Abstract
When the butterfly Eurema hecabe is infected with two different strains (wHecCI2 and wHecFem2) of the bacterial endosymbiont Wolbachia, genetic males are transformed into functional females, resulting in production of all-female broods. In an attempt to understand how and when the Wolbachia endosymbiont feminizes genetically male insects, larval insects were fed an antibiotic-containing diet beginning at different developmental stages until pupation. When the adult insects emerged, strikingly, many of them exhibited sexually intermediate traits in their wings, reproductive organs, and genitalia. The expression of intersexual phenotypes was strong in the insects treated from first instar, moderate in the insects treated from third instar, and weak in the insects treated from fourth instar. The insects treated from early larval instar grew and pupated normally but frequently failed to emerge and died in the pupal case. The dead insects in the pupal case contained lower densities of the feminizing Wolbachia endosymbiont than the successfully emerged insects, although none of them were completely cured of the symbiont infection. These results suggest the following: (i) the antibiotic treatment suppressed the population of feminizing Wolbachia endosymbionts; (ii) the suppression probably resulted in attenuated feminizing activity of the symbiont, leading to expression of intersexual host traits; (iii) many of the insects suffered pupal mortality, possibly due to either intersexual defects or Wolbachia-mediated addiction; and hence (iv) the feminizing Wolbachia endosymbiont continuously acts on the host insects during larval development for expression of female phenotypes under a male genotype. Our finding may prompt reconsideration of the notion that Wolbachia-induced reproductive manipulations are already complete before the early embryonic stage and provide insights into the mechanism underlying the symbiont-induced reversal of insect sex.
Symbiotic associations with microorganisms are common in a diverse array of animals, plants, and other organisms (29, 40). Insects are particularly rich in such examples, reflecting their enormous diversity in the terrestrial ecosystem (4, 5, 6). These symbiotic microorganisms often influence the physiology, biochemistry, development, morphogenesis, reproduction, and other features of their host insects and can contribute to host fitness either positively or negatively (2, 4, 5, 10, 38). The mechanisms by which these microscopic endosymbionts affect the macroscopic phenotypes of their host insects are of great interest.
In the majority of insects, sex is genetically determined. For example, dipteran insects like the fruit fly Drosophila melanogaster have a male-heterogametic sex chromosome constitution, in which XX zygotes become females and XY zygotes develop into males. Lepidopteran insects like the silkworm, Bombyx mori, have a female-heterogametic chromosomal constitution, in which ZZ zygotes become males and ZW zygotes develop into females. Hymenopteran insects like the honeybee, Apis mellifera, have a haplodiploid sex determination system, in which fertilized (2n) eggs become females while unfertilized (n) eggs develop into males (7, 52). The molecular mechanisms underlying sex determination and differentiation in the model insect D. melanogaster are well understood. At a very early embryonic stage, each cell determines its sex independently, and once determined, the sex of each cell is maintained during later development through a gene expression cascade consisting of Sex lethal, transformer, doublesex, and other genes, in which sex-specific mRNA splicing plays an important role (42). Sex determination at a very early embryonic stage in a cell-autonomous manner is believed to be widespread among insects, on the grounds that sexually mosaic individuals often occur in a diverse array of insects (27), although molecular mechanisms of sex determination in those non-Drosophila systems are very poorly understood.
In some animal taxa, various environmental factors, such as temperature, photoperiod, population density, and others, have been shown to affect sex determination or sex differentiation during development or even after maturation (7, 52). In insects, endosymbiotic microorganisms are among the factors that drastically affect their sex determination or reproduction. For example, in parasitic wasps and thrips, endosymbionts of the genera Wolbachia and Rickettsia induce parthenogenesis, wherein unfertilized haploid eggs that are normally destined to be males develop into diploid embryos and become females (1, 14, 45). In a wide variety of insects and other terrestrial arthropods, endosymbionts of the genera Wolbachia and “Candidatus Cardinium” cause cytoplasmic incompatibility (CI), wherein zygotes between infected males and uninfected females suffer embryonic mortality (4, 38). In lady beetles, fruit flies, butterflies, and other insects, endosymbionts of the genera Wolbachia, Spiroplasma, Rickettsia, and others are the causative agents of male killing, wherein male embryos are selectively killed (4, 19, 38). Since these endosymbionts are maternally inherited through host generations, these reproductive phenotypes effectively increase the frequency of infected females in the host populations, often at the expense of the host fitness (4, 38). Although the mechanisms underlying these reproductive phenotypes are not yet understood, considering that these aberrations are expressed as early embryonic events (egg diploidization in parthenogenesis induction; zygote mortality in CI and male killing), some molecular or cellular components, putatively related to sex determination during embryogenesis, have been suggested as targets of these selfish endosymbionts (8, 50).
A different type of reproductive phenotype, feminization of genetic males, is known to occur with Wolbachia endosymbionts of lepidopteran insects, like the common yellow butterfly Eurema hecabe, and terrestrial crustaceans, like the woodlouse, Armadillidium vulgare. This phenotype causes all-female broods of the host arthropods (17, 39). In Japanese populations of E. hecabe, two distinct Wolbachia strains have been identified. One strain, called wHecCI2 (or wHecFem1 in reference 17), is prevalent throughout Japanese populations except northernmost ones, showing almost 100% infection frequencies and causing CI. Another strain, designated wHecFem2, is restricted to southwestern island populations, showing low infection frequencies and causing feminization (17, 18, 35). In lepidopteran insects including E. hecabe, the sex determination system is generally female heterogametic (i.e., ZZ males and ZW females) and cell autonomous (i.e., occurrence of sexual mosaics) (12, 48). Hence, as in the cases of Wolbachia-induced parthenogenesis, CI, and male killing, it appears that Wolbachia-induced feminization occurs as an early embryonic event. Contrary to expectation, however, here we demonstrate that the feminizing Wolbachia endosymbiont continuously acts on genetic males of E. hecabe during larval development for expression of female phenotypes, which provides insights into the mechanism underlying the symbiont-mediated reversal of insect sex.
MATERIALS AND METHODS
Insect materials.
All-female-producing matrilines of E. hecabe infected with wHecCI2 and wHecFem2 were established from adult females collected at Tanegashima, Japan, in September 2005. Normal matrilines of E. hecabe that showed 1:1 sex ratios in their offspring and were infected with wHecCI2 only were established from adult females collected at Tanegashima, Japan, in June 2005. The insects were reared in the laboratory at 25°C under a long-day regimen (16 h light:8 h dark). Larvae were reared on an artificial diet containing cuttings of dry leaves of Albizia julibrissin as described previously (24). Newly emerged adults were kept individually in plastic cups and fed with 10% sucrose solution. The sex ratio of each brood was determined at the point of adult emergence.
Diagnostic PCR detection of Wolbachia endosymbionts.
The Wolbachia strain wHecCI2 was detected as a 398-bp wsp gene segment by using the primers wsp81F (5′-TGGTCCAATAAGTGATGAAGAAAC-3′) (54) and WHecFem1 (5′-ACTAACGTCGTTTTTGTTTAG-3′) (designed by Y. Tagami). The Wolbachia strain wHecFem2 was detected as a 232-bp wsp gene segment by using the primers WHecFem2 (5′-TTACTCACAATTGGCTAAAGAT-3′) (designed by Y. Tagami) and wsp691R (5′-AAAAATTAAACGCTACTCCA-3′) (54). The PCR temperature profile was 35 cycles of 95°C for 1 min, 58°C for 1.5 min, and 72°C for 1.5 min followed by 72°C for 7 min.
Observation of sex chromatin bodies.
The Malpighian tubule and bursa copulatrix were dissected from adult females, fixed in methanol-acetic acid (3:1) for 1 min, transferred onto microscope slides, stained and mounted in lactic acetic orcein, and observed under a light microscope as described previously (22).
Antibiotic treatment.
Larvae of the all-female matrilines were fed continuously with the artificial diet containing 0.05% tetracycline hydrochloride beginning at different larval stages (first, second, third, or fourth instar) until pupation. As controls, larvae of the normal matrilines were fed with the tetracycline-containing artificial diet throughout the larval stages (i.e., from first instar until pupation).
Survival rate.
Effects of the antibiotic treatments on survival were evaluated by counting numbers of pupated individuals, numbers of adults that successfully emerged, and numbers of adults that failed to emerge. Among the adults that failed to emerge, those that completed adult body formation (often actively motile in the pupal case) and those that died without recognizable adult morphogenesis were distinguished and counted separately.
Observation of adult wings, reproductive organs, and genitalia.
Adult wings were examined under a binocular microscope for sex brands and ground color. Testes, ovaries, and/or bursa copulatrices were dissected from adult insects in physiological saline with fine forceps under a binocular microscope. Genital parts were dissected from adult insects, macerated in 10% potassium hydroxide for 2 h, freed of scales, and observed under a light microscope.
Quantitative PCR.
Infection densities of the Wolbachia endosymbionts were quantified by a real-time fluorescent quantitative PCR technique using the SYBR Green and Mx3000P QPCR system (Stratagene) in terms of symbiont wsp gene copies per copy of the gene for host elongation factor 1α (ef1α). Two legs from each of the adult insects were subjected to DNA extraction by using a conventional sodium dodecyl sulfate-proteinase K digestion and phenol-chloroform extraction procedure (41), and each ethanol-precipitated DNA sample was suspended in 100 μl of Tris-EDTA buffer. For each of the DNA samples, wHecCI2 was quantified by using the primers WHecFem1 and wsp81F, whereas wHecFem2 was quantified by using the primers WHecFem2 and wsp691R. For normalization, ef1α copies in each host insect DNA sample were also quantified by using the primers EFS599 (5′-CCGGTTTGAACTCAGATCATGT-3′) and EFA923 (5′-CGCCTGTTTAACAAAAACAT-3′). Each of the PCR mixtures consisted of 2.5 μl of 10× TaqGold buffer (Applied Biosystems), 3 μl of 25 mM MgCl2, 2.5 μl of nucleotide mixture solution (2 mM each of dATP, dTTP, dGTP, and dCTP), 0.25 μl of SYBR Green I (1/1,000-diluted solution) (Molecular Probes), 1.5 μl of primer mixture solution (5 μM each of forward and reverse primers), 0.1 μl of AmpliTaqGold DNA polymerase (Applied Biosystems), 13 μl of distilled water, and 2.5 μl of DNA sample solution. The PCR temperature profile was 94°C for 1 min, 35 cycles of 94°C for 1 min, 53°C for 1.5 min, and 72°C for 1.5 min, followed by 72°C for 7 min. Standard curves for each of the genes were drawn by using standard samples that contained 102, 103, 104, 105, and 106 copies per μl of the target PCR product.
Quality of DNA samples from dead insects.
In total, 61 successfully emerged adult insects and 37 dead adult insects in the pupal case were subjected to the quantitative PCR analyses. The titers of ef1α were 2.26 × 104 ± 1.67 × 104 (mean ± standard deviation) in the emerged adults and 1.73 × 104 ± 1.76 × 104 in the dead adults. Since the difference was not statistically significant (generalized linear model; P = 0.109), the quality of the DNA samples prepared from the dead adults was regarded as sufficient for quantitative PCR. Note that rapid desiccation has been widely used for archival preservation of plant and insect tissues for DNA analyses (15). However, the possibility cannot be excluded that, although the insect DNA had not deteriorated, the symbiont DNA might be somehow affected in the dead insects.
RESULTS
Establishment of normal and all-female matrilines.
We collected adult E. hecabe females from Tanegashima Island, Japan, and generated isofemale lines. Of six isofemale lines established, three exhibited normal sex ratios, whereas the other three produced all-female broods. The striking variation in sex ratios was inherited: five isofemale offspring of the normal broods (i.e., one or two broods from each of the three normal matrilines) also exhibited normal sex ratios, while six isofemale offspring of the all-female broods (i.e., two broods from each of the three all-female matrilines) also produced all-female broods. These matrilines (in total eight normal broods and nine all-female broods spanning two generations) were subjected to the following experiments.
Diagnostic PCR of Wolbachia strains associated with normal and all-female matrilines.
Diagnostic PCR analysis revealed that the normal matrilines harbored Wolbachia strain wHecCI2 only, whereas the all-female matrilines were doubly infected with the strains wHecCI2 and wHecFem2 (data not shown).
Sex chromosome constitution of normal and all-female matrilines.
In many lepidopteran species, including E. hecabe, sex chromosome constitution is female heterogametic (i.e., ZZ males and ZW females), and the W chromosome is cytologically observable as a condensed sex chromatin body in interphase nuclei (49). Our cytological observations of Malpighian tubule cells and bursa copulatrix cells revealed that the sex chromatin bodies were present in females of the normal broods but not in females of the all-female broods (Fig. 1). Hence, the single-strain-infected normal broods produced ZW females, whereas the double-strain-infected all-female broods consisted of ZZ females, confirming that the all-female broods were caused by infection with the feminizing Wolbachia strain wHecFem2.
FIG. 1.
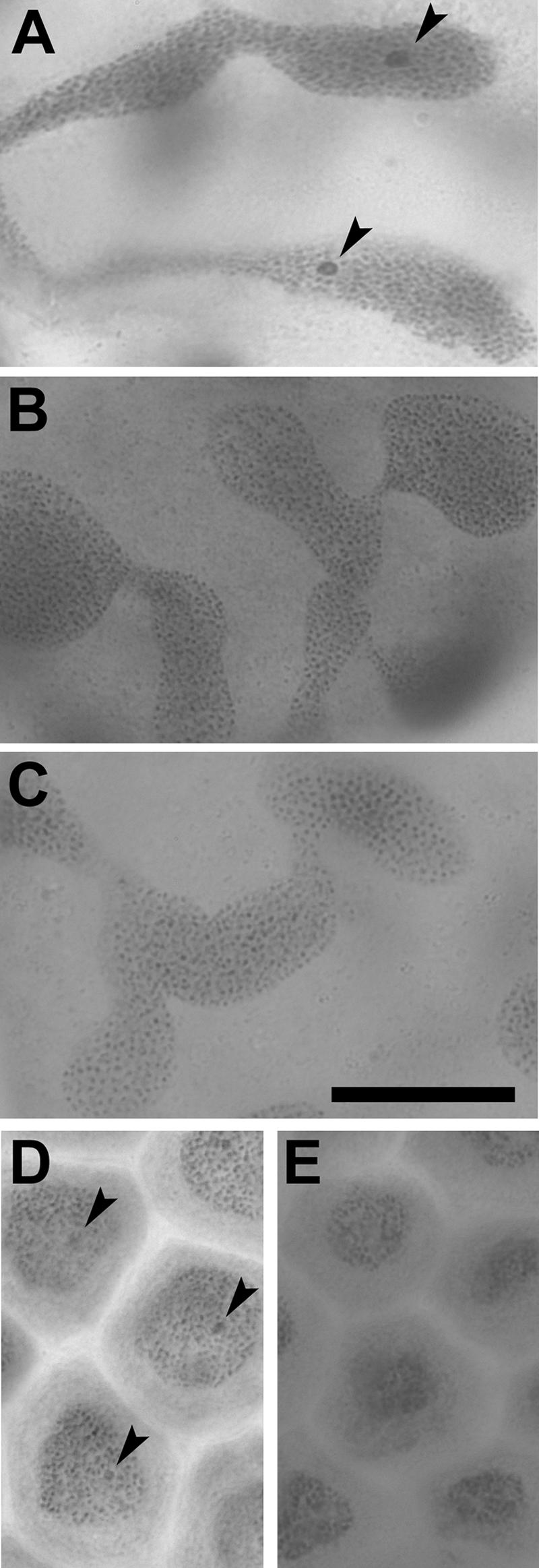
Observation of sex chromatin bodies in interphase nuclei of E. hecabe. (A to C) Nuclei of Malpighian tubule cells from a doubly infected female (A), a singly infected female (B), and a singly infected male. (D and E) Nuclei of bursa copulatrix cells from a doubly infected female (D) and a female singly infected with wHecCI2 (E). Note that Malpighian tubule cells contain highly polyploid branched nuclei. Arrows indicate sex chromatin bodies. Bar, 20 μm.
Pupal mortality specific to all-female matrilines caused by antibiotic treatment at larval stages.
The insects whose larvae were reared normally on an artificial diet were fed with a tetracycline-supplemented diet beginning at different larval instars (first, second, third, or fourth) until pupation, and their growth and phenotypes were monitored. In the all-female matrilines, all the treated and untreated insects exhibited good larval development, attaining pupation rates of 72 to 86%. The insects treated from third or fourth instar showed normal emergence rates of 55% and 71%, and so did the untreated insects, at a rate of 54%. By contrast, most of the insects treated from first or second instar did not emerge, exhibiting only 2% and 9% emergence rates (Table 1). In the normal matrilines, both first-instar treatment and no treatment resulted in normal emergence rates of 49% and 61% (Table 1).
TABLE 1.
Survival rates of antibiotic-treated E. hecabe individualsa
| Brood and treatment | No. of treated larvae | No. of pupae | Pupation rateb | No. of dead pupae
|
Adult formation ratee | No. of emerged adults | Adult emergence ratef | |
|---|---|---|---|---|---|---|---|---|
| Mummiesc | Dead adultsd | |||||||
| Doubly infected, all female | ||||||||
| From 1st instar until pupation (whole larval stage) | 87 | 65 | 0.75 | 25 | 38 | 0.47*** | 3 | 0.03*** |
| From 2nd instar until pupation | 11 | 8 | 0.73 | 5 | 2 | 0.27* | 1 | 0.09** |
| From 3rd instar until pupation | 81 | 73 | 0.90 | 1 | 15 | 0.64 | 37 | 0.46 |
| From 4th instar until pupation | 7 | 6 | 0.86 | 0 | 1 | 0.86 | 5 | 0.71 |
| No treatment | 312 | 225 | 0.72 | 17 | 40 | 0.67 | 168 | 0.54 |
| Singly infected, normal | ||||||||
| From 1st instar until pupation (whole larval stage) | 63 | 46 | 0.73 | 2 | 13 | 0.70 | 31 | 0.49 |
| No treatment | 376 | 262 | 0.70 | ND | ND | ND | 229 | 0.61 |
Asterisks indicate significantly lower value compared to nontreated doubly infected broods (Fisher's exact probability test): *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Number of pupae/number of treated insects.
Dead pupae in which adult morphogenesis was unrecognizable.
Dead pupae in which adult morphogenesis was completed.
(Number of emerged adults + number of dead adults in pupae)/number of treated insects.
Number of emerged adults/number of treated insects.
Appearance of adult insects with sexually intermediate traits specifically in antibiotic-treated all-female matrilines.
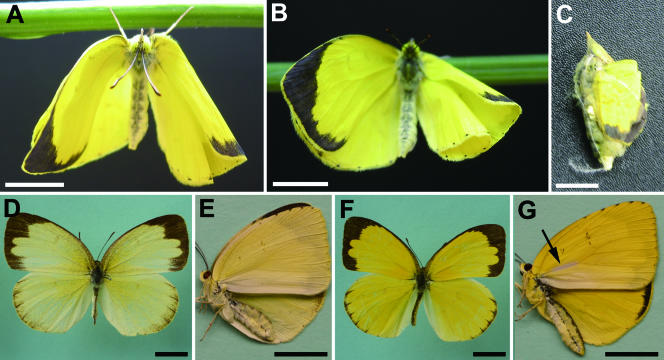
In the antibiotic-treated all-female matrilines, morphologically abnormal adult insects frequently appeared. Most of the emerged adults had abnormal wings, e.g., curled, folded, or asymmetric (Fig. 2A and B). Insects with normally spread wings (only seven individuals) were unable to fly. Dissection of the apparently dead pupae in the antibiotic-treated feminized matrilines revealed that many of them had actually completed adult morphogenesis but failed to escape from the pupal case (Fig. 2C). These adult insects, both emerged ones and ones confined in the pupal case, were closely examined for their morphological traits. Interestingly, many of them exhibited sexually intermediate phenotypes.
FIG. 2.
E. hecabe adults that emerged after larval antibiotic treatment. (A and B) Emerged adult insects with deformed wings, obtained by treatment from third to fourth instar of a doubly infected insect line; (C) adult insect that failed to escape from the pupal case, obtained by treatment from first to fourth instar of a doubly infected insect line; (D and E) normal adult females, pale in ground color and without sex brands, representing a nontreated insect line singly infected with wHecCI2; (F and G) normal adult males, bright in ground color and with sex brands (arrows), representing a nontreated insect line singly infected with wHecCI2. Bars, 10 mm.
Sexually intermediate traits in wing morphology.
In E. hecabe, female wings are soft yellow and without sex brands (Fig. 2D and E), while male wings are bright yellow and have sex brands (Fig. 2F and G). Hence, the ground color and the sex brands were inspected as representatives of sexually dimorphic wing morphology. The pattern observed was that the earlier the insects were treated with the antibiotic, the more male-like wing phenotypes appeared (Table 2). Specifically, the insects treated from first or second instar tended to express male-like wing traits, the insects treated from third instar expressed both male-like and female-like phenotypes, and the insects treated from fourth instar mostly expressed female phenotypes. None of the insects without the antibiotic treatment exhibited such male-like wing traits (Table 2).
TABLE 2.
Sexually intermediate phenotypes in wing morphology of antibiotic-treated E. hecabea
| Treated stage | No of individuals with:
|
Total | |||
|---|---|---|---|---|---|
| Feminine color, sex brand 0 | Masculine color, sex brand 0 | Masculine color, sex brand ? | Masculine color, sex brand 2 | ||
| From 1st instar until pupation (whole larval stage) | 0 | 1 | 0 | 3 | 4 |
| From 2nd instar until pupation | 0 | 0 | 1 | 1 | 2 |
| From 3rd instar until pupation | 11 | 17 | 2 | 13 | 43 |
| From 4th instar until pupation | 3 | 2 | 1 | 0 | 6 |
| No treatment | 168 | 0 | 0 | 0 | 168 |
Feminine color, soft yellow typical of normal females; masculine color, bright yellow typical of normal males. 0, absence of sex brand; ?, sex brand not examined or unrecognized; 2, sex brand present in both forewings. The phenotypes presented range from most feminine to most masculine (left to right).
Sexually intermediate traits in reproductive organs.
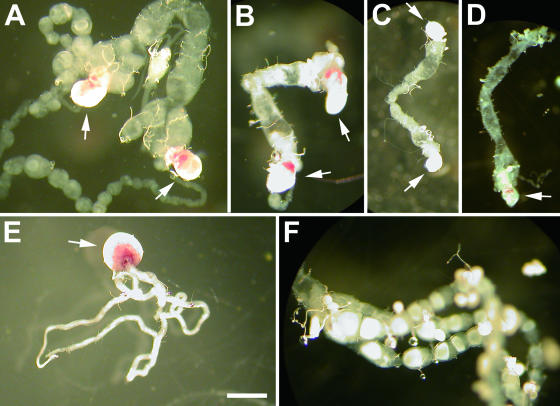
These insects were dissected and examined for two female-specific organs, the bursa copulatrix and ovary, and a male-specific organ, the testis. The pattern observed was that the earlier the insects were treated with the antibiotic, the more male-like reproductive organs appeared (Table 3). Specifically, some insects treated from first to third instar lacked either the bursa copulatrix or ovaries (3/63), and others lacked both (7/63). Strikingly, some insects treated from first to third instar developed deformed testes in association with ovarioles (6/63) (Fig. 3A to D). The insects treated from fourth instar possessed normal female reproductive organs (Table 3). Such phenotypes were not observed in untreated feminized matrilines or in treated normal matrilines (data not shown).
TABLE 3.
Sexually intermediate phenotypes in reproductive organs of antibiotic-treated E. hecabe
| Treated stage | No. of individuals with bursaa/ovaryb/testisc phenotyped
|
Total | ||||||
|---|---|---|---|---|---|---|---|---|
| +/+/0 | +/?/0 | −/+/0 | −/−/0 | +/−/1 | −/+/2 | −/−/2 | ||
| From 1st instar until pupation (whole larval stage) | 4 | 2 | 1 | 1 | 0 | 1 | 4 | 13 |
| From 2nd instar until pupation | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| From 3rd instar until pupation | 36 | 10 | 0 | 1 | 1 | 0 | 0 | 48 |
| From 4th instar until pupation | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| No treatment | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 20 |
Presence (+) or absence (−) of the bursa copulatrix.
Presence (+) or absence (−) of the ovary. ?, not examined or unrecognized.
Number (0, 1, or 2) of deformed testes. In normal male adults, a pair of testes is fused into one (Fig. 2E); however, in antibiotic-treated insects, it is often found that a pair of testes is deformed and not fused (Fig. 2A to D).
The phenotypes presented range from most feminine to most masculine (left to right).
FIG. 3.
Reproductive organs of E. hecabe adults that emerged after larval antibiotic treatment. (A) Two deformed testes coexisting with a mature ovary, obtained by treatment from first to fourth instar of a doubly infected insect line; (B and C) two deformed testes, obtained by treatment from first to fourth instar of a doubly infected insect line; (D) a deformed testis, obtained by treatment from first to fourth instar of a doubly infected insect line; (E) a normal testis, from a nontreated insect line singly infected with wHecCI2; (F) a normal ovary, from a nontreated insect line singly infected with wHecCI2. Note that a pair of testes is often fused into one in lepidopteran adult insects. Arrows indicate testes. Bar, 1 mm.
Sexually intermediate traits in genitalia.
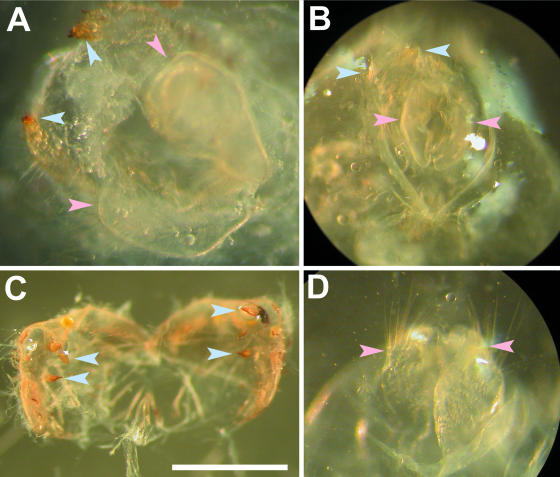
We inspected microscopic specimens of the genitalia prepared from these insects. Again, the pattern observed was that the earlier the insects were treated with the antibiotic, the more male-like genital traits appeared (Table 4). All the insects treated from first instar (16/16) exhibited genitalia with both female and male traits (Fig. 4). Some of the insects treated from third instar (5/53) and none of the insects treated from fourth instar (6/6) had sexually intermediate genitalia. Such phenotypes were not observed in untreated feminized matrilines or in treated normal matrilines (data not shown).
TABLE 4.
Sexually intermediate phenotypes in genitalia of antibiotic-treated E. hecabe
FIG. 4.
Genitalia preparations of E. hecabe adults that emerged after larval antibiotic treatment. (A and B) Sexually intermediate genitalia, obtained by treatment from first to fourth instar of a doubly infected insect line; (C) male genitalia from a nontreated insect line singly infected with wHecCI2; (D) female genitalia from a nontreated insect line singly infected with wHecCI2. Blue arrowheads indicate male traits (bicuspid apex of valva), while pink arrowheads indicate female traits (papilla analis). Bar, 1 mm.
Quantification of Wolbachia densities.
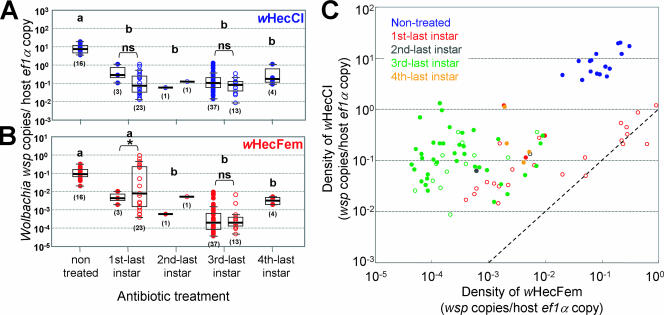
The antibiotic-treated and untreated insects, including both emerged adults and dead adults in the pupal case, were individually subjected to quantitative PCR assay for the Wolbachia strains wHecCI2 and wHecFem2. Two legs from each of the insects were subjected to DNA extraction. Copy numbers of the Wolbachia wsp gene and those of the insect ef1α gene were quantified, and Wolbachia density was calculated in terms of wsp gene copies per ef1α gene copy. Statistical analyses were conducted with the data for insects receiving no treatment and first-, third-, and fourth-instar treatments. The data from the second-instar treatment were excluded from the analyses because of the small sample size (Fig. 5).
FIG. 5.
(A and B) Infection densities of Wolbachia endosymbionts wHecCI2 (A) and wHecFem2 (B) in E. hecabe adults that emerged after larval antibiotic treatment. Each dot represents an individual. Filled dots and open dots indicate emerged adults and dead adults in the pupal case, respectively. Mean, standard deviation, and sample size are shown above or below the dots. Different letters (a and b) indicate statistically significant differences by the generalized linear model after Bonferroni correction (P < 0.05). Asterisks indicate statistically significant differences by the generalized linear model (P < 0.05). (C) Relationship between infection densities of wHecCI2 and wHecFem2 coinfecting the same host insects.
Comparison between wHecCI2 and wHecFem2 in the same host insects.
Regardless of the antibiotic treatments, infection densities of wHecCI2 were consistently higher than those of wHecFem2. The differences were statistically significant (generalized linear model; P < 0.001) except for dead adults of the first-instar treatment (P = 0.369). No adults derived from antibiotic-treated larvae were completely cured of the Wolbachia infection (Fig. 5).
Comparison between antibiotic-treated and untreated insects.
As for wHecCI2, the antibiotic-treated insects consistently exhibited lower infection densities than the untreated insects. The differences were statistically significant (generalized linear model after Bonferroni correction; P < 0.05) (Fig. 5A). As for wHecFem2, the infection densities were also lower in the antibiotic-treated insects than in the untreated insects (generalized linear model after Bonferroni correction; P < 0.05) except for the first-instar treatment (P = 0.814) (Fig. 5B).
Comparison between insects treated with antibiotic from different larval stages.
wHecCI2 exhibited no significant differences in infection density between the first-instar, third-instar, and fourth-instar treatments (generalized linear model after Bonferroni correction; P > 0.05) (Fig. 5A). As for wHecFem2, the insects receiving first-instar treatment showed significantly higher infection density than the insects receiving the other treatments (generalized linear model after Bonferroni correction; P < 0.05) (Fig. 5B).
Comparison between successfully emerged insects and dead insects in the pupal case.
In the first-instar treatment, insects that successfully emerged had higher densities of both wHecCI2 and wHecFem2 than those that did not (generalized linear model; P < 0.05). In the third-instar treatment, on the other hand, no significant differences in infection densities were detected between insects that successfully emerged and those that did not (generalized linear model; P > 0.05) (Fig. 5).
DISCUSSION
Sexually intermediate traits due to attenuation of Wolbachia-induced feminization.
In this study, we demonstrated that antibiotic treatments of E. hecabe at various larval stages frequently induced sexually intermediate traits in wings (Fig. 2; Table 2), reproductive organs (Fig. 3; Table 3), and genitalia (Fig. 4; Table 4) of adult insects. Such phenotypes were specifically expressed in antibiotic-treated all-female lines of E. hecabe that were infected with the feminizing Wolbachia strain wHecFem2 (Tables 2 to 4). Cytological observations confirmed that these all-female lines consisted of phenotypically female but genetically male individuals (Fig. 1). All these results strongly suggest that these sexually intermediate traits are caused by attenuated feminization due to suppression of the Wolbachia endosymbiont by the antibiotic treatment.
Intersexuality or sexual mosaicism?
By definition, sexual mosaicism (or gynandromorphism) is genetic mosaicism wherein an individual consists of both genetically male parts and genetically female parts, while intersexuality is a phenotypic intermediate wherein an individual exhibits both male phenotypes and female phenotypes but possesses a single genotype (11, 27). Hence, the sexually intermediate traits induced by the antibiotic treatment are probably due to intersexuality rather than to sexual mosaicism, on account of the ZZ sex chromosome constitution of the all-female lines of E. hecabe (Fig. 1). Intersexuality has been observed in various lepidopteran insects, such as the gypsy moth, Lymantria dispar (11, 32), the bagworm, Solenobia triquetrella (43, 44), the adzuki bean borer, Ostrinia scapulalis (22, 23), the silkworm, Bombyx mori (16), and others. In O. scapulalis, intersexual individuals with sexually mosaic phenotypes were induced by antibiotic treatment of Wolbachia-infected insects (22, 23).
Timing of antibiotic treatment and expression of sexually intermediate traits.
Sexually intermediate traits were frequently observed with antibiotic treatment from first instar and were also found, although less frequently, with treatment from third instar. The treatment from fourth instar rarely caused such phenotypes. The absence of such phenotypes in the treatment from second instar is probably due to the small number of emerged adults that we managed to examine (Tables 2 to Table 4). These results indicate that continuous infection with the feminizing Wolbachia endosymbiont during the period from first to third instar is needed for complete expression of female phenotypes under the male genotype.
Feminizing Wolbachia continuously acting on E. hecabe during larval development for maintenance of female phenotypes.
On the grounds of the well-understood molecular mechanisms underlying sex determination in D. melanogaster (42) and the universal occurrences of sexual mosaicism in diverse insects (27), it has been supposed that sex determination in insects generally occurs at an early embryonic stage in a cell-autonomous manner. Wolbachia-induced parthenogenesis makes unfertilized eggs develop into female embryos (1, 14, 45). Wolbachia-induced CI results in arrested embryogenesis in incompatible crosses (4, 38). Wolbachia-induced male killing causes male-specific embryonic mortality (4, 19, 38). From these circumstantial lines of evidence, it seems natural to assume that Wolbachia-induced feminization should involve transformation of genetic males into phenotypic females at an early embryonic stage. Our discovery that the feminizing Wolbachia continuously acts on the larvae of E. hecabe for the maintenance of female phenotypes is quite unexpected in this context.
Insights into the mechanism of Wolbachia-induced feminization in E. hecabe.
The sex of lepidopterans is generally determined by a female-heterogametic sex chromosomal system, wherein ZZ zygotes become males and ZW zygotes develop to females (49). By using the genetics of B. mori, epistatic sex determinant loci were identified on the W chromosome, and larval sex was shown to be determined before hatching (48). Sexually mosaic individuals have been observed in a wide variety of butterflies and moths (27). In B. mori, a homolog of doublesex, a sex-related gene of D. melanogaster located downstream of the sex determination cascade, is certainly transcribed in a sex-specific manner (36, 46). Hence, although the mechanism is unknown, lepidopteran sex determination is probably completed very early in embryogenesis, as in many other insects and animals (7, 52). Notwithstanding this, Wolbachia-induced feminization in E. hecabe requires larval infection, clearly indicating that the process of embryonic sex determination cannot be the target of reproductive manipulation. Our finding will provide some insights into the mechanism underlying Wolbachia-induced feminization. Although this explanation is speculative, the feminizing Wolbachia may interact with some female- or male-specific molecular mechanism that is located downstream of the sex determination system and is responsible for the expression of female-specific phenotypes. To confirm this idea, of course, we have to understand the mechanisms underlying lepidopteran sex determination, to which information on the B. mori genome (31, 53) may provide important clues.
Wolbachia-induced feminization in insects and crustaceans.
Interestingly, Wolbachia-induced feminization has also been observed in terrestrial crustaceans like the woodlouse, A. vulgare (39). The genetic basis of sex determination in A. vulgare is female heterogamety (ZZ in males and ZW in females), as in the majority of lepidopteran insects, including E. hecabe. Genetically male individuals of A. vulgare possess an endocrine organ, the androgenic gland, from which a polypeptide factor, androgenic gland hormone (AGH), is secreted (30, 37). On the grounds that both genetic males and genetic females of A. vulgare develop into phenotypic females in the presence of AGH (28, 47), it has been hypothesized that Wolbachia infection might somehow block the formation of the androgenic gland, so that the infected individuals develop into functional females irrespective of their genetic sex in the absence of AGH (39). In insects, on the other hand, sex is generally determined in a cell-autonomous manner, and no sex-differentiating hormones have been identified thus far. At present, it is unclear whether Wolbachia-induced feminization is caused by the same mechanism in butterflies and in crustaceans.
All-male broods and sexually intermediate traits caused by antibiotic treatment.
In a study by Hiroki et al. (17), females from all-female broods of E. hecabe were fed with an antibiotic-containing sucrose solution and were mated with males from normal broods, which resulted in the production of all-male broods. This striking result was interpreted as follows: (i) the continuous parental antibiotic treatment substantially eliminated infection with and therefore the feminizing effect of Wolbachia in the eggs; (ii) the ZZ eggs from feminized females and ZZ sperm from normal males produced ZZ offspring only; and (iii) in the absence of the feminizing Wolbachia infection, the ZZ zygotes all developed into males. On the other hand, we observed that larval antibiotic treatment resulted in adult insects with sexually intermediate traits (Tables 2 to 4; Fig. 2 to 4). These different results are probably attributable to different developmental stages of the antibiotic treatment and/or different intensities of the antibiotic treatment. In this study, no insects were completely cured of the Wolbachia infection (Fig. 5), which may be relevant to the expression of sexually intermediate phenotypes.
Different infection densities of CI-inducing and feminizing Wolbachia strains in the same host insect.
Although the two Wolbachia strains coinfected the same host insect, the CI-inducing strain wHecCI2 consistently exhibited higher infection densities than the feminizing strain wHecFem2 (Fig. 5). The different infection densities may be relevant to their reproductive phenotypes (CI versus feminization), their different proliferation patterns in the host tissues (germ, soma, etc.), or their different levels of adaptation to the host insect (widespread wHecCI2 versus infrequent wHecFem2). The controlling mechanisms underlying the differences in infection density are currently unknown. In parasitoid wasps, coinfecting Wolbachia strains consistently exhibited different infection levels, and the infection densities were independently regulated and were scarcely affected by other coexisting strains (33, 34). In a bruchid beetle, coinfecting Wolbachia strains consistently exhibited different infection levels and tissue tropism, and the infection densities were suppressed by another coexisting strain (21, 25, 26). In D. melanogaster, infection densities of a Wolbachia strain were negatively affected by a coinfecting Spiroplasma strain, and the suppression was observed at particular developmental stages (13). To investigate such aspects of symbiont density regulation in E. hecabe, spatial and temporal population dynamics of these Wolbachia strains during the development of doubly and singly infected insects should be analyzed by using in situ hybridization and tissue-specific quantitative PCR techniques.
Reason for higher mortality with earlier antibiotic treatment: intersexual defects or Wolbachia-mediated addiction?
When the antibiotic treatment began at first or second instar, the insects suffered high pupal mortality (Table 1). In the first-instar treatment, the infection densities of the feminizing Wolbachia strain wHecFem2 in dead adult insects in the pupal case were significantly lower than those in successfully emerged adult insects, while the coexisting strain wHecCI2 did not exhibit such a difference (Fig. 5). These results suggest that the high mortality in the first-instar treatment may be related to the suppressed infection density of wHecFem2. This idea appears to be concordant with the observation that insects treated from first instar exhibited more severely sexually abnormal phenotypes than insects treated from third or fourth instar (Tables 2 to Table 4).
Then why do the antibiotic-treated, symbiont-depleted insects die? A possible explanation for why antibiotic-treated, symbiont-depleted insects die is the so-called “intersexual defects hypothesis.” It seems likely that the abnormal sexually intermediate individuals suffer various malfunctions, possibly leading to mortality upon adult emergence. An alternative explanation is so-called “Wolbachia-mediated addiction hypothesis.” Although the mechanism whereby Wolbachia endosymbionts induce reproductive aberrations of their host insects is unknown, the “modification-rescue” (or “poison-antidote”) model has been proposed to explain CI and other reproductive phenomena (8, 20, 51). In this model, a modifying function of the CI-inducing symbiont modifies the sperm of an infected male, and, when the modified sperm fertilizes an uninfected egg, development of the zygote aborts due to loss of paternal chromosomes. On the other hand, when the modified sperm fertilizes an infected egg, normal development of the zygote is restored by a rescuing function of the symbiont in the egg. Here, given that not the sperm but some essential somatic component of the host is modified by the symbiont and at the same time rescued by the symbiont, the action of the symbiont is expected to result in an addiction-like phenotype, such as that caused by the Medea factor in flour beetles (3). That is, even though the host originally does not require the symbiont, once infected, the host becomes dependent on the symbiont infection. It appears possible, although speculative, that the antibiotic-treated insects suffered pupal mortality due to a Wolbachia-mediated addiction mechanism. In a parasitoid wasp, elimination of a Wolbachia strain inhibits oogenesis and thus results in sterility of the host insect, possibly due to such a mechanism (9). In order to address which of these hypotheses, intersexual defects or Wolbachia-mediated addiction, is appropriate, artificial transfer of the feminizing Wolbachia strain into uninfected insects could provide pivotal experimental data.
Concluding remarks.
What genetic and molecular mechanisms underlie the sex determination in a butterfly? How are the developmental pathways to male and female switched on and off? At present, it is difficult to answer these challenging questions. However, our finding of the unexpected action of the feminizing Wolbachia endosymbiont on E. hecabe, together with information on lepidopteran genomes, could provide a unique approach to resolve these enigmas.
Acknowledgments
We thank C. Suzuki and N. Haruyama for their help in collecting E. hecabe.
S.N. was supported by a Japan Society for the Promotion of Science (JSPS) Fellowship for Young Scientists.
Footnotes
Published ahead of print on 11 May 2007.
REFERENCES
- 1.Arakaki, N., T. Miyoshi, and H. Noda. 2001. Wolbachia-mediated parthenogenesis in the predatory thrips Franklinothrips vespiformis. Proc. R. Soc. Lond. B 268:1011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann, P., N. A. Moran, and L. Baumann. 2000. Bacteriocyte-associated endosymbionts of insects, p. 1-55. In M. Dworkin (ed.), The prokaryotes. Springer, New York, NY.
- 3.Beeman, R. W., K. S. Friesen, and R. E. Denell. 1992. Maternal-effect, selfish genes in flour beetles. Science 256:89-92. [DOI] [PubMed] [Google Scholar]
- 4.Bourtzis, K., and T. A. Miller. 2003. Insect symbiosis. CRC Press, Boca Raton, FL.
- 5.Bourtzis, K., and T. A. Miller. 2006. Insect symbiosis II. CRC Press, Boca Raton, FL.
- 6.Buchner, P. 1965. Endosymbiosis of animals with plant microorganisms. Interscience, New York, NY.
- 7.Bull, J. J. 1983. Evolution of sex determining mechanisms. Benjamin/Cummings Publishing Company, Menlo Park, CA.
- 8.Charlat, S., G. D. Hurst, and H. Mercot. 2003. Evolutionary consequences of Wolbachia infections. Trends Genet. 19:217-223. [DOI] [PubMed] [Google Scholar]
- 9.Dedeine, F., F. Vavre, F. Fleury, B. Loppin, M. E. Hochberg, and M. Bouletreau. 2001. Removing symbiotic Wolbachia bacteria specifically inhibits oogenesis in a parasitic wasp. Proc. Natl. Acad. Sci. USA 98:6247-6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas, A. E. 1998. Nutritional interactions in insect-microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43:17-37. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt, R. 1934. Lymantria. Bibliogr. Genet. 11:1-186. [Google Scholar]
- 12.Goldsmith, M. R., T. Shimada, and H. Abe. 2005. The genetics and genomics of the silkworm, Bombyx mori. Annu. Rev. Entomol. 50:71-100. [DOI] [PubMed] [Google Scholar]
- 13.Goto, S., H. Anbutsu, and T. Fukatsu. 2006. Asymmetrical interactions between Wolbachia and Spiroplasma endosymbionts coexisting in the same insect host. Appl. Environ. Microbiol. 72:4805-4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagimori, T., Y. Abe, and K. Miura. 2006. The first finding of a Rickettsia bacterium associated with parthenogenesis-inducing among insects. Curr. Microbiol. 52:97-101. [DOI] [PubMed] [Google Scholar]
- 15.Hillis, D. M., C. Moritz, and B. K. Mable. 1996. Molecular systematics, 2nd ed. Sinauer Associates, Sunderland, MA.
- 16.Hirokawa, M. 1995. Studies on sex control in the silkworm, Bombyx mori. Bull. Fukushima Seric. Exp. Stn. 28:1-104. (In Japanese.) [Google Scholar]
- 17.Hiroki, M., Y. Kato, T. Kamito, and K. Miura. 2002. Feminization of genetic males by a symbiotic bacterium in a butterfly, Eurema hecabe (Lepidoptera: Pieridae). Naturwissenschaften 89:167-170. [DOI] [PubMed] [Google Scholar]
- 18.Hiroki, M., Y. Tagami, K. Miura, and Y. Kato. 2004. Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe. Proc. R. Soc. Lond. B 271:1751-1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurst, G. D. D., and F. M. Jiggins. 2000. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerg. Infect. Dis. 6:329-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurst, L. D., and G. T. McVean. 1996. Clade selection, reversible evolution and the persistence of selfish elements: the evolutionary dynamics of cytoplasmic incompatibility. Proc. R. Soc. Lond. B 263:97-104. [Google Scholar]
- 21.Ijichi, N., N. Kondo, R. Matsumoto, M. Shimada, H. Ishikawa, and T. Fukatsu. 2002. Internal spatiotemporal population dynamics of infection with three Wolbachia strains in the adzuki bean beetle, Callosobruchus chinensis (Coleoptera: Bruchidae). Appl. Environ. Microbiol. 68:4074-4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kageyama, D., and W. Traut. 2004. Opposite sex-specific effects of Wolbachia and interference with the sex determination of its host Ostrinia scapulalis. Proc. R. Soc. Lond. B 271:251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kageyama, D., S. Ohno, S. Hoshizaki, and Y. Ishikawa. 2003. Sexual mosaics induced by tetracycline treatment in the Wolbachia-infected adzuki bean borer, Ostrinia scapulalis. Genome 46:983-989. [DOI] [PubMed] [Google Scholar]
- 24.Kato, Y., and F. Sakakura. 1994. Artificial diet rearing of Eurema blanda and some notes on its host-plant. Trans. Lepid. Soc. Jpn. 45:21-26. (In Japanese with English summary.) [Google Scholar]
- 25.Kondo, N., M. Shimada, and T. Fukatsu. 2005. Infection density of Wolbachia endosymbionts affected by co-infection and host genotype. Biol. Lett. 1:488-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kondo, N., N. Ijichi, M. Shimada, and T. Fukatsu. 2002. Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol. Ecol. 11:167-180. [DOI] [PubMed] [Google Scholar]
- 27.Laugé, G. 1985. Sex determination: genetic and epigenetic factors, p. 295-318. In G. A. Kerkut and L. I. Gilbert (ed.), Comprehensive insect physiology, biochemistry and pharmacology, vol. 1. Embryogenesis and reproduction. Pergamon Press, Oxford, United Kingdom. [Google Scholar]
- 28.Legrand, J. J., E. Legrand-Hamelin, and P. Juchault. 1987. Sex determination in Crustacea. Biol. Rev. 62:439-470. [Google Scholar]
- 29.Margulis, L., and R. Fester. 1991. Symbiosis as a source of evolutionary innovation. MIT Press, Cambridge, United Kingdom. [PubMed]
- 30.Martin, G., O. Sorokine, M. Moniatte, P. Bulet, C. Hetru, and A. Van Dorsselaer. 1999. The structure of a glycosylated protein hormone responsible for sex determination in the isopod, Armadillidium vulgare. Eur. J. Biochem. 262:727-736. [DOI] [PubMed] [Google Scholar]
- 31.Mita, K., M. Kasahara, S. Sasaki, Y. Nagayasu, T. Yamada, H. Kanamori, N. Namiki, M. Kitagawa, H. Yamashita, Y. Yasukochi, K. Kadono-Okuda, K. Yamamoto, M. Ajimura, G. Ravikumar, M. Shimomura, Y. Nagamura, T. Shin-I, H. Abe, T. Shimada, S. Morishita, and T. Sasaki. 2004. The genome sequence of silkworm, Bombyx mori. DNA Res. 11:27-35. [DOI] [PubMed] [Google Scholar]
- 32.Mosbacher, G. C. 1973. Die Intersexualität bei Lymantria dispar L. (Lepidoptera). Z. Morphol. Tiere. 76:1-96. [Google Scholar]
- 33.Mouton, L., H. Henri, M. Bouletreau, and F. Vavre. 2003. Strain-specific regulation of intracellular Wolbachia density in multiply infected insects. Mol. Ecol. 12:3459-3465. [DOI] [PubMed] [Google Scholar]
- 34.Mouton, L., F. Dedeine, H. Henri, M. Bouletreau, N. Profizi, and F. Vavre. 2004. Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168:181-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narita, S., M. Nomura, Y. Kato, and T. Fukatsu. 2006. Genetic structure of sibling butterfly species affected by Wolbachia infection sweep: evolutionary and biogeographical implications. Mol. Ecol. 15:1095-1108. [DOI] [PubMed] [Google Scholar]
- 36.Ohbayashi, F., M. G. Suzuki, and T. Shimada. 2002. Sex determination in Bombyx mori. Curr. Sci. 83:466-471. [Google Scholar]
- 37.Okuno, A., Y. Hasegawa, T. Ohira, Y. Katakura, and H. Nagasawa. 1999. Characterization and cDNA cloning of androgenic gland hormone of the terrestrial isopod Armadillidium vulgare. Biochem. Biophys. Res. Commun. 264:419-423. [DOI] [PubMed] [Google Scholar]
- 38.O'Neill, S. L., A. A. Hoffmann, and J. H. Werren. 1997. Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, New York, NY.
- 39.Rigaud, T. 1997. Inherited microorganisms and sex determination of arthropod hosts, p. 81-101. In S. L. O'Neill, A. A. Hoffmann, and J. H. Werren (ed.), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, New York, NY.
- 40.Ruby, E., B. Henderson, and M. McFall-Ngai. 2004. We get by with a little help from our (little) friends. Science 303:1305-1307. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 42.Schutt, C., and R. Nöthiger. 2000. Structure, function and evolution of sex-determining systems in dipteran insects. Development 127:667-677. [DOI] [PubMed] [Google Scholar]
- 43.Seiler, J. 1965. Sexuality as developmental process, p. 199-207. In S. J. Geerts (ed.), Genetics today. Proceedings of the XI International Congress of Genetics, The Hague 1963. Pergamon, Oxford, United Kingdom.
- 44.Seiler, J., O. Puchta, and E. Brunold. 1958. Die Entwicklung des Genitalapparates bei triploiden Intersexen von Solenobia triquetrella F.R. (Lepid. Psychidae). Deutung des Intersexualitetsphänomens. Wilhelm Roux's Arch. Entwicklungsmech. Org. 150:190-372. [DOI] [PubMed] [Google Scholar]
- 45.Stouthamer, R. 1997. Wolbachia-induced parthenogenesis, p. 102-124. In S. L. O'Neill, A. A. Hoffmann, and J. H. Werren (ed.), Influential passengers: inherited microorganisms and arthropod reproduction. Oxford University Press, New York, NY.
- 46.Suzuki, M. G., S. Funaguma, T. Kanda, T. Tamura, and T. Shimada. 2005. Role of the male BmDSX protein in the sexual differentiation of Bombyx mori. Evol. Dev. 7:58-68. [DOI] [PubMed] [Google Scholar]
- 47.Suzuki, S., and K. Yamasaki. 1997. Sexual bipotentiality of developing ovaries in the terrestrial isopod Armadillidium vulgare (Malacostraca, Crustacea). Gen. Comp. Endocrinol. 107:136-146. [DOI] [PubMed] [Google Scholar]
- 48.Tazima, Y. 1964. The genetics of the silkworm. Academic Press, London, United Kingdom.
- 49.Traut, W., and F. Marec. 1996. Sex chromatin in Lepidoptera. Q. Rev. Biol. 71:239-256. [DOI] [PubMed] [Google Scholar]
- 50.Veneti, Z., J. K. Bentley, T. Koana, H. R. Braig, and G. D. D. Hurst. 2005. A functional dosage compensation complex required for male killing in Drosophila. Science 307:1461-1463. [DOI] [PubMed] [Google Scholar]
- 51.Werren, J. H. 1997. Biology of Wolbachia. Annu. Rev. Entomol. 42:587-607. [DOI] [PubMed] [Google Scholar]
- 52.Werren, J. H., and L. W. Beukeboom. 1998. Sex determination, sex ratios, and genetic conflict. Annu. Rev. Ecol. Syst. 29:233-261. [Google Scholar]
- 53.Xia, Q., Z. Zhou, C. Lu, D. Cheng, F. Dai, B. Li, P. Zhao, X. Zha, T. Cheng, C. Chai, G. Pan, J. Xu, C. Liu, Y. Lin, J. Qian, Y. Hou, Z. Wu, G. Li, M. Pan, C. Li, Y. Shen, X. Lan, L. Yuan, T. Li, H. Xu, G. Yang, Y. Wan, Y. Zhu, M. Yu, W. Shen, D. Wu, Z. Xiang, J. Yu, J. Wang, R. Li, J. Shi, H. Li, G. Li, J. Su, X. Wang, G. Li, Z. Zhang, Q. Wu, J. Li, Q. Zhang, N. Wei, J. Xu, H. Sun, L. Dong, D. Liu, S. Zhao, X. Zhao, Q. Meng, F. Lan, X. Huang, Y. Li, L. Fang, C. Li, D. Li, Y. Sun, Z. Zhang, Z. Yang, Y. Huang, Y. Xi, Q. Qi, D. He, H. Huang, X. Zhang, Z. Wang, W. Li, Y. Cao, Y. Yu, H. Yu, J. Li, J. Ye, H. Chen, Y. Zhou, B. Liu, J. Wang, J. Ye, H. Ji, S. Li, P. Ni, J. Zhang, Y. Zhang, H. Zheng, B. Mao, W. Wang, C. Ye, S. Li, J. Wang, G. K. Wong, and H. Yang; Biology Analysis Group. 2004. A draft sequence for the genome of the domesticated silkworm (Bombyx mori). Science 306:1937-1940. [DOI] [PubMed] [Google Scholar]
- 54.Zhou, W., F. Rousset, and S. O'Neill. 1998. Phylogeny and PCR based classification of Wolbachia strains using wsp gene sequences. Proc. R. Soc. Lond. B 265:509-515. [DOI] [PMC free article] [PubMed] [Google Scholar]