Abstract
Clinical microbiology laboratories need to communicate results of antibacterial susceptibility testing to prescribers. Sophisticated prescribers who are knowledgeable of the pharmacokinetics and pharmacodynamics of antibacterials may desire no more information than the MIC of the drug in question. However, most prescribers require interpretation of antibacterial susceptibility testing results. Breakpoints can assist in determining if an antibacterial is potentially useful in the treatment of a bacterial infection. Breakpoints should be set prior to an antibacterial being used clinically. Breakpoint setting requires integration of knowledge of the wild-type distribution of MICs, assessment of the pharmacokinetics/pharmacodynamics of the antibacterial, and study of the clinical outcome of infections when the antibacterial is used. It is mandatory that breakpoints be reviewed when antibacterial agents have been in clinical use for some time, particularly if mechanisms of bacterial resistance to the drug have been described. In general, greater amounts of information on the pharmacokinetics and pharmacodynamics of an antibacterial are available when breakpoints need to be revised. However, the opportunity to conduct randomized clinical studies of an antibacterial declines after the drug has been released commercially. Well-designed observational clinical studies are therefore necessary in order to provide reliable data to inform those reevaluating breakpoints. Breakpoint-setting organizations may also play a role in developing phenotypic tests for detection of resistance mechanisms, as this information may complement use of the breakpoint in some circumstances.
INTRODUCTION
Breakpoints are an integral part of modern microbiology laboratory practice and are used to define susceptibility and resistance to antibacterials. Depending on the testing method, they are expressed as either a concentration (in mg/liter or μg/ml) or a zone diameter (in mm). In general, all susceptibility testing methods require breakpoints, also known as interpretive criteria, so that the results of the tests can be interpreted as susceptible, intermediate, or resistant and reported as such to a broad range of clinicians. It is acknowledged that sophisticated prescribers may not require (or desire) breakpoints but rather utilize the MIC and knowledge of the pharmacodynamics (PD) of the antibacterial in question to optimize antibacterial selection and dosing. However, given the volume of specimens that a typical clinical microbiology laboratory receives and the diversity of clinicians that a laboratory serves, categorical interpretation of antibacterial susceptibility testing results is a practical necessity and is preferred by most clinicians.
The term “breakpoint” has been used in a variety of ways in the literature (141). The first and most obvious one refers to the MIC for any given antibacterial that distinguishes wild-type populations of bacteria from those with acquired or selected resistance mechanisms (“wild-type breakpoints;” sometimes called microbiological breakpoints). Data for deriving this type of breakpoint are generated from moderate to large numbers of in vitro MIC tests, sufficient to describe the wild-type population. In this context, the wild-type strain is defined as a strain of a bacterium which does not harbor any acquired or selected resistance to the particular antibacterial being examined or to antibacterials with the same mechanism/site of action. The second are so-called clinical breakpoints, which refer to those concentrations (MICs) that separate strains where there is a high likelihood of treatment success from those bacteria where treatment is more likely to fail. In their simplest form, these breakpoints are derived from prospective human clinical studies comparing outcomes with the MICs of the infecting pathogen. The third use of the term “breakpoint” refers to antibacterial concentrations calculated from knowledge of a PD parameter and the dimension of that parameter that predicts efficacy in vivo. These are the pharmacokinetic/PD (PK/PD) breakpoints, where data that have been generated in an animal model are extrapolated to humans by using mathematical or statistical techniques. Recently, in an attempt to reduce confusion about the meaning of the term “breakpoint,” the European Committee on Antimicrobial Susceptibility Testing proposed the use of the term “epidemiological (or wild-type) cutoff value” to replace the term “microbiological breakpoint” (82). We propose that the term “cutoff” be used more widely to describe the three types of “breakpoints” and that the term “breakpoint” be reserved for the final selected value to be applied in the clinical laboratory. Hence, we prefer the terms wild-type cutoff, PK/PD cutoff, and clinical cutoff to describe these entities.
DEFINITIONS OF SUSCEPTIBILITY CATEGORIES
Breakpoints are used to define susceptibility and resistance. (In this review, the term “susceptibility” is preferred over “sensitivity”). While these terms should be universally understood, they are frequently used ambiguously because they can refer to the direct interaction between the antibacterial agent and the organism or to the likelihood that the patient will respond to treatment. The first can be measured simply in vitro, while the second involves in vivo complexities such as the dose and dosing schedule, the site of infection, PK of the antibacterial in the individual, and a range of other factors, including the adequacy of host defenses. In some methods, breakpoints are set in such a manner as to create a third category, i.e., intermediate (susceptibility). This category has multiple purposes, including (i) providing a “buffer” between the resistant and susceptible categories to prevent serious interpretive errors and (ii) implying that the organism is susceptible if the antibacterial is concentrated at the site of infection (e.g., in urine) or suggesting that higher doses of antibacterial should be used where it is safe to do so to achieve efficacy.
Two sets of category definitions are given below to accommodate the two types of meanings. In vitro definitions are as follows: susceptible, growth of the bacterial strain is inhibited by an antibacterial agent concentration in the range found for wild-type strains; resistant, growth of the bacterial strain is inhibited by an antibacterial agent concentration higher than the range seen for wild-type strains; and wild type, strains that harbor no acquired resistance mechanism to the antibacterial under question, specifically no resistance attributable to (i) mutation, (ii) acquisition of foreign DNA, (iii) up-regulation of an efflux pump, (iv) up-regulation of target production, or (v) any combination of these. PD and clinical definitions, currently listed in the newly developed international reference method ISO/DIS 20776-1 (78), are as follows: susceptible, the bacterial strain is inhibited by a concentration of an antibacterial agent that is associated with a high likelihood of therapeutic success; intermediate, the bacterial strain is inhibited by a concentration of an antibacterial agent that is associated with an uncertain therapeutic effect; and resistant, the bacterial strain is inhibited by a concentration of an antibacterial agent that is associated with a high likelihood of therapeutic failure.
While intuitively appealing, these definitions do not capture all of the concepts embedded in susceptibility categories. A more encompassing set of definitions is provided by the Clinical and Laboratory Standards Institute (CLSI) (28), as follows. The “susceptible” category implies that isolates are inhibited by the usually achievable concentrations of antimicrobial agent when the recommended dosage (dosage regimen) is used for that site of infection. The “intermediate” category includes isolates with antimicrobial agent MICs that approach usually attainable blood and tissue levels and for which response rates may be lower than those for susceptible isolates. The intermediate category implies clinical efficacy in body sites where the drugs are physiologically concentrated (e.g., quinolones and β-lactams in urine) or when a higher-than-normal dosage of a drug can be used (e.g., β-lactams). The category also includes a buffer zone which should prevent small, uncontrolled technical factors from causing major discrepancies in interpretations, especially for drugs with narrow pharmacotoxicity margins. The “resistant” category implies that isolates are not inhibited by the usually achievable concentrations of the agent with normal dosage schedules and/or demonstrate MICs/zone diameters that fall in the range where specific microbial resistance mechanisms (e.g., β-lactamases) are likely and that clinical efficacy against the isolate has not been shown reliably in treatment studies.
ORGANIZATIONS THAT SET BREAKPOINTS
The processes by which breakpoints are determined can vary widely between susceptibility testing methods. Frequently, these processes are not made explicit in the documentation of the method. For those methods that describe breakpoints without explanation of how the breakpoints are derived, it is assumed that the categories of susceptible, intermediate, and resistant are set using the wild-type cutoff. Methods with recently published breakpoints are outlined in Table 1.
TABLE 1.
Susceptibility testing methods with recently published breakpoints
| Organization or test [method reference(s)] | Method(s)—principal mediaa | Breakpoint-setting parameters [reference(s)] |
|---|---|---|
| Arbeidsgruppen for antibiotikaspørsmål (Norwegian Working Group on Antibiotics [APA]) (18) | Disk diffusion—Mueller-Hinton or Iso-Sensitest | Resistance markers, MIC distributions, PK/PD, clinical and bacteriological outcomes (13, 14) |
| British Society for Antimicrobial Chemotherapy (BSAC) (1, 11) | Agar dilution, broth dilution, broth microdilution, disk diffusion—IsoSensitest agar and broth | PK and protein binding (formula), MIC distributions (95) |
| Calibrated dichotomous sensitivity test (CDS; promulgated by a single laboratory in Sydney, Australia) (16, 17) | Disk diffusion—Sensitest agar | Principally zone diameter distributions (16) |
| Clinical and Laboratory Standards Institute (CLSI) (25, 26, 27, 28) and the U.S. Food and Drug Administration | For aerobic and facultative bacteria, broth dilution, broth microdilution, disk diffusion—Mueller-Hinton agar and broth; for anaerobic bacteria, agar dilution, broth microdilution—supplemented Brucella agar and broth | MIC distributions, PK/PD, clinical/bacteriological outcome correlations (24) |
| Commissie Richtlijnen Gevoeligheidsbepalingen (CRG) (30, 138, 139, 140) | Disk diffusion—Iso-Sensitest | MIC distributions, PK/PD, clinical and bacteriological outcome correlates (31, 104) |
| Comité de l'Antibiogramme de la Société Française de Microbiologie (CA-SFM) (29) | Agar dilution, broth microdilution, disk diffusion—Mueller-Hinton | MIC distributions, PK, correlation with clinical and bacteriological outcome (95) |
| Deutches Institut für Normung (DIN) (44, 45, 46, 49, 50, 51, 52, 53) | Agar dilution, broth microdilution, disk diffusion—Mueller-Hinton | MIC distributions, PK, correlation with clinical and bacteriological outcome (47, 48, 54) |
| EUCAST (65, 66, 67) | Agar dilution, broth dilution, broth microdilution—Mueller-Hinton | In vitro drug characteristics, MIC distributions, PK/PD, clinical outcome correlations (64) |
| Japanese Society for Chemotherapy (JSC) (79, 80) | Broth microdilution—Mueller-Hinton | MIC-clinical outcome correlations (12, 115, 116) |
| Rosco Diagnostica (a commercial company based in Denmark) (114) | Disk (pressed tablet) diffusion—Mueller-Hinton, Iso-Sensitest, PDM, and Danish blood agar | Zone diameters are calibrated against a range of different national and international MIC breakpoints as well as unique breakpoints for tests performed on Danish blood agar (114) |
| Mesa Española de Normalización de la Sensibilidad y Resistencia a los Antimicrobianos (MENSURA [Spain]) (121) | Disk diffusion, broth dilution, agar dilution—Mueller-Hinton | MIC distributions, PK/PD, clinical and bacteriological outcomes (15, 98) |
| Swedish Reference Group for Antibiotics (SRGA) (123) | Agar dilution, disk diffusion, gradient diffusion—Iso-Sensitest | “Pharmacological breakpoints” with species-related adjustments |
PDM, paper disk method.
Only two international standard-setting groups, the Clinical and Laboratory Standards Institute (CLSI; formerly known as the NCCLS) and the European Union Committee on Antimicrobial Susceptibility Testing (EUCAST), have published guidelines on which data are required for, and how these data are applied to, breakpoint setting (24, 81). The U.S. Food and Drug Administration also sets breakpoints for antibacterials at the time of their approval for use. Unfortunately, breakpoints developed by various organizations may differ, creating confusion for clinical microbiologists, antibacterial susceptibility testing device manufacturers, and clinicians. Harmonization of breakpoints among these organizations should clearly be the aim, taking into account possible differences in doses and dosing schedules used in different parts of the world.
THE NATURE OF MICs
MICs, as currently measured, are presently the simplest estimates we have of the antibacterial effect in vitro. They are only semiquantitative (see below), yet they have significant utility. There is currently no better measure of antibacterial effect.
All breakpoints are either MICs or zone diameter values correlated with MICs. As a consequence, an understanding of the nature of the MIC is fundamental to breakpoint setting. The central concept of an MIC is that it is a measurement of the activity of an antibacterial agent against an individual strain of an organism. It has become the reference measuring tool for susceptibility testing. The value of MIC measurement is frequently criticized because of the “unnatural” conditions under which it is performed, but that criticism misses the point. It is unnecessary for it to reflect exactly the conditions at the site of infection, and of course in most circumstances it cannot. Hence, the common practice of comparing MICs with levels measured in various body compartments is qualitative at best. The true value of an MIC is as a measuring tool that generates values to which other parameters, such as PD end points and clinical outcomes, can be reliably compared. This requires that MICs have a reasonable level of reproducibility, a subject that has not received a great deal of attention over the years. Indeed, it is frequently quoted that the “error” associated with measuring an MIC is “plus or minus one twofold dilution.” While this can work as a rule of thumb, results from so-called “tier 2 studies” described by the CLSI (24) for establishing quality control ranges show that precision of MIC measurements can be less than or greater than this, depending on the organism-antibacterial combination (131).
The origins of the MIC can be traced back to the original Fleming paper on penicillin (strictly, on cultures of a Penicillium strain) (70). Introduced in this paper were the ideas of (i) serial twofold dilution of an antibacterial agent in broth to measure its activity against different species and (ii) reading the end point by “noting the opacity of the broth.” For decades, the conventional method of determining MICs was in normal test tubes containing 1 to 2 ml of broth, the so-called “macro method” (27). In the 1960s, the method was adapted to microtiter trays (23), and this has become the preferred method for performing MIC tests in broth. MICs can also be determined by agar dilution, where the antibacterial is incorporated into agar, again in a twofold dilution series, and the inoculum is spotted onto the agar surface prior to incubation (10, 27).
The development and adoption of the serial twofold dilution series for MIC measurement, while originally done for convenience in the macro method, have serendipitously turned out to be valuable from at least one point of view. When the MICs of a particular antibacterial for a large number of strains of a single species are plotted on a histogram, it appears that the wild-type population follows a log-normal distribution (132) (Fig. 1). This means that wild-type MICs appear to be normally distributed on a logarithmic scale; the logarithm to base 2 is the simplest of these scales. Furthermore, strains with the same type of acquired resistance also have a log-normal distribution of MICs. For species in which a single resistance mechanism to an antibacterial predominates, it is therefore usual to see a bimodal distribution.
FIG. 1.
MIC distributions for four organism-antimicrobial pairs. In each case, the wild type appears as the log-normally distributed population at the lower MICs. COWT, calculated wild-type cutoff value. (Generated using data from http://217.70.33.99/Eucast2/ and reference 132.)
Another important feature of the MIC as we currently measure it is that it actually represents a range of MICs. By way of example, Fig. 1 shows that 51,082 of 71,360 strains of Staphylococcus aureus have a vancomycin MIC of 1 mg/liter. In reality, this represents the individual MICs for those strains, each of which is >0.5 mg/liter and ≤1 mg/liter. In other words, there are 51,082 strains whose MICs lie in the range of >0.5 to ≤1 mg/liter. Indeed, it is quite possible to determine MICs between the two conventional twofold dilution series values by setting up such concentrations or by using gradient diffusion products (e.g., Etest [AB Biodisk, Solna, Sweden]).
From the clinical and PD perspective, such a discriminatory ability may be quite useful. Indeed, in some settings, it would actually be preferable to have a more finely divided range of MICs than conventional twofold dilutions. For example, “actual” MICs for amikacin for a Pseudomonas aeruginosa strain of >4 to ≤8 μg/ml will be recorded, using serial twofold dilution series, as 8 μg/ml. Yet if the PD parameter that best predicts amikacin success for P. aeruginosa is a ratio of peak concentration achieved to MIC (103), a measured amikacin peak concentration of 40 μg/ml and a recorded MIC of 8 μg/ml will result in a ratio of 5. However, the true ratio may actually be closer to 10 if the “actual” MIC was just over 4 μg/ml. No commercially available antibacterial susceptibility testing products give a more suitable finely divided range of MICs, and space limitations preventing long ranges of dilutions are an important reason for this. However, their development for research purposes is hindered by a misunderstanding of the precision of current MIC tests, often stated to be “plus or minus one twofold dilution,” as discussed above.
The values generated by MIC tests will of necessity be influenced by the method employed (90). The results may differ by choice of technique (broth macrodilution, broth microdilution, agar dilution, or gradient diffusion), medium (Mueller-Hinton, Iso-Sensitest, or Sensitest medium, lot-to-lot variation, divalent cation concentrations, and the effects of additives, such as blood), inoculum size and concentration, incubation conditions (temperature and duration of incubation), and precision in the preparation of different concentrations of the antibacterial being used. Thus, an MIC is only meaningful when the methods and conditions of the test are known.
It is for these reasons that the development of an international standard reference method for determining MICs was recently proposed. The recognition of the effectiveness of international standardization in other areas of scientific measurement has stimulated the development of an International Organization for Standards reference method for antibacterial susceptibility testing using broth microdilution and cation-adjusted Mueller-Hinton medium (78). It was published as an approved standard in late 2006. This is just the beginning, as the reference method will not work for all bacteria for which we might want to have MICs. Nevertheless, it is MICs generated using this reference methodology and its future enhancements that will become the standards for MICs in future.
DATA NEEDS FOR SETTING BREAKPOINTS
A range of data are used to assist breakpoint-setting organizations in selecting breakpoints, including in vitro microbiological data, animal and human PK/PD data, and clinical/bacteriological outcome data from prospective clinical studies. No single set of data provides all the information necessary to make decisions. As pointed out above, some methods concentrate mainly on in vitro data to establish breakpoints. Undoubtedly, human PK is factored into the decision, but how this occurs is not made explicit. We believe that the following four main types of data are necessary for the establishment of appropriate breakpoints: (i) MIC distributions and wild-type cutoffs; (ii) in vitro resistance markers, both phenotypic and genotypic; (iii) PK/PD data from animal models and human studies; and (iv) outcome data, both clinical and bacteriological, from appropriate clinical studies and the MICs of the causative pathogens in those studies. For establishing zone diameter breakpoints, additional data establishing the relationship between zone diameters and MICs are required.
The most important and difficult challenge in using these data is ensuring that there is an appropriate balance between the various forms of data, with consideration being given to the different pathogens and the types and severity of the infections associated with those pathogens. At present, there is no formula that can assist in deciding which data are more important in a particular circumstance. Instead, the final choice of emphasis on the different types of data is made by consensus among standard-setting committees and groups. Such an approach can be divisive, with individuals weighting different data types differently according to their training and skill base. It is essential that the membership of any breakpoint-setting committee include a mix of skills in all four main data areas.
Since infections occur in different body compartments, e.g., blood, soft tissues, subarachnoid space, bladder, bone, lungs, or mucosal surfaces, breakpoints, at least in theory, should be developed for each infection syndrome, e.g., bloodstream infection, cellulitis, meningitis, lower urinary tract infection, osteomyelitis, pneumonia, or pharyngitis. This would vastly increase the complexity of breakpoint setting, and therefore almost all methods choose only one set of breakpoints or, sometimes, other sets of breakpoints adjusted to the type of infection in cases where drug concentrations are substantially different, such as urinary tract infection or meningitis. In general, bloodstream infections are considered most generally representative of serious infections, and therefore the PK of the drug in blood is used to set breakpoints.
MIC Distributions and Wild-Type Cutoff Values
Constructing MIC distributions for organism-antibacterial combinations is the first step in the development of breakpoints for that antibacterial. Broth and/or agar dilution MICs are determined for all organisms of interest, and histograms such as those in Fig. 1 are constructed. Inspection of a histogram gives an immediate picture of whether only wild-type strains are present or whether strains with abnormally elevated MICs are also included. Ideally, these histograms should be constructed on a species-by-species basis because it is unlikely that even related species will have exactly the same modal MIC or wild-type range of MICs (or same mean and standard deviation on the log-normal scale). Often, species are combined for convenience, such as coagulase-negative staphylococci or Enterobacter species, but in general it is better to avoid this where possible.
It is also desirable that the full range of the wild-type MIC distribution is included in the antibacterial dilution series and that the MICs are not truncated at one or the other end of the distribution. From the full range of the wild-type MIC distribution, simple inspection will often allow one to estimate where the upper end of the wild-type distribution ends and thus to define wild-type cutoff values, with the wild type being defined as above. EUCAST has released tables and histograms showing a range of wild-type MIC distributions for many organisms and antibacterials. They are freely available on the Internet at www.eucast.org. The data are a collation of MICs collected from a wide array of national and international studies using defined methods.
If the full span of wild-type MICs is available, it is also amenable to statistical analysis. Using this type of data, statistical techniques that can reliably determine wild-type cutoff values have recently been developed, thus eliminating the need to estimate cutoff values by inspection (92, 132). This is particularly useful for distributions where there is apparent overlap between the wild-type distribution and the distribution of abnormal strains, such as the example of Pseudomonas aeruginosa and gentamicin in Fig. 1. The first of these methods uses an iterative process to find the optimum fit of the cumulative distribution MICs and works readily on standard twofold MICs. The second method uses a reflection of the lower half of the wild-type distribution about the estimated mean and requires intermediate values between the standard twofold dilution series to be effective. It was adapted from an original method used to determine zone diameter ranges and interpretive criteria of susceptible strains (92, 91).
In setting breakpoints, it is generally considered inappropriate to select values that fall inside the wild-type range. Placing breakpoints above or below wild-type distributions creates no problems with interpretation. However, when breakpoints fall inside wild-type distributions, it creates the somewhat anomalous splitting of strains into “susceptible” and “resistant” strains even though the latter do not have an acquired resistance mechanism. In some cases, such a split cannot be avoided, because the desire is to have as few breakpoints as possible for a given antibacterial agent and for some species the wild-type MIC distribution is elevated compared to that for most other “susceptible” species. A typical example of this type of splitting is seen with Stenotrophomonas maltophilia and ceftazidime, where the wild-type distribution ranges from values within reach of those achievable clinically to values well above those that can be achieved with maximum doses.
Phenotypic and Genotypic Resistance Markers
It is frequently possible to identify the existence of a resistance mechanism in a bacterial isolate by methods other than measuring the MIC (or zone diameter). These methods can be phenotypic or genotypic.
Phenotypic methods include (i) direct detection of degrading enzymes (e.g., β-lactamase testing); (ii) screening plates by using a concentration lower than the breakpoint (e.g., extended-spectrum β-lactamase screening); (iii) medium modification to enhance resistance expression (e.g., use of brain heart infusion agar to detect vancomycin-resistant enterococci); (iv) modification of incubation conditions to enhance resistance expression (e.g., incubation at ≤30 to 35°C to detect methicillin resistance in staphylococci); (v) β-lactamase confirmation by the use of specific inhibitors, such as clavulanate or EDTA; (vi) induction tests (e.g., macrolide induction of clindamycin resistance); (vii) direct detection of the protein conferring resistance (e.g., agglutination detection of penicillin-binding protein 2a in Staphylococcus aureus); and (vii) detection of resistance to high levels of an antibacterial (e.g., high-level aminoglycoside resistance in enterococci) (124).
Genotypic tests are usually reserved for confirmation of phenotypic resistance (112). The one common exception is the detection of mecA in staphylococci by PCR methods. This gene encodes the altered penicillin-binding protein 2a and is associated with methicillin “resistance.” This association holds strongly for S. aureus, where the detection of mecA correlates strongly with MICs that are greater than the wild-type cutoff value (oxacillin MIC, 2 mg/liter [CLSI]). Problems with the correlation in coagulase-negative staphylococci led to the significant lowering of the oxacillin breakpoint to 0.5 mg/liter by the CLSI to achieve greater correlation with the presence of mecA (28). This was precautionary, as there are no clinical data available to confirm or refute whether strains of coagulase-negative staphylococci with a MIC of 1 or 2 mg/liter will respond to treatment.
If simple special phenotypic (i.e., other than the MIC itself) or genotypic methods can be developed to detect acquired resistance, then ideally these should be used to validate the choice of wild-type cutoff values. Strains with MICs at and to either side of the wild-type cutoff should be subjected to those special phenotypic and/or genotypic tests to ensure correlation. If necessary, the wild-type cutoff should be adjusted to that defined by the presence of the resistance mechanism.
Most methods that recommend the use of special phenotypic and/or genotypic tests also recommend that the isolate be reported as resistant regardless of the conventional MIC-based susceptibility result. Put another way, the recommendation is that the isolate should be interpreted as resistant, the usual breakpoint does not apply, and the MIC result should be ignored. The validity of this approach has rarely been tested and would be difficult to test except in animal models. Instead, the conservative view is taken that in vitro detection of resistance to an antibacterial by additional special phenotypic and/or genotypic tests should preclude the use of that antibacterial (see the example above with mecA and coagulase-negative staphylococci). Fortunately, for many organism-antibacterial combinations, the relationship between special phenotypic and/or genotypic tests, elevated MICs, and poor treatment outcomes appears convincing. There are some notable exceptions, such as Streptococcus pneumoniae and penicillin, where the resistance phenotype and genotype, that is, the presence of altered penicillin-binding proteins and/or the genes encoding them, do not appear to correlate well with outcomes (68, 108, 142), except in cases of meningitis.
This situation provides a conundrum when breakpoints have already been set for a particular organism-antibacterial combination. New resistance mechanisms are frequently detected only after an antibacterial has been in clinical use for some time. These may represent examples of “hidden resistance” whereby resistance mechanisms are detected genotypically in organisms that have MICs within the “susceptible” range. Examples of “hidden resistance” which have not been addressed completely by organizations which set breakpoints include quinolone target mutations in gram-negative bacilli (42) and Streptococcus pneumoniae (43), extended-spectrum beta-lactamases in organisms that also constitutively produce AmpC (125), and metallo-beta-lactamase production by gram-negative bacilli (73). At the local level, clinical laboratories may be faced with a dilemma whereby resistance mechanisms are confirmed to be occurring by genotypic methods yet no review of breakpoints has been made by CLSI or EUCAST. In some circumstances, phenotypic methods may have been described to assist in detection of these resistance mechanisms. However, we caution that such methods are often based on organisms from a limited geographic area and may not be universally applicable. Their greatest use may be for epidemiologic purposes rather than for communication to prescribers.
PK/PD Considerations
With the development of our understanding in the PD of antibacterials, one of the major benefits has been its application to the setting of breakpoints (4, 60, 105). PD is the study of drug effects over time and is therefore intimately linked with the changes in drug concentrations over time, namely, PK. The terms are usually linked to create the term PK/PD. For antibacterials, PK/PD is the study of the relationship between PK variables and microbial inhibition or killing in vivo and, by extension, clinical outcome (41, 58). Extensive studies in animal and in vitro PK models over the last 20 years have led to a clear understanding of PK/PD relationships of many classes of antibacterials, including the β-lactams, aminoglycosides, quinolones, macrolides, lincosamides, tetracyclines, and glycopeptides. Clinical studies supporting the evidence generated in animal models have been conducted for β-lactams, aminoglycosides, and fluoroquinolones (2).
In vitro studies.
The PD properties of an antibacterial agent are initially explored in vitro. Bacterial inhibition and killing are initially examined by exposing species of interest to a range of fixed drug concentrations, e.g., different multiples of the MIC, and measuring viable counts over a number of hours (33). The following two major patterns are found: concentration-dependent killing, where killing becomes more rapid and profound with increasing drug concentrations; and concentration-independent or time-dependent killing, where no further increase in the rate of killing is seen for concentrations much above the MIC (33). The second in vitro phenomenon of importance is the so-called postantibiotic effect (PAE). This is a period of delayed regrowth, following drug removal, after brief periods of exposure to an antibacterial in vitro. Again, two major patterns emerge, as follows: a moderate to long delay in regrowth (prolonged PAE) and immediate regrowth or only a short delay (minimal PAE) (40). Other persistent antibacterial effects have been described, such as postantibiotic leukocyte enhancement (97) and postantibiotic sub-MIC effects (106). None of these other effects have added greatly to our understanding of the action of antibacterials in vivo or provided information that would be predictive of clinical efficacy. Demonstration of in vitro PAE requires validation in an animal model, because the persistent effect observed in vitro may occasionally be absent in vivo (1, 134). However, in most circumstances, a combination of the bacterial killing and PAE patterns is sufficient to describe in vitro PD properties and to make predictions about in vivo PK/PD properties, as summarized in Table 2.
TABLE 2.
PD properties of various antibacterial classes
| Killing/inhibition pattern | PAE | In vivo PK/PD parameter predicting efficacy | Antibiotic class(es)a |
|---|---|---|---|
| Time dependent | Minimal | %T>MIC | β-Lactams |
| Minimal | AUC/MIC ratio | Linezolid* | |
| Prolonged | AUC/MIC ratio | Macrolides, lincosamides, tetracyclines | |
| Prolonged | Cmax/MIC ratio | Glycopeptides* | |
| Concentration dependent | Minimal | AUC/MIC ratio | Polymyxins |
| Prolonged | AUC/MIC ratio and/or Cmax/MIC ratio | Aminoglycosides, quinolones, streptogramins, ketolides, daptomycin |
In vitro studies can be extended to sophisticated PD models, where the PK of drugs in humans are simulated and the effects on bacterial killing measured (96). Most studies using this technique have focused on addressing specific questions rather than defining the fundamental PD properties of drugs. In other cases, they have confirmed earlier findings from animal models. In vitro PD model studies have been helpful in one area, namely, defining the importance of a regimen in preventing the emergence of resistant subpopulations (19, 69). For a small number of bacterium-drug interactions, selection of resistant subpopulations present in the original bacterial population is a problem. The most notable of these is aminoglycosides and Pseudomonas aeruginosa (76).
Animal model studies.
Studies with animal models have been critical to our understanding of PK/PD relationships. The earliest work, by Eagle and colleagues, showed the importance of the dosing interval in determining the efficacy of penicillin (61). It took another 35 years before progress was made in this area, when the neutropenic mouse thigh and pneumonia models were employed for the first time to define possible PD parameters predicting efficacy (94, 135). These studies were the first to clearly define the relationship between PK parameters and bacterial killing in tissues by using PD principles. They successfully showed that different classes of antibacterials could have different predictors of efficacy. The principal value of these two models has been to assist in defining PK/PD parameters for the different drug classes, as they were not designed to directly reflect the clinical picture in humans.
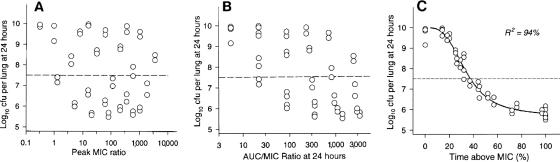
The three PK/PD predictors of efficacy for which relationships have been shown are (i) time above the MIC (T>MIC), usually expressed as a percentage of the dosing interval; (ii) the ratio of the area under the curve over 24 h to the MIC (AUC24/MIC); and (iii) the peak level-to-MIC ratio (Cmax/MIC). In this context, the AUC is the area found under the plasma concentration-time profile, and the Cmax is the maximum plasma concentration after each dose. The choice of the three PK components T>MIC, AUC, and Cmax rather than other PK components relates to the fact that these components have relatively low interdependence, i.e., they can vary significantly without greatly affecting each other. Thus, dosing regimens can be varied in animal model studies so widely that it is possible to minimize the effect of interdependence and thereby maximize the chance that only one of the components will be highly statistically correlated. An example of this is shown in Fig. 2, showing significant scatter and poor correlation between AUC24/MIC or Cmax/MIC and killing but excellent correlation to T>MIC for one organism-antibacterial pair in the mouse pneumonia model.
FIG. 2.
Relationship between PK/PD parameters and killing of Klebsiella pneumoniae by cefotaxime in the mouse pneumonia model. (Reprinted from reference 37 with permission from Elsevier.)
Note that “MIC” appears in each of the PK/PD parameters. For the utility of PK/PD data in setting breakpoints, this was a pivotal finding. When the PK/PD parameters are “controlled for” by using MIC in the denominator, it is possible to show that similar agents have similar parameter magnitudes for the same degree of killing when MIC differences between these agents are factored into the analysis (36, 37, 137).
Another important feature of the PK/PD parameters is that correspondences between drugs are best when protein binding is taken into account (1, 37). For instance, the T>MIC percentages for maximum killing of Staphylococcus aureus in the mouse thigh model are identical for cefotaxime and ceftriaxone, but only when free drug concentrations are used in the analysis (37). This is strong evidence that protein binding must be taken into account when applying PK/PD findings to the setting of breakpoints.
Questions arise regarding the applicability of PK/PD parameter data generated in animal models such as the mouse thigh model, where the end point is bacteriostasis or bacterial killing after 24 h. Some confidence about the parameters and their magnitudes can be taken from analyses that have compared them with mortality in a range of animal models (39) and across different organisms (41).
The magnitudes of the relevant parameters predicting efficacy are thought to vary depending on a range of factors, with the most important being the class of drug and the infection compartment. T>MIC percentages to achieve bacteriostasis (no net growth or killing at the site of infection in the animal model) vary between β-lactam classes, with cephalosporins requiring the highest percentage and carbapenems the lowest. Variation between bacterial species is sometimes seen.
Clinical studies of PD.
There are few clinical studies that have been conducted deliberately to confirm the findings of in vitro and animal model PD. This is understandable because in order to confirm the choice of PK/PD parameters, it is necessary to have both clinical and microbiological failures, as well as sufficient variation in dosing regimens, to separate out the correct PK/PD parameter. Planning for failures in studies is generally considered unethical, and wide variation in dosing regimens is impractical. Hence, most studies in this area have been retrospective, although a few key ones have analyzed outcomes prospectively based on PK/PD parameters (2). Those that have been conducted prospectively have confirmed the analyses made in vitro and with animal PK/PD models.
A number of clinical studies have shown a relationship between MIC and treatment outcome. Conditions and agents studied include suspected gram-negative bacteremia and cefoperazone (56), multiple different infections and cefoperazone (36) or cefotaxime (55), acute otitis media and cefuroxime axetil (75), Bacteroides fragilis and cefoxitin (120), methicillin-resistant Staphylococcus aureus bacteremia and vancomycin (117), and extended-spectrum β-lactamase-producing gram-negative bacteremia and third-generation cephalosporins (83). By itself, this relationship is informative about the importance of the MIC but otherwise unhelpful because it is not possible to know which of the three PK/PD parameters is relevant. This comes about because dosage regimens in these studies are usually fixed or vary little, making it impossible to control for the level of interdependence of the PK/PD variables. Furthermore, the MIC of the infecting pathogen and the dosing schedule of the antibacterial agent are not the only determinants of efficacy, and the host response can be important or even dominant in the resolution of infection. Most importantly, we cannot determine from such studies whether the dosing schedules used were optimal. If the PK/PD parameters are known, the effects of changes in the dosing schedule can be predicted and dosing schedules optimized.
Fortunately, there are some studies that have been able to examine which of the PK/PD parameters are important. These are listed in Table 3. In each case, the selected parameter and its magnitude correlate reasonably well with those found in animal models. These data strengthen the case for using PK/PD parameters and their magnitudes for estimation of breakpoints that have clinical meaning.
TABLE 3.
Prospective clinical studies of PK/PD parameters
| Agent | Infection(s)c | PK/PD parameter selected | Magnitude of parameter for maximum efficacy (clinical cure rate)f | Reference(s) |
|---|---|---|---|---|
| β-Lactams | ||||
| Cefmenoxime | Nosocomial pneumonia | Time above DRCa | 70-100% | 118 |
| Cefepime | Hospitalized, various | Time above 4.3× MIC | 100% | 126 |
| Penicillins and cephalosporinsb | Otitis media caused by Streptococcus pneumoniae or Haemophilus influenzae | Time above MIC | 60% | 38 |
| Fluoroquinolones | ||||
| Ciprofloxacin | Mainly nosocomial pneumonia | AUC24/MIC ratio | ≥125 | 71 |
| Levofloxacin | Serious community-acquired infection | Cmax/MIC ratio | ≥12.2 | 111 |
| Gatifloxacin and levofloxacin | Streptococcus pneumoniae community-acquired pneumonia and AECB | fuAUC24/MIC ratiod | ≥33.7 | 1 |
| Grepafloxacin | AECB | AUC24/MIC ratio | ≥175 | 72 |
| Garenoxacin | Community-acquired pneumonia, AECB, and sinusitis | Nonee | 133 | |
| Levofloxacin | Nosocomial pneumonia | AUC24/MIC ratio | ≥87 | 59 |
| Ciprofloxacin | Pseudomonas aeruginosa bacteremia | Cmax/MIC ratio | ≥8 | 143 |
| Other agents | ||||
| Gentamicin | Nosocomial pneumonia | Cmax/MIC ratio | ≥10 | 85 |
| Gentamicin and tobramycin | Pseudomonas aeruginosa bacteremia | Cmax/MIC ratio | ≥8 | 143 |
| Vancomycin | Staphylococcus aureus lower respiratory tract infection | AUC24/MIC ratio | ≥350 | 101, 102 |
DRC was used as a surrogate of the MIC per reference 128.
This was an analysis of data from multiple clinical studies.
AECB, acute exacerbations of chronic bronchitis.
fu, fraction unbound.
Due to the very high activity of the agent, fuAUC24/MIC ratios were >200 in more than 90% of patients.
Magnitudes are expressed in terms of total drug, rather than unbound drug, unless stated otherwise.
Estimation of target attainment.
Initial attempts at using PK/PD data to estimate breakpoints used average PK values (129). This is unsatisfactory because of significant intersubject variability in PK, even in young healthy subjects (58). PK values can vary even more during illness and as a consequence of underlying diseases. Both kinds of variation need to be included in any estimation of breakpoints based on PK/PD data. The method for factoring in variation is known as Monte Carlo simulation and was first successfully employed in antibacterial PD by Drusano et al. (57, 58).
Monte Carlo simulation is a statistical technique whereby a population of values of interest is simulated using existing data, such as the mean and standard deviation from a standard (small) PK/PD study, and then randomly generating a large number of patient values according to an underlying statistical distribution, such as the normal (Gaussian) or, sometimes, log-normal distribution. In this way, the variation in time above a certain value, the AUC24, and Cmax, as found for patients given a defined dosage regimen, can be “created” and used to estimate the probabilities of reaching certain values when large numbers of patients are treated with that regimen. In the context of antibacterials, these “certain values” will be determined by the magnitude of the PK/PD parameter predicting maximum efficacy, for example, 50% for %T>MIC of a β-lactam, 100 for an AUC24/MIC ratio of a fluoroquinolone, or 10 for a Cmax/MIC ratio of an aminoglycoside. With these values, it is possible to find the probability that these magnitudes will be reached at different MICs. This whole process is called target attainment analysis. When target attainment rates fall below 90%, the probability of that dosing regimen being effective is significantly diminished, and the PK/PD cutoff becomes the highest MIC for which the target attainment exceeds 90%. The figure of 90% has been chosen arbitrarily and has not been validated clinically but is widely used in target attainment analysis.
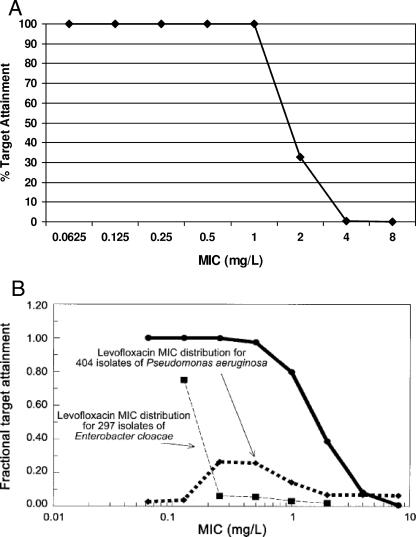
By way of example, using healthy volunteer PK/PD data for intravenous levofloxacin at a dose of 500 mg daily (22), the fractional probabilities of achieving an AUC24/MIC ratio of at least 100 for different MICs are shown in Fig. 3A. Improvements on this basic process can be made when (i) PK data from prospective clinical studies are used, rather than conventional PK data from volunteer studies (58); and (ii) MIC distributions, as described above, are factored into the analysis (59). These improvements are shown in Fig. 3B, from a study of intravenous levofloxacin (750 mg once daily) for treatment of nosocomial pneumonia (59).
FIG. 3.
(A) Fractional probability of target attainment (expressed as a percentage) of intravenous levofloxacin at 500 mg daily, based on PK in healthy volunteers. (Generated using data from reference 22.) (B) Fractional probability of target attainment of intravenous levofloxacin at 750 mg daily, based on PK in a clinical efficacy study, compared to MIC distributions of two major nosocomial pneumonia pathogens. (Reprinted from reference 59 with permission. © 2004 by the Infectious Diseases Society of America. All rights reserved.)
Monte Carlo simulation is being used increasingly to assist in developing clinical breakpoints (5, 105). It is now recognized that including between-patient variation in PK and between-organism variation in MICs is an essential component that must be taken into account when setting breakpoints. It is no longer appropriate to use average PK/PD values or susceptibility measures such as the MIC90 (1).
Results of Monte Carlo simulation may vary according to the input variables and underlying assumptions used. The input variables, as mentioned above, include a variety of PK parameters and the extent of protein binding of the antibacterial. Clearly, such variables may differ from source study to study. The adequacy of these studies may influence the outcome of the Monte Carlo simulation. Even the use of apparently equivalent parameters, such as half-life versus clearance, in different models may greatly alter the results. The number of patient simulations is also of relevance; typically, 5,000 to 10,000 such simulations are used. A number of different programs are used to conduct Monte Carlo simulation (such as Adapt, Crystal Ball, etc.), but it is unlikely that the use of different programs will substantially alter the results of target attainment analysis. We believe that standard, predefined methodologies for Monte Carlo simulation should be used by organizations setting breakpoints and that these methods should be internationally harmonized.
Outcome Data from Clinical Studies
Some organizations give the greatest weight to prospective clinical studies to define breakpoints, especially those trials done for regulatory purposes. Such trials are typically randomized controlled trials with predefined dosage regimens, clinical end points, and bacteriologic end points. An advantage of such studies is that concomitant antibacterial use is typically minimal so that the effects of the antibacterial under study can be studied independently. Most often, the appropriate correlation in such studies is clinical and/or bacteriological outcome versus the MIC for the infecting pathogens. Clinical studies may have their greatest value in providing a “reality check” for cutoffs derived from microbiologic and PK/PD studies. Clinical failure rates in excess of those predicted by microbiological and PK/PD data should trigger a reassessment of breakpoints prior to an antibacterial's commercial introduction.
It is important to note that there is a range of problems with regulatory clinical studies that limit our ability to always correctly interpret these data for breakpoint setting. These limitations are as follows. (i) The great majority of studies predefine “resistance” and exclude/withdraw patients with “resistant” strains. The way “resistance” is predefined is often not clear to the investigators but often appears to be based, at least in part, on MIC distributions, i.e., the wild-type cutoff for pathogens likely to be involved in the infection under study. (ii) As a consequence of point i and the nature of MIC distributions, it is likely that for some species, there will be few infections included in the study caused by strains at the top end of the distribution, especially because sample sizes for an individual species are likely to be small. This greatly enhances the chance of error in estimating a clinical breakpoint. (iii) There is little consensus on which rates of cure and/or eradication are acceptable. From a regulatory standpoint, such studies typically test noninferiority compared to a drug that has previously received regulatory approval. It is not clear whether this is always the appropriate end point for assessment of breakpoints. Indeed, regulators have recently recognized the limitations of comparative noninferiority studies and in some cases are now requesting studies designed to show superiority or to be placebo controlled. (iv) No numerical account is taken of natural response rates, which can be quite high for some common bacterial infections. (v) Not all species of interest in a particular infection necessarily get included in prospective studies. Thus, breakpoints may be extrapolated inappropriately from commoner species.
Ideally, clinical studies would recruit significant numbers of cases where the infecting pathogen has an MIC on either side of the wild-type and PK/PD cutoff values to assist in the clinical validation of final breakpoints. However, this is unlikely to happen for a range of reasons, including point ii above and the fact that the only studies with sufficient recruitment are those conducted on new, potent antibacterial agents for regulatory registration purposes. Of interest, the use of clinical outcome data to define breakpoints has been popular in the past in Japan. Examination of outcomes by MIC has led to the selection of a breakpoint estimated to be the lowest MIC where maximum or near-maximum efficacy has been achieved (115,116).
COMBINING CUTOFFS TO SET BREAKPOINTS
General Principles
The most comprehensive process for setting MIC breakpoints involves a comparison between PK/PD cutoffs, clinical cutoffs, and wild-type cutoffs. Ideally, it is based on a full set of data, as outlined in Table 4. Combining cutoffs into a single breakpoint is still largely a matter of judgment. The process, unfortunately, can be swayed by the composition of skills and biases of the group making the breakpoint decisions. It is largely a question of how to merge the three cutoffs in such a way as to ensure that (i) strains that are likely to respond to treatment with the chosen dosage schedule at the likely site of infection are classified as susceptible and (ii) strains that are unlikely to respond to treatment with the chosen dosage schedule at the likely site of infection are classified as resistant.
TABLE 4.
Preferred data sets for establishing breakpoints of an antibacterial
| Area of investigation | Data item |
|---|---|
| In vitro activity | Construct MIC distributions of bacterial species of interest |
| Determine wild-type cutoffs for individual species or closely related species | |
| If possible, determine resistance mechanisms if there is evidence of acquired resistance in any species and correlate them with wild-type cutoffs | |
| In vitro PD | Determine if killing in vitro is concentration or time dependent |
| Determine duration of the PAE | |
| Measure protein binding in animals used in the animal model for PK/PD and in humans | |
| Animal model PD | Determine PK in animal model |
| Determine PD parameter that best predicts efficacy (bacterial killing) in animal model (e.g., mouse thigh), i.e., either %T>MIC, AUC24/MIC, or Cmax/MIC | |
| Estimate the magnitude of the PD parameter based on unbound drug (target) that produces bacteriostasis or near-maximum killing | |
| Human PD | Determine PK in humans (usually with volunteer studies), including nature of population kinetic model (normal or log-normal) and means and standard deviations of volumes of distribution, elimination half-lives, AUCs, and Cmax values (depending on the relevant PD parameter) |
| Using Monte Carlo simulation, calculate target attainment rates for unbound drug at different MICs for bacteriostasis and near-maximum killing | |
| Set PD cutoff at the highest MIC where target attainment exceeds 90% | |
| Clinical outcome studies | Collect outcome data from prospective clinical studies by infection type and by bacterial species (clinical efficacy and bacteriological efficacy) |
| Perform PK studies with at least a subset of patients and compare results with those of previous volunteer studies (as described for human PD studies) | |
| Tabulate both types of outcomes by infection type, by bacterial species, and by MIC | |
| Select clinical cutoff (by species and infection type if necessary) as the highest MIC giving maximum efficacy | |
| Disk diffusion zone diameters | Construct scattergrams of zone diameters versus MICs of closely related species by species |
It is our opinion that the initial step in breakpoint determination should be an assessment of the MIC distribution for a contemporary collection of isolates obtained from global sources. The PK/PD cutoff should be applied to this collection. We believe that the PK/PD cutoff provides the greatest amount of “value” in this situation because it includes much of the relevant data in its construction, including (i) the MIC as it is measured in vitro; (ii) the relevant PD parameter and its magnitude, which predicts in vivo efficacy; (iii) human PK and its intersubject variation; and (iv) the dosing regimen. PK/PD breakpoints are sometimes criticized on the basis that they are established using PK/PD data from plasma/serum, which do not necessarily reflect what is happening to the drug in tissues where infections are located. This criticism misses the point. When the PD parameter that predicts efficacy is determined with an animal model, it is by using the plasma levels as a surrogate for what is happening at the site of infection. If a robust relationship can be found between bacterial inhibition and killing and plasma PK/PD (which is almost always true), then that is all that is needed to validate the model. This is true for both bactericidal and bacteriostatic agents.
Clinical cutoffs should be considered a validation tool for PK/PD cutoffs and, because of the limitations stated above, should not necessarily receive greater weighting. Clinical cutoffs are most important when they fall below PK/PD cutoffs. If this is the case, it suggests that further PK/PD work is required to understand the relationship between PD and outcomes. Different results arising from application of clinical versus PK/PD cutoffs may arise when differing dosage regimens of the drug are licensed or used in clinical practice. PK/PD cutoffs apply to specific dosage regimens. The most conservative approach is to generate PK/PD cutoffs based on the lowest approved dosage regimen for the drug. It would be logical to apply different breakpoints to different dosage regimens—unfortunately, communication of such information by clinical microbiology laboratories to prescribers may be difficult. Ideally, authorities setting breakpoints for susceptibility testing should specify the dosage regimen on which their breakpoints are based.
The principal application of wild-type cutoff values is to examine whether the PK/PD and clinical cutoffs fall below them and inside the wild-type MIC distribution. If this does occur, then problems will be encountered in testing and interpretation, as some wild-type strains will be “susceptible” and others “intermediate” or “resistant,” and because of day-to-day variations in testing results, some strains could readily end up in any of the three categories. A general solution to the possible breakpoint splitting of wild-type distributions has not been agreed upon yet. In practice, if the split is at the high end of the distribution, then the breakpoint is not modified and it is accepted that a small number of strains with wild-type MICs will test as “intermediate” or “resistant.” If the split occurs in the center or lower end of the MIC distribution, the conservative approach is to nominate that species as intermediate or resistant.
Breakpoints by Infection Site
Breakpoints are usually established on the basis that they are relevant at all sites of infection. However, there are body compartments where antibacterials can be concentrated or have restricted penetration.
Urinary tract.
Many antibacterials are excreted primarily in urine and achieve concentrations substantially higher than those seen in plasma. In many methods, it is conventional not to adjust breakpoints for urinary tract infections for the majority of agents in this category in order to reduce the complexity of multiple interpretative criteria. Whether this is warranted is unclear. Conversely, for those methods that do supply different breakpoints for urinary tract isolates causing lower urinary tract infection, it is usually not possible for the laboratory to distinguish isolates associated with lower versus upper urinary tract infection. Previously, it was believed that urinary concentrations predicted the outcome better than did serum concentrations, at least for lower urinary tract infection (122). Prospective clinical data on the outcome of treating urinary tract infections caused by organisms with various levels of susceptibility and resistance are scarce. Those studies that do examine this have found that the current CLSI breakpoints for trimethoprim-sulfamethoxazole, which are based on systemic PK, effectively separate clinical and microbiological successes and failures or relapse (20, 77, 113) and that failure rates with strains defined as resistant using systemic PK are the same as those seen with placebo (100).
Recent attempts to examine the PD of urinary tract infection treatment have shown a modest relationship between the total time that aminopenicillins exceed the MIC in plasma and treatment outcomes in clinical studies (74). Maximum efficacy is achieved when the total time is ≥30 h. Further studies with a mouse model of ascending urinary tract infection have been able to show a correlation between the MIC and bacterial killing for sulfamethizole but not amdinocillin (87). Despite all the uncertainties, some methods, such as that of the British Society for Antimicrobial Chemotherapy, do provide higher breakpoints for more than 10 drugs used for urinary tract infection (95). Other authorities, such as the CLSI and Comité de l'Antibiogramme de la Société Française de Microbiologie, provide “urinary” breakpoints for agents whose primary role is the treatment of urinary tract infection, for instance, nitrofurantoin, sulfonamides, trimethoprim, norfloxacin and some other quinolones, fosfomycin, and amdinocillin (28). More work will be required in the area of urinary tract levels, PD, and outcomes.
Cerebrospinal fluid.
Although the penetration of many drugs into cerebrospinal fluid is known to be restricted, even in the presence of inflammation, breakpoints are not usually adjusted to take the specialized PK of antibacterials in the subarachnoid space into account, principally because it is standard practice to use much higher doses for bacterial meningitis to compensate for restricted penetration. Almost all of the information relevant to breakpoint setting has been generated by observing treatment failures, mostly to expanded-spectrum cephalosporins, of pneumococcal meningitis (84, 86, 107). A number of organizations have adjusted their breakpoints for pneumococci when they are associated with meningitis, but mainly for expanded-spectrum cephalosporins. Most methods already had low breakpoints for penicillin, and these did not require adjustment when strains with reduced susceptibility emerged.
Breakpoint Setting before Resistance Has Emerged
When antibacterial agents with novel mechanisms of action are developed, it is usual for there to be no resistance among the bacteria within their spectrum. It is therefore not possible to be confident of a correlation between susceptibility and treatment outcome from clinical studies, as no patients will have been infected with “resistant” strains. Under these circumstances, in vitro data, animal PD data, and human PK data (with Monte Carlo simulation) are used to define breakpoints. If the calculated PK/PD cutoff is substantially greater than the wild-type cutoff for any species, the conservative decision is usually to set the MIC breakpoint only one or two doubling dilutions above the wild-type cutoff value. Some authorities, such as the CLSI, will choose to set only a single breakpoint, with that being “susceptible.” When resistance has emerged, it is then possible to reexamine the tentative breakpoint and to establish a resistance breakpoint and, if necessary, an intermediate range.
Reevaluation of Breakpoints Years after the Commercial Release of an Antibacterial Agent
A difficult situation arises when new mechanisms of bacterial antibacterial resistance are detected a significant time after breakpoints were initially determined. Such a situation has occurred with the discovery of β-lactamase types such as extended-spectrum β-lactamases (109) and metallo-β-lactamases (136). When these new mechanisms of resistance are found in organisms susceptible to an antibacterial potentially subject to these resistance mechanisms, a case may be made for reevaluating breakpoints. Such a signal to reevaluate breakpoints is often hastened by clinical case reports describing failure of the antibacterial in question when used in treatment of an organism harboring the new mechanism of resistance. In other circumstances, a renewed understanding of the PK/PD of an antibacterial may serve as the trigger for breakpoint reevaluation (7).
Breakpoint organizations in the United States, in particular CLSI, do reevaluate breakpoints when it is deemed necessary. However, their implementation is more difficult because of the role of the regulatory authority in setting and altering breakpoints. Additionally, there may be commercial reluctance to invest funds in the provision of new clinical data assessing the need to reevaluate breakpoints. In particular, it is highly unlikely that prospective clinical studies will be performed in an environment similar to that in which regulatory studies are conducted. Finally, antibacterials undergoing reevaluation may be generically available; in this situation, there is unlikely to be any commercial support for provision of new clinical data.
It is our opinion that the discovery of new antibacterial resistance mechanisms occurring in organisms which are “susceptible” to an antibacterial using previously established breakpoints should be the prompt that necessitates reevaluation of antibacterial breakpoints. It is our belief that the regulatory authorities should mandate that the manufacturer of the drug undergo collection of PK/PD and clinical data relevant to the breakpoint reevaluation. The PK/PD data should be of the standard we have previously defined. It is impractical to suggest that the clinical data should come from new prospective randomized trials. Rather, such clinical data should come from large data sets for consecutive patients treated with the relevant antibacterial. In general and where relevant, bloodstream infection is the most useful infection type in such a reevaluation since there can be little debate as to the clinical relevance of such an infection. Clinical and bacteriologic end points (i.e., rates of cure, improvement and failure, or eradication and persistence) should be predefined rather than determined after preliminary exploration of the data. Potential confounders to an association with clinical outcome include the dosing regimen, organism type, portal of entry of the organism, comorbid illnesses, severity of illness, presence of immunosuppression, and concomitant antibacterial therapy. The clinical data set should be of sufficient size that the assessment is adequately powered to determine statistically significant differences in outcome from infections due to organisms with different MICs. A further layer of substantiation of clinical outcome may come from comparison of outcomes from infections with the target organism/MIC but with different antibacterials used for treatment.
An example is provided in order to illustrate such an approach. A variety of β-lactamases which can hydrolyze cefepime yet occur in organisms with cefepime MICs within the susceptible range have been detected (110). PK/PD analysis suggests that target attainment may be suboptimal using regulator-approved doses of cefepime for some MICs previously regarded as susceptible (127). A data set of consecutive patients with gram-negative bacteremia treated with cefepime would be collected, including the acquisition of data on confounding variables. The outcomes for patients infected with organisms of different MICs would be compared and analyzed using logistic regression or other multivariate analysis. If statistically significant differences in outcomes at different MICs occur, this would provide strong support for a change in breakpoint.
Case reports or small case series (particularly of nonconsecutive patients) may be a “signal” to reevaluate breakpoints but should not themselves be regarded as satisfactory evidence when breakpoints are being reevaluated. If inadequate clinical data are present to support or refute breakpoint revisions based on PK/PD analysis, either the breakpoints based on PK/PD analysis should be accepted or phenotypic screening and confirmatory tests for the resistance mechanism might be developed. It is appreciated that the latter approach is more “conservative,” but many involved in breakpoint setting may prefer to “play it safe” and avoid risking patient safety by properly categorizing a potentially compromised antibacterial agent. In many cases, development of screening and confirmatory tests for new resistance mechanisms can be undertaken prior to acquisition of solid clinical data relevant to breakpoint assessment. The downside of this approach is that broader-spectrum agents are often inadvertently “promoted” by the testing laboratory.
SETTING ZONE DIAMETER BREAKPOINTS FOR DISK DIFFUSION TESTING
Susceptibility testing by the disk diffusion method rather than the MIC-based method is still very widely used. Although there have been attempts to establish disk diffusion interpretive criteria, usually by defining resistance as zone diameters smaller than those of the wild type (91), the only valid method is to correlate zone diameters with MICs as described below. Hence, once MIC breakpoints have been set, zone diameter breakpoints can be developed. The simplest approach is to plot a “scattergram” of zone diameters versus MICs for strains tested by both methods (130) (Fig. 4). Scattergrams allow visual inspection of the correlation between zone diameters and MICs. Originally, it was considered appropriate to combine similar species on a single scattergram and fit a regression line through the data points (63). However, this does not readily lead to the setting of criteria to discriminate between susceptible and resistant strains. Furthermore, MIC and zone diameter data are not evenly distributed along a continuum for a species or related species, but tend to cluster. Hence, the validity of applying regression is questionable.
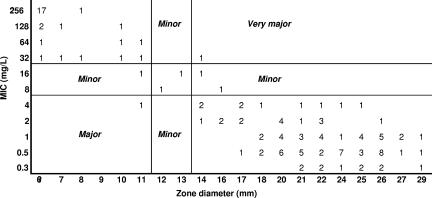
FIG. 4.
“Scattergram” of MICs versus zone diameters. Numbers represent the number of isolates at each MIC/zone diameter pair (e.g., there were 17 isolates whose MICs were ≥256 mg/liter and whose zone diameters were ≤6 mm). (Reprinted from reference 130 with permission of the publisher.)
The first effective statistical method for setting zone diameter interpretive criteria based on scattergram data was developed by Metzler and DeHaan (99). They developed the so-called error-rate-bounded method, which involves the selection of zone diameter values defining resistance and susceptibility from predefined acceptable rates of error. Tolerance of “very major” errors, where strains known to be resistant on MIC testing but whose zone diameter criterion would define them as susceptible, was set very low, at ≤1% of all MIC-zone diameter pairs (Fig. 4). This was justified because calling resistant strains susceptible in a laboratory test could cause serious adverse consequences for the patient. Tolerance of “major” errors, i.e., susceptible by MIC data but resistant by zone diameter data, was accepted at a level of ≤5%. These authors did not comment on acceptable rates of “minor” errors. Their analysis was also based on the use of a single MIC breakpoint defining susceptibility and resistance only on the basis of MICs, an infrequent situation for most susceptibility testing methods.
The error-rate-bounded method has been enhanced through the work of Brunden et al. (21). They adapted the method to two MIC breakpoints defining intermediate susceptibility as well as susceptibility and resistance and introduced an accepted “minor” error rate of ≤5%. Minor errors are zone diameter results that categorize either resistant or susceptible MIC results as intermediate or intermediate MIC results as resistant or susceptible (Fig. 4). They also introduced the concept of iteration to find the best fit of zone diameters that would minimize errors overall. This was achieved through the development of an index (index = [possible susceptible zone diameter − possible proposed resistant zone diameter]/percentage error). By trying different possible zone diameter pairs to define susceptible and resistant, the aim is to maximize the value of the index. While their accommodation of an intermediate range of MICs is now widely accepted, the iterative method of fitting has yet to be widely adopted.
An alternative method, again based on the concept of error rate bounding, has been proposed by the CLSI (24). In this system, discrepancy rates for very major, major, and minor errors are established for three different bands within the MIC range, whose widths vary according to the range of the intermediate MIC category. For example, in Fig. 4, the bands are ≤2 mg/liter, 4 to 16 mg/liter, and ≥32 mg/liter. Adjustments to discrepancy rates are made if there is only a single MIC breakpoint with no intermediate range. Readers are referred to the CLSI M23 document for further explanation about the method and its application (24). The method offers greater flexibility in establishing zone diameter interpretive criteria, but possibly at the cost of reduced correlation with MICs.
Kronvall et al. proposed a different approach altogether in setting zone diameter breakpoints (91, 92). They noted the resemblance of zone diameter distributions of the wild-type population to a normal distribution and developed a proprietary method for defining the distribution and setting a single zone diameter breakpoint. The method takes advantage of the fact that the upper end of the normal distribution is easily recognized graphically, and the data can be used statistically to define the lower end of the wild-type distribution by, in a sense, “reflecting” the upper end. MICs or breakpoints are not used in the analysis, and it cannot be used to determine intermediate zone diameter breakpoints.
An entirely different alternative to establishing zone diameter interpretive criteria has been proposed by Craig (32). The method is based on detailed statistical modeling of the spread and error of MICs and zone diameters and was designed to reduce the sometimes arbitrary choice of zone diameter breakpoints which can still occur when error-rate-bounded methods are used. Although this is the most sophisticated of the methods developed so far, it has yet to become adopted by any standard-setting body.
In summary, there is no current consensus on the optimum method for determining zone diameter breakpoints. While the method of Craig is the most robust statistically, it is not in a form, such as a computer program, that can be used readily by those charged with determining these breakpoints. One or another error-rate-bounded method will continue to be the standard for the immediate future. That recommended by Brunden et al. would serve the breakpoint setting community well for the foreseeable future.
UNANSWERED QUESTIONS AND FUTURE NEEDS
In spite of significant progress being made in recent years, breakpoint setting is still not an exact science. There are quite a few questions whose answers will improve the decision-making process and increase the interpretive value of breakpoints, including the following. (i) Should strains possessing an acquired resistance mechanism but having MICs below the PK/PD breakpoint be considered resistant and reported as such, regardless of the MIC? (ii) What are the best magnitudes to choose for the various PK/PD parameters? (iii) Do the magnitudes of the PK/PD parameters apply to all infection sites, or do they vary by site? (iv) Do the magnitudes of the PK/PD parameters apply to all bacteria, or do they vary by species? (v) Should breakpoints be linked to a specific dosage regimen? (vi) Is there utility in considering the MIC in conjunction with the breakpoint (e.g., the “breakpoint quotient,” or the MIC of the infecting pathogen divided by the breakpoint MIC), similar to the method proposed long ago for comparing blood or tissue levels with the MIC of the infecting pathogen (62)? (vii) If not all data are available, which data would be considered a minimum for setting a breakpoint? (viii) What systems need to be established to ensure the timely review of breakpoints as resistance emerges? (ix) Can MIC measurements be improved to give greater precision and reproducibility?
We look forward to the work of our colleagues to provide answers to these and other questions so that the art and science of breakpoint setting might converge in the not too distant future.
CONCLUSIONS
Breakpoints can be used in several ways. They allow communication from the clinical laboratory to the prescriber regarding the likelihood that a particular antibacterial regimen will be clinically useful in the treatment of patients with infections. They also allow the epidemiologic study of changing resistance patterns in a defined institution or geographic area. Breakpoints should be set prior to an antibacterial being used clinically and must be reviewed when mechanisms of resistance to the antibacterial become apparent. Breakpoint setting is not an exact science. It requires knowledge of the wild-type distribution of MICs, assessment of the PK/PD of the antibacterial, and study of the clinical outcome of infections when the antibacterial is used. Our current state of knowledge about each of these facets of breakpoint setting is imperfect. Breakpoint-setting organizations must utilize experts in microbiology, PD, and clinical infectious diseases in order to come to a consensus regarding the most appropriate breakpoint to be utilized. If appropriately developed and revised, breakpoints have greater relevance to the prescriber than does phenotypic detection of resistance mechanisms. However, breakpoint-setting organizations may also play a role in developing phenotypic tests for detection of resistance mechanisms, as this information may have epidemiologic and clinical importance that complements use of the breakpoint.
REFERENCES
- 1.Ambrose, P. G., and R. Quintiliani. 2000. Limitations of single point pharmacodynamic analysis. Pediatr. Infect. Dis. J. 19:769. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamics of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ambrose, P. G. 2005. Antimicrobial susceptibility breakpoints: PK-PD and susceptibility breakpoints. Treat. Respir. Med. 4(Suppl. 1):5-11. [PubMed] [Google Scholar]
- 5.Ambrose, P. G. 2006. Monte Carlo simulation in the evaluation of susceptibility breakpoints: predicting the future: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 26:129-134. [DOI] [PubMed] [Google Scholar]
- 6.Amsterdam, D. 2005. Susceptibility testing of antimicrobials in liquid media, p. 61-143. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 7.Andes, D., and W. A. Craig. 2005. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin. Microbiol. Infect. 11(Suppl. 6):10-17. [DOI] [PubMed] [Google Scholar]
- 8.Andes, D., R. Walker, S. Ebert, and W. Craig. 1994. Increasing protein binding of cefonicid enhances its in vivo activity in an animal model of infection, abstr. A93. Abstr. 34th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 9.Andes, D., M. L. van Ogtrop, J. Peng, and W. A. Craig. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484-3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrews, J. 2001. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 48(Suppl. S1):5-16. [DOI] [PubMed] [Google Scholar]
- 11.Andrews, J. January 2005, posting date. BSAC disc diffusion method for antimicrobial susceptibility testing, version 4, January 2005. British Society for Antimicrobial Chemotherapy, Birmingham, United Kingdom. www.bsac.org.uk. Accessed 5 February 2006.
- 12.Arakawa, S., T. Matsui, S. Kamidono, Y. Kawada, H. Kumon, K. Hirai, T. Hirose, T. Matsumoto, K. Yamaguchi, T. Yoshida, K. Watanabe, K. Ueno, A. Saito, and T. Teranishi. 1998. Derivation of a calculation formula for breakpoints of antimicrobial agents in urinary tract infections J. Infect. Chemother. 4:97-106. [Google Scholar]
- 13.Arbeidsgruppen for Antibiotikaspørsmål. 2006. AFAs brytningspunkter for bakteriers antibiotikafølsomhet, versjon 1.9. Arbeidsgruppen for Antibiotikaspørsmål, Tromso, Norway. www.unn.no/category10274. Accessed 1 November 2006.
- 14.Arbeidsgruppen for Antibiotikaspørsmål. 29 May 2005, posting date. Nødvendig bakgrunnsmateriale og prosedyrer for etablering og justering av mm-brytningssoner. Arbeidsgruppen for Antibiotikaspørsmål, Tromso, Norway. www.unn.no/article19775-927.html.
- 15.Baquero, F., J. Martínez-Beltrán, R. Cánton, and the remaining members of the Mesa Española de Normalización de la Sensibilidad y Resistencia a los Antimicrobianos (MENSURA). 1997. Criterios del grupo MENSURA para la definiciónde los puntos críticos de sensibilidad a los antibióticos. Rev. Esp. Quimioter. 10:103-313. [Google Scholar]
- 16.Bell, S. M. 1975. The CDS disc method of antibiotic sensitivity testing (calibrated dichotomous sensitivity test). Pathology 7(Suppl.):1-48. [DOI] [PubMed] [Google Scholar]
- 17.Bell, S. M., B. J. Gatus, J. N. Pham, and D. L. Rafferty. Accessed 1 November 2006. Antibiotic susceptibility testing by the CDS method. The CDS Users Group, Randwick, Australia. www.med.unsw.edu.au/pathology-cds.2004.
- 18.Bergan, T., J. N. Bruun, A. Digranes, E. Lingaas, K. K. Melby, and J. Sander. 1997. Susceptibility testing of bacteria and fungi. Scand. J. Infect. Dis. 103(Suppl.):1-36. [PubMed] [Google Scholar]
- 19.Blaser, J., B. B. Stone, M. C. Groner, and S. H. Zinner. 1985. Impact of netilmicin regimens on the activities of ceftazidime-netilmicin combinations against Pseudomonas aeruginosa in an in vitro pharmacokinetic model. Antimicrob. Agents Chemother. 28:64-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown, P. D., A. Freeman, and B. Foxman. 2002. Prevalence and predictors of trimethoprim-sulfamethoxazole resistance among uropathogenic Escherichia coli isolates in Michigan. Clin. Infect. Dis. 34:1061-1066. [DOI] [PubMed] [Google Scholar]
- 21.Brunden, M. N., G. E. Zurenko, and B. Kapik. 1992. Modification of the error-rate bounded classification scheme for use with two MIC break points. Diagn. Microbiol. Infect. Dis. 15:135-140. [DOI] [PubMed] [Google Scholar]
- 22.Chien, S.-C., M. C. Rogge, L. G. Gibson, C. Curtin, F. Wong, J. Natarajan, R. R. Williams, C. L. Fowler, W. K. Cheung, and A. Chow. 1997. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral and intravenous doses. Antimicrob. Agents Chemother. 41:2256-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitwood, L. A. 1969. Tube dilution antimicrobial susceptibility testing: efficacy of a microtechnique applicable to diagnostic laboratories. Appl. Microbiol. 17:707-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clinical and Laboratory Standards Institute. 2001. Development of in vitro susceptibility testing criteria and quality control parameters; approved guideline—2nd ed. CLSI document M23-A2. CLSI, Wayne, PA.
- 25.Clinical and Laboratory Standards Institute. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard—6th ed. CLSI document M11-A6. CLSI, Wayne, PA.
- 26.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial disk susceptibility tests; approved standard—9th ed. CLSI document M2-A9. CLSI, Wayne, PA.
- 27.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—7th ed. CLSI document M7-A7. CLSI, Wayne, PA.
- 28.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. CLSI document M100-S17. CLSI, Wayne, PA.
- 29.Comité de l'Antibiogramme de la Société Française de Microbiologie. February 2006, accession date.Communiqué 005. Société Française de Microbiologie, Paris, France. www.sfm.asso.fr.
- 30.Commissie Richtlijnen Gevoelighheidsbepalingen. 1996. Standaadisatie van gevoelighheidsbepalingen. Ned. Tijschr. Med. Microbiol. 4:104-106. [Google Scholar]
- 31.Commissie Richtlijnen Gevoelighheidsbepalingen. 2000. Interpretie van gevoelighheidsonderzoek en gevoelighheidscriteria voor antibacteriële middelen in Nederland. Ned. Tijdschr. Med. Microbiol. 8:79-81. [Google Scholar]
- 32.Craig, B. A. 2000. Modeling approach to diameter breakpoint determination. Diagn. Microbiol. Infect. Dis. 36:193-202. [DOI] [PubMed] [Google Scholar]
- 33.Craig, W. A., and S. C. Ebert. 1991. Killing and regrowth of bacteria in vitro: a review. Scand. J. Infect. Dis. 74(Suppl.):63-70. [PubMed] [Google Scholar]
- 34.Craig, W. A., Y. Watanabe, and S. Ebert. 1992. AUC/MIC ratio is the unifying parameter for comparison of the in vivo activity among aminoglycosides, abstr. 49. Abstr. 32nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 35.Craig, W. A., S. Ebert, and Y. Watanabe. 1993. Differences in time above MIC required for efficacy of beta-lactams in animal infection models, abstr. 86. Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 36.Craig, W. A. 1993. Qualitative susceptibility tests versus quantitative MIC tests. Diagn. Microbiol. Infect. Dis. 16:231-236. [DOI] [PubMed] [Google Scholar]
- 37.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 38.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 39.Craig, W. A. 1996. Antimicrobial resistance issues of the future. Diagn. Microbiol. Infect. Dis. 25:213-217. [DOI] [PubMed] [Google Scholar]
- 40.Craig. W. A., and S. Gudmundsson. 1996. Postantibiotic effect, p. 296-329. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD.
- 41.Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 42.Crump, J. A., T. J. Barrett, J. T. Nelson, and F. J. Angulo. 2003. Reevaluating fluoroquinolone breakpoints for Salmonella enterica serotype Typhi and for non-Typhi salmonellae. Clin. Infect. Dis. 37:75-81. [DOI] [PubMed] [Google Scholar]
- 43.Davies, T. A., A. Evangelista, S. Pfleger, K. Bush, D. F. Sahm, and R. Goldschmidt. 2002. Prevalence of single mutations in topoisomerase II genes among levofloxacin-susceptible clinical strains of Streptococcus pneumoniae isolated in the United States in 1992 to 1996 and 1999 to 2000. Antimicrob. Agents Chemother. 46:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 3. Agar diffusion test. DIN document 58940-. Beuth Verlag GmbH, Berlin, Germany.
- 45.Deutches Institut für Normung. 2000. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 3. Agar diffusion test—data for the interpretation of inhibition zone diameters. DIN document 58940-supplement 1. Beuth Verlag GmbH, Berlin, Germany.
- 46.Deutches Institut für Normung. 1989. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 3. Agar diffusion test—zone diameters with control strains. DIN document 58940-supplement 2. Beuth Verlag GmbH, Berlin, Germany.
- 47.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 4. Evaluation classes of the minimum inhibitory concentration. DIN document 58940-. Beuth Verlag GmbH, Berlin, Germany.
- 48.Deutches Institut für Normung. 2004. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 4. Evaluation classes of the minimum inhibitory concentration—MIC breakpoints of antibacterial agents. DIN document 58940-supplement 1. Beuth Verlag GmbH, Berlin, Germany.
- 49.Deutches Institut für Normung. 2003. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 6. Determination of the minimum inhibitory concentration (MIC) with the agar dilution method. DIN document 58940-. Beuth Verlag GmbH, Berlin, Germany.
- 50.Deutches Institut für Normung. 1989. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 6. Determination of the minimum inhibitory concentration (MIC) with the agar dilution method—MICs of agents with control strains for the agar dilution method. DIN document 58940-supplement 1. Beuth Verlag GmbH, Berlin, Germany.
- 51.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 81. Microdilution—special requirements for testing non-fastidious bacteria. DIN document 58940-1 1. Beuth Verlag GmbH, Berlin, Germany.
- 52.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 82. Microdilution—special requirements for testing fastidious bacteria. DIN document 58940-1. Beuth Verlag GmbH, Berlin, Germany.
- 53.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 83. Microdilution—special requirements for testing strictly anaerobic bacteria. DIN document 58940-3. Beuth Verlag GmbH, Berlin, Germany.
- 54.Deutches Institut für Normung. 2002. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. 10. Criteria for the evaluation of the in vitro efficacy and the admission of new antimicrobial agents. DIN document 58940-10. Beuth Verlag GmbH, Berlin, Germany.
- 55.Doern, G. V. 1995. The in vitro activity of cefotaxime versus bacteria involved in selected infections of hospitalized patients outside the intensive care unit. Diagn. Microbiol. Infect. Dis. 22:13-17. [DOI] [PubMed] [Google Scholar]
- 56.Drusano, G. L. 1988. Role of pharmacokinetics in the outcome of infections. Antimicrob. Agents Chemother. 32:289-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drusano, G. L., S. L. Preston, C. Hardalo, R. Hare, C. Banfield, D. Andes, O. Vesga, and W. A. Craig. 2001. Use of preclinical data for selection of a phase II/III dose for evernimicin and identification of a preclinical MIC breakpoint. Antimicrob. Agents Chemother. 45:13-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drusano, G. L. 2004. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat. Rev. Microbiol. 2:289-300. [DOI] [PubMed] [Google Scholar]
- 59.Drusano, G. L., S. L. Preston, C. Fowler, M. Corrado, B. Weisenger, and J. Kahn. 2004. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in eradication of the infecting pathogen, in patients with nosocomial pneumonia. J. Infect. Dis. 189:1590-1597. [DOI] [PubMed] [Google Scholar]
- 60.Dudley, M. N., and P. G. Ambrose. 2000. Pharmacodynamics in the study of drug resistance and establishing in vitro susceptibility breakpoints: ready for prime time. Curr. Opin. Microbiol. 3:515-521. [DOI] [PubMed] [Google Scholar]
- 61.Eagle, H., R. Fleischman, and M. Levy. 1953. Continuous versus discontinuous therapy with penicillin: the effect of interval between injections on therapeutic efficacy. N. Engl. J. Med. 248:481-488. [DOI] [PubMed] [Google Scholar]
- 62.Ellner, P. D., and H. C. Neu. 1981. The inhibitory quotient. A method for interpreting minimum inhibitory concentration data. JAMA 246:1575-1578. [DOI] [PubMed] [Google Scholar]
- 63.Ericsson, H., and J. C. Sherris. 1971. Antibiotic sensitivity testing. Report of an international collaborative study. Acta Pathol. Microbiol. Scand. 217(Suppl. B):1-90. [PubMed] [Google Scholar]
- 64.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. Determination of antimicrobial susceptibility test breakpoints. Clin. Microbiol. Infect. 6:570-572. [DOI] [PubMed] [Google Scholar]
- 65.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin. Microbiol. Infect. 6:509-515. [DOI] [PubMed] [Google Scholar]
- 66.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 6:503-508. [DOI] [PubMed] [Google Scholar]
- 67.European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). 2000. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9:1-7. [DOI] [PubMed] [Google Scholar]
- 68.Falcó, V., B. Almirante, Q. Jordano, L. Calonge, O. del Valle, C. Pigrau, A. M. Planes, J. Gavaldà, and A. Pahissa. 2004. Influence of penicillin resistance on outcome in adult patients with invasive pneumococcal pneumonia: is penicillin useful against intermediately resistant strains? J. Antimicrob. Chemother. 54:481-488. [DOI] [PubMed] [Google Scholar]
- 69.Firsov, A. A., S. N. Vostrov, I. Y. Lubenko, K. Drlica, Y. A. Portnoy, and S. H. Zinner. 2003. In vitro pharmacodynamic evaluation of the mutant selection window hypothesis using four fluoroquinolones against Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1604-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fleming, A. 1929. On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br. J. Exp. Pathol. 10:226-236. [Google Scholar]
- 71.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forrest, A., S. Chodosh, M. A. Amantea, D. A. Collins, and J. J. Schentag. 1997. Pharmacokinetics and pharmacodynamics of oral grepafloxacin in patients with acute bacterial exacerbations of chronic bronchitis. J. Antimicrob. Chemother. 40(Suppl. A):45-57. [DOI] [PubMed] [Google Scholar]
- 73.Franklin, C., L. Liolios, and A. Y. Peleg. 2006. Phenotypic detection of carbapenem-susceptible metallo-β-lactamase-producing gram-negative bacilli in the clinical laboratory. J. Clin. Microbiol. 44:3139-3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frimodt-Møller, N. 2002. Correlation between pharmacokinetic/pharmacodynamic parameters and efficacy for antibiotics in the treatment of urinary tract infection. Int. J. Antimicrob. Agents 19:546-553. [DOI] [PubMed] [Google Scholar]
- 75.Gehanno, P., G. Lenoir, and P. Berche. 1995. In vivo correlates for Streptococcus pneumoniae penicillin resistance in acute otitis media. Antimicrob. Agents Chemother. 39:271-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gerber, A. U., A. P. Vastola, J. Brandel, and W. A. Craig. 1982. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J. Infect. Dis. 146:691-697. [DOI] [PubMed] [Google Scholar]
- 77.Gupta, K., T. M. Hooton, and W. E. Stamm. 2001. Increasing antimicrobial resistance and the management of uncomplicated community-acquired urinary tract infections. Ann. Int. Med. 135:41-50. [DOI] [PubMed] [Google Scholar]
- 78.International Organization for Standards. 15 November 2006, posting date. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility testing devices. 1. Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing anaerobic bacteria involved in infectious diseases. ISO 20776-1. International Organization for Standards, Geneva, Switzerland. http://www.iso.org/iso/en/CatalogueDetailPage.CatalogueDetail?CSNUMBER=41630.
- 79.Japanese Society for Chemotherapy. 1990. Method for the determination of minimum inhibitory concentration (MIC) of aerobic bacteria by microdilution method. Chemotherapy (Tokyo) 38:102-105. [Google Scholar]
- 80.Japanese Society for Chemotherapy. 1993. Method for the determination of minimum inhibitory concentration (MIC) of fastidious bacteria and anaerobic bacteria by microdilution method. Chemotherapy (Tokyo) 41:183-189. [Google Scholar]
- 81.Kahlmeter, G. 16 October 2006, accession date. EUCAST procedure for harmonising and defining breakpoints. EUCAST, Basel, Switzerland. http://www.srga.org/EUCAST/bpsetting.htm.
- 82.Kahlmeter, G., D. F. J. Brown, F. W. Goldstein, A. P. MacGowan, J. W. Mouton, A. Österland, A. Rodloff, M. Steinbakk, P. Urbaskova, and A. Vatopoulos. 2003. European harmonisation of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J. Antimicrob. Chemother. 52:145-148. [DOI] [PubMed] [Google Scholar]
- 83.Kang, C.-I., S.-H. Kim, W. B. Park, K.-D. Lee, H.-B. Kim, E.-C. Kim, M.-Y.Oh, and K.-W. Choe. 2004. Bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob. Agents Chemother. 48:4574-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaplan, S. L. 2002. Management of pneumococcal meningitis. Pediatr. Infect. Dis. J. 21:589-592. [DOI] [PubMed] [Google Scholar]
- 85.Kashuba, A. D. M., A. N. Nafziger, G. L. Drusano, and J. S. Bertino. 1999. Optimizing aminoglycoside therapy for nosocomial pneumonia. Antimicrob. Agents Chemother. 43:623-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kellner, J. D., D. W. Scheifele, S. A. Halperin, M. C. Lebel, D. Moore, N. Le Saux, E. L. Ford-Jones, B. Law, W. Vaudry, and Other Members of the Canadian Paediatric Society/Centre for Infectious Disease Prevention and Control, Immunization Monitoring Program. 2002. Outcome of penicillin-nonsusceptible Streptococcus pneumoniae meningitis: a nested case-control study. Pediatr. Infect. Dis. J. 21:903-909. [DOI] [PubMed] [Google Scholar]
- 87.Kerrn, M. B., N. Frimodt-Møller, and F. Espersen. 2003. Effects of sulfamethizole and amdinocillin against Escherichia coli strains (with various susceptibilities) in an ascending urinary tract infection mouse model. Antimicrob. Agents Chemother. 47:1002-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knudsen, J. D., K. Fuursted, F. Espersen, and N. Frimodt-Møller. 1997. Activities of vancomycin and teicoplanin against penicillin-resistant pneumococci in vitro and in vivo and correlation to pharmacokinetics parameters in the mouse peritonitis model. Antimicrob. Agents Chemother. 41:1910-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Knudsen, J. D., K. Fuursted, S. Raber, F. Espersen, and N. Frimodt-Møller. 2000. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob. Agents Chemother. 44:1247-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Koeth, L. M., A. King, H. Knight, J. May, L. A. Miller, I. Phillips, and J. A. Poupard. 2000. Comparison of cation-adjusted Mueller-Hinton broth with Iso-Sensitest broth for the NCCLS broth microdilution method. J. Antimicrob. Chemother. 46:369-376. [DOI] [PubMed] [Google Scholar]
- 91.Kronvall, G., G. Kahlmeter, E. Myrne, and M. F. Galas. 2003. A new method for normalized interpretation of antimicrobial resistance from disk test results for comparative purposes. Clin. Microbiol.Infect. 9:120-132. [DOI] [PubMed] [Google Scholar]
- 92.Kronvall, G. 2003. Determination of the real standard distribution of susceptible strains in zone histograms. Int. J. Antimicrob. Agents 22:7-13. [DOI] [PubMed] [Google Scholar]
- 93.Kronvall, G., I. Karlsson, M. Walder, M. Sörberg, and L. E. Nisson. 2006. Epidemiological MIC cut-off values for tigecycline calculated from Etest values using normalized resistance interpretation. J. Antimicrob. Chemother. 57:498-505. [DOI] [PubMed] [Google Scholar]
- 94.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relations at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-282. [DOI] [PubMed] [Google Scholar]
- 95.MacGowan, A., and R. Wise. 2001. Establishing MIC breakpoints and the interpretation of in vitro susceptibility tests. J. Antimicrob. Chemother. 48(Suppl. S1):17-28. [DOI] [PubMed] [Google Scholar]
- 96.MacGowan, A., C. Rogers, and K. Bowker. 2003. In vitro models, in vivo models, and pharmacokinetics: what can we learn from in vitro models? Clin. Infect. Dis. 33(Suppl. 3):S214-S220. [DOI] [PubMed] [Google Scholar]
- 97.McDonald, P. J., B. H. Wetherall, and H. Pruul. 1981. Postantibiotic leukocyte enhancement: increased susceptibility of bacteria pretreated with antibiotics to activity of leukocytes. Rev. Infect. Dis. 3:38-44. [DOI] [PubMed] [Google Scholar]
- 98.Mesa Española de Normalización de la Sensibilidad y Resistencia a los Antimicrobianos (MENSURA). 2000. Recommedaciones del grupo MENSURA para la selecciónde antimicrobianos en el studio de la sensibilidad y criterios para la interpretacióndel antibiograma. Rev. Esp. Quimioter. 13:73-86. [PubMed] [Google Scholar]
- 99.Metzler, C. M., and R. M. DeHaan. 1974. Susceptibility tests of anaerobic bacteria: statistical and clinical considerations. J. Infect. Dis. 30:88-594. [DOI] [PubMed] [Google Scholar]
- 100.Miller, L. G., and A. W. Tang. 2004. Treatment of uncomplicated urinary tract infections in an era of increasing antimicrobial resistance. Mayo Clin. Proc. 79:1048-1054. [DOI] [PubMed] [Google Scholar]
- 101.Moise, P. A., A. Forrest, S. M. Bhavnani, M. C. Birmingham, and J. J. Schentag. 2000. Area under the inhibitory curve and a pneumonia scoring system for predicting outcomes of vancomycin therapy for respiratory infections by Staphylococcus aureus. Am. J. Hosp. Pharm. 57(Suppl. 2):S4-S9. [DOI] [PubMed] [Google Scholar]
- 102.Moise-Broder, P. A., A. Forrest, M. C. Birmingham, and J. J. Schentag. 2004. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharmacokinet. 43:925-942. [DOI] [PubMed] [Google Scholar]
- 103.Moore, R. D., P. S. Lietman, and C. R. Smith. 1987. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimum inhibitory concentration. J. Infect. Dis. 155:93-99. [DOI] [PubMed] [Google Scholar]
- 104.Mouton, J. W., J. E. Degener, B. van Klingeren, A. J. de Neeling, and Commissie Richtlijnen Gevoelighheidsbepalingen. 2000. Het vastellen van gevoelighehidscriteria voor antibcateriële middelen in Nederland: verleden, heden en toekomst. Ned. Tijdschr. Med. Microbiol. 8:73-78. [Google Scholar]
- 105.Mouton, J. W. 2003. Impact of pharmacodynamics on breakpoint selection for susceptibility testing. Infect. Dis. Clin. N. Am. 17:579-598. [DOI] [PubMed] [Google Scholar]
- 106.Odenholt Tornqvist, I., S. E. Holm, and O. Cars. 1991. Pharmacodynamic effects of subinhibitory antibiotic concentrations. Scand. J. Infect. Dis. 74(Suppl.):94-101. [PubMed] [Google Scholar]
- 107.Olivier, C., R. Cohen, P. Begué, and D. Floret. 2000. Bacteriologic outcome of children with cefotaxime- or ceftriaxone-susceptible and -nonsusceptible Streptococcus pneumoniae meningitis. Pediatr. Infect. Dis. J. 19:1015-1017. [DOI] [PubMed] [Google Scholar]
- 108.Pallares, R., J. Liñares, M. Vadillo, C. Cabellos, F. Manresa, P. F. Viladrich, R. Martin, and F. Gudiol. 1995. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N. Engl. J. Med. 333:474-480. [DOI] [PubMed] [Google Scholar]
- 109.Paterson, D. L., and R. A. Bonomo. 2005. Extended-spectrum beta-lactamases: a clinical update. Clin. Microbiol. Rev. 18:657-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Paterson, D. L., W.-C. Ko, A. Von Gottberg, J. M. Casellas, L. Mulazimoglu, K. P. Klugman, R. A. Bonomo, L. B. Rice, J. G. McCormack, and V. L. Yu. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical laboratory. J. Clin. Microbiol. 39:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Preston, S. L., G. L. Drusano, A. L. Berman, C. L. Fowler, A. T. Chow, B. Dornseif, V. Reichl, J. Natarajan, and M. Corrado. 1998. Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials. JAMA 279:125-129. [DOI] [PubMed] [Google Scholar]
- 112.Rasheed, J. K., and F. C. Tenover. 2003. Detection and characterization of antimicrobial resistance genes in bacteria, p. 1196-1212. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 113.Raz, R., B. Chazan, Y. Kennes, R. Colodner, E. Rottensterrich, M. Dan, W. Stamm, and the Israeli Urinary Tract Infection Group. 2002. Empiric use of trimethoprim-sulfamethoxazole (TMP-SMX) in the treatment of women with uncomplicated urinary tract infections, in a geographical area with a high prevalence of TMP-SMX-resistant uropathogens. Clin. Infect. Dis. 24:1165-1169. [DOI] [PubMed] [Google Scholar]
- 114.Rosco Diagnostica. 2006 2005. User's guide. Neo-Sensitabs susceptibility testing, 17th ed. Rosco Diagnostica A/C, Taastrup, Denmark.
- 115.Saito, A. 1995. Clinical breakpoints for antimicrobial agents in pulmonary infections and sepsis: report of the Committee for Japanese Standards for Antimicrobial Susceptibility Testing of Bacteria. J. Infect. Chemother. 1:83-88. [Google Scholar]
- 116.Saito, A., T. Inamatsu, J. Okada, T. Oguri, H. Kanno, N. Kusano, H. Kumon, K. Yamaguchi, A. Watanabe, and K. Watanabe. 1999. Clinical breakpoints in pulmonary infections and sepsis: new antimicrobial agents and supplemental information for some agents already released. J. Infect. Chemother. 5:223-226. [DOI] [PubMed] [Google Scholar]
- 117.Sakoulas, G., P. A. Moise-Broder, J. Schentag, A. Forrest, R. C. Moellering, Jr., and G. M. Eliopoulos. 2004. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob. Agents Chemother. 42:2398-2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schentag, J. J., I. L. Smith, D. J. Swanson, C. DeAngelis, J. E. Fracasso, A. Vari, and J. W. Vance. 1984. Role of dual individualization with cefmenoxime. Am. J. Med. 77:43-50. [DOI] [PubMed] [Google Scholar]
- 119.Sirot, J., P. Courvalin, and C.-J. Soussy. 1996. Definition and determination of in vitro antibiotic susceptibility breakpoints for bacteria. Clin. Microbiol. Infect. 2(Suppl. 1):S5-S10. [DOI] [PubMed] [Google Scholar]
- 120.Snydman, D. R., G. J. Cuchural, L. McDermott, and M. Gill. 1992. Correlation of various in vitro testing methods with clinical outcomes in patients with Bacteroides fragilis group infections treated with cefoxitin: a retrospective study. Antimicrob. Agents Chemother. 36:540-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sociedad Españolade Enfermedades Infecciosas y Microbiología Clínica. 2000. Procedimientos en microbiología clínica: métados básicos para el estudio de la sensibilidad a los antimicrobianos. Sociedad Españolade Enfermedades Infecciosas y Microbiología Clínica, Madrid, Spain. http://www.seimc.org/protocolos/microbiologia/.
- 122.Stamey, T. A., W. R. Fair, M. M. Timothy, M. A. Millar, G. Mihara, and Y. C. Lowery. 1974. Serum versus urinary antimicrobial concentrations in cure of urinary-tract infections. N. Engl. J. Med. 291:1159-1163. [DOI] [PubMed] [Google Scholar]
- 123.Swedish Reference Group on Antibiotics. 11 February 2004, revision date. The principles behind SRGA breakpoints. Swedish Reference Group on Antibiotics, Vaxjo, Sweden. www.srga.org. Accessed 5 February 2006.
- 124.Swenson, J. M., J. F. Hindler, and J. H. Jorgensen. 2003. Special phenotypic methods for detecting antibacterial resistance, p. 1178-1195. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed., vol. 1. ASM Press, Washington, DC. [Google Scholar]
- 125.Szabó, D., R. A. Bonomo, F. Silveira, A. W. Pasculle, C. Baxter, P. K. Linden, A. M. Hujer, K. M. Hujer, K. Deeley, and D. L. Paterson. 2005. SHV-type extended-spectrum beta-lactamase production is associated with reduced cefepime susceptibility in Enterobacter cloacae. J. Clin. Microbiol. 43:5058-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tam, V. H., P. S. McKinnon, R. L. Akins, M. L. Ryback, and G. L. Drusano. 2002. Pharmacodynamics of cefepime in patients with gram-negative infections. J. Antimicrob. Chemother. 50:425-428. [DOI] [PubMed] [Google Scholar]
- 127.Tam, V. H., P. S. McKinnon, R. L. Akins, G. L. Drusano, and M. L. Ryback. 2003. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob. Agents Chemother. 47:1853-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Turnidge, J. D. 1998. The pharmacodynamics of β-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 129.Turnidge, J. 1999. Pharmacokinetics and pharmacodynamics of fluoroquinolones. Drugs 58(Suppl. 2):29-36. [DOI] [PubMed] [Google Scholar]
- 130.Turnidge, J. D., and J. M. Bell. 2005. Antimicrobial susceptibility on solid media, p. 8-60. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 131.Turnidge, J., and G. Bordash. 2007. Statistical methods for establishing quality control ranges for antibacterial agents in Clinical and Laboratory Standards Institute susceptibility testing. Antimicrob. Agents Chemother. 51:2483-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Turnidge, J., G. Kahlmeter, and G. Kronvall. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin. Microbiol. Infect. 12:418-425. [DOI] [PubMed] [Google Scholar]
- 133.Van Wart, S., L. Phillips, E. A. Ludwig, R. Russo, D. A. Gajjar, A. Bello, P. G. Ambrose, C. Costanzo, T. H. Grasela, R. Echols, and D. M. Grasela. 2004. Population pharmacokinetics of garenoxacin in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 48:4766-4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vogelman, B., S. Gudmundsson, J. Turnidge, J. Leggett, and W. A. Craig. 1988. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J. Infect. Dis. 157:287-298. [DOI] [PubMed] [Google Scholar]
- 135.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 136.Walsh, T. R., M. A. Toleman, L. Poirel, and P. Nordmann. 2005. Metallo-β-lactamases: the quiet before the storm? Clin. Microbiol. Rev. 18:306-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Watanabe, Y., S. Ebert, and W. Craig. 1992. AUC/MIC ratio is the unifying parameter for comparison of the in-vivo activity among fluoroquinolones, abstr. 42. Abstr. 32nd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 138.Werkgroep Richtlijnen Gevoelighheidsbepalingen. 1981. Standaadisatie van gevoelighheidsbepalingen. RIVM, Bilthoven, The Netherlands.
- 139.Werkgroep Richtlijnen Gevoelighheidsbepalingen. 1990. Standaadisatie van gevoelighheidsbepalingen, supplement e. RIVM, Bilthoven, The Netherlands.
- 140.Werkgroep Richtlijnen Gevoelighheidsbepalingen. 1991. Standaadisatie van gevoelighheidsbepalingen, supplement e, bijlage. RIVM, Bilthoven, The Netherlands.
- 141.Wikler, M. A., and P. G. Ambrose. 2005. The breakpoint, p. 1-7. In V. Lorian (ed.), Antibiotics in laboratory medicine, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 142.Yu, V. L., C. C. C. Chiou, C. Feldman, A. Ortqvist, J. Rello, A. J. Morris, L. M. Baddour, C. M. Luna, D. R. Snydman, M. Ip, W. C. Ko, B. F. Chedid, A. Andremont, and K. Klugman. 2003. An international prospective study of pneumococcal bacteremia: correlation with in vitro resistance, antibiotics administered, and clinical outcome. Clin. Infect. Dis. 37:320-327. [DOI] [PubMed] [Google Scholar]
- 143.Zelenitsky, S. A., G. K. Harding, S. Sun, K. Ubhi, and R. E. Ariano. 2003. Treatment and outcome of Pseudomonas aeruginosa bacteraemia: an antibiotic pharmacodynamic analysis. J. Antimicrob. Chemother. 52:668-674. [DOI] [PubMed] [Google Scholar]