Abstract
Dampness in buildings has been linked to adverse health effects, but the specific causative agents are unknown. Mycotoxins are secondary metabolites produced by molds and toxic to higher vertebrates. In this study, mass spectrometry was used to demonstrate the presence of mycotoxins predominantly produced by Aspergillus spp. and Stachybotrys spp. in buildings with either ongoing dampness or a history of water damage. Verrucarol and trichodermol, hydrolysis products of macrocyclic trichothecenes (including satratoxins), and trichodermin, predominately produced by Stachybotrys chartarum, were analyzed by gas chromatography-tandem mass spectrometry, whereas sterigmatocystin (mainly produced by Aspergillus versicolor), satratoxin G, and satratoxin H were analyzed by high-performance liquid chromatography-tandem mass spectrometry. These mycotoxin analytes were demonstrated in 45 of 62 building material samples studied, in three of eight settled dust samples, and in five of eight cultures of airborne dust samples. This is the first report on the use of tandem mass spectrometry for demonstrating mycotoxins in dust settled on surfaces above floor level in damp buildings. The direct detection of the highly toxic sterigmatocystin and macrocyclic trichothecene mycotoxins in indoor environments is important due to their potential health impacts.
Microorganisms are thought to be involved in health problems connected to damp buildings. However, the causative microbiological agents are unknown (22). Many molds that thrive in damp indoor environments are potent mycotoxin producers and may play a role in the reported adverse health effects ( 1, 5, 17, 23, 24, 26, 30). Mycotoxins are secondary metabolites, e.g., produced to give molds strategic advantages over encroaching organisms. Examples are sterigmatocystin (STRG), a carcinogenic mycotoxin produced mainly by Aspergillus versicolor; satratoxin G (SATG) and satratoxin H (SATH), which are cytotoxic mycotoxins produced by Stachybotrys chartarum; and citrinin, gliotoxin, and patulin, produced by, e.g., Aspergillus spp. and Penicillium spp. The latter three mycotoxins have been shown to be immunomodulatory, causing a polarization in cytokine production towards a Th2 phenotype (36), and citrinin caused depletion of intracellular glutathione at nontoxic concentrations (18). Based on spore counts, the airborne mycotoxin concentrations found in damp buildings have been estimated to be insufficient for causing adverse health effects (20). However, indoor molds may fragment into very small airborne mycotoxin-containing particles, resulting in up to a 500-fold larger exposure than assumed previously (4, 11, 21, 32). In addition, Cho et al. (7) showed that the respiratory deposition of S. chartarum fragments was over 200-fold higher than that of spores in adults and an additional 4 to 5 times higher in infants. These aerosolized fragments could potentially also be the source of allergens (13).
S. chartarum and A. versicolor are two commonly encountered molds in buildings with moisture problems (9, 12, 15, 28) and are prominent mycotoxin producers. Thin-layer chromatography, high-performance liquid chromatography (HPLC), and enzyme-linked immunosorbent assay are techniques that have been applied for detecting some of these mycotoxins, e.g., in ceiling materials (8, 10, 15), in paper materials (19, 26, 29), in a gypsum board liner (2), in airborne dust (4, 6), and in airborne particles in a home where an infant developed pulmonary hemorrhage (35). However, many have preferred to use mass spectrometry (MS)-based methods, especially tandem MS (MSMS), because of the high analytical specificity offered. Thus, HPLC-MSMS was used to demonstrate SATs and STRG in mold-affected interior materials and carpet dust from buildings with a history of water damage (9, 33, 34). Gas chromatography (GC)-MS and GC-MSMS were used to detect verrucarol (VER) and trichodermol (TRID), hydrolysis products of, respectively, macrocyclic trichothecenes and trichodermin of S. chartarum, in mold-affected building materials (3, 14, 27) and settled house dust (3).
In the present study, we used GC-MSMS for determining the amounts of VER and TRID and HPLC-MSMS for determining the amounts of SATG, SATH, and STRG in samples from water-damaged indoor environments. The goal was to apply state-of-the-art MS technology to direct analysis of building materials, settled dust, and cultivated airborne fungal particles for some mycotoxins mainly produced by S. chartarum and A. versicolor. We demonstrate that, by applying these complementary MS methods, mycotoxins may be detected not only in building materials, but also in cultivable airborne fungal particles and settled house dust in damp buildings.
MATERIALS AND METHODS
Chemicals and standards.
The solvents and reagents were of analytical or HPLC grade and used without any further purification. The buffers were degassed and filtered through 0.45-μm filters (Millipore, Bedford, MA) before use. Water was distilled and deionized. Methanol, dichloromethane, and sodium hydroxide were purchased from Fischer Chemicals (Leicester, United Kingdom) and acetonitrile, toluene, and acetone from Lab Scan (Dublin, Ireland). N-Heptafluorobutyrylimidazole (HFBI), STRG, and VER were purchased from Sigma (Schnelldorf, Germany). 1,12-Dodecanediol, ammonium acetate, and sodium acetate were purchased from Fluka (Schnelldorf, Germany). Reserpine (5 ng/μl) was purchased from Varian, Inc. (Walnut Creek, CA). The trichodermin was a kind gift from Poul Rasmussen (Leo-Pharma, Denmark), and TRID was derived from trichodermin by hydrolysis. Crude SATG and SATH mycotoxin standards were kindly provided by Bruce B. Jarvis (Dept. of Chemistry and Biotechnology, University of Maryland).
Building material and dust samples.
Pieces (4 to 6 cm2) of paper (n = 39) collected from gypsum boards at 31 different locations were analyzed (Table 1). Stachybotrys was identified in all gypsum paper samples by conventional microscopic examination (31). Other materials with visible mold growth that were sampled were wood (n = 8), concrete (n = 3), paper (n = 6), masonite, linoleum, carpet, and tile (n = 7 altogether). These samples were found to be positive for Stachybotrys spp. and/or Aspergillus spp. by using a combination of microscopy and culture (on malt extract agar) identification.
TABLE 1.
Mycotoxins detected in the building material and dust samples studieda
| Mycotoxin(s) | No. of samples of the indicated building material in which mycotoxin(s) was detected
|
No. of samples of dust in which mycotoxin(s) was detected
|
|||||
|---|---|---|---|---|---|---|---|
| Gypsum paper (n = 39) | Wood based (n = 8) | Concrete/stone (n = 3) | Paper (n = 6) | Otherb (n = 7) | Settled dust (n = 8) | Airborne dust culture (n = 8) | |
| TRID | 1 | ND | 1 | 1 | ND | ND | 3 |
| VER | ND | 1 | ND | ND | 2 | 1 | ND |
| STRG | 2 | 4 | ND | 1 | ND | ND | ND |
| TRID, VER | 10 | ND | 1 | 2 | ND | 1 | 1 |
| TRID, STRG | 6 | ND | ND | ND | ND | 1 | ND |
| VER, STRG | ND | ND | ND | ND | ND | ND | 1 |
| TRID, VER, STRG | 6 | 1 | ND | 1 | ND | ND | ND |
| TRID, VER, SATG, SATH | 1 | ND | ND | ND | ND | ND | ND |
| TRID, VER, STRG, SATG | 1 | ND | ND | ND | ND | ND | ND |
| TRID, VER, STRG, SATG, SATH | 3 | ND | ND | ND | ND | ND | ND |
ND, not detected; n, number of samples analyzed.
Includes five linoleum samples and two synthetic material samples.
Settled dust (n = 8) was sampled in four homes with histories of water damage (Table 2). In one home (object 1), the damage was caused by flooding in the apartment above and further worsened by leaky waste pipes. Growths of S. chartarum (approximately 2 to 3 m2) were found on indoor wall surfaces. One dust sample from the top of a doorframe (460 mg) and another from the floor (560 mg) were collected by using a vacuum cleaner (3). In another house (object 2), moisture load from an outer wall caused water damage inside the construction. One dust sample each from the floor (1,020 mg), from surfaces above floor level (420 mg), and from the inlet (50 mg) and outlet (390 mg) of an air ventilation duct where dust had previously been found to be culture-positive for S. chartarum was collected by using a vacuum cleaner (3). Dust samples were collected on cotton swabs from two additional dwellings; one sample was collected from the outlet of an air ventilation duct in a school (object 3) where Stachybotrys spp. were found both in air samples and inside the wall construction, and the other sample from the top of a bedroom skirting board in a private home (object 4). The latter swab contained large amounts of dark-pigmented fragments of hyphae and spores, mainly of Chaetomium spp. and Stachybotrys spp. (unpublished results).
TABLE 2.
Mycotoxin contents in the settled dust samples studied
| Building | Sampling date | Sampling location | Mycotoxin content (pg/mg sample)a
|
||
|---|---|---|---|---|---|
| VER | TRID | STRG | |||
| Object 1 | 4 Oct. | Top of a doorframe | ND | 3.4 | 17 |
| Object 1 | 4 Oct. | Floor | ND | ND | ND |
| Object 2 | 5 Oct. | Floor | ND | ND | ND |
| Object 2 | 5 Oct. | Surfaces above floor level | ND | ND | ND |
| Object 2 | 5 Oct. | Air duct leading from inside to outside of house | ND | ND | ND |
| Object 2 | 5 Oct. | Air duct leading to inside from outside of house | ND | ND | ND |
| Object 3 | 4 Jan. | Outlet of an air ventilation duct | 43 | ND | ND |
| Object 4 | 4 Jan. | Top of a bedroom skirting board | 19 | 2.4 | ND |
ND, not detected.
Cultures (n = 8) of airborne cultivable fungal particles collected by using a Reuter centrifugal sampler (RCS; Folex-Biotest-Schleussner Inc., Farfield, NJ) during a 4-min sampling period (40 liters/min) were analyzed. The rose bengal agar strips (agar strip HS; Biotest-Serum Institute GmbH, Frankfurt/Main, Germany) were cultivated at 25°C for approximately 12 days before microscopic examination and kept in plastic bags at 4°C before sample preparation and chemical analysis. The sampling sites included two private homes (apartments), a shop, an office, a room in a municipal hall, a kindergarten, a school, and an indoor ice rink. The numbers of cultivable airborne fungal particles in these locations ranged from 31 to 281 CFU/m3 air (Table 3).
TABLE 3.
Mycotoxin contents in cultures of the airborne dust samples studied
| Building | Sampling date | CFU/m3 | Mycoflora in culturea | Mycotoxin in culture (pg/cm2 agar)b
|
||
|---|---|---|---|---|---|---|
| VER | TRID | STRG | ||||
| Municipal hall | 4 Jan. | 106 | 88% Stachybotrys spp., 12% Mycelia sterila | ND | 330 | ND |
| Private home 1 | 4 Jan. | 281 | 36% Penicillium spp., 24% Mycelia sterila, 20% Chaetomium spp., 18% Stachybotrys spp., 2% Cladosporium spp. | ND | ND | ND |
| Kindergarten | 3 Jan. | 44 | 43% Stachybotrys spp., 29% Mycelia sterila, 14% Aspergillus spp., 14% Penicillium spp. | ND | 1,500 | ND |
| Private home 2 | 14 Feb. | 44 | 44% yeasts, 14% Stachybotrys spp., 14% Cladosporium spp., 14% Penicillium spp., 14% Mycelia sterila | 2,900 | 790 | ND |
| School | 13 Feb. | 19 | 34% Stachybotrys spp. 33% Geomyces spp., 33% Mycelia sterila | ND | 1,900 | ND |
| Office | 8 Mar. | 38 | 83% Mycelia sterila, 7% Stachybotrys spp. | ND | ND | ND |
| Shop | 2 Mar. | 31 | 40% Penicillium spp., 20% Aspergillus spp., 20% Cladosporium spp., 20% Mycelia sterila | ND | ND | ND |
| Indoor ice rink | 18 Jan. | 100 | 38% Mycelia sterila, 19% Cladosporium spp., 19% Penicillium spp., 12% Aspergillus spp., 12% Stachybotrys spp. | 250 | ND | 130 |
Mycelia sterila is a nonsporulating mycelium.
ND, not detected.
Sample preparation, extraction, and purification.
Pieces of agar cultures (approximately 5 cm2), dust samples (∼0.4 g), and building material samples (0.3 to 3 g) were prepared for chemical analysis as described elsewhere (3). In brief, the samples were covered with methanol (3 to 5 ml) in 10-ml glass test tubes with Teflon-lined screw caps and stored in the dark for 72 h at room temperature. After the extraction, the samples were centrifuged (3,200 rpm, 5 min) and the supernatants were decanted into new tubes. One-hundred-microliter amounts of sterile water were added, and the mixtures were extracted twice with 2 ml heptane. The methanolic phases were evaporated under a gentle stream of nitrogen, dissolved in dichloromethane, and applied to polyethyleneimine (1 ml)-bonded silica gel columns (JT Baker, Phillipsburg, NJ) that had been preconditioned with 4 ml each of methanol and dichloromethane. The samples were eluted with 5 ml dichloromethane, evaporated under nitrogen, redissolved in 1 ml methanol, filtered through 0.45-μm Millex syringe filters (polytetrafluoroethylene; Millipore, Bedford, MA) into new Teflon-capped analysis vials, and kept at −20°C until HPLC analysis or further preparation.
HPLC-MS.
A ProStar HPLC/1200L triple-quadrupole MSMS system (Varian Inc., Walnut Creek, CA) was used. Twenty microliters of each sample was injected, using an autosampler (model 410; Varian), into a Polaris 5-μM C18-A 150- by 2.0-mm RP-18 column equipped with a MetaGuard 2.0-mm Polaris 5-μM C18-A precolumn (Varian). Reserpine was used as the internal standard. The column was maintained at 25°C, and the flow rate was 0.2 ml/min. A supplement of 10 mM ammonium acetate and 20 μM sodium acetate was added to the methanol-aqueous buffer to increase the cationization in the electrospray ionization mode. An initial methanol concentration of 20% methanol was held for 1 min, after which it was raised linearly (9 min) to 70% and held for 8 min before it was again raised linearly (1 min) to 95% and held for 5 min. At the end of the run, the concentration of methanol was linearly lowered again (1 min) to 20% and kept there for 12 min for stabilization. Ten microliters of methanol was injected in between samples to minimize cross-contamination. Nitrogen from a nitrogen generator (Domnick Hunter, Ltd., Tyne and Wear, United Kingdom) was used as both the nebulizing gas (50 lb/in2) and the drying gas (20 lb/in2), and argonium (1.75 mTorr) was used for collision-induced dissociation. The capillary temperature was 310°C, the capillary voltage 40 V, the needle voltage 5,000 V, and the electron multiplier voltage 2,000 V. The MS spectra were collected as centroid data from m/z 100 to 800, with a scan time of 0.5 s and a scan width of 0.7 s.
The MS was tuned through direct injection of polypropylene glycol tuning solution with a syringe, according to the manufacturer's protocol. Standards and reserpine were included in each batch of samples analyzed in order to assure instrument performance. Two calibration curves were constructed by injecting STRG (n = 3) (0, 25, 50, 100, 250, 500, and 1,000 pg and 0.5, 1, 2.5, 5, 10, and 25 ng) together with reserpine (1 and 10 ng). The coefficient of variation was calculated by dividing the standard deviation by the mean peak area ratio of the STRG standard (1-ng injections) to the internal standard (n = 9), and the recovery value was calculated by dividing the mean peak area from 1-ng injections of the STRG standards (n = 9) that had passed the sample preparation procedure by the corresponding STRG standards that did not pass this procedure.
GC-MS.
The sample preparation was performed essentially as described previously (3). In brief, 200 μl of the methanolic sample extracts were mixed with 500 pg of internal standard (1,12-dodecanediol), evaporated, hydrolyzed in 0.2 M methanolic NaOH, and extracted with water and dichloromethane. The organic phases were transferred to new tubes, evaporated to dryness, and placed in a desiccator overnight. The dried extracts were then subjected to derivatization by adding 80 μl of acetonitrile-toluene (1:6, vol/vol) and 20 μl of HFBI followed by heating at 70°C for 60 min. Then, samples were left standing in an excess of derivatizing agent at room temperature for a minimum of 4 h before analysis. The derivatives were analyzed by using MSMS in negative-ion chemical ionization mode, at an energy of 70 eV and an ion source temperature of 150°C, and with ammonia as the ionization gas (0.4 kPa). Sample volumes of 1 to 2 μl were injected in the splitless mode. The injector syringe was washed five times with acetone and toluene, before and after, respectively, each sample injection. A mix of HFBI and acetone (1:3, vol/vol) was injected in between samples to eliminate any trace of un- or semiderivatized VER/TRID. The performance of the instrument was assured by including TRID/VER standards and 1,12-dodecanediol (internal standard) in each batch of samples analyzed. Two calibration curves were constructed by injecting VER/TRID (n = 3) (0, 25, 50, 100, 250, 500, and 1,000 pg and 0.5, 1, 2.5, 5, 10, and 25 ng) together with the internal standard (250 pg and 2.5 ng). The coefficient of variation was calculated by dividing the standard deviation by the mean peak area ratio of the VER/TRID standard to the internal standard (n = 9), and the recovery value was calculated by dividing the mean peak area from 1-ng injections of VER/TRID standards (n = 9) that had passed the sample preparation procedure by corresponding VER/TRID standards that did not pass this procedure.
RESULTS
HPLC-MS standards.
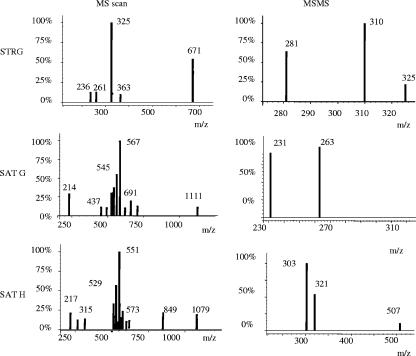
The electrospray ionization MS parameters, optimized to achieve maximal detection sensitivity for STRG, SATG, and SATH, have been summarized in Table 4. The mass spectra of the standards are shown in Fig. 1. The spectrum of STRG showed prominent ions of m/z 671 [2M + Na]+, m/z 363 [M + K]+, and m/z 325 [M + H]+. Ion m/z 325 was chosen for fragmentation in MSMS, and the product ions of m/z 310 and m/z 281 were monitored; the detection limit was 0.2 pg (injected amount monitoring m/z 310, signal-to-noise ratio [peak-to-peak value] ≥4). The peak area ratio of the STRG standard/internal standard (reserpine) versus the amounts of STRG standard followed the equations y = 0.0675x + 0.714 (R2 = 0.992) for the 0- to 1,000-pg amounts injected and y = 0.0035x + 1.742 (R2 = 0.992) for the 0.5 to 25 ng injected; the recovery value was 53% ± 6%, and the coefficient of variation was 11.2%. The SATG mass spectrum showed dominant ions of m/z 1,111 [2M + Na]+, 567 [M + Na]+, and 545 [M + H]+. Ion m/z 567 was chosen for fragmentation in MSMS, and its product ions m/z 263 and m/z 231 were monitored. The dominant ions in the SATH mass spectrum were m/z = 1,079 [2M + Na]+, 551 [M + Na]+, and 529 [M + H]+. Ion m/z 551 was used for fragmentation in MSMS, and its product ions of m/z 321 and 303 were monitored. SATH and SATG could not be quantified, since the purity of these crude mycotoxin preparations was unknown. For reserpine, m/z 609 [M]+ was used as the parent ion in MSMS and its product ion m/z 195 was monitored.
TABLE 4.
Optimized electrospray ionization MS parameters for the studied mycotoxins and the internal standard
| Mycotoxin | Parent ion (m/z) | Product ion(s) (m/z)a | Collision-induced dissociation value (V) |
|---|---|---|---|
| SATG | 567 [M + Na]+ | 263; 231 | −31 |
| SATH | 551 [M + Na]+ | 321; 303 | −31 |
| STRG | 325 [M]+ | 310; 281 | −25 |
| Reserpine | 609 [M + Na]+ | 195 | −45 |
The values in boldface represent the main product ions.
FIG. 1.
Positive electrospray MS (left) and MSMS (right) spectra of the STRG, SATG, and SATH standards.
GC-MS standards.
The MS characteristics of VER-diheptafluorobutyryl (HFB2) and TRID-heptafluorobutyryl (HFB), including the ions used for monitoring in MSMS and detection sensitivity, have been described previously (3). The peak area ratios of the mycotoxin standards/internal standard versus the amounts of the mycotoxin standards in the samples followed the equations y = 0.0016x + 0.069 (R2 = 0.991) for the 0- to 1,000-pg amounts of VER injected and y = 0.0002x + 0.032 (R2 = 0.995) for the 0.5- to 25-ng amounts injected. Accordingly, the equations for TRID were y = 0.0021x + 0.035 (R2 = 0.990) and y = 0.0002x + 0.116 (R2 = 0.999), respectively. The recovery values were 13% ± 1% for VER and 29% ± 4% for TRID, and the coefficients of variation were 5.3% and 3.2% for VER and TRID, respectively.
Building material and dust samples.
The mycotoxin analysis results (with amounts adjusted according to recovery values) are summarized in Tables 1 to 3. In the building material samples, the amounts of STRG were 1.9 to 1,100 pg/mg (mean, 110; median, 14); of TRID, 3.4 to 18,000 pg/mg (mean, 660; median, 5.9); and of VER, 7.7 to 600 pg/mg (mean, 16; median, 25). The amounts of STRG (17 pg/mg; 130 pg/cm2), TRID (2.4 to 3.4 pg/mg; 330 to 1,900 pg/cm2), and VER (19 to 43 pg/mg; 250 to 2,900 pg/cm2) in settled dust samples and cultured agar strips, respectively, from RCS samplings are given in Tables 2 and 3.
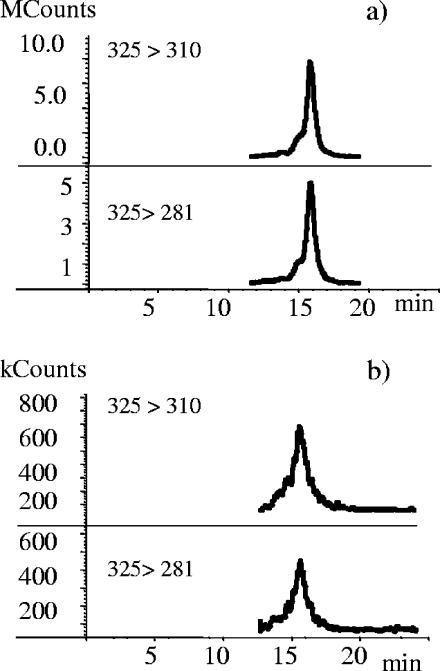
STRG was detected in 25 of the 62 building material samples studied. It was usually found together with two or more other mycotoxins; in fact, it was the sole mycotoxin found in only seven samples. In particular, STRG was frequently found together with TRID and never with VER, SATG, or SATH in the absence of TRID. A representative chromatogram demonstrating STRG in a paper sample is shown in Fig. 2a. One settled dust sample collected from the top of a doorframe (Fig. 2b) and one dust sample collected with an RCS were positive for STRG.
FIG. 2.
HPLC-positive electrospray MSMS chromatograms demonstrating the presence of STRG (m/z 325 > m/z 310 and m/z 325 > m/z 281) in a paper sample culture positive (>30,000 CFU/m2) for Aspergillus spp., including A. versicolor (a), and in a settled dust sample from the top of a doorframe (b).
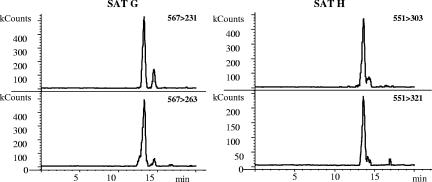
SATG was found in five and SATH in four building material samples; all were gypsum board papers that were also positive for VER and TRID. Representative chromatograms are shown in Fig. 3. SATG and SATH were not identified in any of the dust samples.
FIG. 3.
HPLC-positive electrospray MSMS chromatograms demonstrating the presence of SATG (m/z 567 > m/z 231 and m/z 567 > m/z 263) and SATH (m/z 551 > m/z 303 and m/z 551 > m/z 321) in a gypsum board paper sample.
VER was the sole mycotoxin in only 3 out of 29 building material samples. It was usually found together with TRID or a combination of TRID and STRG, and always where SATG and SATH were found. VER was identified in two dust samples, once as the sole mycotoxin and once together with TRID (Table 1). VER was also found in two cultured agar strips from the RCS samplings, once together with TRID and once with STRG.
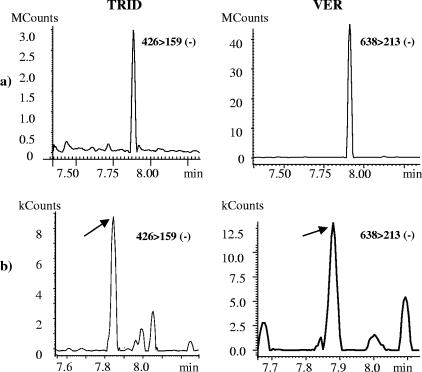
TRID also was rarely the sole mycotoxin found in the building material samples. In total, TRID was found in 35 of the 62 building materials studied, in two of the eight settled dust samples (one of the latter samples, collected from the top of a doorframe, has been described previously [3]), and in four of the eight cultured dust samples. Representative chromatograms demonstrating the presence of VER and TRID in building material samples and settled and cultured dust samples are shown in Fig. 4.
FIG. 4.
GC-MSMS (negative-ion chemical ionization) chromatograms of TRID-HFB and VER-HFB2 (indicated by arrows in panel b) in a gypsum board paper sample (a), a settled dust sample collected on a cotton swab (b, left), and a cultured RCS-obtained air sample (b, right).
DISCUSSION
S. chartarum and A. versicolor are water-associated indoor molds in Scandinavia and many other parts of the world (12, 15). Since both of these species are potent mycotoxin producers, they were the foci of the present study. The general criterion for including a building material in this investigation was visible mold growth; the gypsum board-derived paper samples were contaminated with Stachybotrys spp., while the other samples, in general, contained a diverse mycoflora where A. versicolor (besides S. chartarum) was a dominating species. The dust samples included were all collected from indoor environments with severe moisture damage.
Our results demonstrate that molds in these sampled indoor environments regularly produce mycotoxins, since 45 of the total of 62 building material samples (73%) were positive for at least one of the studied mycotoxins. By comparison, Toumi et al. (34) found STRG in 19 of 79 (24%) crude building material samples, plus VER, SATG, and SATH in 5 samples, by using HPLC-ion trap MSMS analysis; the recovery of STRG was in accordance with our results. One reason for the high prevalence of mycotoxins found in our study may be the high detection sensitivity offered by triple-quadrupole mass spectrometers in MSMS mode. This type of instrument offers ready detection of subpicogram amounts of STRG, VER, and TRID (3). While the analysis of underivatized STRG by using HPLC-MSMS is straightforward, we initially experienced several problems with the GC-MSMS analyses. These problems (carryover and ghost peak formation), particularly noticeable for VER, were occurring despite frequent syringe washings, changes of solvents and columns, and cleaning of the MS, and were found to depend largely upon adsorption of non- or semiderivatized VER in the GC injector. The problems were overcome by regular injections of an HFBI-acetone (1:3, vol/vol) mixture, by avoiding washing the preparations with water after HFBI derivatization (to prevent degradation of the derivative), and by injecting a maximum 1-μl sample in order to minimize the risk of injector contamination (unpublished results). The GC-MS hydrolysis product method (where VER and TRID are detected) has previously been successfully applied to screen authentic water-damaged building material samples for S. chartarum mycotoxins (3, 12, 27).
In most, but not all, cases, the natural producer of a certain mycotoxin was identified in the sample by cultivation and/or microscopy; this was also found in other studies (34). However, Stachybotrys was not identified in a small number of building material samples that were positive for VER and/or TRID, viz., in 1 of 28 gypsum papers, in one of two wood-based materials, and in one linoleum sample. Likewise, Aspergillus spp. were not identified in all STRG-positive samples, viz., in 4 of 18 gypsum board papers, and in two of five wood-based materials (Table 1).
The CFU counts and mycotoxin contents from the RCS samplings did not correlate, probably because only a small fraction of the molds may have been cultivable. The composition of colonizers and secondary metabolite production may vary over time, even within a single isolate (16), probably due to fluctuations in water activity, nutrition, and coexisting microbial flora. The amounts of mycotoxins present may also have been below the detection limits in certain instances, due to low recovery amounts or instrument limitations.
The five gypsum paper materials that were positive for SATG and SATH were also positive for VER. In addition, VER was identified in an additional 24 building material samples plus two dust samples. It is likely that the detection sensitivity for VER is higher than for SATG or SATH. Also, although VER is thought to derive mainly from SATG and SATH (8, 19), VER is a hydrolysis product also of other macrocyclic trichothecenes and could therefore theoretically represent other SATs or verrucarins, etc.
The STRG/TRID mycotoxin combination was found in six of the building material samples; notably, STRG was never found together with VER only. It can be speculated whether A. versicolor has a capability of, or benefits from, growing together with or succeeding S. chartarum strains of chemotype A, rather than of chemotype S, due to the strongly cytotoxic mycotoxins produced by the latter. As recent reports have shown synergistic effects in cytotoxicity and apoptosis mechanisms in mouse macrophages challenged by spore extracts from cocultures of A. versicolor and S. chartarum (25), it is also interesting to speculate whether the two chemotypes play different roles in these mechanisms.
In this study, HPLC-MSMS and GC-MSMS have proven to be complementary analytical tools for detecting some of the most potent mycotoxins produced by molds frequently encountered in damp indoor environments. These methods are so sensitive that STRG, VER, and TRID can be detected not only in mold-affected building materials, but also in house dust. In fact, to the best of our knowledge, this is the first report on the use of MSMS for demonstrating mycotoxins in dust settled on surfaces above floor level in damp buildings. The methods used are important tools for further research aiming to shed some light on the role of molds in building-associated illnesses. In the future, we plan to expand our battery of mycotoxin analytes and to evaluate the health relevance of mycotoxins in indoor environments. Such work is in progress in our laboratory.
Acknowledgments
We thank Poul Rasmussen (Leo-Pharma, Denmark) for providing trichodermin and Bruce B. Jarvis (Department of Chemistry and Biochemistry, University of Maryland) for mycotoxin standards. We thank the Centre of Complex Environmental Monitoring and Environmental Risk Assessment (CEMERA; University of Warsaw) for collaboration.
The Development Fund of the Swedish Construction Industry (SBUF), the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (FORMAS), the Swedish Asthma and Allergy Association's Research Foundation, and Queen Silvia's Jubilee Fund for Research on Children and Children's Disabilities are gratefully acknowledged for financial support.
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.American Academy of Pediatrics Committee on Environmental Health. 1998. Toxic effects of indoor molds. Pediatrics 101:712-714. [PubMed] [Google Scholar]
- 2.Andersson, M. A., M. Nikulin, U. Köljalg, M. C. Andersson, F. Rainey, K. Reijula, E.-L. Hintikka, and M. S. Salkinoja-Salonen. 1997. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloom, E., K. Bal, E. Nyman, and L. Larsson. 2007. Optimizing a GC-MS method for screening of Stachybotrys mycotoxins in indoor environments. J. Environ. Monit. 9:151-156. [DOI] [PubMed] [Google Scholar]
- 4.Brasel, T. L., J. M. Martin, C. G. Carriker, S. C. Wilson, and D. C. Straus. 2005. Detection of airborne Stachybotrys chartarum macrocyclic trichothecene mycotoxins in the indoor environment. Appl. Environ. Microbiol. 71:7376-7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, R. K., J. M. Portnoy, A. Saxon, A. I. Terr, and R. A. Wood. 2006. The medical effects of mold exposure. J. Allergy Clin. Immunol. 117:326-333. [DOI] [PubMed] [Google Scholar]
- 6.Charpin-Kadouch, C., G. Maurel, R. Felipo, J. Queralt, M. Ramadour, H. Dumon, M. Garans, A. Botta, and D. Charpin. 2006. Mycotoxin identification in moldy dwellings. J. Appl. Toxicol. 26:475-479. [DOI] [PubMed] [Google Scholar]
- 7.Cho, S.-H., S.-C. Seo, D. Schmechel, S. A. Grinshpun, and T. Reponen. 2005. Aerodynamic characteristics and respiratory deposition of fungal fragments. Atmos. Environ. 39:5454-5465. [Google Scholar]
- 8.Croft, W. A., B. B. Jarvis, and C. S. Yatawara. 1986. Airborne outbreak of trichothecene toxicosis. Atmos. Environ. 20:49-552. [Google Scholar]
- 9.Engelhart, S., A. Loock, D. Skutlarek, H. Sagunski, A. Lommel, H. Farber, and M. Exner. 2002. Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Appl. Environ. Microbiol. 68:3886-3890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flappan, S. M., J. Portnoy, P. Jones, and C. Barnes. 1999. Infant pulmonary hemorrhage in suburban home with water damage and mold (Stachybotrys atra). Environ. Health Perspect. 107:927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Górny, R. L., T. Reponen, K. Willeke, D. Schmechel, E. Robine, M. Boissier, and S. A. Grinshpun. 2002. Fungal fragments as indoor air biocontaminants. Appl. Environ. Microbiol. 68:3522-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravesen, S., P. A. Nielsen, R. Iversen, and K. F. Nielsen. 1999. Microfungal contamination of damp buildings—examples of risk constructions and risk materials. Environ. Health Persp. 107(Suppl. 3):505-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green, B., E. Tovey, J. Sercombe, F. Blachere, D. Beezhold, and D. Schmechel. 2006. Airborne fungal fragments and allergenicity. Med. Microbiol. 44:S245-S255. doi: 10.1080/13693780600776308. [DOI] [PubMed] [Google Scholar]
- 14.Hinkley, S. F., and B. B. Jarvis. 2001. Chromatographic method for Stachybotrys toxins. Methods Mol. Biol. 157:173-194. [PubMed] [Google Scholar]
- 15.Hodgson, M. J., P. Morey, L. Wing-Yan, L. Morrow, D. Miller, B. B. Jarvis, H. Robbins, J. F. Halsey, and E. Storey. 1998. Building-associated pulmonary disease from exposure to Stachybotrys chartarum and Aspergillus versicolor. JOEM 40:241-248. [DOI] [PubMed] [Google Scholar]
- 16.Jarvis, B. B., J. Salemme, and A. Morais. 1995. Stachybotrys toxins. Nat. Toxins 3:10-16. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis, B. B., and J. D. Miller. 2005. Mycotoxins as harmful indoor air contaminants. Appl. Microbiol. Biotechnol. 66:367-372. [DOI] [PubMed] [Google Scholar]
- 18.Johannessen, L. N., A. M. Nilsen, and M. Løvik. 2007. Mycotoxin-induced depletion of intracellular glutathione and altered cytokine production in the human alveolar epithelial cell line A549. Toxicol. Lett. 168:103-112. doi: 10.1016/j.toxlet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Johanning, E., R. Biagini, H. DeLon, P. Morey, B. B. Jarvis, and P. Landsbergis. 1996. Health and immunology study following exposure to toxigenic fungi (Stachybotrys chartarum) in water-damaged office environment. Int. Arch. Occup. Environ. Health 68:207-218. [DOI] [PubMed] [Google Scholar]
- 20.Kelman, B. J., C. A. Robbins, L. J. Swenson, and B. D. Hardin. 2004. Risk from inhaled mycotoxins in indoor office and residential environments. Int. J. Toxicol. 23:3-10. [DOI] [PubMed] [Google Scholar]
- 21.Kildesø, J., H. Wurtz, K. F. Nielsen, P. Kruse, K. Wilkins, U. Thrane, S. Gravesen, P. A. Nielsen, and T. Schneider. 2003. Determination of fungal spore release from wet building materials. Indoor Air 13:148-155. [DOI] [PubMed] [Google Scholar]
- 22.Mazur, L. J., J. Kim, and the Committee on Environmental Health. 2006. Spectrum of noninfectious health effects from molds. Pediatrics 118:1909-1926. doi: 10.1542/peds.2006-2829. [DOI] [PubMed] [Google Scholar]
- 23.Miller, J. D. 1992. Fungi as contaminants in indoor air. Atmos. Environ. 26A:2163-2172. [Google Scholar]
- 24.Müller, A., I. Lehmann, A. Seiffart, U. Diez, H. Wetzig, M. Borte, and O. Herbarth. 2002. Increased incidence of allergic sensitisation and respiratory diseases due to mold exposure: results of the Leipzig Allergy Risk children Study (LARS). Int. J. Environ. Health 204:363-365. [DOI] [PubMed] [Google Scholar]
- 25.Murtoniemi, T., P. Penttinen, A. Nevalainen, and M.-R. Hirvonen. 2005. Effects of microbial cocultivation on inflammatory and cytotoxic potential of spores. Inhal. Toxicol. 17:681-693. [DOI] [PubMed] [Google Scholar]
- 26.Nevalainen, A., and M. Seuri. 2005. Of microbes and men. Indoor Air 15(Suppl. 9):58-64. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen, K. F., M. O. Hansen, T. O. Larsen, and U. Thrane. 1998. Production of trichothecene mycotoxins on water damaged gypsum boards in Danish buildings. Int. Biodeterior. Biodegrad. 42:1-7. [Google Scholar]
- 28.Nielsen, K. F., U. Thrane, T. O. Larsen, P. A. Nielsen, and S. Gravesen. 1998. Production of mycotoxins on artificially inoculated building materials. Int. Biodeterior. Biodegrad. 42:9-16. [Google Scholar]
- 29.Nielsen, K. F., S. Gravesen, P. A. Nielsen, B. Andersen, U. Thrane, and J. C. Frisvad. 1999. Production of mycotoxins on artificially and naturally infested building materials. Mycopathologia 145:43-56. [DOI] [PubMed] [Google Scholar]
- 30.Salo, P. M., S. J. Arbes, Jr., M. Sever, R. Jaramillo, R. D. Cohn, S. J. London, and D. C. Zeldin. 2006. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J. Allergy Clin. Immunol. 118:892-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samson, R. A., E. S. Hoekstra, J. C. Frisvad, and O. Filtenborg (ed.). 1995. Introduction to food-borne fungi. Centraalbureau voor Schimmelcultures, Baarns, The Netherlands.
- 32.Sorenson, W. G., D. G. Frazer, B. B. Jarvis, J. Simpson, and V. A. Robinson. 1987. Trichothecene mycotoxins in aerosolized conidia of Stachybotrys atra. Appl. Environ. Microbiol. 53:1370-1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuomi, T., L. Saarinen, and K. Reijula. 1998. Detection of polar and macrocyclic trichothecene mycotoxins from indoor environments. Analyst 123:1835-1841. [DOI] [PubMed] [Google Scholar]
- 34.Tuomi, T., K. Reijula, T. Johnsson, K. Hemminki, E.-L. Hintikka, O. Lindroos, S. Kalso, P. Koukila-Kähkölä, H. Mussalo-Rauhamaa, and T. Haahtela. 2000. Mycotoxins in crude building materials from water- damaged buildings. Appl. Environ. Microbiol. 66:1899-1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vesper, S., D. G. Dearborn, I. Yike, T. Allan, J. Sobolewski, S. F. Hinkley, B. B. Jarvis, and R. A. Haugland. 2000. Evaluation of Stachybotrys chartarum in the house of an infant with pulmonary hemorrhage: quantitative assessment before, during, and after remediation. J. Urban Health 77:68-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wichmann, G., O. Herbarth, and I. Lehmann. 2002. The mycotoxins citrinin, gliotoxin, and patulin affect interferon-gamma rather than interleukin-4 production in human blood cells. Environ. Toxicol. 17:211-218. [DOI] [PubMed] [Google Scholar]