinski, K. W., B. K. Tischer, S. Trapp, and N. Osterrieder. 2006. Marek's disease virus: lytic replication, oncogenesis and control. Expert Rev. Vaccines 5:761-772. [DOI] [PubMed] [Google Scholar]
- 82.Johnson, K. S., G. B. Harrison, M. W. Lightowlers, K. L. O'Hoy, W. G. Cougle, R. P. Dempster, S. B. Lawrence, J. G. Vinton, D. D. Heath, and M. D. Rickard. 1989. Vaccination against ovine cysticercosis using a defined recombinant antigen. Nature 338:585-587. [DOI] [PubMed] [Google Scholar]
- 83.Kahn, L. H. 2006. Confronting zoonoses, linking human and veterinary medicine. Emerg. Infect. Dis. 12:556-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kelly, C., M. Bugg, C. Robinson, Z. Mitchell, N. Davis-Poynter, J. R. Newton, K. A. Jolley, M. C. Maiden, and A. S. Waller. 2006. Sequence variation of the SeM gene of Streptococcus equi allows discrimination of the source of strangles outbreaks. J. Clin. Microbiol. 44:480-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kennedy, N. J., T. W. Spithill, J. Tennent, P. R. Wood, and D. Piedrafita. 2006. DNA vaccines in sheep: CTLA-4 mediated targeting and CpG motifs enhance immunogenicity in a DNA prime/protein boost strategy. Vaccine 24:970-979. [DOI] [PubMed] [Google Scholar]
- 86.Killian, G., L. Miller, J. Rhyan, and H. Doten. 2006. Immunocontraception of Florida feral swine with a single-dose GnRH vaccine. Am. J. Reprod. Immunol. 55:378-384. [DOI] [PubMed] [Google Scholar]
- 87.Kirkbride, C. A. 1993. Diagnoses in 1,784 ovine abortions and stillbirths. J Vet. Diagn. Investig. 5:398-402. [DOI] [PubMed] [Google Scholar]
- 88.Kocan, K. M., J. de la Fuente, A. A. Guglielmone, and R. D. Melendez. 2003. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 16:698-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kreeger, T. J., W. E. Cook, W. H. Edwards, P. H. Elzer, and S. C. Olsen. 2002. Brucella abortus strain RB51 vaccination in elk. II. Failure of high dosage to prevent abortion. J. Wildl. Dis. 38:27-31. [DOI] [PubMed] [Google Scholar]
- 90.Kroll, J. J., M. B. Roof, and S. McOrist. 2004. Evaluation of protective immunity in pigs following oral administration of an avirulent live vaccine of Lawsonia intracellularis. Am. J. Vet. Res. 65:559-565. [DOI] [PubMed] [Google Scholar]
- 91.Kuiken, T., G. Rimmelzwaan, D. van Riel, G. van Amerongen, M. Baars, R. Fouchier, and A. Osterhaus. 2004. Avian H5N1 influenza in cats. Science 306:241. [DOI] [PubMed] [Google Scholar]
- 92.Ladd, A. E., C. Y. Wang, and T. J. Zamb. January 1998. Immunogenic LHRH peptide constructs and synthetic universal immune stimulators for vaccines. U.S. patent 5843446.
- 93.Lambert, P. H., M. Liu, and C. A. Siegrist. 2005. Can successful vaccines teach us how to induce efficient protective immune responses? Nat. Med. 11:S54-S62. [DOI] [PubMed] [Google Scholar]
- 94.Larche, M., C. A. Akdis, and R. Valenta. 2006. Immunological mechanisms of allergen-specific immunotherapy. Nat. Rev. Immunol. 6:761-771. [DOI] [PubMed] [Google Scholar]
- 95.Lea, I. A., E. E. Widgren, and M. G. O'Rand. 2002. Analysis of recombinant mouse zona pellucida protein 2 (ZP2) constructs for immunocontraception. Vaccine 20:1515-1523. [DOI] [PubMed] [Google Scholar]
- 96.Lehman, J. R., R. M. Weigel, A. M. Siegel, L. G. Herr, A. C. Taft, and W. F. Hall. 1993. Progress after one year of a pseudorabies eradication program for large swine herds. J. Am. Vet. Med. Assoc. 203:118-121. [PubMed] [Google Scholar]
- 97.Liang, R., J. V. van den Hurk, L. A. Babiuk, and S. van Drunen Littel-van den Hurk. 2006. Priming with DNA encoding E2 and boosting with E2 protein formulated with CpG oligodeoxynucleotides induces strong immune responses and protection from bovine viral diarrhea virus in cattle. J. Gen. Virol. 87:2971-2982. [DOI] [PubMed] [Google Scholar]
- 98.Lightowlers, M. W., and D. D. Heath. 2004. Immunity and vaccine control of Echinococcus granulosus infection in animal intermediate hosts. Parasitologia 46:27-31. [PubMed] [Google Scholar]
- 99.Lorenzen, N., and S. E. LaPatra. 2005. DNA vaccines for aquacultured fish. Rev. Sci. Tech. 24:201-213. [PubMed] [Google Scholar]
- 100.Mackowiak, M., J. Maki, L. Motes-Kreimeyer, T. Harbin, and K. Van Kampen. 1999. Vaccination of wildlife against rabies: successful use of a vectored vaccine obtained by recombinant technology. Adv. Vet. Med. 41:571-583. [DOI] [PubMed] [Google Scholar]
- 101.Maes, R. K., M. D. Sussman, A. Vilnis, and B. J. Thacker. 1997. Recent developments in latency and recombination of Aujeszky's disease (pseudorabies) virus. Vet. Microbiol. 55:13-27. [DOI] [PubMed] [Google Scholar]
- 102.Maizels, R. M., A. Balic, N. Gomez-Escobar, M. Nair, M. D. Taylor, and J. E. Allen. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89-116. [DOI] [PubMed] [Google Scholar]
- 103.Marcelino, I., M. F. Sousa, C. Verissimo, A. E. Cunha, M. J. Carrondo, and P. M. Alves. 2006. Process development for the mass production of Ehrlichia ruminantium. Vaccine 24:1716-1725. [DOI] [PubMed] [Google Scholar]
- 104.Marsh, A. E., J. Lakritz, P. J. Johnson, M. A. Miller, Y. W. Chiang, and H. J. Chu. 2004. Evaluation of immune responses in horses immunized using a killed Sarcocystis neurona vaccine. Vet. Ther. 5:34-42. [PubMed] [Google Scholar]
- 105.Martens, A., A. De Moor, L. Vlaminck, F. Pille, and M. Steenhaut. 2001. Evaluation of excision, cryosurgery and local BCG vaccination for the treatment of equine sarcoids. Vet. Rec. 149:665-669. [DOI] [PubMed] [Google Scholar]
- 106.McKeever, D. J., E. L. Taracha, W. I. Morrison, A. J. Musoke, and S. P. Morzaria. 1999. Protective immune mechanisms against Theileria parva: evolution of vaccine development strategies. Parasitol. Today 15:263-267. [DOI] [PubMed] [Google Scholar]
- 107.McOrist, S., S. Jasni, R. A. Mackie, N. MacIntyre, N. Neef, and G. H. Lawson. 1993. Reproduction of porcine proliferative enteropathy with pure cultures of ileal symbiont intracellularis. Infect. Immun. 61:4286-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Medina, E., and C. A. Guzman. 2001. Use of live bacterial vaccine vectors for antigen delivery: potential and limitations. Vaccine 19:1573-1580. [DOI] [PubMed] [Google Scholar]
- 109.Meeusen, E. N., and D. Piedrafita. 2003. Exploiting natural immunity to helminth parasites for the development of veterinary vaccines. Int. J. Parasitol. 33:1285-1290. [DOI] [PubMed] [Google Scholar]
- 110.Reference deleted.
- 111.Reference deleted.
- 112.Miller, L. A., J. C. Rhyan, and M. Drew. 2004. Contraception of bison by GnRH vaccine: a possible means of decreasing transmission of brucellosis in bison. J. Wildl. Dis. 40:725-730. [DOI] [PubMed] [Google Scholar]
- 113.Minke, J. M., J. C. Audonnet, and L. Fischer. 2004. Equine viral vaccines: the past, present and future. Vet. Res. 35:425-443. [DOI] [PubMed] [Google Scholar]
- 114.Monath, T. P., J. Arroyo, C. Miller, and F. Guirakhoo. 2001. West Nile virus vaccine. Curr. Drug Targets Infect. Disord. 1:37-50. [DOI] [PubMed] [Google Scholar]
- 115.Monath, T. P., J. Liu, N. Kanesa-Thasan, G. A. Myers, R. Nichols, A. Deary, K. McCarthy, C. Johnson, T. Ermak, S. Shin, J. Arroyo, F. Guirakhoo, J. S. Kennedy, F. A. Ennis, S. Green, and P. Bedford. 2006. A live, attenuated recombinant West Nile virus vaccine. Proc. Natl. Acad. Sci. USA 103:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Moormann, R. J., A. Bouma, J. A. Kramps, C. Terpstra, and H. J. De Smit. 2000. Development of a classical swine fever subunit marker vaccine and companion diagnostic test. Vet. Microbiol. 73:209-219. [DOI] [PubMed] [Google Scholar]
- 117.Moreau, Y., E. Vidor, G. Bissuel, and N. Dubreuil. 1989. Vaccination against canine babesiosis: an overview of field observations. Trans. R. Soc. Trop. Med. Hyg. 83(Suppl.):95-96. [DOI] [PubMed] [Google Scholar]
- 118.Moriyon, I., M. J. Grillo, D. Monreal, D. Gonzalez, C. Marin, I. Lopez-Goni, R. C. Mainar-Jaime, E. Moreno, and J. M. Blasco. 2004. Rough vaccines in animal brucellosis: structural and genetic basis and present status. Vet. Res. 35:1-38. [DOI] [PubMed] [Google Scholar]
- 119.Morrow, C. J., J. F. Markham, and K. G. Whithear. 1998. Production of temperature-sensitive clones of Mycoplasma synoviae for evaluation as live vaccines. Avian Dis. 42:667-670. [PubMed] [Google Scholar]
- 120.Mortensen, S., R. Stryhn, A. Boklund, K. D. Stark, J. Christensen, and P. Willeberg. 2002. Risk factors for infection of sow herds with porcine reproductive and respiratory syndrome (PRRS) virus. Prev. Vet. Med. 53:83-101. [DOI] [PubMed] [Google Scholar]
- 121.Murphy, F. A., E. P. J. Gibbs, M. C. Horzinek, and M. J. Studdert. 1999. Veterinary virology, 3rd ed. Academic Press, London, United Kingdom.
- 122.Murtaugh, M. P., M. R. Elam, and L. T. Kakach. 1995. Comparison of the structural protein coding sequences of the VR-2332 and Lelystad virus strains of the PRRS virus. Arch. Virol. 140:1451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Newton, S. E., and E. N. Meeusen. 2003. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. 25:283-296. [DOI] [PubMed] [Google Scholar]
- 124.Nielsen, K., D. R. Ewalt, B. Garin-Bastuji, A. P. MacMillan, J. Stack, and P. Wright. 2004. Bovine brucellosis, p. 409-438. In Office International des Epizooties (ed.), Manual of diagnostic tests and vaccines for terrestrial animals. Office International des Epizooties, Paris, France.
- 125.Nogueira, F. S., M. A. Moreira, G. P. Borja-Cabrera, F. N. Santos, I. Menz, L. E. Parra, Z. Xu, H. J. Chu, C. B. Palatnik-de-Sousa, and M. C. Luvizotto. 2005. Leishmune vaccine blocks the transmission of canine visceral leishmaniasis: absence of Leishmania parasites in blood, skin and lymph nodes of vaccinated exposed dogs. Vaccine 23:4805-4810. [DOI] [PubMed] [Google Scholar]
- 126.Nuttall, P. A., A. R. Trimnell, M. Kazimirova, and M. Labuda. 2006. Exposed and concealed antigens as vaccine targets for controlling ticks and tick-borne diseases. Parasite Immunol. 28:155-163. [DOI] [PubMed] [Google Scholar]
- 127.O'Hern, P. A., C. S. Bambra, M. Isahakia, and E. Goldberg. 1995. Reversible contraception in female baboons immunized with a synthetic epitope of sperm-specific lactate dehydrogenase. Biol. Reprod. 52:331-339. [DOI] [PubMed] [Google Scholar]
- 128.Olson, M. E., H. Ceri, and D. W. Morck. 2000. Giardia vaccination. Parasitol. Today 16:213-217. [DOI] [PubMed] [Google Scholar]
- 129.Olson, M. E., D. W. Morck, and H. Ceri. 1997. Preliminary data on the efficacy of a Giardia vaccine in puppies. Can. Vet. J. 38:777-779. [PMC free article] [PubMed] [Google Scholar]
- 130.Paillot, R., D. Hannant, J. H. Kydd, and J. M. Daly. 2006. Vaccination against equine influenza: quid novi? Vaccine 24:4047-4061. [DOI] [PubMed] [Google Scholar]
- 131.Paoletti, E. 1996. Applications of pox virus vectors to vaccination: an update. Proc. Natl. Acad. Sci. USA 93:11349-11353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pardo, M. C., and M. Mackowiak. 1999. Efficacy of a new canine-origin, modified-live virus vaccine against canine coronavirus. Canine Practice 24:6-8. [Google Scholar]
- 133.Park, M. S., J. Steel, A. Garcia-Sastre, D. Swayne, and P. Palese. 2006. Engineered viral vaccine constructs with dual specificity: avian influenza and Newcastle disease. Proc. Natl. Acad. Sci. USA 103:8203-8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11:S63-S68. [DOI] [PubMed] [Google Scholar]
- 135.Pastoret, P. P., and B. Brochier. 1999. Epidemiology and control of fox rabies in Europe. Vaccine 17:1750-1754. [DOI] [PubMed] [Google Scholar]
- 136.Pastoret, P. P., and P. Jones. 2004. Veterinary vaccines for animal and public health. Dev. Biol. (Basel) 119:15-29. [PubMed] [Google Scholar]
- 137.Pensaert, M., G. Labarque, H. Favoreel, and H. Nauwynck. 2004. Aujeszky's disease vaccination and differentiation of vaccinated from infected pigs. Dev. Biol. (Basel) 119:243-254. [PubMed] [Google Scholar]
- 138.Perrin, B., M. Perrin, A. Moussa, and M. Coudert. 1996. Evaluation of a commercial gE blocking ELISA test for detection of antibodies to infectious bovine rhinotracheitis virus. Vet. Rec. 138:520. [DOI] [PubMed] [Google Scholar]
- 139.Philippon, V. 2006. Modified vaccinia Ankara (MVA) vector vaccines. University of California San Francisco School of Medicine, San Francisco, CA. http://chi.ucsf.edu/vaccines/vaccines?page=vc-01-04.
- 140.Pipano, E., and V. Shkap. 2000. Vaccination against tropical theileriosis. Ann. N. Y. Acad. Sci. 916:484-500. [DOI] [PubMed] [Google Scholar]
- 141.Plowright, W. 1972. The production and use of rinderpest cell culture vaccine in developing countries. World Anim. Rev. 1:14-18. [Google Scholar]
- 142.Pluimers, F. H. 2004. Foot-and-mouth disease control using vaccination: the Dutch experience in 2001. Dev. Biol. (Basel) 119:41-49. [PubMed] [Google Scholar]
- 143.Powell, K. 2004. DNA vaccines—back in the saddle again? Nat. Biotechnol. 22:799-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pulendran, B., and R. Ahmed. 2006. Translating innate immunity into immunological memory: implications for vaccine development. Cell 124:849-863. [DOI] [PubMed] [Google Scholar]
- 145.Rank, R. G. 1999. Models of immunity, p. 239-295. In R. S. Stephens (ed.), Chlamydia: intracellular biology, pathogenesis, and immunity. American Society for Microbiology, Washington, DC.
- 146.Rautenschlein, S., C. Kraemer, J. Vanmarcke, and E. Montiel. 2005. Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis. 49:231-237. [DOI] [PubMed] [Google Scholar]
- 147.Robbins, S. C., M. D. Jelinski, and R. L. Stotish. 2004. Assessment of the immunological and biological efficacy of two different doses of a recombinant GnRH vaccine in domestic male and female cate (Felis catus). J. Reprod. Immunol. 64:107-119. [DOI] [PubMed] [Google Scholar]
- 148.Rodolakis, A. 1983. In vitro and in vivo properties of chemically induced temperature-sensitive mutants of Chlamydia psittaci var. ovis: screening in a murine model. Infect. Immun. 42:525-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rodolakis, A., and A. Souriau. 1986. Response of goats to vaccination with temperature-sensitive mutants of Chlamydia psittaci obtained by nitrosoguanidine mutagenesis. Am. J. Vet. Res. 47:2627-2631. [PubMed] [Google Scholar]
- 150.Roeder, P. 2006. Rinderpest eradication—is it feasible? p. 61-62. In I. Olsen and T. Gjøen (ed.), Proceedings of the International Veterinary Vaccine and Diagnostics Conference, Oslo, Norway. Reprosentralen, University of Oslo, Oslo, Norway.
- 151.Romero, J. J., E. Perez, and K. Frankena. 2004. Effect of a killed whole Neospora caninum tachyzoite vaccine on the crude abortion rate of Costa Rican dairy cows under field conditions. Vet. Parasitol. 123:149-159. [DOI] [PubMed] [Google Scholar]
- 152.Rupprecht, C. E., C. A. Hanlon, and D. Slate. 2004. Oral vaccination of wildlife against rabies: opportunities and challenges in prevention and control. Dev. Biol. (Basel) 119:173-184. [PubMed] [Google Scholar]
- 153.Rutten, V. P., W. R. Klein, W. A. De Jong, W. Misdorp, P. A. Steerenberg, W. H. De Jong, W. Den Otter, and E. J. Ruitenberg. 1991. Immunotherapy of bovine ocular squamous cell carcinoma by repeated intralesional injections of live bacillus Calmette-Guerin (BCG) or BCG cell walls. Cancer Immunol. Immunother. 34:186-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scheerlinck, J. P., and D. L. Greenwood. 2006. Particulate delivery systems for animal vaccines. Methods 40:118-124. [DOI] [PubMed] [Google Scholar]
- 155.Scheerlinck, J. P., J. Karlis, T. E. Tjelle, P. J. Presidente, I. Mathiesen, and S. E. Newton. 2004. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine 22:1820-1825. [DOI] [PubMed] [Google Scholar]
- 156.Schetters, T. 2005. Vaccination against canine babesiosis. Trends Parasitol. 21:179-184. [DOI] [PubMed] [Google Scholar]
- 157.Schurig, G. G., R. M. Roop II, T. Bagchi, S. Boyle, D. Buhrman, and N. Sriranganathan. 1991. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet. Microbiol. 28:171-188. [DOI] [PubMed] [Google Scholar]
- 158.Schurig, G. G., N. Sriranganathan, and M. J. Corbel. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479-496. [DOI] [PubMed] [Google Scholar]
- 159.Schynts, F., E. Baranowski, M. Lemaire, and E. Thiry. 1999. A specific PCR to differentiate between gE negative vaccine and wildtype bovine herpesvirus type 1 strains. Vet. Microbiol. 66:187-195. [DOI] [PubMed] [Google Scholar]
- 160.Sedgmen, B. J., S. A. Lofthouse, and E. N. Meeusen. 2006. The ovine nasal mucosa: an alternative tissue site for mucosal immunization. Methods 38:112-116. [DOI] [PubMed] [Google Scholar]
- 161.Seregin, A., R. Nistler, V. Borisevich, G. Yamshchikov, E. Chaporgina, C. W. Kwok, and V. Yamshchikov. 2006. Immunogenicity of West Nile virus infectious DNA and its noninfectious derivatives. Virology 356:115-125. [DOI] [PubMed] [Google Scholar]
- 162.Seya, T., T. Akazawa, J. Uehori, M. Matsumoto, I. Azuma, and K. Toyoshima. 2003. Role of Toll-like receptors and their adaptors in adjuvant immunotherapy for cancer. Anticancer Res. 23:4369-4376. [PubMed] [Google Scholar]
- 163.Shams, H. 2005. Recent developments in veterinary vaccinology. Vet. J. 170:289-299. [DOI] [PubMed] [Google Scholar]
- 164.Shirley, M. W., A. L. Smith, and F. M. Tomley. 2005. The biology of avian Eimeria with an emphasis on their control by vaccination. Adv. Parasitol. 60:285-330. [DOI] [PubMed] [Google Scholar]
- 165.Shkap, V., and E. Pipano. 2000. Culture-derived parasites in vaccination of cattle against tick-borne diseases. Ann. N. Y. Acad. Sci. 916:154-171. [DOI] [PubMed] [Google Scholar]
- 166.Shryock, T. R. 2004. The future of anti-infective products in animal health. Nat. Rev. Microbiol. 2:425-430. [DOI] [PubMed] [Google Scholar]
- 167.Simard, N., C. Lyngoy, V. Funk, G. Traxler, S. LaPatra, and K. Salonius. 2006. Research to market: meeting safety and efficacy requirements for a DNA vaccine used in Atlantic salmon, p. 46. In I. Olsen and T. Gjøen (ed.), Proceedings of the International Veterinary Vaccine and Diagnostics Conference, Oslo, Norway. Reprosentralen, University of Oslo, Oslo, Norway.
- 168.Simms, M. S., D. P. Scholfield, E. Jacobs, D. Michaeli, P. Broome, J. E. Humphreys, and M. C. Bishop. 2000. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br. J. Cancer 83:443-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh, M., and D. T. O'Hagan. 2003. Recent advances in veterinary vaccine adjuvants. Int. J. Parasitol. 33:469-478. [DOI] [PubMed] [Google Scholar]
- 170.Slate, D., C. E. Rupprecht, J. A. Rooney, D. Donovan, D. H. Lein, and R. B. Chipman. 2005. Status of oral rabies vaccination in wild carnivores in the United States. Virus Res. 111:68-76. [DOI] [PubMed] [Google Scholar]
- 171.Stevens, V. C. 1997. Some reproductive studies in the baboon. Hum. Reprod. Update 3:533-540. [DOI] [PubMed] [Google Scholar]
- 172.Stoffregen, W. C., S. C. Olsen, and B. J. Bricker. 2006. Parenteral vaccination of domestic pigs with Brucella abortus strain RB51. Am. J. Vet. Res. 67:1802-1808. [DOI] [PubMed] [Google Scholar]
- 173.Stout, T. A., and B. Colenbrander. 2004. Suppressing reproductive activity in horses using GnRH vaccines, antagonists or agonists. Anim. Reprod. Sci. 82-83:633-643. [DOI] [PubMed] [Google Scholar]
- 174.Sweeney, C. R., J. F. Timoney, J. R. Newton, and M. T. Hines. 2005. Streptococcus equi infections in horses: guidelines for treatment, control, and prevention of strangles. J. Vet. Intern. Med. 19:123-134. [PubMed] [Google Scholar]
- 175.Taylor, G. 2000. Castration—an abbreviated history of western manhood. Routledge, New York, NY.
- 176.Taylor, J., B. Meignier, J. Tartaglia, B. Languet, J. VanderHoeven, G. Franchini, C. Trimarchi, and E. Paoletti. 1995. Biological and immunogenic properties of a canarypox-rabies recombinant, ALVAC-RG (vCP65) in non-avian species. Vaccine 13:539-549. [DOI] [PubMed] [Google Scholar]
- 177.Timoney, J. F., and D. Eggers. 1985. Serum bactericidal responses to Streptococcus equi of horses following infection or vaccination. Equine Vet. J. 17:306-310. [DOI] [PubMed] [Google Scholar]
- 178.Tshewang, U., K. F. Dowsett, L. M. Knott, and T. E. Trigg. 1997. Preliminary study of ovarian activity in fillies treated with a GnRH vaccine. Aust. Vet. J. 75:663-667. [DOI] [PubMed] [Google Scholar]
- 179.Tung, K. S., P. Primakoff, L. Woolman-Gamer, and D. G. Myles. 1997. Mechanism of infertility in male guinea pigs immunized with sperm PH-20. Biol. Reprod. 56:1133-1141. [DOI] [PubMed] [Google Scholar]
- 180.Umetsu, D. T., and R. H. DeKruyff. 2006. The regulation of allergy and asthma. Immunol. Rev. 212:238-255. [DOI] [PubMed] [Google Scholar]
- 181.van Aarle, P. 2003. Suitability of an E2 subunit vaccine of classical swine fever in combination with the E(rns)-marker-test for eradication through vaccination. Dev. Biol. (Basel) 114:193-200. [PubMed] [Google Scholar]
- 182.van den Bosch, H., and J. Frey. 2003. Interference of outer membrane protein PalA with protective immunity against Actinobacillus pleuropneumoniae infections in vaccinated pigs. Vaccine 21:3601-3607. [DOI] [PubMed] [Google Scholar]
- 183.van Drunen Littel-van den Hurk, S., S. Babiuk, and L. A. Babiuk. 2006. Needle-free delivery of veterinary DNA vaccines. Methods Mol. Med. 127:91-105. [DOI] [PubMed] [Google Scholar]
- 184.Van Immerseel, F., U. Methner, I. Rychlik, B. Nagy, P. Velge, G. Martin, N. Foster, R. Ducatelle, and P. A. Barrow. 2005. Vaccination and early protection against non-host-specific Salmonella serotypes in poultry: exploitation of innate immunity and microbial activity. Epidemiol. Infect. 133:959-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.van Oirschot, J. T., M. J. Kaashoek, and F. A. Rijsewijk. 1996. Advances in the development and evaluation of bovine herpesvirus 1 vaccines. Vet. Microbiol. 53:43-54. [DOI] [PubMed] [Google Scholar]
- 186.Van Overbeke, I., K. Chiers, R. Ducatelle, and F. Haesebrouck. 2001. Effect of endobronchial challenge with Actinobacillus pleuropneumoniae serotype 9 of pigs vaccinated with a vaccine containing Apx toxins and transferrin-binding proteins. J. Vet. Med. B Infect. Dis. Vet. Public Health 48:15-20. [DOI] [PubMed] [Google Scholar]
- 187.Veits, J., D. Wiesner, W. Fuchs, B. Hoffmann, H. Granzow, E. Starick, E. Mundt, H. Schirrmeier, T. Mebatsion, T. C. Mettenleiter, and A. Romer-Oberdorfer. 2006. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc. Natl. Acad. Sci. USA 103:8197-8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Vemulapalli, R., J. R. McQuiston, G. G. Schurig, N. Sriranganathan, S. M. Halling, and S. M. Boyle. 1999. Identification of an IS711 element interrupting the wboA gene of Brucella abortus vaccine strain RB51 and a PCR assay to distinguish strain RB51 from other Brucella species and strains. Clin. Diagn. Lab. Immunol. 6:760-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vercruysse, J., D. P. Knox, T. P. Schetters, and P. Willadsen. 2004. Veterinary parasitic vaccines: pitfalls and future directions. Trends Parasitol. 20:488-492. [DOI] [PubMed] [Google Scholar]
- 190.Vretou, E., E. Psarrou, M. Kaisar, I. Vlisidou, V. Salti-Montesanto, and D. Longbottom. 2001. Identification of protective epitopes by sequencing of the major outer membrane protein gene of a variant strain of Chlamydia psittaci serotype 1 (Chlamydophila abortus). Infect. Immun. 69:607-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Walker, J. A., and J. F. Timoney. 2002. Construction of a stable non-mucoid deletion mutant of the Streptococcus equi Pinnacle vaccine strain. Vet. Microbiol. 89:311-321. [DOI] [PubMed] [Google Scholar]
- 192.Wallach, M. 1997. The importance of transmission-blocking immunity in the control of infections by apicomplexan parasites. Int. J. Parasitol. 27:1159-1167. [DOI] [PubMed] [Google Scholar]
- 193.Wallach, M., N. C. Smith, M. Petracca, C. M. Miller, J. Eckert, and R. Braun. 1995. Eimeria maxima gametocyte antigens: potential use in a subunit maternal vaccine against coccidiosis in chickens. Vaccine 13:347-354. [DOI] [PubMed] [Google Scholar]
- 194.Whetstone, C. A., J. M. Miller, B. S. Seal, L. J. Bello, and W. C. Lawrence. 1992. Latency and reactivation of a thymidine kinase-negative bovine herpesvirus 1 deletion mutant. Arch. Virol. 122:207-214. [DOI] [PubMed] [Google Scholar]
- 195.Willadsen, P. 2004. Anti-tick vaccines. Parasitology 129:S367-S387. [DOI] [PubMed] [Google Scholar]
- 196.Williams, R. B. 2002. Fifty years of anticoccidial vaccines for poultry (1952-2002). Avian Dis. 46:775-802. [DOI] [PubMed] [Google Scholar]
- 197.Yen, H. H., J. P. Scheerlinck, S. Gekas, and P. Sutton. 2006. A sheep cannulation model for evaluation of nasal vaccine delivery. Methods 38:117-123. [DOI] [PubMed] [Google Scholar]
- 198.Zweygarth, E., A. I. Josemans, M. F. Van Strijp, L. Lopez-Rebollar, M. Van Kleef, and B. A. Allsopp. 2005. An attenuated Ehrlichia ruminantium (Welgevonden stock) vaccine protects small ruminants against virulent heartwater challenge. Vaccine 23:1695-1702. [DOI] [PubMed] [Google Scholar]