Abstract
The human immunodeficiency virus (HIV) epidemic emerged in the early 1980s with HIV infection as a highly lethal disease among men who have sex with men and among frequent recipients of blood product transfusions. Advances in the treatment of HIV infection have resulted in a fundamental shift in its epidemiology, to a potentially chronic and manageable condition. However, challenges in the prevention of this infection remain. In particular, increasing evidence suggests that transmission of drug-resistant virus is becoming more common and that the epidemic is having a profound impact on morbidity and mortality in ethnic and racial minority subgroups in the United States. New population-based data collection systems designed to describe trends in behaviors associated with HIV transmission and better methods for measuring the true incidence of transmission will better elucidate the characteristics of HIV infection in the United States and inform future public health policies.
INTRODUCTION
Advances in the treatment of human immunodeficiency virus (HIV) infection have resulted in a fundamental shift in its epidemiology, from a highly lethal infectious disease to a potentially chronic and manageable condition (46). Thus, overall rates of the most severe clinical presentations of the infection, i.e., AIDS, have declined compared to those in the early 1990s (35). The result is that more people are living with HIV infection, and deaths from AIDS remain low relative to historical levels (33). Unfortunately, successes in preventing new infections appear to have stalled during the late 1990s (33). Therefore, achieving a clear understanding of the sociobehavioral determinants of HIV infection will be essential for successfully controlling the disease. Moreover, racial and ethnic disparities clearly exist both in access to effective treatments for HIV and in rates of new infections (15). Given that the success of prevention and treatment efforts depends on accurate and complete identification of at-risk populations, collection, analysis, and interpretation of surveillance data remain critical for keeping abreast of the evolving nature of the epidemic (12, 50). In particular, the increase in the time from diagnosis of HIV to the onset of AIDS resulting from the use of effective antiretrovirals underscores the need for ongoing enumeration and evaluation of the extents and patterns of diagnosed HIV infections as well as AIDS cases (25).
In 1999, the Centers for Disease Control and Prevention (CDC) recommended national implementation of name-based HIV reporting, which had previously been implemented in only 34 areas (11). To date, all states and the District of Columbia have mandated some form of reporting for individuals diagnosed with HIV infection, even in the absence of AIDS, and efforts are under way to standardize methods to comply with CDC-recommended confidential name-based reporting and to integrate data into a national HIV/AIDS surveillance system (17). An essential role of such a surveillance system is to safeguard against duplication of case reports among different states to provide the most accurate and complete picture of the HIV epidemic across the United States. The purpose of this paper is to describe the current state of HIV infection, using national HIV/AIDS surveillance data.
BACKGROUND AND EARLY HISTORY
The first decade of the HIV epidemic was characterized by steady and sharp increases in the incidence of AIDS cases, punctuated by diagnostic and therapeutic milestones as well as changes in the methods used to monitor the clinical manifestations of the infection. These changes were precipitated by increases in the number of new infections and advances in the understanding of the biology of the virus. By the peak of the epidemic in 1993, AIDS had become the leading cause of death in men and women aged 25 to 44 years and was the eighth leading cause of death overall, accounting for 2% of all deaths in the United States (10). Age-adjusted death rates attributed to HIV increased in a linear fashion from 6 deaths per 100,000 in 1987 to 17 deaths per 100,000 in 1995, when deaths due to the disease peaked. Between 1995 and 1998, however, the numbers of reported AIDS cases and deaths in the United States declined precipitously, at rates of 28%, 45%, and 18% each year, after which they remained relatively stable, at approximately 40,000 each year through 2004 (7). Widespread use of improved antiretroviral therapy leading to longer incubation periods and survival after infection is believed to explain most of the rapid reduction in mortality in the mid-1990s (21). However, other factors, such as increased use of prophylaxis for opportunistic infections and primary prevention, are also likely to have contributed to the observed decline (28).
Models that indirectly measured the incidence of HIV infection, using AIDS case reports and knowledge about the distribution of HIV incubation periods, indicated that the transmission incidence peaked around the mid-1980s, approximately a decade earlier than did cases of AIDS (33).
In the early years of the epidemic, HIV/AIDS, defined as all HIV infections, with and without AIDS, was characterized as a disease of white men who have sex with men (MSM), the group in whom the disease was first recognized (8). Injecting drug users (IDUs) represented approximately 20% of cases, while heterosexuals and females from all transmission categories accounted for about 5% and 8% of all reported cases, respectively (10). Because direct socioeconomic data were not routinely collected as part of the AIDS national surveillance system, little to no information is available at the national level to describe the socioeconomic characteristics of the early patients.
DATA SOURCES AND METHODS
HIV/AIDS National Surveillance
The national HIV/AIDS surveillance system, supported by the CDC, is the primary national source of information used to track the changing characteristics and patterns of the epidemic. With ongoing technical assistance from the CDC, state and local health departments collect surveillance data for analysis at the local level (41). Local surveillance data are routinely reported to the CDC, where they are analyzed in the aggregate to provide national population-based estimates aimed at elucidating the epidemic. Surveillance data include data on all persons diagnosed with HIV in the entire U.S. population, including institutionalized individuals and noncivilian (i.e., military) persons.
Prior to the widespread use of highly active antiretroviral therapy (HAART) in 1996, HIV prevalence was estimated indirectly through mathematical models designed to “back-calculate” numbers of HIV diagnoses (1, 4). The validity of these estimates depended not only on the number of reported AIDS cases but also on knowledge of the HIV incubation period distribution needed to estimate the probability of developing AIDS for each year following HIV infection (23). HAART can differentially affect the incubation period distribution for users, depending on access, adherence, and effectiveness. This situation renders the back-calculation method based on the incidence of AIDS inaccurate for deriving the infection incidence for HIV after the advent of HAART. Since 1994, the number of states using an integrated HIV/AIDS surveillance system has increased (23). Currently, data are available from 33 states with surveillance systems that integrate ascertainment of new HIV diagnoses with monitoring of AIDS cases, using confidential name-based reporting. Sixty-three percent of all new U.S. AIDS cases each year are diagnosed in these states (23). It is important, however, that current surveillance data are limited to persons with a diagnosed HIV infection and do not directly represent the number of infections because some HIV-infected individuals may not get tested.
Population-Based Serosurveys (NHANES)
The National Health and Nutrition Examination Surveys (NHANES) are a series of population-based cross-sectional surveys designed to provide nationally representative estimates of the health and nutritional status of the civilian noninstitutionalized U.S. population (9). NHANES data are collected through personal interviews and physical examinations of survey participants, with the latter including biologic samples used for anonymous HIV antibody testing from 1988 to 1994 and for confidential name-based HIV antibody testing from 1999 to 2002 (42). As such, NHANES is the only nationally representative survey from which HIV seroprevalence can be estimated. Moreover, data from the 1999-2002 NHANES can be used to examine associations between HIV serostatus and various demographic and risk characteristics for a more thorough investigation of the impact of these factors on the disease (39).
Other Methods
Mortality data collected through the HIV/AIDS surveillance system are sensitive to changes in the HIV and AIDS case definitions because surveillance reporting is limited to cases that meet the definition in effect at the time of a person's death. For example, the annual numbers of deaths among persons with HIV infection or AIDS reported to CDC's HIV/AIDS surveillance program by state and local health departments may be affected by expansion of the CDC case definition for AIDS and by expansion of the policies of the health departments for reporting of non-AIDS HIV infection cases. Reporting may be less complete for AIDS cases meeting the expanded criteria of the current AIDS case definition but not meeting the narrower criteria of earlier case definitions, particularly for cases diagnosed years before the case definition was expanded in 1993. Further complicating the interpretation of mortality for cases reported to CDC's HIV/AIDS surveillance program is the fact that no distinction is made between deaths due to HIV/AIDS and deaths due to other causes. In contrast, death certificate data on deaths attributed to HIV disease (regardless of whether it is AIDS or non-AIDS) as the underlying cause are less likely to have been affected by changes in case reporting policies and practices since 1987 and are more likely to reflect true long-term trends in mortality caused by HIV. Therefore, estimates of trends and distributions of deaths due to HIV in the United States are based on data compiled by the National Center for Health Statistics (NCHS) from death certificates of U.S. residents in the 50 states and the District of Columbia. The underlying cause of each death is selected from the conditions reported by physicians, medical examiners, and coroners in the cause-of-death section of the death certificate. The mortality data from NCHS are the sole source of information on all causes of death in the national population, allowing comparison of deaths due to HIV disease and deaths due to other causes (7, 29).
Smaller serosurveys lack either power (if population-based) or representativeness (if targeted to special high-risk populations) and thus cannot be generalized to the U.S. population. Also, in the case of the latter design, the size and characteristics of the population from which samples were drawn (denominator) are often unknown.
Longitudinal cohort studies to determine the incidence of HIV infection are not practical due to the associated costs and loss to follow-up and may also lack representativeness.
CURRENT PICTURE
The most recent estimates indicate that 40 million people are currently infected with HIV (with and without AIDS) worldwide, 1.2 million of whom are in the United States and thus represent 2.5% of the global disease burden (24, 48).
Mortality Patterns
After years of steady increase followed by precipitous declines, deaths of persons with HIV/AIDS reported to the national HIV/AIDS surveillance system and U.S. Vital Statistics began to stabilize to approximately 5 per 100,000 in 1998 (7, 29). Between 2000 and 2004, in contrast to the steep annual decreases of up to 45% in the latter part of the 1990s, deaths of persons with HIV/AIDS declined only a modest 8%. The lower rates of decline in HIV-related mortality in recent years are largely attributed to the emergence of resistance to currently available HAART regimens and to differential access to treatment. When these medications are appropriately prescribed and the regimens are followed, the progression of the infection can be arrested effectively and substantial immune reconstitution can ensue. However, such treatment cannot eradicate or cure HIV infection. Therefore, ongoing therapy is necessary to arrest disease progression. Late diagnosis, erratic adherence, and adverse side effects of the medications result in poorer outcomes and the emergence of drug resistance. All of these health service challenges are compounded by the fact that HIV is increasingly impacting communities that lack access to treatment, including racial and ethnic minorities and the socioeconomically disadvantaged (3, 15).
Although death rates have followed similar patterns across most demographic and behavioral strata, including gender, age, geographic distribution, and race/ethnicity, substantial variation exists in the percentages of decline among different subgroups. For example, although the number of deaths attributed to HIV has declined in both men and women, it has always been and continues to be higher among males (Fig. 1). This reflects the higher incidence rate of the infection in males. However, over time, the ratio of male to female deaths fell from 10:1 in 1987 to 3:1 in 1998 (7, 29). Similarly, although HIV-related deaths declined rapidly in the 1990s in all racial groups, the percent decline was significantly lower for non-Hispanic blacks than for any other race/ethnic group, suggesting race/ethnic disparities (Fig. 2). Further stratification of race/ethnicity by gender illustrated similar lower percent decreases in nonwhite men and women. Black women had the smallest percent decline of any category. Moreover, between 2001 and 2004, while the number of deaths continued to decrease steadily, if less dramatically, for non-Hispanic whites, they either stayed the same or increased slightly from year to year among the other race/ethnic groups. Percent declines also differed by geographic region and by poverty level. Geographic comparison of mortality patterns across the U.S. Census Bureau-designated regions of the country (Northeast, South, West, and Midwest) showed the highest death rates in the Northeast and South, with coastal states and large cities carrying the heaviest burden of deaths due to HIV (Fig. 3). Racial and ethnic disparities in death rates were observed in all regions, with non-Hispanic blacks showing the lowest percent decline compared to other race/ethnic groups in all four regions of the country. Another factor associated with death rates was poverty, with the poorest areas (defined by the percentage of residents living below the poverty level in 1990) experiencing the lowest percent decreases in mortality. Finally, mortality data suggest an important shift in deaths toward older age groups, such that the median age at death due to HIV infection increased from 36 years in 1987 to the older median age of 43 years in 2002 (Fig. 4). Only 61 deaths due to AIDS were reported for children of <13 years in 2004 (12). The age-related trends are reflective of changes in survival after diagnosis due to HAART as well as of a decreasing incidence of infection from perinatal exposure and transfusions with contaminated blood products for general medical indications as well as for hemophiliacs. Intergroup differences are also evident in the prevalence and diagnosis of HIV/AIDS, as presented below.
FIG. 1.
Trends in annual age-adjusted (to 2000 U.S. population) rate of death due to HIV disease in the United States by sex for the period 1987-2002. For comparison with data for 1999 and later years, data for 1987-1988 were modified to account for ICD-10 rules instead of ICD-9 rules. (Reprinted from the CDC website [http://www.cdc.gov/hiv/graphics/mortalit.htm].)
FIG. 2.
Trends in age-adjusted (to 2000 U.S. population) annual rates of death due to HIV disease in the United States by race/ethnicity for the period 1990-2002. For comparison with data for 1999 and later years, data for 1987-1988 were modified to account for ICD-10 rules instead of ICD-9 rules. (Reprinted from the CDC website [http://www.cdc.gov/hiv/graphics/mortalit.htm].)
FIG. 3.
Trends in age-adjusted (to 2000 U.S. population) annual rates of death due to HIV disease in the United States by geographic region for the period 1987-2002. For comparison with data for 1999 and later years, data for 1987-1988 were modified to account for ICD-10 rules instead of ICD-9 rules. The age distribution of the U.S. population was used as the standard for age adjustment. (Reprinted from the CDC website [http://www.cdc.gov/hiv/graphics/mortalit.htm].)
FIG. 4.
Trends in age-adjusted (to 2000 U.S. population) annual rates of death due to HIV disease in the United States by age group for the period 1987-2002. For comparison with data for 1999 and later years, data for 1987-1988 were modified to account for ICD-10 rules instead of ICD-9 rules. (Reprinted from the CDC website [http://www.cdc.gov/hiv/graphics/mortalit.htm].)
AIDS
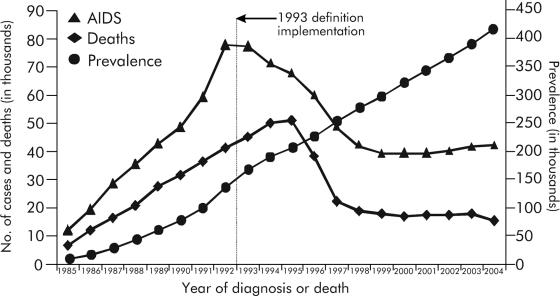
As increasing use of HAART leads to increased survival times and decreasing numbers of HIV-related deaths, the total number of persons living with the infection has increased (Fig. 5) (35). Moreover, as long as the number of new infections decreases or remains the same, the biggest increase in persons living with HIV likely will be among those who would otherwise have died. Results of the surveillance data indicate that despite rapid declines in AIDS diagnoses between 1993 and 2001, estimated AIDS diagnoses increased modestly each year from 2001 through 2004, supporting the assumptions described above. In 2004, the annual incidence of AIDS was estimated to be 14.1 per 100,000 nationally (12). Almost all of the reported AIDS cases in 2004 were in adults (Table 1). Children under the age of 13 years accounted for only 48 of the newly diagnosed cases, representing a very small fraction of the total cases of AIDS and, more importantly, reflecting a substantial decrease of 61% in pediatric AIDS since 2000. Among the mostly adult AIDS diagnoses reported in 2004, males represented almost three times as many cases (∼31,000) as females (∼11,000) and were diagnosed with AIDS at higher rates (25.6 per 100,000) than females (9.0 per 100,000) between 2000 and 2004. However, females had the largest increase in absolute numbers of AIDS (10%) compared to their male counterparts (7%) in the same time period. Also, during 2000-2004, the number of AIDS cases increased slightly in all racial/ethnic categories. However, almost double the number of AIDS cases (∼21,000) occurred among non-Hispanic blacks compared to non-Hispanic whites (∼12,000), with almost three times as many as those among Hispanics (∼8,700). In 2004, non-Hispanic blacks also represented the highest rates of AIDS (56.4 per 100,000), followed by Hispanics (18.6 per 100,000), American Indians/Alaska Natives (7.9 per 100,000), whites (6.0 per 100,000), and Asians/Pacific Islanders (3.7 per 100,000).
FIG. 5.
Estimated numbers of AIDS cases, deaths, and persons living with AIDS in the United States for the period 1985-2004. Data have been adjusted for reporting delays. (Reprinted from the CDC website [http://www.cdc.gov/hiv/topics/surveillance/resources/slides/trends/index.htm].)
TABLE 1.
Estimated numbers of AIDS cases, by year of diagnosis and selected characteristics, in the United States in 2000-2004a
| Characteristic | No. of cases in yr of diagnosisb
|
||||
|---|---|---|---|---|---|
| 2000 | 2001 | 2002 | 2003 | 2004 | |
| Sex | |||||
| Male | 28,974 | 28,743 | 29,730 | 30,578 | 31,024 |
| Female | 10,415 | 10,348 | 10,429 | 11,184 | 11,442 |
| Age at diagnosis (yr) | |||||
| <13 | 124 | 115 | 109 | 69 | 48 |
| 13-19 | 351 | 353 | 383 | 359 | 386 |
| 20-29 | 4,761 | 4,582 | 4,746 | 4,940 | 5,364 |
| 30-39 | 15,427 | 14,907 | 14,726 | 14,766 | 13,817 |
| 40-49 | 12,730 | 12,903 | 13,481 | 14,393 | 14,992 |
| 50-59 | 4,535 | 4,766 | 5,154 | 5,526 | 6,011 |
| ≥60 | 1585 | 1579 | 1668 | 1777 | 1897 |
| Race/ethnicity | |||||
| White, not Hispanic | 11,378 | 11,052 | 11,604 | 11,657 | 12,013 |
| Black, not Hispanic | 19,510 | 19,473 | 19,934 | 20,685 | 20,965 |
| Hispanic | 7,957 | 7,974 | 7,907 | 8,632 | 8,672 |
| Asian/Pacific Islander | 350 | 381 | 440 | 478 | 488 |
| American Indian/Alaska Native | 175 | 169 | 186 | 189 | 193 |
| Transmission category | |||||
| Male-to-male sexual contact | 15,374 | 15,510 | 16,442 | 17,139 | 17,691 |
| IDU | 10,429 | 9,622 | 9,255 | 9,281 | 9,152 |
| Male-to-male sexual contact and IDU | 2,102 | 2,056 | 1,982 | 1,996 | 1,920 |
| Heterosexual contact | 10,947 | 11,370 | 11,952 | 12,826 | 13,128 |
| Perinatal | 122 | 113 | 105 | 68 | 47 |
| Otherc | 537 | 533 | 528 | 520 | 577 |
| Region of residence | |||||
| Northeast | 12,105 | 11,212 | 10,395 | 11,149 | 11,158 |
| Midwest | 3,968 | 3,949 | 4,303 | 4,495 | 4,498 |
| South | 15,841 | 16,598 | 17,751 | 18,612 | 19,792 |
| West | 6,443 | 6,258 | 6,745 | 6,474 | 6,083 |
| U.S. dependency | 1,156 | 1,190 | 1,073 | 1,100 | 982 |
| Totald | 39,513 | 39,206 | 40,267 | 41,831 | 42,514 |
Data from reference 12.
These numbers do not represent reported case counts. Rather, these numbers are point estimates, which result from adjustments of reported case counts. The reported case counts are adjusted for reporting delays and for redistribution of cases for persons initially reported without an identified risk factor. The estimates do not include adjustment for incomplete reporting.
Includes hemophilia, blood transfusion, perinatal transmission, and risk factor not reported or not identified.
Includes persons of unknown race or multiple races and persons of unknown sex. The cumulative total includes 2,308 persons of unknown race or multiple races and 2 persons of unknown sex. Because column totals were calculated independently of the values for the subpopulations, the values in each column may not sum to the column total.
Although the estimated annual number of AIDS diagnoses increased among persons exposed through heterosexual contact as well as among MSM between 2000 and 2004, the number decreased among IDUs and MSM who were also IDUs in the same time period. The largest number of AIDS cases in 2004 was attributed to male-male sex, followed by heterosexual sex and then IDU. Regionally, the number of reported AIDS cases increased 25% in the South and 13% in the Midwest while decreasing 8% in the Northeast, 6% in the West, and 15% in the U.S. dependencies, possessions, and associated nations (12).
Results from the 1999-2002 NHANES indicate racial disparities in treatment that may be partially responsible for the proportional disparities in AIDS deaths and prevalence among non-Hispanic blacks (39). First, by comparing CD4 counts among seropositive respondents who reported HAART use to those among seropositive respondents who did not, NHANES analysis confirmed previous results of HAART effectiveness in slowing disease progression in a population-based sample. Next, the analysis indicated that only 17% of seropositive black respondents reported using HAART medication, in contrast to 78% of respondents in all other categories. Furthermore, among seropositive respondents who were aware of their HIV status, only 67% of blacks reported HAART use, in contrast to a full 100% among all other race/ethnic groups (39).
HIV Infection (Including AIDS)
Of the estimated 1.2 million people in the United States living with HIV infection, approximately 34% have progressed to AIDS, 42% are classified as having HIV only (not AIDS), and 24% remain undiagnosed and therefore may be at any stage along the spectrum of the disease. Table 2 summarizes the demographic and transmission characteristics of persons newly diagnosed with HIV in 2004. The data suggest that HIV-infected individuals who are diagnosed can be characterized as predominantly male (73%) and nonwhite (69%) (24). Non-Hispanic blacks comprise the greatest percentage of HIV infections (50%), while Hispanics represent 18% of all cases. Male-male sex is the most frequently reported transmission category (47%), followed by heterosexual contact (33%) and IDU (5%). Similarly, among non-Hispanic blacks, the most frequently reported transmission route is male-male sex (45%), followed by heterosexual contact (27%) and IDU (22%) (24).
TABLE 2.
Estimated numbers and proportions of persons with HIV infection diagnosis by selected characteristics in 33 states with confidential name-based HIV infection reporting in 2004a
| Characteristic | No. (%) with HIV diagnosisb |
|---|---|
| Sex | |
| Male | 28,143 (72.7) |
| Female | 10,410 (26.9) |
| Age at diagnosis (yr) | |
| <13 | 174 (0.4) |
| 13-19 | 1,121 (2.9) |
| 20-29 | 8,366 (21.6) |
| 30-39 | 12,281 (31.7) |
| 40-49 | 10,880 (28.1) |
| 50-59 | 4,370 (11.3) |
| ≥60 | 1,538 (4.0) |
| Race/ethnicity | |
| White, not Hispanic | 11,806 (30.5) |
| Black, not Hispanic | 19,206 (49.6) |
| Hispanic | 6,970 (18.0) |
| Asian/Pacific Islander | 394 (1.0) |
| American Indian/Alaska Native | 208 (0.5) |
| Transmission category | |
| Male-to-male sexual contact | 18,203 (47.1) |
| IDU | 5,962 (15.4) |
| Male-to-male sexual contact and IDU | 1,372 (3.5) |
| Heterosexual contact | 12,683 (32.8) |
| Perinatal | 145 (0.4) |
| Otherc | 335 (0.9) |
| Total | 38,685 (100) |
Data from reference 12. Since 2000, the following 33 states have had laws or regulations requiring confidential name-based HIV infection reporting: Alabama, Alaska, Arizona, Arkansas, Colorado, Florida, Idaho, Indiana, Iowa, Kansas, Louisiana, Michigan, Minnesota, Mississippi, Missouri, Nebraska, Nevada, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, South Carolina, South Dakota, Tennessee, Texas, Utah, Virginia, West Virginia, Wisconsin, and Wyoming. Since July 1997, Florida has had confidential name-based HIV infection reporting only for new diagnoses.
These numbers do not represent reported case counts. Rather, these numbers are point estimates, which result from adjustments of reported case counts. The reported case counts are adjusted for reporting delays and for redistribution of cases for persons initially reported without an identified risk factor. The estimates do not include adjustment for incomplete reporting. Data include persons with a diagnosis of HIV infection. This includes persons with a diagnosis of HIV infection only, a diagnosis of HIV infection and a later AIDS diagnosis, and concurrent diagnoses of HIV infection and AIDS.
Includes hemophilia, blood transfusion, perinatal transmission, and risk factor not reported or not identified.
Health care personnel are potentially at risk of occupationally acquired HIV infection. However, many health care workers also have behavioral risk factors for HIV that make it difficult to differentiate whether the source of infection was attributable specifically to workplace exposures. In general, few HIV infections have been rigorously documented as occurring due to exposures to infectious fluids or tissues from patients. Data from the National Surveillance for Occupationally Acquired HIV Infection and national AIDS surveillance systems identified 57 cases of documented occupationally acquired infection between 1981 and 2001. Specific regimens have been recommended to provide postexposure prophylaxis (PEP) for workers with significant exposures, and among the three cases that occurred after 1996, recommended PEP failed in the only person who received treatment with a two-drug regimen; the other two refused PEP (19, 44).
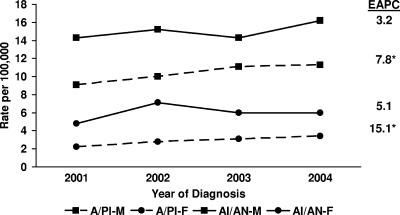
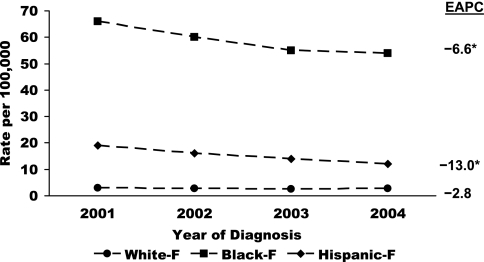
Geographically, HIV infection remains predominantly an urban phenomenon, and southern and northeastern regions of the country contain a disproportionate burden of the disease (24). While the overall trends in new HIV diagnoses suggested no significant change in annual rates between 2001 and 2004 (Fig. 6), subgroup analyses revealed statistically significant increases in annual rates for both males and females in the Asian race/ethnic category (7.8% and 15.1% per year, respectively) (Fig. 7). Annual rates declined significantly in the IDU and heterosexual transmission categories (−9.1% and −3.9% per year, respectively) as well as in non-Hispanic black males and females (Fig. 8 and 9) and Hispanic females (−4.4%, −6.8%, and −13.0% per year, respectively) during the same time period (12, 22). Although the reductions in rates of HIV infection in certain subgroups are encouraging, important intergroup rate differences exist and must also be considered. In particular, members of nonwhite race/ethnic groups, especially females in these groups, are disproportionately affected by the epidemic. Between 2001 and 2004, 51% of all new HIV diagnoses were in non-Hispanic blacks, who accounted for only 13% of the population from which the data were collected (15). In the same time period, annual HIV diagnosis rates were highest for non-Hispanic black males, followed by non-Hispanic black females, who had higher rates of infection than males of all other race/ethnic categories (12).
FIG. 6.
Estimated numbers and rates of HIV/AIDS diagnoses in 33 states for the period 2001-2004. EAPC, estimated annual percent change.
FIG. 7.
Estimated annual rates of HIV/AIDS diagnoses in 33 states by sex and race/ethnicity for the period 2001-2004. EAPC, estimated annual percent change; A/PI, Asian/Pacific Islander; AI/AN, American Indian/Alaska Native. Statistically significant percent changes are indicated with asterisks.
FIG. 8.
Estimated annual rates of HIV/AIDS diagnoses among males in 33 states by race/ethnicity for the period 2001-2004. EAPC, estimated annual percent change. Percentages of change were not statistically significant.
FIG. 9.
Estimated annual rates of HIV/AIDS diagnoses among females in 33 states by race/ethnicity for the period 2001-2004. EAPC, estimated annual percent change. Statistically significant percent changes are indicated with asterisks.
The 1999-2002 NHANES results are consistent with surveillance data and indicate a high prevalence of HIV in non-Hispanic blacks aged 40 to 49 years old (3.58%; 95% confidence interval, 1.88% to 6.71%), with 40- to 49-year-old non-Hispanic black males having the highest prevalence (4.54%; 95% confidence interval, 2.24% to 8.97%) (39). Additionally, surveillance results indicate racial/ethnic differences in transmission characteristics. Analyses of case surveillance data show that male-male sex was the most commonly reported transmission category for all race/ethnic groups (12). However, the proportion of black men reportedly infected through heterosexual contact was over four times greater than that for white men (27% versus 6%). Also, a higher proportion of black men (18%) reported IDU, compared to 10% of white men (12).
Infection attributed to heterosexual exposure was reported with greatest frequency among females in all race/ethnic categories. However, infections among white women were almost twice as likely to be attributed to IDU (30%) as those among black women (17%). Therefore, a higher proportion of black women are reportedly infected through heterosexual contact (80%) than are white women (67%) (CDC, unpublished data). Stratification of the estimated prevalence of cases of diagnosed and undiagnosed infections by stage of disease (HIV [not AIDS] or AIDS) also reveals some interesting differences. First, females represent a higher proportion of all cases in the HIV (not AIDS) category than in the AIDS category (29% versus 23%). Likewise, non-Hispanic blacks comprise a higher percentage of those with HIV (not AIDS) than those in the AIDS category (47% versus 43%). Finally, 29% of HIV (not AIDS) cases are reported for heterosexuals versus 23% of AIDS cases. In contrast, whites, MSM, and IDUs represent a larger proportion of persons living with AIDS than of those in the HIV (not AIDS) group (24). These proportions, although not statistically significantly different, are suggestive of more new infections in women, nonwhites, and heterosexuals due to a higher percentage of them representing an earlier stage of the disease. However, these differences may be an artifact of differential testing patterns for the various subgroups and must be investigated further using methods to measure HIV incidence.
While people of color currently experience a disproportionate burden of HIV infection, the numbers of children aged <13 years with HIV and of new perinatal infections have declined dramatically. Between 1992 and 2004, the estimated annual number pediatric AIDS cases in the United States decreased steadily each year from 945 to 57, a 94% decrease in just over a decade (12). The reduction of pediatric and perinatal HIV infection has resulted from successful public health interventions targeted to pregnant women. Specifically, since antiretroviral therapy was demonstrated to be effective in reducing mother-to-child transmission of HIV, most professional groups and the CDC have recommended that pregnant women in the United States be provided with universal counseling, voluntary testing, and appropriate treatment to prevent such transmission. If testing and/or treatment is refused or proves ineffective in blocking transmission to a newborn, the child is placed on treatment early to increase survival and decrease the risk of morbidity and mortality due to HIV. These measures have led to the significant reductions in HIV-related infant and child mortality observed in recent years (13).
Studies of sexual transmission of HIV suggest that persons who are unaware of their HIV infection are 3.5 times as likely to transmit the virus to a partner as individuals who are aware of their HIV status (37). Therefore, providing opportunities for widespread testing and early detection would greatly benefit HIV prevention efforts. However, because of the stigma traditionally associated with HIV and AIDS, recommendations and policies surrounding the use of HIV testing have usually included requirements for extensive counseling about the disease, with the solicitation of explicit consent before obtaining the test. This approach is known as the “opt-in” method. However, in the context of screening pregnant women for HIV, the imperatives for optimizing early detection in order to guide therapy and prevent transmission to the newborn have resulted in most recommendations from professional organizations, including the CDC, encouraging an “opt-out” approach to testing of pregnant women. This approach allows for testing unless the mother explicitly objects. Currently, five states (Delaware, Florida, Indiana, Oregon, and Texas) authorize an “opt-out” approach to HIV testing in pregnant women, and two states (New York and Connecticut) mandate HIV testing for all newborns (26). Recently, the CDC extended the recommendation to testing all persons being evaluated in clinical settings. Persons being tested for HIV can be informed either verbally without extensive counseling or by soliciting written consent (3).
Taken together, results from national surveillance and population-based survey data reveal some important trends, underscoring successes while serving as reminders of significant challenges that remain in the context of social, economic, and health care access disparities. Therapeutic advances have resulted in reduced mortality, longer survival, and improved life quality among HIV-infected individuals. Public health campaigns to provide education, testing, and treatment to pregnant women with HIV have led to declines in the number of infants suffering from the disease. Finally, transfusion-transmitted and hemophilia-associated HIV infections have been virtually eliminated in the United States. On the other hand, surveillance data indicate that racial/ethnic, gender, and possibly socioeconomic disparities in HIV infection persist. However, current prevalence estimates from surveillance data cannot discriminate between new and long-standing infections and therefore do not adequately explain the impact of these disparities on the future of the epidemic.
FUTURE DIRECTIONS
Clinical and Epidemiologic Effects of HAART
During the past decade, the introduction of HAART has been responsible for increased length and quality of life among many HIV-infected individuals and has contributed to significant reductions in HIV-related morbidity and mortality (27, 43). As illustrated in Fig. 10, following the dramatic proportional increase in the number of AIDS cases without opportunistic infections (OIs) relative to those with OIs that resulted from the change in the AIDS case definition in 1993 (which was expanded to include all HIV-infected persons with a CD4+ T-lymphocyte count of <200 cells/μl or a CD4+ T-lymphocyte percentage of total lymphocytes of <14%), the percentage of AIDS cases without OIs continued to increase coincident with the widespread use of HAART in 1996. The absolute number of AIDS cases without OIs also began to increase after 1996, such that 71% of all reported AIDS cases in 2003 were classified on the basis of low CD4 counts without OI, compared to only 35% in 1993.
FIG. 10.
Annual distribution of AIDS cases in the United States by case definition category for the period 1981-2003.
Despite obvious successes, however, widespread use of HAART has also led to new challenges in clinical management of HIV disease due to its failure to completely suppress viral replication, thus resulting in the emergence of HIV drug resistance (5). Most cases of drug resistance are thought to arise as a result of random mutations in the presence of drug-selective pressures in treated individuals. However, although the use of HAART has been shown to decrease HIV infectivity by suppressing the viral load (2), transmission of viruses resistant to all classes of antiretroviral drugs has also been demonstrated clearly for all transmission categories. Moreover, transmission of drug-resistant virus appears to be increasing (47). Given that 25% of patients reportedly discontinue treatment within 1 year due to drug toxicities and inconsistent adherence, the long-term success of HAART must be evaluated in the context of this important and rising phenomenon of drug resistance. The drug-associated mutations in most transmitted quasi-species appear to persist as the dominant variant over time, unlike the situation where mutations in a wild-type variant are selected by drug pressure (4). In the latter situation, there is generally rapid reemergence and dominance of archived wild-type quasi-species when medications are discontinued. Although strains with drug resistance-associated mutations persist in the setting of transmitted resistance, such mutations usually result in reduced viral fitness, as measured by replicative capacity (19). Nevertheless, despite compromised viral fitness and a probable reduced transmissibility of HIV type 1 (HIV-1) drug-resistant variants relative to that of the wild type, primary transmission to drug-naïve individuals can and does occur, and resistant variants persist for years even in the absence of treatment. Persons with acquired or transmitted drug resistance do not respond as well to treatment and take 2 to 8 weeks longer to achieve viral suppression than their drug-susceptible counterparts (18).
The prevalence of resistance mutations in drug-naïve individuals newly diagnosed with HIV-1 infection has been shown to vary with place and time. Resistance to all classes of HAART drugs has been documented worldwide, as has resistance to multiple drugs. Various studies have estimated the prevalence of at least one resistance mutation to range from 8% to 28% in the United States (47). Analysis of surveillance data representing a large population-based sample of persons in the United States revealed a prevalence of at least one mutation in 10% of the overall sample. The prevalence of resistance to all classes of drugs was detected and varied by state from 6% to 13% (49). In addition, more sensitive assays are now available to detect minority mutations that were not detectable using bulk sequence detection methods (32). Nevertheless, it is difficult to predict the future trends in transmitted HIV drug resistance. At present, transmitted resistance accounts for only a small fraction of the drug resistance population, and there is evidence of continuing benefits of antiretroviral therapy even among persons with persistent drug-resistant variants (18).
HAART medications are also associated with substantial side effects that can result in minor as well as substantial metabolic, dermatologic, and neurologic dysfunctions (40). It is beyond the scope of this paper to review the impact of specific HAART medications. However, up to 25% of patients reportedly discontinue treatment within the first 8 months from HAART initiation, in part due to toxic effects of the drugs. Adverse effects of HAART therapy range from mild nuisances, such as fatigue, headaches, and skin rashes, to life-threatening effects, such as metabolic disruption and hepatotoxicity (40). Given the potential morbidity and mortality associated with HAART therapy and the impact of poor adherence on the development of drug resistance, management of HAART-related adverse outcomes is imperative.
On the population level, increased use of HAART must also be considered in the context of HIV incidence and changes in risky behaviors. The impacts of HAART use on the epidemic are decreased mortality and incidence rates (43). However, potential increases in risky behaviors due to treatment availability and complacence among high-risk groups have been shown to negatively impact the amount by which these parameters are reduced (2, 20). Therefore, the potential tradeoff between treatment benefits and negative consequences must be closely monitored. Specifically, continued prevention efforts targeted to high-risk populations combined with identification and monitoring of viral resistance are critical needs in the next phase of the epidemic. The CDC is currently supporting several surveillance sites to monitor the prevalence of HIV-1 subtypes and primary drug resistance patterns among new cases of HIV infection. As part of the Variant, Atypical, and Resistant Strains of HIV Surveillance System, four cities and 18 state heath departments are currently funded to conduct drug resistance surveillance on remnant sera collected from persons newly diagnosed with HIV infection (1).
HIV Incidence
According to back-calculations, 40,000 people are newly infected with HIV in the United States annually. However, the accuracy of the statistical models used to derive this estimate is unclear, given their sensitivity to the effects of the change in the AIDS case definition in 1993 and the introduction of HAART in 1996. Yet no alternative was available since, historically, systematic monitoring of new cases of HIV infection was not a part of the national surveillance infrastructure, primarily due to unavailability of a marker able to distinguish newly infected (incident) cases from infections of longer duration. As recommended by the Institute of Medicine in 2001, assessment of HIV incidence trends is a priority for evaluation of current prevention efforts as well as for future resource allocation (30). Incidence estimates are also necessary to evaluate the goal of reducing the estimated annual 40,000 new HIV infections in the United States to 20,000 per year, as outlined in the CDC HIV Prevention Strategic Plan (6). Toward this end, the CDC developed and has begun to implement a National HIV Incidence Surveillance System, which consists of two equally important components (36). The first is an innovative serologic testing strategy called the Serologic Testing Algorithm for Recent HIV Seroconversion (STARHS), which was developed and validated for ascertaining seroincidence (31). This method provided the breakthrough necessary to differentiate between recent and long-standing infections by using a tandem sensitive/less sensitive testing method. The less sensitive assay was subsequently replaced by an enzyme immunoassay that is able to accurately capture immunoglobulin G from infections with HIV subtypes B, E, and D. This test is known as the BED assay and was developed specifically for detecting recent HIV infection (45). However, due to various factors, including widespread use of HAART, that can affect antibody levels measured by these tests, the STARHS test results must be interpreted in the context of supplementary testing history information on each individual tested in order to obtain valid incidence estimates. Collection of the testing history information is the second component of the new surveillance system, which is designed to be fully integrated within the existing case surveillance system. Currently, 34 areas that cover approximately 85% of the epidemic in the United States are implementing this system.
Monitoring HIV Risk Behavior
In addition to enumerating new HIV infections, ongoing assessment of risk correlates, especially for subgroups at increased risk of infection, is an integral part of the goal to reduce the burden of disease. The CDC is coordinating the National HIV Behavioral Surveillance System (NHBS) to examine HIV-related behaviors in three high-risk groups, including MSM, IDUs, and heterosexual adults, in areas with high rates of HIV by using population-based surveys (14, 22). Data collection began in 2003 among MSM aged ≥18 years, the group with the highest rates of HIV infection. As of 2005, surveys have been conducted among eligible MSM in 25 metropolitan statistical areas. Results of surveys conducted between November 2003 and April 2005 indicate that the majority of MSM participants had previously been tested (>90%) for HIV infection, with 77% percent reporting testing during the preceding 12 months and 80% having received free condoms during the preceding 12 months (14). In contrast, 58% of respondents reported unprotected anal intercourse with a main partner, while 34% reported this same behavior with a casual partner. Moreover, noninjection drug use was reported by 42% of respondents, and few individual-level (15%) or group-level (8%) HIV prevention programs were utilized by respondents. These data suggest that although HIV testing is common practice among this group, MSM continue to be at increased risk of HIV through sexual and drug-related behaviors (14). Interestingly, data collected between 1990 and 1999 in New York City indicated a 15% decline in HIV seroprevalence among male IDUs who have sex with other men, despite their engaging in these high-risk behaviors. The same study also showed HIV seroprevalence to have decreased in male IDUs who did not report sex with other men (38).
Another risk group for whom national HIV-related behavior data have been collected is adolescents. Data from the national Youth Risk Behavior Survey indicate a decline in the percentage of high school students engaging in high-risk sexual behavior during 1991-2005 (16). Overall, self-reported sexual experience decreased 13%, from 54% to 47%; multiple sex partners (defined as four or more in the preceding 3 months) decreased 24%, from 19% to 14%; current sexual activity decreased 9%, from 37% to 34%; and condom use increased 36%, from 46% to 63%. Despite overall declines, however, disparities that must be addressed further remained among subgroups. For example, black students were more likely than white and Hispanic students to report high-risk sexual behaviors, while no decrease was observed in the prevalence of sexual experience, having had multiple sex partners, or current sexual activity among Hispanic students. Moreover, the percentage of high school students reporting injection drug use remained stable, at 4%, during the same time period.
The second group assessed by the NHBS was IDUs (34). The next phase of NHBS focuses on high-risk heterosexuals and is under way. Data generated from NHBS and other national and local surveys will provide more information on HIV-related behaviors and help us to move toward more effective and targeted prevention strategies to reduce the burden of HIV disease.
CONCLUSION
The emergence of the HIV epidemic in the United States has been characterized by many transitions, initially presenting as a rapidly lethal disease primarily affecting white MSM in a limited number of urban areas. Systematic public health surveillance data have served as the foundation for extensive clinical, epidemiologic, viral, behavioral, and pharmacologic research. The result of these efforts was the rapid identification of a novel retroviral infection with a long incubation period as the cause of a severe, cellular immunodeficiency. The development of diagnostic tests, effective behavioral preventive interventions for adults, and the implementation of treatment protocols that decrease perinatal transmission were followed, within a decade, by the introduction of pharmacologic therapies that arrest disease progression and allow for immune restoration. Despite the generally encouraging evolution of these events, the epidemic presents persistent challenges. HIV now exacts enormous morbidity and mortality in minority communities, particularly among African-American men and women. The infection has also disseminated into rural areas. Finally, although treatable, HIV infections are incurable. The result has been the emergence of drug resistance among treated persons as well as drug-naïve, newly diagnosed persons. Surveillance efforts must continue to adapt to the changing characteristics of the epidemic to accurately capture the molecular, clinical, and behavioral dimensions of the epidemic so that activities focused on prevention, treatment, and care can continue to progress.
Acknowledgments
We are grateful to Richard Selik for his input and assistance in summarizing the HIV mortality data.
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Bennett, D. 2005. HIV [corrected] genetic diversity surveillance in the United States. J. Infect. Dis. 192:4-9. [DOI] [PubMed] [Google Scholar]
- 2.Blower, S., E. Bodine, J. Kahn, and W. McFarland. 2005. The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS 19:1-14. [DOI] [PubMed] [Google Scholar]
- 3.Branson, B. M., H. H. Handsfield, M. A. Lampe, R. S. Janssen, A. W. Taylor, S. B. Lyss, and J. E. Clark. 2006. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. Morb. Mortal. Wkly. Rep. Recomm. Rep. 55:1-17. [PubMed] [Google Scholar]
- 4.Brookmeyer, R. 2003. Temporal factors in epidemics: the role of the incubation period, p. 127-145. In R. Brookmeyer and D. F. Stroup (ed.), Monitoring the health of populations: statistical principles and methods for public health surveillance. Oxford University Press, Oxford, United Kingdom.
- 5.Cane, P. A. 2005. Stability of transmitted drug-resistant HIV-1 species. Curr. Opin. Infect. Dis. 18:537-542. [DOI] [PubMed] [Google Scholar]
- 6.CDC. August 2003, posting date. HIV prevention strategic plan through 2005. CDC, Atlanta, GA. http://www.cdc.gov/hiv/partners/PSP/Goal4-Objective1-2-3.htm#obj1.
- 7.CDC. 2002, posting date. HIV/AIDS mortality surveillance slides. CDC, Atlanta, GA. http://www.cdc.gov/hiv/graphics/mortalit.htm.
- 8.CDC. 1981. Kaposi's sarcoma and Pneumocystis pneumonia among homosexual men—New York City and California. Morb. Mortal. Wkly. Rep. 30:305-308. [PubMed] [Google Scholar]
- 9.CDC. 1994. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat. 1:1-407. [PubMed] [Google Scholar]
- 10.CDC. 1996. Update: mortality attributable to HIV infection among persons aged 25-44 years—United States, 1994. Morb. Mortal. Wkly. Rep. 45:121-125. [PubMed] [Google Scholar]
- 11.CDC. December 2000, posting date. HIV/AIDS surveillance report, 1999, vol. 11. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/stats/hasr1102.pdf.
- 12.CDC. December 2005, posting date. HIV/AIDS surveillance report, 2004, vol. 17. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA. http://www.cdc.gov/hiv/topics/surveillance/resources/reports/2004report/pdf/2004SurveillanceReport.pdf.
- 13.CDC. 2006. Achievements in public health. Reduction in perinatal transmission of HIV infection—United States, 1985-2005. Morb. Mortal. Wkly. Rep. 55:592-597. [PubMed] [Google Scholar]
- 14.CDC. 2006. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors—United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003-April 2005. Morb. Mortal. Wkly. Rep. 55:1-16. [PubMed] [Google Scholar]
- 15.CDC. 2006. Racial/ethnic disparities in diagnoses of HIV/AIDS—33 states, 2001-2004. Morb. Mortal. Wkly. Rep. 55:121-125. [PubMed] [Google Scholar]
- 16.CDC. 2006. Trends in HIV-related risk behaviors among high school students—United States, 1991-2005. Morb. Mortal. Wkly. Rep. 55:851-854. [PubMed] [Google Scholar]
- 17.CDC. 2006. Twenty-five years of HIV/AIDS—United States, 1981-2006. Morb. Mortal. Wkly. Rep. 55:585-589. [PubMed] [Google Scholar]
- 18.Deeks, S. G., T. Wrin, T. Liegler, R. Hoh, M. Hayden, J. D. Barbour, N. S. Hellmann, C. J. Petropoulos, J. M. McCune, M. K. Hellerstein, and R. M. Grant. 2001. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N. Engl. J. Med. 344:472-480. [DOI] [PubMed] [Google Scholar]
- 19.Do, A. N., C. A. Ciesielski, R. P. Metler, T. A. Hammett, J. Li, and P. L. Fleming. 2003. Occupationally acquired human immunodeficiency virus (HIV) infection: national case surveillance data during 20 years of the HIV epidemic in the United States. Infect. Control Hosp. Epidemiol. 24:86-96. [DOI] [PubMed] [Google Scholar]
- 20.Fenton, K. A., and J. Imrie. 2005. Increasing rates of sexually transmitted diseases in homosexual men in Western Europe and the United States: why? Infect. Dis. Clin. N. Am. 19:311-331. [DOI] [PubMed] [Google Scholar]
- 21.Fleming, P. L., J. W. Ward, J. M. Karon, D. L. Hanson, and K. M. De Cock. 1998. Declines in AIDS incidence and deaths in the USA: a signal change in the epidemic. AIDS 12(Suppl. A):S55-S61. [PubMed] [Google Scholar]
- 22.Gallagher, K., P. S. Sullivan, A. Lansky, and I. Onorato. 2007. Behavioral surveillance among persons at risk for HIV infection in the U.S.: The National HIV Behavioral Surveillance System. Public Health Rep. 122(Suppl. 1):32-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glynn, M. K., L. M. Lee, and M. T. McKenna. 2007. The status of national HIV case surveillance, United States 2006. Public Health Rep. 122(Suppl. 1):63-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glynn, M. K., and P. Rhodes. Estimated HIV prevalence in the United States at the end of 2003, abstr. T1-B1101. 2005 Natl. HIV Prev. Conf., Atlanta, GA, 14 June 2005.
- 25.Hall, H. I., K. McDavid, Q. Ling, and A. Sloggett. 2006. Determinants of progression to AIDS or death after HIV diagnosis, United States, 1996 to 2001. Ann. Epidemiol. 16:824-833. [DOI] [PubMed] [Google Scholar]
- 26.Health Research and Education Trust. 2006, posting date. Cross-state comparison tables. Health Research and Education Trust, Chicago, IL. http://www.hret.org/hret/about/crossstate.html.
- 27.Hogg, R. S., B. Yip, C. Kully, K. J. Craib, M. V. O'Shaughnessy, M. T. Schechter, and J. S. Montaner. 1999. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ 160:659-665. [PMC free article] [PubMed] [Google Scholar]
- 28.Holtgrave, D. R. 2005. Causes of the decline in AIDS deaths, United States, 1995-2002: prevention, treatment or both? Int. J. Sex Transm. Dis. Acquir. Immune Defic. Syndr. 16:777-781. [DOI] [PubMed] [Google Scholar]
- 29.Hoyert, D. L., M. P. Heron, S. L. Murphy, and H. Kung. April 2006, posting date. Deaths: final data for 2003. National vital statistics reports, 2006, vol. 54. National Center for Health Statistics, Hyattsville, MD. http://www.cdc.gov/nchs/data/nvsr/nvsr54/nvsr54_13.pdf. [PubMed]
- 30.Institute of Medicine. 2001, posting date. No time to lose. Institute of Medicine, Washington, DC. http://www.iom.edu/Object.File/Master/4/131/HIV8pager.pdf.
- 31.Janssen, R. S., G. A. Satten, S. L. Stramer, B. D. Rawal, T. R. O'Brien, B. J. Weiblen, F. M. Hecht, N. Jack, F. R. Cleghorn, J. O. Kahn, M. A. Chesney, and M. P. Busch. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280:42-48. [DOI] [PubMed] [Google Scholar]
- 32.Johnson, J., J. F. Li, X. Wei, J. Lipscomb, A. Smith, C. Stone, D. Irlbeck, M. Monsour, E. Lanier, and W. Heneine. 2007. Low frequency mutations substantially increase the prevalence of transmitted drug resistance and greatly strengthen the relationship between resistance mutations and virologic failure, abstr. 639. 14th Conf. Retrovir. Opportun. Infect., Los Angeles, CA, February 2007.
- 33.Karon, J. M., P. L. Fleming, R. W. Steketee, and K. M. De Cock. 2001. HIV in the United States at the turn of the century: an epidemic in transition. Am. J. Public Health 91:1060-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lansky, A., A. S. Abdul-Quader, M. Cribbin, T. Hall, T. J. Finlayson, R. S. Garfein, L. S. Lin, and P. S. Sullivan. 2007. Developing an HIV behavioral sruveillance system for injecting drug users: a national application of venue-based, time-space sampling. Public Health Rep. 122(Suppl. 1):48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, L. M., J. M. Karon, R. Selik, J. J. Neal, and P. L. Fleming. 2001. Survival after AIDS diagnosis in adolescents and adults during the treatment era, United States, 1984-1997. JAMA 285:1308-1315. [DOI] [PubMed] [Google Scholar]
- 36.Lee, L. M., and M. T. McKenna. 2007. Monitoring the incidence of HIV infection in the United States. Public Health Rep. 122(Suppl. 1):72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marks, G., N. Crepaz, and R. S. Janssen. 2006. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS 20:1447-1450. [DOI] [PubMed] [Google Scholar]
- 38.Maslow, C. B., S. R. Friedman, T. E. Perlis, R. Rockwell, and J. D. C. Des. 2002. Changes in HIV seroprevalence and related behaviors among male injection drug users who do and do not have sex with men: New York City, 1990-1999. Am. J. Public Health 92:382-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McQuillan, G. M., D. Kruszon-Moran, B. J. Kottiri, L. A. Kamimoto, L. Lam, M. F. Cowart, M. Hubbard, and T. J. Spira. 2006. Prevalence of HIV in the US household population: the National Health and Nutrition Examination Surveys, 1988 to 2002. J. Acquir. Immune Defic. Syndr. 41:651-656. [DOI] [PubMed] [Google Scholar]
- 40.Montessori, V., N. Press, M. Harris, L. Akagi, and J. S. Montaner. 2004. Adverse effects of antiretroviral therapy for HIV infection. CMAJ 170:229-238. [PMC free article] [PubMed] [Google Scholar]
- 41.Nakashima, A. K., and P. L. Fleming. 2003. HIV/AIDS surveillance in the United States, 1981-2001. J. Acquir. Immune Defic. Syndr. 32(Suppl. 1):S68-S85. [PubMed] [Google Scholar]
- 42.National Center for Health Statistics. October 2006, posting date. National Health and Nutrition Examination Survey (NHANES). National Center for Health Statistics, Hyattsville, MD. http://www.cdc.gov/nchs/about/major/nhanes/nhanes01-02.htm.
- 43.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 44.Panlilio, A. L., D. M. Cardo, L. A. Grohskopf, W. Heneine, and C. S. Ross. 2005. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. Morb. Mortal. Wkly. Rep. Recomm. Rep. 54:1-17. [PubMed] [Google Scholar]
- 45.Parekh, B. S., M. S. Kennedy, T. Dobbs, C. P. Pau, R. Byers, T. Green, D. J. Hu, S. Vanichseni, N. L. Young, K. Choopanya, T. D. Mastro, and J. S. McDougal. 2002. Quantitative detection of increasing HIV type 1 antibodies after seroconversion: a simple assay for detecting recent HIV infection and estimating incidence. AIDS Res. Hum. Retrovir. 18:295-307. [DOI] [PubMed] [Google Scholar]
- 46.Pomerantz, R. J., and D. L. Horn. 2003. Twenty years of therapy for HIV-1 infection. Nat. Med. 9:867-873. [DOI] [PubMed] [Google Scholar]
- 47.Tang, J. W., and D. Pillay. 2004. Transmission of HIV-1 drug resistance. J. Clin. Virol. 30:1-10. [DOI] [PubMed] [Google Scholar]
- 48.UNAIDS. December 2006, posting date. AIDS epidemic update. UNAIDS, Geneva, Switzerland. http://data.unaids.org/pub/EpiReport/2006/2006_EpiUpdate_en.pdf.
- 49.Wheeler, W., K. Mahle, U. Bodnar, R. Kline, I. Hall, and M. McKenna. Antiretroviral drug-resistance mutations and subtypes in drug-naive persons newly diagnosed with HIV-1 infection, US, March 2003-October 2006, abstr. 648. 14th Conf. Retrovir. Opportun. Infect. Los Angeles, CA, February 2007.
- 50.World Health Organization. 2006, posting date. Second generation surveillance for HIV/AIDS. World Health Organization, Geneva, Switzerland. http://www.who.int/hiv/topics/surveillance/2ndgen/en/.